Abstract
Organic manure and grass mulching are widely recognized as modifiers of soil microbial communities and nutrient dynamics; however, the combined effects of these practices on nitrogen fractionation and microbial functionality in orchard ecosystems remain poorly understood. This study conducted a comprehensive evaluation of soil nitrogen fractions, enzymatic activity, microbial diversity and functional traits in walnut orchards under three management practices: organic manure (OM), grass mulching combined with manure (GM), and chemical fertilization (CF) in China’s Loess Plateau. The results revealed that OM and GM significantly enhanced soil nutrient pools, with GM elevating total nitrogen by 1.96-fold, soil organic carbon by 97.79%, ammonium nitrogen by 128%, and nitrate nitrogen by 54.56% relative to CF. Furthermore, the OM significantly increased the contents of total hydrolysable nitrogen, amino sugar nitrogen, amino acid nitrogen, ammonia nitrogen, hydrolysable unidentified nitrogen, non-acid-hydrolyzable nitrogen compared to the CF and GM treatments. Meanwhile, ASN and AN had significant effects on mineral and total nitrogen. The OM and GM had higher activities of leucine aminopeptidase enzymes (LAP), α-glucosidase enzyme, β-glucosidase enzyme (βG), and N-acetyl-β-D-glucosidase enzyme (NAG). Microbial community analysis revealed distinct responses to different treatments: OM and GM enhanced bacterial Shannon index, while suppressing fungal diversity, promoting the relative abundance of copiotrophic bacterial phyla such as Proteobacteria and Chloroflexi. Moreover, GM favored the enrichment of lignocellulose-degrading Ascomycota fungi. Functional annotation indicated that Chemoheterotrophy (43.54%) and Aerobic chemoheterotrophy (42.09%) were the dominant bacterial metabolic pathways. The OM significantly enhanced the abundance of fermentation-related genes. Additionally, fungal communities under the OM and GM showed an increased relative abundance of saprotrophic taxa, and a decrease in the relative abundances of potential animal and plant pathogenic taxa. The Random forest model further confirmed that βG, LAP, and NAG, as well as Basidiomycota, Mortierellomycota, and Ascomycota served as pivotal mediators of soil organic nitrogen fraction. Our findings demonstrated that combined organic amendments and grass mulching can enhance soil N retention capacity, microbial functional redundancy, and ecosystem stability in semi-arid orchards. These insights support the implementation of integrated organic management as a sustainable approach to enhance nutrient cycling and minimize environmental trade-offs in perennial fruit production systems.
1. Introduction
The Loess Plateau region has emerged as a significant cultivation area for fruit trees, including pears and apples, in China, attributed to its distinctive geographical and climatic conditions. Nevertheless, the soil in this region is characterized by a loose texture, poor structure, susceptibility to erosion and degradation, and low levels of nutrients and organic matter, which present substantial challenges to the sustainable development of the fruit industry [1]. In recent years, efforts to achieve high orchard yields have led to the prolonged and excessive use of chemical fertilizers, resulting in a reduction in soil organic matter, soil compaction, diminished microbial community diversity and activity, and decreased fertility levels [2,3,4]. This has further exacerbated soil quality degradation and impeded the sustainable advancement of the local fruit industry. Consequently, the development of strategies for appropriate fertilization and enhancement of soil fertility in orchards is a critical issue requiring immediate attention.
The application of organic fertilizer can effectively increase soil organic matter content, improve soil structure, significantly enhance the abundance and diversity of soil microorganisms, optimize microbial community composition, strengthen soil nutrient supply capacity, and thereby comprehensively enhance soil productivity [5,6]. However, the environmental consequences of organic amendments require thorough evaluation and careful consideration. Toselli et al. [7] reported that while manure application can increase soil nitrogen reserves by 22–35%, it may paradoxically raise the risk of nitrogen leaching by up to 40% compared to conventional systems when mineralization rates exceed plant uptake capacity. Nitrogen dynamics in agricultural systems present significant challenges, as dissolved N constitutes over 60% of total nitrogen losses through hydrological pathways [8]. This dichotomy underscores the pressing need for advanced nutrient management strategies to reconcile agronomic productivity with environmental sustainability in orchard ecosystems. Grass mulching has emerged as an effective strategy for mitigating soil erosion, achieving reductions of 45–60%, and enhancing soil multifunctionality indices by 56% compared to conventional tillage systems [9,10]. Notably, grass mulching has demonstrated superior efficacy in improving soil physicochemical properties relative to natural vegetation, particularly in terms of enhancing aggregate stability and water infiltration rates [10]. It is important to note that the present study primarily aims to evaluate the interactive effects between organic fertilization and grass mulching practices, rather than comparing their absolute benefits against an unfertilized control. Therefore, a no-fertilization control treatment was not included in the experimental design. This approach allows for a more focused investigation into the synergies or trade-offs between these two management strategies, which is particularly relevant for orchard systems transitioning from conventional to organic or integrated management.
Soil organic nitrogen (SON) accounted for over 90% of the total nitrogen content in soil, consisting of chemically distinct fractions that possess varying potentials for mineralization [11,12]. The composition of SON was significantly altered by organic amendments [13]. For instance, the application of manure has been shown to increase the pools of amino acid nitrogen (AAN) and hydrolyzable ammonium nitrogen (HAN) by 30–50%, both of which were closely linked to nitrogen mineralization fluxes [13]. In contrast, amino sugar nitrogen (ASN) contributed minimally to mineralization, despite its association with potentially mineralizable nitrogen [14]. Recent studies have identified hydrolyzable unidentified nitrogen (UHN) as a significant yet understudied factor in the mineralization dynamics of SON, accounting for 25–40% of the mineralized nitrogen in agricultural soils [15]. These fraction-specific behaviors underscored the necessity for a mechanistic understanding of SON transformations under various management practices [16,17]. The transformation dynamics of active SON into plant-available forms were critical for optimizing nitrogen use efficiency while minimizing environmental losses. However, the current understanding of these processes remains incomplete, especially concerning the interaction between SON fractionation and microbial mediation in orchard agroecosystems.
Microorganisms play crucial roles in regulating nitrogen transformations [18]. The application of organic fertilizers has been shown to significantly modify microbial community structures, resulting in a 35–50% increase in microbial biomass carbon and a two- to threefold enhancement in extracellular enzyme activities related to carbon and nitrogen cycling [19,20]. Recent metagenomic studies have revealed that manure application selectively enriches copiotrophic taxa, such as Bradyrhizobium spp., which exhibit an increase in abundance of 1.5–2 log units and possess enhanced nitrogen mineralization capabilities, while simultaneously suppressing oligotrophic groups [21,22]. Fungal communities demonstrated similar responses, with organic management practices leading to a 30–40% increase in the abundance of Fusarium spp., a genus known to participate in both nitrogen mineralization and denitrification pathways [23,24].
Despite these advances, significant knowledge gaps remain concerning: (1) the temporal dynamics of SON fractionation under combined organic amendment and grass mulching management practices, and (2) the specific microbial functional community driving the distribution of SON fraction in orchard ecosystems. To address these knowledge gaps, we conducted a seven-year field experiment in an orchard system, employing a factorial design of organic fertilization and grass mulching. The present study was structured to achieve three principal objectives: (1) to quantify the differential effects of organic management and grass mulching on SON fractionation and its associated mineralization potentials; (2) to characterize alterations in microbial community structure and functional profiles utilizing high-throughput sequencing in conjunction with enzymatic activity profiling; and (3) to identify key biological determinants influencing SON fractionation through multivariate statistical analyses. This research offers essential insights for optimizing organic orchard management by enhancing the mechanistic understanding of soil nitrogen cycling and microbial ecological processes.
2. Materials and Methods
2.1. Experimental Site and Design
The study was conducted in a Juglans regia L. (walnut) orchard at the Pomology Institute of Shanxi Agricultural University, Taigu County, Shanxi Province, China (112°32′ N, 37°23′ E; elevation 820–900 m). The region exhibits a temperate continental monsoon climate with a mean annual temperature of 10.6 °C and annual precipitation of 400–600 mm. The soil is classified as Calcic Haplustalfs, with pre-experiment physicochemical properties (0–20 cm depth) as follows: pH 7.8 (soil:water = 1:2.5), soil organic carbon (SOC) 8.08 g kg−1, total nitrogen (TN) 0.70 mg kg−1, available phosphorus (AP) 30.1 mg kg−1, and available potassium (AK) 130.5 mg kg−1. The orchard, established in 2011, had a planting density of 4 m between plants and 5 m between rows, equivalent to 495 trees per hectare. A randomized complete block design was implemented from spring 2016, consisting of three treatments with three replicates each, resulting in nine experimental plots (each plot measuring 48 m2; arranged as two rows with six trees per row).
Chemical Fertilizer (CF): Annual application of chemical fertilizer (N:P2O5:K2O = 18:18:18) at a rate of 330 kg ha−1 in November, combined with manual weed removal conducted in April, August, and October. Manure + Perennial Grass Mulch (GM): Annual application of sheep manure (N:P2O5:K2O = 15:11:9) at 6 × 104 kg ha−1 in November; inter-row planting of Orychophragmus violaceus (February orchid) at a sowing rate of 1.0 g m−2 with a row width of 3 m; and post-emergence pruning carried out in July, August, and September produced biomass (3.20 kg m−2), which was applied as surface mulch. Manure (OM): application of the same amount of manure as in the GM treatment, along with manual weed removal.
2.2. Sample Collection
In May 2023, composite soil samples (0–20 cm depth) were collected from five equidistant points within each plot using a five-point sampling method, avoiding rhizosphere proximity (>20 cm from tree trunks). Surface litter was removed prior to sampling. Samples were homogenized and sieved through a 2 mm mesh to remove roots and debris, then divided into three aliquots for different analyses: (1) microbial DNA analysis—stored at −80 °C; (2) physicochemical analysis—air-dried and stored at room temperature; and (3) enzyme activity and microbial metabolic profiling—stored at −20 °C.
2.3. Soil Physicochemical and Organic Nitrogen Fraction Analysis
Soil organic carbon (SOC) was determined using the potassium dichromate oxidation-ferrous sulfate titration method. The total nitrogen content (TN) was determined using the semi-micro Kjeldahl method. The available phosphorus content (AP) was measured by extracting the soil with 0.5 M NaHCO3 solution (pH 8.5) and analyzing it via the molybdenum-antimony colorimetric method. The available potassium content (AK) was measured by extracting the soil with 1 M NH4OAc solution and measuring it using Atomic flame absorption spectrometry. Soil pH was measured potentiometrically. Nitrate () and ammonium () concentrations were quantified using continuous flow analysis of SKALAR San++® (Skalar Analytical B.V., Breda, The Netherlands) (Method S1).
Microbial biomass carbon and nitrogen (MBC and MBN) were determined using the chloroform fumigation-K2SO4 extraction method. Following chloroform fumigation of the soil sample, extraction was carried out with 0.5 mol L−1 K2SO4 solution. The extracted solution was then analyzed for carbon and nitrogen content using a total carbon and nitrogen analyzer (2100S, multi N/C, Analytik Jena AG, Jena, Germany). The MBC and MBN were then determined by subtracting the carbon and nitrogen concentrations measured after fumigation from those obtained before fumigation.
Soil organic nitrogen (SON) fractions were characterized using sequential acid hydrolysis. Total hydrolysable nitrogen (THN)—Sum of all hydrolysable fractions; amino sugar nitrogen (ASN) —colorimetric determination with ninhydrin; amino acid nitrogen (AAN) —steam distillation after phosphate-borate buffer treatment; ammonia nitrogen (AN) —magnesium oxide steam distillation; hydrolysable unidentified nitrogen (HUN) —calculated as THN - (ASN + AAN + AN); non-acid-hydrolyzable nitrogen (NHN) was calculated as TN - THN (Method S2).
2.4. Soil Enzyme Activity and Microbial Community Analysis
Soil enzyme activity: α-glucosidase (αG), β-glucosidase (βG), leucine aminopeptidase (LAP), N-acetyl-β-D-glucosaminidase (NAG), polyphenol oxidase (PPO), and cellobiohydrolase (S-C1) were extracted using Solarbio activity assay kits (Beijing Solarbio Science & Technology Co., Ltd. Beijing, China). The enzymatic activities were subsequently measured using a multi-functional microplate reader (BioTek Synergy LX, Agilent, Santa Clara, CA, USA) (Method S3).
Microbial metabolic potential: fresh soil suspensions (0.85% NaCl) were inoculated into plates and incubated at 15 °C in darkness, which was assessed using Biolog EcoPlates™ (Biolog Inc., Hayward, CA, USA) containing 31 carbon substrates. Absorbance (595 nm and 750 nm) was measured daily for 7 days (SpectraMax i3x, Molecular Devices, San Jose, CA, USA). Average well color development (AWCD) was calculated to normalize substrate utilization [25] (Method S4).
Microbial Community Composition: Soil specimens were submitted to Shanghai Meiji Biotechnology Co., Ltd. (Shanghai, China), for the extraction of total bacterial and fungal DNA, followed by high-throughput sequencing. The DNA extraction was carried out using the FastDNA Spin Kit (MP Biomedicals, Irvine, CA, USA). Specific regions of bacterial 16S rRNA (V3–V4; primers 338F/806R) and fungal ITS (primers ITS1F/ITS2R) were amplified through polymerase chain reaction (PCR). Library preparation was conducted with the TruSeq™ DNA Kit (Illumina, San Diego, CA, USA), and sequencing was performed on the Illumina MiSeq system. The raw sequence data were analyzed using Vsearch v2.4.2 with a similarity cutoff of 97%, and taxonomic classification was achieved using the RDP classifier. Functional annotation of gene sequences was performed by aligning them to the KEGG database via BLAST+ (version 2.13.0).
2.5. Statistical Analysis
The normality of the data was evaluated using the Shapiro–Wilk test. Treatment effects on soil parameters were analyzed using a one-way ANOVA, followed by LSD post hoc tests (SPSS 26.0, p < 0.05). The redundancy analysis (RDA) was applied to assess the effects of soil organic nitrogen fraction on total nitrogen, , and (Canoco 5.0). The microbial community structure was visualized through non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarities. Mantel tests (vegan R package, version 4.2.4) was employed to determine the key drivers of microbial diversity and function. Random forest prediction model (rfPermute R package, version 4.2.4) was employed to assess the influence of microbial community composition and enzyme activity on the SON fraction. The importance of each predictor variable was quantified by the percentage increase in mean squared error (MSE), with higher MSE% values indicating a stronger influence on predictive performance. Figures were generated using Origin 2024 software.
3. Results
3.1. Soil Physicochemical, Microbial Biomass, Nitrogen Fractions
The soil physicochemical characteristics under different treatments were presented in Table 1. Overall, the OM exhibited significantly higher levels of soil organic carbon (SOC), total nitrogen (TN), ammonium nitrogen (), nitrate nitrogen (), available phosphorus (AP), available potassium (AK), microbial biomass carbon (MBC) and nitrogen (MBN) compared to the CF and GM. However, no significant differences in pH levels were detected among the different treatments.

Table 1.
Effect of manure and grass mulching on soil physicochemical properties and microbial biomass.
The composition of organic nitrogen was significantly influenced by the application of organic fertilizers and the implementation of grass mulching, as shown in Table 2. The THN, ASN, AAN, AN, HUN, and NHN contents in the OM were significantly higher than that in both the CF and GM treatments. The GM showed higher levels of THN, ASN, AAN, HUN, and NHN compared to the CF; however, these differences did not reach statistical significance.

Table 2.
Effect of manure and grass mulching on soil nitrogen fractions (mg kg−1).
The Redundancy analyses (RDA) was employed to examine the relationships between organic nitrogen (ASN, AN, HUN, AAN, and NHN) and both total nitrogen and mineral nitrogen (Figure 1). The first and second RDA axes explained 98.25% and 0.74% of the total variation in total nitrogen and mineral nitrogen, respectively. The OM treatment can be separated from the CF and GM based on total and mineral nitrogen contents. Moreover, the ASN and AN were determined to be the key factors affecting the variation in soil mineral nitrogen and total nitrogen, accounting for 80.2% (p < 0.01) and 12.6% (p < 0.05) of the explanatory power, respectively.
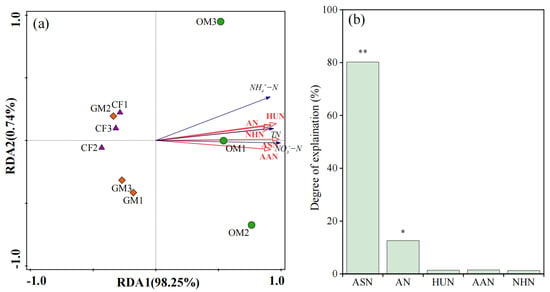
Figure 1.
Redundancy analyses (RDA) of organic nitrogen (ASN, AN, HUN, AAN, and NHN) with total nitrogen and mineral nitrogen (, and ). (a): redundancy analyses figure; (b) explanation degrees of organic nitrogen to total nitrogen and mineral nitrogen. ASN, amino sugar nitrogen; AAN, amino acid nitrogen; AN, ammonia nitrogen; HUN, hydrolysable unidentified nitrogen; NHN, non-acid-hydrolyzable nitrogen; TN, total nitrogen; CF, Chemical Fertilizer + Conventional Tillage; GM, Manure + Perennial Grass Mulch; OM, Manure + Conventional Tillage treatments. ** p < 0.01, * p < 0.05.
3.2. Soil Enzyme Activity
Different treatments had a significant effect on soil enzyme activities (Table 3). Specifically, the LAP activity under the OM was significantly higher than that under the CF and GM, showing increases of 47.1% and 25.0%, respectively. Similarly, βG activity in the OM was markedly elevated compared to the CF and GM, with increases of 96.2% and 54.1%, respectively. In contrast, the S-C1 activity in the GM was significantly higher compared to both the CF and OM, showing increases of 15.1% and 15.6%, respectively. Regarding the NAG, there was no notable variation in activity between the GM and OM; however, a significantly higher level was detected when compared to the CF, showing increases of 54.0% and 56.3%, respectively.

Table 3.
Effect of manure and grass mulching on soil enzyme activity (μmol∙d−1∙g−1).
3.3. Microbial Community Composition and Functional Diversity
Different treatments significantly influenced soil bacterial and fungal diversity (Table 4). The Chao1 index was higher for both bacteria and fungi in GM than in CF and OM, though not significantly. The bacterial Shannon index was higher in OM and GM than in CF, while the fungal Shannon index was lowest in OM compared to GM and CF. The Simpson index showed higher bacterial dominance in OM compared to CF and GM, and greater fungal dominance in OM than in GM or CF.

Table 4.
Effect of manure and grass mulching on microbial community diversity.
The relative capacity for substrate utilization in soils from different treatments was presented in Figure 2. A significantly higher value of AWCD was observed in the OM treatment compared to the GM and CF treatments (Figure 1a) starting from 48 h. Furthermore, a significant difference occurred from 144 h between the treatments with organic fertilizer (OM and GM) and CF. As shown in Figure 1b, carbon sources were divided into six categories based on their utilization. During the 144-h incubation, amino acids, carboxylic acids, and polymers were the primary carbon sources used. Soil microbes in the CF showed a lower substrate utilization rate than those in the OM and GM (Figure 1b). Furthermore, AWCD values for all six carbon substrates were significantly higher in the OM and GM compared to CF, with amino acids exhibiting the greatest difference.
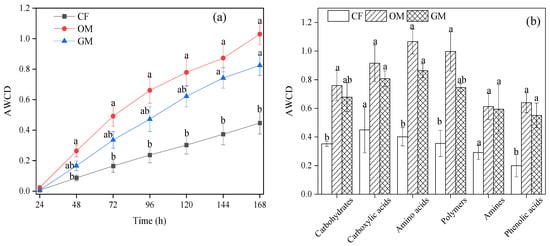
Figure 2.
The average well color development (AWCD) of soil microbia (a) and carbon substrate category utilization by soil microbia (b) under different treatments. The lowercase letters above the error bar indicate significant differences among treatments at p < 0.05. CF, GM, and OM represent Chemical Fertilizer + Conventional Tillage, Manure + Perennial Grass Mulch, and Manure + Conventional Tillage treatments, respectively.
Significant differences were observed in the bacterial and fungal community structures across the various treatments (Figure 3 and Figure 4). NMDS analysis showed distinct differences in bacterial and fungal communities among CF, OM, and GM treatments (Figure 3a and Figure 4a). Regarding bacteria, Actinobacteriota exhibited the highest abundance at the phylum level in soil samples, followed by Proteobacteria, Chloroflexi, and Acidobacteriota (Figure 3c). Among these, ASN and MBC were identified as the primary environmental factors influencing the soil bacterial community structure at the phylum level (Figure S1a). Furthermore, Micrococcaceae, Microvirga, and Vicinamibacteraceae dominated at the genus level (Figure 3d), with SOC and MBC being the main environmental drivers shaping the bacterial community structure (Figure S1b).
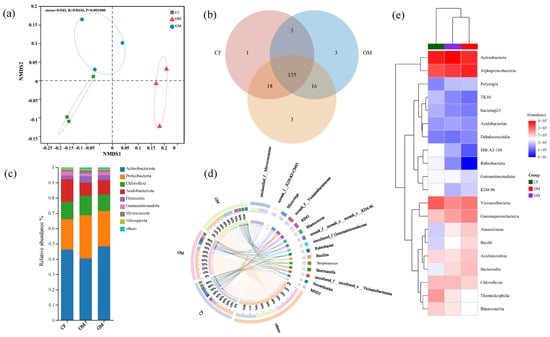
Figure 3.
The effect of manure and grass mulching on bacterial communities. (a) NMDS analysis; (b) A Venn diagram displaying common and distinct ASVs; (c) Relative abundances (%) of dominant bacterial phyla; (d) Circos diagram depicting the relationship between treatments and the abundance of various bacterial genera; (e) Heatmap illustrating the prevalent bacterial classes across all treatment groups. CF, GM, and OM represent Chemical Fertilizer + Conventional Tillage, Manure + Perennial Grass Mulch, and Manure + Conventional Tillage treatments, respectively.
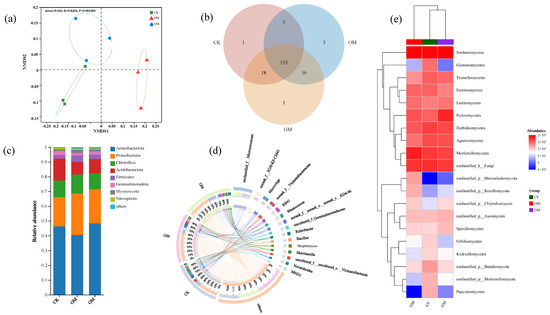
Figure 4.
The effect of manure and grass mulching on fungal communities. (a) NMDS analysis; (b) A Venn diagram displaying common and distinct ASVs; (c) Relative abundances (%) of dominant bacterial phyla; (d) Circos diagram depicting the relationship between treatments and the abundance of various bacterial genera; (e) Heatmap illustrating the prevalent bacterial classes across all treatment groups. CF, GM, and OM represent Chemical Fertilizer + Conventional Tillage, Manure + Perennial Grass Mulch, and Manure + Conventional Tillage treatments, respectively.
In terms of fungi (Figure 4c), Ascomycota was found to be most abundant at the phylum level across all treatments in soils, followed by Mortierellomycota and Basidiomycota, AN, HUN, and MBN were identified as the primary environmental factors influencing the soil fungal community structure at the phylum level (Figure S1c). At the genus level, Mortierella, Lophotrichus, and Gibberella prevailed as dominant species (Figure 4d). Notably, OM significantly increased abundances of Proteobacteria and Chloroflexi (Figure 3c), as well as Ascomycota (Figure 4c). NHN, SOC, and MBC were found to be the main environmental drivers shaping the fungal community structure at the genus level (Figure S1d).
3.4. Microbial Functional Characteristics
Fertilization treatments significantly affected the relative abundances of bacterial and fungal functional genes (Figure 5). The dominant bacterial functional categories included Chemoheterotrophy (40–44%), Aerobic_chemoheterotrophy (41–47%), Fementation (3–6%), Nitrate_reduction (1–9%), Aromatic_compound_degradation (2–3%) (Figure 5a). Additionally, the relative abundances of Aerobic_chemoheterotrophy and Fementation in OM was higher than the other treatments. Meanwhile, the relative abundances of Nitrogen_fixation and Nitrate_reduction in GM and OM was lower than CF. For the fungi community (Figure 5b), the dominant categories were Undifined saprotroph (21–32%), Animal pathogen (12–20%), Endophyte (11–23%), Dung saprotroph (9–19%) and Plant Pathogen (8–15%). Additionally, the relative abundances of Undefined saprotroph and Dung saprotroph in the OM and GM increased by 23.8% and 52.4%, as well as 22.2% and 111.1% compared to the CF. The relative abundance of Plant and Animal Pathogen was lower in the OM and GM than the CF.
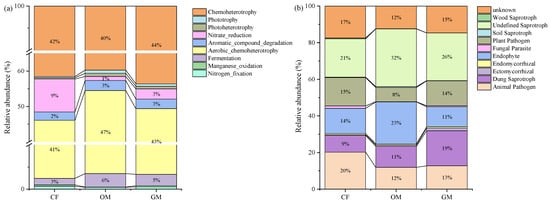
Figure 5.
The effect of manure and grass mulching on relative abundances of functional genes in bacterial (a) and fungal (b) communities. CF, GM, and OM represent Chemical Fertilizer + Conventional Tillage, Manure + Perennial Grass Mulch, and Manure + Conventional Tillage treatments, respectively. The values presented in the figure indicate the relative abundances of functional genes, calculated by dividing the abundance of each functional category by the total abundance of all identified functional genes within the corresponding sample.
3.5. Correlation of Microbial Community and Microbial Functional Characteristics with Soil Physicochemical Properties, Nitrogen Fractions, and Enzyme Activity
Mantel’s test was employed to analyze the correlation of microbial phylum diversity and microbial functional characteristics with soil physico-chemical properties, nitrogen fractions, and enzyme activity (Figure 6). In the microbial phylum diversity, bacterial taxa were significantly and positively correlated with SOC, AP, , AK, LAP, βG, ASN and HUN, while fungal taxa were significantly and positively correlated with AP, AK, LAP, NAG, AN, AAN, ASN, THN and HUN (Figure 6a). In terms of microbial functional characteristics (Figure 6b), bacterial functional genes were significantly and positively correlated with SOC, AP, , AK, LAP, βG, and ASN. Furthermore, fungal functional genes were significantly and positively correlated with AP, , AK, and NAG. The microbial community function based on biolog exhibited a strong positive correlation with LAP and NAG activities.
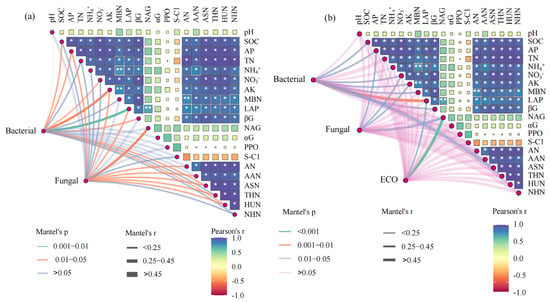
Figure 6.
Mantel’s test for the correlation of microbial phylum diversity (a) and microbial functional characteristics (b) with soil physico-chemical properties, nitrogen fractions, and enzyme activity (n = 9). The edge width corresponds to the R value and edge color denotes the statistical significance. The color gradient indicates Pearson correlation coefficients between soil bio-chemical properties and soil nitrogen fractions. SOC, soil organic carbon; AP, available phosphorus; TN, total nitrogen; AK, available potassium; MBN, microbial biomass nitrogen; LAP, leucine aminopeptidase enzymes; αG, α-glucosidase enzyme; βG, β-glucosidase enzyme; PPO, polyphenol oxidase enzyme; NAG, N-acetyl-β-D-glucosidase enzyme; S-C1, cellobiohydrolase enzyme; THN, total hydrolysable nitrogen; ASN, amino sugar nitrogen; AAN, amino acid nitrogen; AN, ammonia nitrogen; HUN, hydrolysable unidentified nitrogen; NHN, non-acid-hydrolyzable nitrogen; ECO, microbial community function based on biolog. * and ** denote statistically significance at p < 0.05 and p < 0.01 levels, respectively.
3.6. The Key Factors Influencing the Soil Nitrogen Fraction
The random forest model was utilized to examine and determine the primary influencing factors of soil microorganisms and enzyme activities on soil nitrogen fractions (Figure 7). Notably, in the case of AN, the primary contributors to its variation were identified as Basidiomycota (8.6%), MBN (6.6%), Actinobacteria (6.2%), Blastococcus (6%), Mortierella (5.9%). For the AAN, significant influences were attributed to βG (10.9%), Proteobacteria (9.7%), LAP (6.0%), NAG (5.9%), Mortierellomycota (5.9%). The ASN was mainly affected by Proteobacteria (11.9%), Basidiomycota (7.8%), NAG (7.0%), βG (5.9%). The THN was predominantly influenced by Basidiomycota (9.9%), Proteobacteria (8.8%), NAG (6.9%), Firmicutes (6.0%), and Mortierellomycota (5.9%). Regarding the HUN, the most influential factors were the Basidiomycota (11.0%), Streptomyces (10.9%), MBN (6.7%), Mortierellomycota (6.0%). Lastly, the NHN was predominantly influenced by βG (9.6%), Proteobacteria (6.6%), Blastococcus (5.7%).
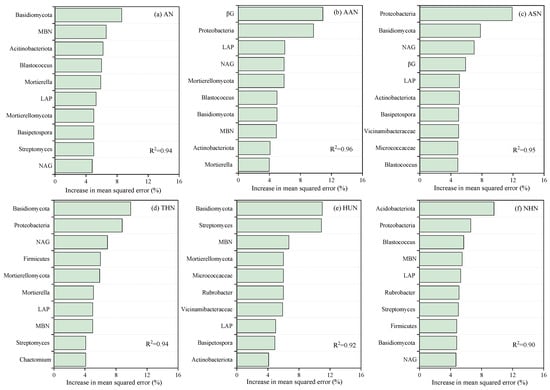
Figure 7.
The random forest model for evaluating the relative importance of microbial community composition and enzyme activity in influencing ammonia nitrogen (AN) (a); amino acid nitrogen (AAN) (b); amino sugar nitrogen (ASN) (c); total hydrolysable nitrogen (THN) (d); hydrolysable unidentified nitrogen (HUN) (e); non-acid-hydrolyzable nitrogen (NHN) (f) (n = 9). MBN, microbial biomass nitrogen; NAG, N-acetyl-β-D-glucosidase enzyme; LAP, leucine aminopeptidase enzymes; αG, α-glucosidase enzyme; βG, β-glucosidase enzyme. Display only the top ten influencing factors.
4. Discussion
4.1. Impacts of Organic Manure and Grass Mulching on Soil Nitrogen Fraction
The manure application in the OM significantly enhanced nutrient contents compared to the CF and GM (Table 1). Among these nutrients, total nitrogen levels were notably higher in OM, exceeding GM and CF by 1.96 and 2.82 times, respectively (Table 1). This is consistent with the results of earlier research emphasizing the advantages of using organic fertilizers and grass mulch in orchard management systems [26]. This enhancement in total nitrogen underscored the role of organic manure in providing a sustained nitrogen source, which was critical for maintaining soil fertility and supporting plant growth. However, the lower total nitrogen content observed in the GM compared to the OM can be attributed to nitrogen uptake by the cover grass [27] in the GM. It is important to note that soil sampling in this study was conducted at the time of grass harvest, before the grass biomass was returned to the field. This timing likely contributed to the observed differences in total nitrogen (Table 1) and other nitrogen fractions (Table 2), which were significantly higher in OM relative to GM. These observations suggested that while organic manure can provide a direct and immediate source of nitrogen, grass mulching can depend on a more gradual nutrient release process mediated by the decomposition of grass residues. Such differences in nitrogen dynamics between OM and GM treatments provided valuable insights into their respective roles in sustainable soil management.
Active N fractions (, , AAN, ASN, and AN) are critical determinants of nitrogen mineralization rates and soil nitrogen supply capacity [28]. In this research, the ratios of and to total nitrogen, as well as the ratios of active organic nitrogen fractions (AN, AAN, and ASN) to total nitrogen, were 12.4% and 18.3% higher, respectively, in GM compared to OM (Table S1). These results suggested that integrating organic manure with cover grass can enhance the proportion of active nitrogen, thereby improving nitrogen availability for plant uptake. This finding aligns with previous studies demonstrating the ability of grass mulching to promote the accumulation of labile nitrogen fractions, which were more readily accessible for microbial and plant utilization [29]. Furthermore, MBN was shown to respond more sensitively to fertilization and grass mulching practices than other N fractions [29]. In this study, MBN content followed the order OM > GM > CF (Table 1). The increase in MBN under OM and GM may result from the sustained application of organic inputs. These inputs serve as a direct supply of SON while also enhancing microbial nitrogen transformation activities by introducing carbon-rich substances [30]. High SOC levels from manure application likely stimulated microbial decomposition of organic materials (Figure 2), thereby promoting the conversion of organic nitrogen into mineral nitrogen. This observation underscored the integral role of soil microbes in mediating nitrogen transformations, particularly under organic amendment practices.
In contrast, the stable organic nitrogen content in the GM was lower compared to the OM (Table 2), likely due to greater nitrogen uptake by grass in the GM treatment. Additionally, stable organic nitrogen in the GM might be more easily converted into active nitrogen fractions via microbial production of proteases and cellulases, thereby transforming it into biologically accessible forms [31]. Notably, NHN, a stable nitrogen reservoir, was significantly higher in the GM and OM compared to the CF (Table 2). This finding highlighted the long-term nitrogen retention benefits associated with organic amendments, as NHN played a critical role in retaining fertilizer-derived nitrogen and mitigating nitrogen losses [16]. The microbial transformation of organic nitrogen into mineral nitrogen was a pivotal process for the retention and availability of SON. Ning et al. [30] utilized a 15N cross-labeling technique to demonstrate that organic amendments enhanced the microbial assimilation of nitrogen, thereby increasing SON retention. This microbially driven process not only contributed to nitrogen cycling but also affected nutrient availability and ecosystem productivity. These findings underscored the importance of understanding the composition and dynamics of soil microbial communities under organic fertilization and grass mulching practices to elucidate changes in SON fractions and their implications for sustainable soil management.
4.2. Effects of Manure and Grass Mulching on Soil Microbial Community and Functions
The OM and GM exhibited superior microbial abundances and diversity compared to CF (Figure 3 and Figure 4). This was because organic amendments promoted microbial community restructuring by facilitating substrate-mediated niche differentiation [32]. Notably, the pronounced proliferation of copiotrophic phyla, including Proteobacteria, Chloroflexi, and Firmicutes, in amended soils (Figure 3c), highlighted their ecological strategy to thrive in environments with high organic carbon content. This finding aligns with the description provided by Li et al. [22], who identified these taxa as specialists with a pronounced response to organic carbon availability. The prevalence of Proteobacteria and Chloroflexi in both orchard soils [4] and manure composts [32]—the principal manure source in this study—indicated a conserved pattern of microbial recruitment across systems utilizing organic amendments. At a more refined taxonomic level, the enrichment of Alphaproteobacteria in the OM and GM treatments (Figure 3e) indicated substrate-driven selection, as this class possesses specialized metabolic pathways for the degradation of complex organic compounds. Previous research by Wang et al. [33] identified Alphaproteobacteria as keystone taxa in environments rich in organic matter, a conclusion supported by our findings of increased substrate utilization efficiency for amines and phenolic acids in the OM and GM (Figure 2b). This functional enhancement was likely due to genomic adaptations in Alphaproteobacteria, particularly their expanded array of gene clusters for the degradation of aromatic compounds [34]. Additionally, KEGG pathway analysis demonstrated an increased fermentation potential in amended soils (Table 3), which can be attributed to the synergistic effects of enhanced substrate availability [32,35] and community-level metabolic cooperation.
The OM and GM exhibited a substantial decrease in fungal Shannon diversity compared to CF, aligning with the findings of Yang et al. [36] and Li et al. [22], who reported manure-induced suppression of fungal communities. This suppression may occur through several mechanisms: (1) direct inhibition of pathogenic fungi by antimicrobial compounds present in manure; (2) increased resource competition that favors fast-growing copiotrophic fungi; and (3) niche exclusion mediated by changes in pH. Our findings corroborated these mechanisms, as evidenced by the decreased abundance of animal and plant pathogens, and ectomycorrhizal fungi, in soils amended with manure (Figure 5). This suggested a functional trade-off between pathogen suppression and symbiotic associations. The proliferation of Ascomycota under the OM and GM (Figure 4c) indicated their ecological adaptability to nutrient-rich environments, supported by their enzymatic capabilities for lignocellulose decomposition [37,38]. Notably, the superior relative abundance of Ascomycota in GM compared to OM (Figure 4c) was likely attributable to their enhanced cellulolytic or hemicellulolytic abilities to degrade grass residues, as demonstrated in the comparative study of organic amendment types by Meng et al. [39]. The functional shift towards saprotrophic dominance in the GM and OM treatments, particularly among Undefined saprotrophs (8.74–47.98%) and Dung saprotrophs (23.50–83.11%), underscored the substrate-driven reorganization of fungal communities (Figure 5). This dominance of saprotrophs, alongside a reduction in ectomycorrhizal associations in the GM and OM, indicated a fundamental restructuring of plant-microbe interactions under conditions of high nutrient availability in the GM and OM. The N mediation hypothesis proposed by Correia et al. [40] offered a plausible explanation: elevated soil nitrogen levels in OM and GM treatments may disrupt the mutualistic balance between plants and ectomycorrhizal fungi, thereby favoring saprotrophs that are better adapted to nutrient-rich environments.
4.3. Contribution of Soil Microbial Communities and Enzyme Activities to Organic Nitrogen Fractions
The soil microbial communities and enzyme activities were closely associated with the organic nitrogen fraction (Figure 6 and Figure 7). Actinobacteria, Proteobacteria, and Firmicutes were the dominant bacterial phyla, with Blastococcus, Vicinamibacteraceae, and Rubrobacter being the predominant genera associated with variations in soil organic nitrogen components. Among fungi, Basidiomycota, Mortierellomycota, and Ascomycota were the dominant phyla, while Basipetospora, Mortierella, Gibberella, and Chaetomium were the key genera linked to these components (Figure 7). Actinomycetes, Proteobacteria, and Firmicutes played crucial roles in the decomposition of macromolecular organic nitrogen compounds, such as proteins and chitin, through the secretion of extracellular enzymes, including proteases and chitinases. This enzymatic activity facilitated the conversion of these compounds into soluble small-molecule organic nitrogen forms, such as amino acids [41]. Actinobacteria and their subordinate genera, such as Blastococcus and Rubrobacter, harbor functional genes involved in nitrogen mineralization, including deaminase and urease. These genes facilitate the breakdown of amino acids and urea, thereby enhancing the transformation of organic nitrogen into inorganic forms. Additionally, Proteobacteria contributed significantly to the microbial nitrogen pool, comprising 35–60% of the microbial biomass nitrogen. The decomposition of Proteobacteria residues released organic nitrogen, such as amino sugars, which constituted 3–28% of the soil organic nitrogen pool [42]. In environments with high carbon availability, such as those enriched with organic fertilizers and grass mulching, Firmicutes, Actinobacteria, and Proteobacteria enhanced the expression of nitrogen mineralization-related genes, including deaminase and protease genes, through carbon-nitrogen coupled metabolic processes. These processes degraded carbon sources such as cellulose, facilitated nitrogen accumulation in the particulate organic carbon pool, and expedited the conversion of organic nitrogen into mineral forms [43,44].
As the core soil fungal taxa, Basidiomycota, Mortierellomycota, and Ascomycota played crucial roles in the transformation of SON through mechanisms including enzyme-mediated decomposition, regulation of nitrogen cycling, and adaptation to environmental conditions [45]. The mycelia of these three major groups had the capacity to assimilate inorganic nitrogen and converted it into a nitrogen source that can be utilized by microorganisms [46]. Simultaneously, they secreted proteases and chitinases to break down proteins and chitin into small-molecule nitrogen sources, such as amino acids and glucosamine, thereby facilitating the release of organic nitrogen [47]. Notably, Basidiomycetes were capable of degrading complex organic nitrogen compounds by secreting extracellular oxidases [48]. For example, the lignin peroxidase (LiP) and manganese peroxidase (MnP) were secreted by ectomycorrhizal fungi such as Pisolithus and Mortierella in the lignin-degrading enzyme system [49]. These enzymes can cleave lignin-protein complexes, thus releasing bound organic nitrogen, including aromatic nitrogen-containing compounds [50]. Furthermore, certain species within the Ascomycota, such as Basipetospora, Gibberella, and Chaetomium, were capable of secreting organic acids like oxalic acid, which dissolved mineral-bound organic nitrogen and thereby enhance its bioavailability.
The βG, LAP, and NAG were key enzymes involved in shaping the composition of soil organic nitrogen (Figure 7). This was because these enzymes played a crucial role in regulating biogeochemical processes in the soil, especially those associated with N cycling. For example, βG played a central role in breaking down complex carbon compounds into simpler forms [51], thus providing substrates for microbial metabolism [52]. Through the hydrolysis of cellulose, βG produced glucose, which served as a substrate for fermentation and phototrophy, thereby stimulating microbial metabolism. Additionally, the carbon source generated through microbial metabolism can further stimulate nitrate reduction, thus promoting soil nitrogen cycling. The Saprotroph microbia may utilize the glucose released by βG for the degradation of more recalcitrant carbon compounds brought in by grass and manure through a “synergistic decomposition-energy compensation” mode [35]. The LAP can hydrolyze hydrophobic amino acids (like leucine and phenylalanine) at the N-terminus of proteins or polypeptides, releasing free amino acids, promoting organic nitrogen conversion to , and providing a usable nitrogen source for plants and microorganisms. The application of manure and grass mulching introduced a substantial amount of nitrogen-rich organic matter into the soil (Table 2), which provided ample substrates for LAP activity. The hydrolysis of these organic compounds through LAP activity led to a marked increase in and amino acids (Table 1 and Table 2), thus enhancing N cycling and promoting the efficient utilization of nitrogen in manure and grass mulching management systems [53]. The NAG, which plays a crucial role in the degradation of chitin, was found to be significantly associated with fungal diversity and functional activity (Figure 6). Chitin, a primary structural component of fungal cell walls, underwent hydrolysis facilitated by NAG [54]. This enzymatic breakdown of chitin resulted in the release of N-acetylglucosamine, which subsequently promoted the proliferation of saprophytic fungi by enhancing their carbon and nitrogen assimilation processes [55]. Therefore, under the combined influence of manure and grass mulching, a carbon-nitrogen resource-sharing system and cross-kingdom interactions between bacteria and fungi were established, promoting efficient nutrient cycling through enhanced enzyme activities. This led to the formation of an “enzyme-substrate-community” feedback loop, which contributed to sustainable soil health (Figure 8).
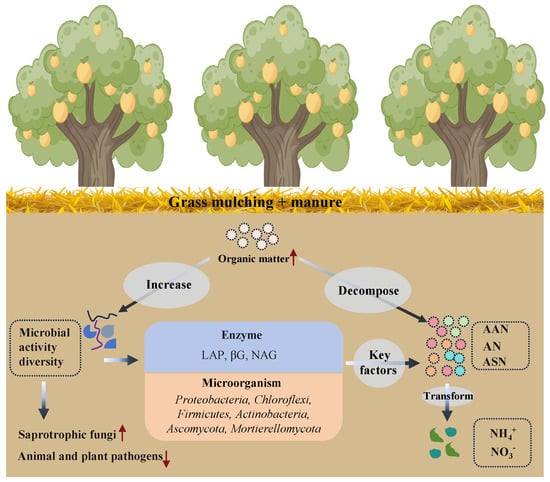
Figure 8.
Conceptual diagram of responses of microbial communities, enzyme activities, and soil organic nitrogen fractions to long-term manure and grass mulching applications in orchard soils. NAG, N-acetyl-β-D-glucosidase enzyme; LAP, leucine aminopeptidase enzymes; αG, α-glucosidase enzyme; βG, β-glucosidase enzyme; ASN, amino sugar nitrogen; AAN, amino acid nitrogen; AN, ammonia nitrogen.
The results presented are predominantly derived from observations at a single time point and should be considered preliminary within this research domain. In real-world agricultural ecosystems, soil nitrogen transformation and microbial community dynamics are subject to a myriad of influences, including climatic conditions, tillage practices, fertilization strategies, and crop types, all of which exhibit significant spatio-temporal variability [56]. To systematically elucidate the mechanisms by which different treatments impact soil microbial characteristics and organic nitrogen components, it is imperative that future studies incorporate continuous dynamic monitoring across multiple growing seasons and developmental stages. For example, repeated sampling during critical phenological stages of fruit trees—such as bud break, flowering, fruit development, and post-harvest—could facilitate a comprehensive assessment of temporal variations in soil physicochemical properties, enzyme activities, microbial community structure, and crop growth indicators. This methodological approach would significantly enhance our understanding of the trends and enduring effects associated with various management practices. Studies incorporating multiple time points will aid in establishing more robust causal relationships and provide stronger scientific evidence to support orchard soil health management and the sustainable development of regional agriculture.
5. Conclusions
A 12-year field study investigated the effect of manure and grass mulching on soil organic nitrogen fraction, enzyme activity, and microbial community structure and function in orchard ecosystems. Organic fertilizers and grass mulching can enhance the contents of soil total nitrogen, microbial nitrogen, and various organic nitrogen components (including total hydrolysable nitrogen, amino sugar nitrogen, amino acid nitrogen, ammonia nitrogen, hydrolysable unidentified nitrogen, and non-acid-hydrolyzable nitrogen). Additionally, they promoted the activities of enzymes such as LAP, αG, βG, NAG, and S-C1, increased the diversity of bacterial communities, and supported the growth and reproduction of key microorganisms, including Proteobacteria, Chloroflexi, Firmicutes, Actinobacteria, as well as fungal groups like Ascomycota and Mortierellomycota. Functional predictions suggested that organic fertilizers and straw mulching can markedly enhance the relative abundances of microorganisms associated with nitrogen metabolism and saprotrophic fungi, while reducing the relative abundances of potential animal and plant pathogens. Furthermore, βG, LAP, and NAG, as well as Basidiomycota, Mortierellomycota, Ascomycota, Actinobacteria, Proteobacteria, and Firmicutes were identified as key biological factors regulating soil organic nitrogen components. Additionally, amino sugar nitrogen and ammonia nitrogen exerted the most significant influences on mineral nitrogen.
The results of this research offer significant understanding into how organic amendments affect nutrient cycling processes within soil. The establishment of an “enzyme-substrate-community” feedback loop, driven by microbial and enzyme activity, enhances soil fertility, accelerates nitrogen cycling, and promotes the efficient utilization of nitrogen by fruit trees. This understanding will help guide the development of more sustainable and productive agricultural systems, ensuring long-term soil health and increased crop yields.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15192084/s1, Methods S1: The Methodology of the Analysis of Physical and Chemical Properties of Soil (Soil Organic Carbon, Total Nitrogen, Soil Organic Nitrogen Fraction, and Fixed Ammonium; Methods S2: The Methodology of the Analysis of Soil Organic Nitrogen Fraction, and Fixed Ammonium; Methods S3: The Methodology of the Analysis of Soil Enzyme Activity Assay; Methods S4: Biolog EcoPlates™ Assay; Methods S5: The Calculations of Chao1, Shannon, and Simpson Indices—(1) Table S1: The ratio of ammonium nitrogen (), nitrate nitrogen (), ammonia nitro-gen (AN), amino acid nitrogen (AAN), and amino sugar nitrogen (ASN) to total nitrogen (%); (2) Figure S1: Redundancy analysis of the relationships between soil bacterial and fungal community structures at the phylum and genus levels and soil physicochemical properties. SOC, soil organic carbon; AP, available phosphorus; TN, total nitrogen; AK, available potassium; , Ammonium nitrogen; , nitrate nitrogen; MBC and MBN, microbial biomass carbon and nitrogen; THN, total hydrolysable nitrogen; ASN, amino sugar nitrogen; AAN, amino acid nitrogen; AN, ammonia nitrogen; HUN, hydrolysable unidentified nitrogen; NHN, non-acid-hydrolyzable ni-trogen; CF, GM, and OM represent Chemical Fertilizer + Conventional Tillage, Manure + Perennial Grass Mulch, and Manure + Conventional Tillage treatments respectively.
Author Contributions
Conceptualization, Q.W.; methodology, Q.W., L.G. and X.G.; software, Q.W. and S.C.; validation, Q.W. and X.S.; formal analysis, Q.W. and F.G.; investigation, Q.W. and W.L.; resources, Q.W.; data curation, Q.W. and H.G.; writing—original draft preparation, Q.W.; writing—review and editing, Q.W., G.W. and X.F.; visualization, Q.W. and W.L.; supervision, X.F.; project administration, Q.W.; funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation Research Project of Shanxi Province, grant number 202203021212445 and the Scientific and Technological Innovation Foundation of Shanxi Agricultural University (Ph.D. Research Startup), grant number 2022BQ15. The earmarked fund for Modern Agro-industry Technology Research System, grant number 2025CYJSTX07-08.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, J.; Fan, L.; Feng, P.; Sun, X.; Xue, S. Response of vegetation evapotranspiration to landscape pattern changes in an arid region: A case study of the Loess Plateau, China. CATENA 2025, 252, 108878. [Google Scholar] [CrossRef]
- Jiao, H.; Yin, Q.; Fan, C.; Wang, L.; Zhao, J.; Wang, X.; Du, K.; Lin, H. Long-term effects of liquid swine manure land surface application in an apple orchard field on soil bacterial community and heavy metal contents in apple (Malus pumila Mill.). Environ. Sci. Pollut. Res. 2021, 28, 49613–49626. [Google Scholar] [CrossRef]
- Kai, T.; Adhikari, D. Effect of organic and chemical fertilizer application on apple nutrient content and orchard soil condition. Agriculture 2021, 11, 340. [Google Scholar] [CrossRef]
- Wan, Y.; Li, W.; Wang, J.; Shi, X. Bacterial diversity and community in response to long-term nitrogen fertilization gradient in citrus orchard soils. Diversity 2021, 13, 282. [Google Scholar] [CrossRef]
- Liao, H.; Li, Y.; Yao, H. Fertilization with inorganic and organic nutrients changes diazotroph community composition and N-fixation rates. J. Soils Sediments 2018, 18, 1076–1086. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Green, S.M.; Dungait, J.A.J.; Wen, X.; Tang, Y.; Guo, Z.; Yang, Y.; Sun, X.; Quine, T.A. Nitrogen functional gene activity in soil profiles under progressive vegetative recovery after abandonment of agriculture at the puding karst critical zone observatory, SW China. Soil Biol. Biochem. 2018, 125, 93–102. [Google Scholar] [CrossRef]
- Toselli, M.; Baldi, E.; Cavani, L.; Mazzon, M.; Quartieri, M.; Sorrenti, G.; Marzadori, C. Soil-plant nitrogen pools in nectarine orchard in response to long-term compost application. Sci. Total Environ. 2019, 671, 10–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Li, C.; Peng, H.; Liu, J.; Luo, Y.; Song, M.; Dai, Y.; Deng, K.; Ji, X. Optimizing fertilizer application and straw return to fields to minimize nitrogen and phosphorus runoff losses in double-rice cropping systems. Agric. Water Manag. 2025, 317, 109601. [Google Scholar] [CrossRef]
- Taguas, E.V.; Vanderlinden, K.; Pedrera-Parrilla, A.; Giráldez, J.V.; Gómez, J.A. Spatial and temporal variability of spontaneous grass cover and its influence on sediment losses in an extensive olive orchard catchment. Catena 2017, 157, 58–66. [Google Scholar] [CrossRef]
- Tang, W.; Yang, H.; Wang, W.; Wang, C.; Pang, Y.; Chen, D.; Hu, X. Effects of living grass mulch on soil properties and assessment of soil quality in chinese apple orchards: A meta-analysis. Agronomy 2022, 12, 1974. [Google Scholar] [CrossRef]
- Ding, S.; Xin, X.; Yang, W.; Zhang, X.; Zhu, A.; Huang, S.; Yang, J.; Ren, G.; Li, M. Transformation of fertilizer nitrogen in fluvo-aquic soils with different textures and its influencing factors. Plant Soil 2022, 471, 541–558. [Google Scholar] [CrossRef]
- Meng, D.; Wang, X.; Tang, J.; Zong, N.; Zhang, J.; He, N. Distributions and controlling factors of soil total nitrogen and nitrogen fractions along an altitude gradient in Qinghai-Tibet Plateau. J. Soils Sediments 2024, 24, 3311–3322. [Google Scholar] [CrossRef]
- Wu, H.; Du, S.; Zhang, Y.; An, J.; Zou, H.; Zhang, Y.; Yu, N. Effects of irrigation and nitrogen fertilization on greenhouse soil organic nitrogen fractions and soil-soluble nitrogen pools. Agric. Water Manag. 2019, 216, 415–424. [Google Scholar] [CrossRef]
- Sharifi, M.; Zebarth, B.J.; Burton, D.L.; Grant, C.A.; Cooper, J.M. Evaluation of some indices of potentially mineralizable nitrogen in soil. Soil Sci. Soc. Am. J. 2007, 71, 1233–1239. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Y.; Liu, D.; Zhang, S.; Lan, B.; He, L.; Yu, Z.; Zhou, S.; Chen, X.; Qu, Y. Characteristics of organic nitrogen fractions in sediments of the water level fluctuation zone in the tributary of the Yangtze River. Sci. Total Environ. 2019, 653, 327–333. [Google Scholar] [CrossRef]
- Lü, H.; He, H.; Zhao, J.; Zhang, W.; Xie, H.; Hu, G.; Liu, X.; Wu, Y.; Zhang, X. Dynamics of fertilizer-derived organic nitrogen fractions in an arable soil during a growing season. Plant Soil 2013, 373, 595–607. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Miao, Y.F.; Li, S.Q. Soil organic nitrogen and its contribution to crop production. J. Integr. Agr. 2014, 13, 2061–2080. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Young, M.D.; Ros, G.H.; de Vries, W. Impacts of agronomic measures on crop, soil, and environmental indicators: A review and synthesis of meta-analysis. Agric. Ecosyst. Environ. 2021, 319, 107551. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Xu, L.; Zhang, H.; Shen, X.; Xu, H.; Jiao, J.; Li, H.; Hu, F. Crop yield-soil quality balance in double cropping in China’s upland by organic amendments: A meta-analysis. Geoderma 2021, 403, 115197. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Zhao, C.; Hu, X.; Yin, C. Nitrogen fertilization increases soil microbial biomass and alters microbial composition especially under low soil water availability. Microb. Ecol. 2023, 86, 536–548. [Google Scholar] [CrossRef]
- Li, K.; Xing, X.; Wang, S.; Liao, R.; Hassan, M.U.; Aamer, M.; Barbanti, L.; Wen, T.; Xu, H. Organic fertilisation enhances network complexity among bacteria, fungi, and protists by improving organic matter and phosphorus in acidic agricultural soils. Eur. J. Soil Biol. 2024, 122, 103649. [Google Scholar] [CrossRef]
- Guerra, V.A.; Beule, L.; Mackowiak, C.L.; Dubeux, J.C.B.; Blount, A.R.S.; Wang, X.B.; Rowland, D.L.; Liao, H.L. Soil bacterial community response to rhizoma peanut incorporation into Florida pastures. J. Environ. Qual. 2022, 51, 55–65. [Google Scholar] [CrossRef]
- Erhunmwunse, A.S.; Queiroz, L.M.D.; Zhang, K.; Mackowiak, C.L.; Blount, A.R.S.; Dubeux, J.C.B.; Liao, H.L. Changes in soil microbial diversity and community composition across bahiagrass and rhizoma peanut pastures. Biol. Fertil. Soils 2023, 59, 285–300. [Google Scholar] [CrossRef]
- Garland, J.L. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, P.; Thomas, B.W.; Zhao, J.; Zhang, X.; Yan, H.; Zhang, Z.; Chen, S.; Shi, X.; Zhang, Y. Organic carbon and nitrogen accumulation in orchard soil with organic fertilization and cover crop management: A global meta-analysis. Sci. Total Environ. 2022, 852, 158402. [Google Scholar] [CrossRef]
- Lyu, H.; Li, Y.; Wang, Y.; Wang, P.; Shang, Y.; Yang, X.; Wang, F.; Yu, A. Drive soil nitrogen transformation and improve crop nitrogen absorption and utilization—A review of green manure applications. Front. Plant Sci. 2023, 14, 1305600. [Google Scholar] [CrossRef]
- Li, L.L.; Li, S.T. Nitrogen mineralization from animal manures and its relation to organic N fractions. J. Integr. Agric. 2014, 13, 2040–2048. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Ye, Y.; Ma, Q.; Guan, Q.; Jones, D.L. Response of nitrogen fractions in the rhizosphere and bulk soil to organic mulching in an urban forest plantation. J. For. Res. 2021, 32, 2577–2588. [Google Scholar] [CrossRef]
- Ning, Y.; Li, S.; Ning, C.; Ren, J.; Xia, Z.; Zhu, M.; Gao, Y.; Zhang, X.; Ma, Q.; Yu, W. Effects of exogenous nitrogen addition on soil organic nitrogen fractions in different fertility soils: Result from a 15N cross-labeling experiment. Agric. Ecosyst. Environ. 2025, 379, 109366. [Google Scholar] [CrossRef]
- Li, H.; Zhou, B.; Zhuo, Z.; Wang, L.; Wang, Z.; Xie, C.; Jiang, F.; Lin, J.; Huang, Y.; Zhang, Y. Effects of cover measures on soil organic nitrogen fractions and total soluble nitrogen pools in citrus orchards of the red soil hilly region of southern China. Agriculture 2024, 14, 1879. [Google Scholar] [CrossRef]
- Wan, J.; Wang, X.; Yang, T.; Wei, Z.; Banerjee, S.; Friman, V.P.; Mei, X.; Xu, Y.; Shen, Q. Livestock manure type affects microbial community composition and assembly during composting. Front. Microbiol. 2021, 12, 621126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Yang, F.; Yaoyao, E.; Raza, W.; Huang, Q.; Shen, Q. Application of bioorganic fertilizer significantly increased apple yields and shaped bacterial community structure in orchard soil. Microb. Ecol. 2017, 73, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Wang, X.; Bao, X.; Zhu, L.; Xie, Z.; Che, Z.; Sun, B. Exogenous substrate quality determines the dominant keystone taxa linked to carbon mineralization: Evidence from a 30-year experiment. Soil Biol. Biochem. 2022, 169, 108683. [Google Scholar] [CrossRef]
- Xiang, Y.; Chang, S.X.; Shen, Y.; Chen, G.; Liu, Y.; Yao, B.; Xue, J.; Li, Y. Grass cover increases soil microbial abundance and diversity and extracellular enzyme activities in orchards: A synthesis across China. Appl. Soil Ecol. 2023, 182, 104720. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liu, J.; Zhou, Z.; Zhang, T.; Wang, X. Fungal community structure in relation to manure rate in red soil in southern China. Appl. Soil Ecol. 2020, 147, 103442. [Google Scholar] [CrossRef]
- Baldrian, P.; Voříšková, J.; Dobiášová, P.; Merhautová, V.; Lisá, L.; Valášková, V. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil 2011, 338, 111–125. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, W.; Men, M.; Bello, A.; Xu, X.; Xu, B.; Deng, L.; Jiang, X.; Sheng, S.; Wu, X.; et al. Microbial community succession and response to environmental variables during cow manure and corn straw composting. Front. Microbiol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Correia, M.; Espelta, J.M.; Morillo, J.A.; Pino, J.; Rodríguez-Echeverría, S. Land-use history alters the diversity, community composition and interaction networks of ectomycorrhizal fungi in beech forests. J. Ecol. 2021, 109, 2856–2870. [Google Scholar] [CrossRef]
- Rozmoš, M.; Bukovská, P.; Hršelová, H.; Kotianová, M.; Dudáš, M.; Gančarčíková, K.; Jansa, J. Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. ISME J. 2022, 16, 676–685. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Li, Y.; Wang, G.; Liu, X.; Tang, C.; Lian, T.; Adams, J.; Liu, J.; Liu, J.D.; et al. Soil microbial metabolism on carbon and nitrogen transformation links the crop-residue contribution to soil organic carbon. npj Biofilms Microbiomes 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Tang, S.; Zhou, J.; Wanek, W.; Gregory, A.S.; Ge, T.D.; Marsden, K.A.; Chadwick, D.R.; Liang, Y.C.; Wu, L.H.; et al. Long-term manure and mineral fertilisation drive distinct pathways of soil organic nitrogen decomposition: Insights from a 180-year-old study. Soil Biol. Biochem. 2025, 207, 109840. [Google Scholar] [CrossRef]
- Bahr, A.; Ellström, M.; Akselsson, C.; Ekblad, A.; Mikusinska, A.; Wallander, H. Growth of ectomycorrhizal fungal mycelium along a Norway spruce forest nitrogen deposition gradient and its effect on nitrogen leakage. Soil Biol. Biochem. 2013, 59, 38–48. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Sieradzki, E.T.; Nuccio, E.E.; Pett-Ridge, J.; Firestone, M.K. Expression of macromolecular organic nitrogen degrading enzymes identifies potential mediators of soil organic N availability to an annual grass. ISME J. 2023, 17, 967–975. [Google Scholar] [CrossRef]
- Chen, D.M.; Taylor, A.F.S.; Burke, R.M.; Cairney, J.W.G. Identification of genes for lignin peroxidases and manganese peroxidases in ectomycorrhizal fungi. New Phytol. 2001, 152, 151–158. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Z.; Shi, J.; Yang, C.; Fang, Y.; Chen, G.; Chen, H.; Tian, C. Enzymatic hydrolysis of corn stover lignin by laccase, lignin peroxidase, and manganese peroxidase. Bioresour. Technol. 2022, 361, 127699. [Google Scholar] [CrossRef]
- Baca-Patiño, B.; González-Rodríguez, A.; García-Oliva, F.; García, A.; Lara-De La Cruz, I.; Garibay-Orijel, R.; Poret-Peterson, A.; Maldonado-López, Y.; Cuevas-Reyes, P.; Gómez-Tagle, A.; et al. Land-use change from native forest to avocado orchards: Effects on soil nutrient transformation and microbial communities. Appl. Soil Ecol. 2025, 205, 105748. [Google Scholar] [CrossRef]
- Chioru, A.; Chirsanova, A. β-Glucans: Haracterization, Extraction Methods, and Valorization. Food Nutr. Sci. 2023, 14, 963–983. [Google Scholar] [CrossRef]
- Sun, X.; Ye, Y.; Ma, Q.; Guan, Q.; Jones, D.L. Variation in enzyme activities involved in carbon and nitrogen cycling in rhizosphere and bulk soil after organic mulching. Rhizosphere 2021, 19, 100376. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; He, H.; Wang, H.; Wu, F. Extracellular enzyme activity and nutrient characteristics of pinus massoniana lamb. families with different growth levels: Insights into the ectomycorrhizal fungal community and rhizosphere soil. Forests 2023, 14, 1447. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).