Assessment of Heavy Metal Transfer from Soil to Forage and Milk in the Tungurahua Volcano Area, Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Sample Preparation and Analysis
2.4. Physicochemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
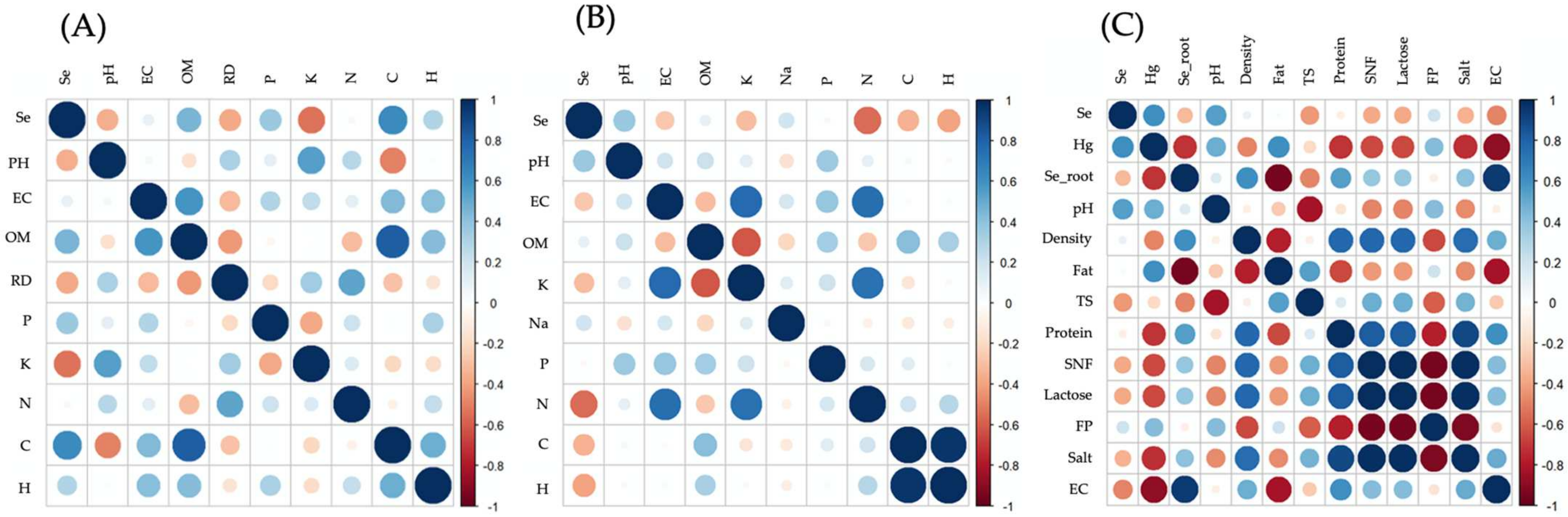
3.1. Physicochemical Parameters in Soil Samples
3.2. Soil Macronutrients
3.3. Physical Parameters of the Forage
3.4. Macronutrients in Forage
3.5. Physicochemical Parameters of Milk
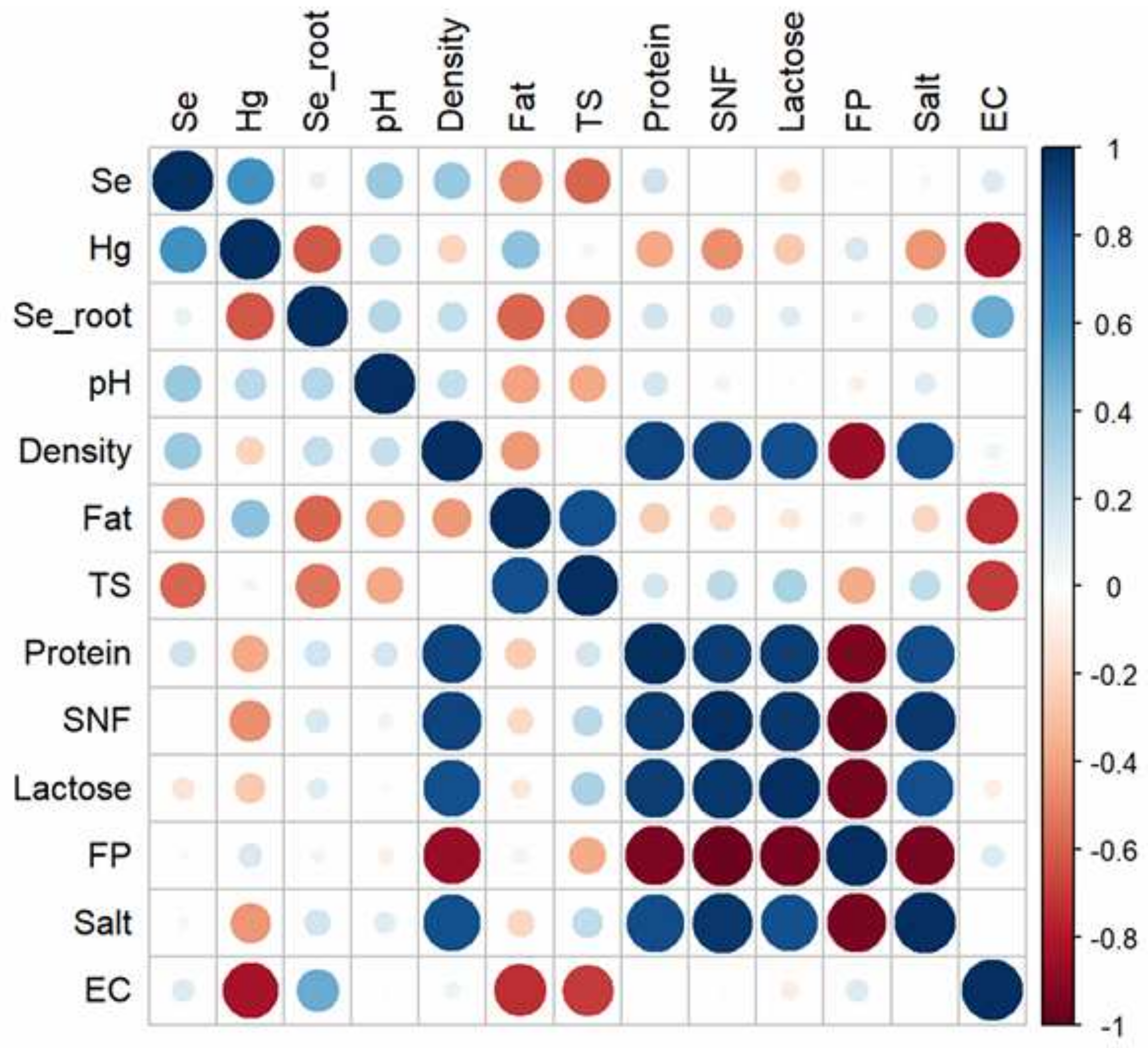
3.6. Metal Analysis
3.6.1. Soils
3.6.2. Forage
3.6.3. Milk
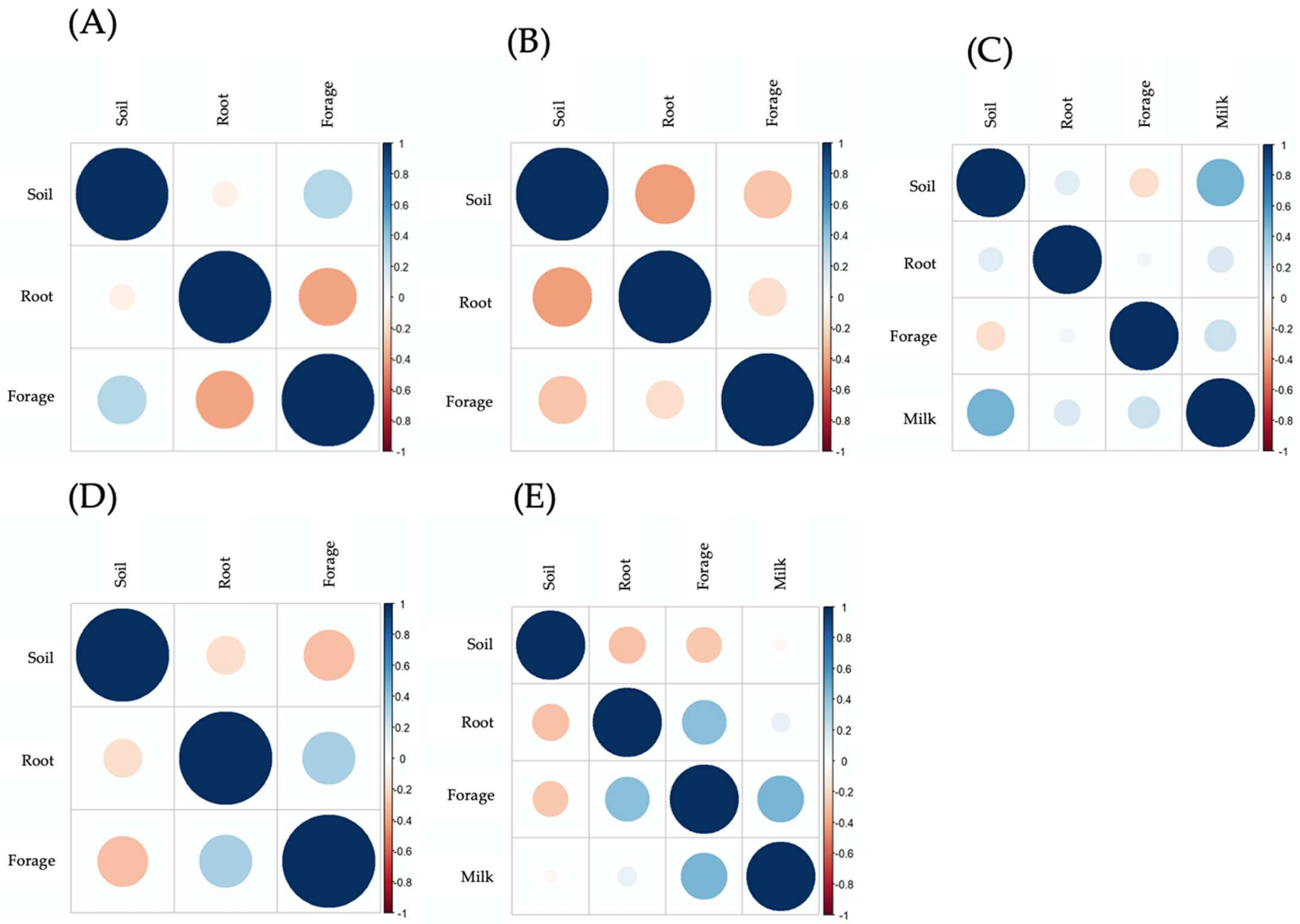
3.6.4. Bioaccumulation and Biotransfer Factors of Heavy Metals
3.7. Limitations of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pb | Lead |
| Cd | Cadmium |
| Hg | Mercury |
| As | Arsenic |
| Se | Selenium |
| P | Phosphorus |
| N | Nitrogen |
| BCF | Root bioconcentration factor |
| TF | Translocation factor |
| BTF | Biotransfer factor of heavy metals from forage to milk |
| EC | Electrical conductivity |
| OM | Organic matter |
| TS | Total solids |
| SNF | Solids not fat |
| SFN | Solids not fat content in milk |
| LOD | Limit of detection |
| BAF | Aerial part bioaccumulation factor |
| pH | Hydrogen potential |
| DR | Real density (for soil) |
| RD | Real density (for soil) |
| K | Potassium |
| C | Carbon |
| H | Hydrogen |
| Na | Sodium |
| Dcorr | Corrected milk density |
| FP | Freezing point (of milk) |
| Salts | Mineral content (in milk) |
| INEN | Ecuadorian Institute for Standardization |
| MAE | Ministry of the Environment of Ecuador |
| Se_root | Selenium concentration in roots |
References
- Tomii, Y.; Shibayama, T.; Nishida, Y.; Nakamura, R.; Okumura, N.; Yamaguchi, H.; Tanokura, Y.; Oshima, Y.; Sugawara, N.; Fujisawa, K.; et al. Estimation of Volcanic Ashfall Deposit and Removal Works Based on Ash Dispersion Simulations. Nat. Hazards 2020, 103, 3377–3399. [Google Scholar] [CrossRef]
- Loughlin, S.C.; Sparks, S.; Brown, S.K.; Jenkins, S.F.; Vye-Brown, C. (Eds.) Global Volcanic Hazards and Risk; Cambridge University Press: Cambridge, UK, 2015; p. 388. ISBN 9781107111752. [Google Scholar]
- Barclay, J.; Haynes, K.; Houghton, B.; Johnston, D. Chapter 69—Social Processes and Volcanic Risk Reduction. In The Encyclopedia of Volcanoes, 2nd ed.; Sigurdsson, H., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 1203–1214. ISBN 978-0-12-385938-9. [Google Scholar]
- Covey, J.; Dominelli, L.; Horwell, C.J.; Rachmawati, L.; Martin-del Pozzo, A.L.; Armienta, M.A.; Nugroho, F.; Ogawa, R. Carers’ Perceptions of Harm and the Protective Measures Taken to Safeguard Children’s Health Against Inhalation of Volcanic Ash: A Comparative Study Across Indonesia, Japan and Mexico. Int. J. Disaster Risk Reduct. 2021, 59, 102194. [Google Scholar] [CrossRef]
- Trejos, E.M.; Silva, L.F.O.; Hower, J.C.; Flores, E.M.M.; González, C.M.; Pachón, J.E.; Aristizábal, B.H. Volcanic Emissions and Atmospheric Pollution: A Study of Nanoparticles. Geosci. Front. 2021, 12, 746–755. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Briceño, J.; Tonato, E.; Silva, M.; Paredes, M.; Armado, A. Metal content evaluation in soils and edible tissues of allium fistulosum l. On crops near the tungurahua volcano. Granja 2020, 32, 112–123. [Google Scholar] [CrossRef]
- Mihai, R.A.; Espinoza-Caiza, I.A.; Melo-Heras, E.J.; Cubi-Insuaste, N.S.; Pinto-Valdiviezo, E.A.; Catana, R.D. Does the Mineral Composition of Volcanic Ashes Have a Significant Effect on Agricultural Crops and Food Security? Toxics 2023, 11, 846. [Google Scholar] [CrossRef]
- Hernández Cevallos, R.E. Análisis del Último Período Eruptivo del Volcán Tungurahua y el Impacto en las Actividades Humanas en las Zonas de Incidencia del Cantón Penipe, Provincia de Chimborazo. Ph.D. Thesis, Universidad de Extremadura, Cáceres, Spain, 2018. [Google Scholar]
- Grijalva, J.; Córdova, J.J. La ceniza volcánica y su relación en el crecimiento de pastos y nutrición de rumiantes. Rev. Inf. 1999, 13, 16–18. [Google Scholar]
- Haro Haro, A.N. Evaluación del Impacto Ambiental en los Pastizales Producidos por el Proceso Eruptivo del Volcán Tungurahua en la Hacienda Choglontus. Master’s Thesis, Escuela Superior Politécnica del Chimborazo, Riobamba Canton, Ecuador, 2011. [Google Scholar]
- Reyes-Cabrera, Y.; Borges-Terrero, Y.; Hernández-Jatib, N.; García-Cruz, S.; Villazón-Gómez, J.A. Caracterización química de suelos de uso agrícola en una unidad de producción agroalimentaria de Moa. Min. Geol. 2023, 39, 44–54. [Google Scholar]
- Kemp, D.D. The Environment Dictionary; Psychology Press: London, UK; New York, NY, USA, 1998; p. 464. ISBN 0415127521, 9780415127523. [Google Scholar]
- Porras, O.N.; Valle, F.C. Cenizas Volcánicas: Contaminación Ambiental. Rev. Inst. Nac. Enfer-Medades Respir. 2004, 17, 232–238. [Google Scholar]
- Ruggieri, F.; Saavedra, J.; Fernandez-Turiel, J.L.; Gimeno, D.; Garcia-Valles, M. Environmental geochemistry of ancient volcanic ashes. J. Hazard. Mater. 2010, 183, 353–365. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Wang, Z.; Gong, J.; Gao, J.; Tang, S.; Ma, S.; Duan, Z. Heavy metal pollution in agricultural soils of a typical volcanic area: Risk assessment and source appointment. Chemosphere 2022, 304, 135340. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Report of the Fifth Session of the Codex Committee on Contaminants in Foods (REP11/CF), Hague, The Netherlands, 21–25 March 2011; FAO/WHO: Rome, Italy, 2011. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Phytoremediation: Halophytes as Promising Heavy Metal Hyperaccumulators. In Heavy Metals; Saleh, H.M., Eid Sayed, R.F., Eds.; IntechOpen: London, UK; Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Peirovi-Minaee, R.; Taghavi, M.; Harimi, M.; Zarei, A. Trace Elements in Commercially Available Infant Formulas in Iran: Determination and Estimation of Health Risks. Food Chem. Toxicol. 2024, 186, 114588. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in Plants on Polluted Soils: Effects of Soil Factors, Hyperaccumulation, and Amend-ments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Zhou, T.; Li, L.; Zhang, X.; Zheng, J.; Zheng, J.; Joseph, S.; Pan, G. Changes in Organic Carbon and Nitrogen in Soil with Metal Pollution by Cd, Cu, Pb and Zn: A Meta—Analysis. Eur. J. Soil Sci. 2016, 67, 237–246. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cadmium Pollution on Food Safety and Human Health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- World Health Organization. Exposure to Lead: A Major Public Health Concern: Preventing Disease Through Healthy Environments, 3rd ed.; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef]
- Basu, M. Impact of Mercury and Its Toxicity on Health and Environment: A General Perspective. In Mercury Toxicity: Challenges and Solutions; Kumar, N., Ed.; Springer Nature: Singapore, 2023; pp. 95–139. ISBN 978-981-99-7719-2. [Google Scholar]
- Pant, R.; Mathpal, N.; Chauhan, R.; Singh, A.; Gupta, A. A Review of Mercury Contamination in Water and Its Impact on Public Health. In Mercury Toxicity Mitigation: Sustainable Nexus Approach; Kumar, N., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 93–115. ISBN 978-3-031-48817-7. [Google Scholar]
- Rizwan, M.; Ali, S.; Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.W.; Tack, F.M.G.; Abbasi, G.H.; Hussain, A.; Igalavithana, A.D.; Lee, B.C.; et al. Effects of Selenium on the Uptake of Toxic Trace Elements by Crop Plants: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2531–2566. [Google Scholar] [CrossRef]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; p. 406. ISBN 0195070119, 9780195070118. [Google Scholar]
- McLaughlin, M.J.; Parker, D.R.; Clarke, J.M. Metals and micronutrients—Food safety issues. Field Crops Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- Castro-González, N.P.; Moreno-Rojas, R.; Calderón-Sánchez, F.; Moreno-Ortega, A.; Tamariz-Flores, J.V. Metales pesados en leche de vacas alimentadas con alfalfa producida en suelos irrigados con aguas residuales en Puebla y Tlaxcala, México. Rev. Mex. Cienc. Pecu. 2018, 9, 466–485. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef]
- Miclean, M.; Cadar, O.; Levei, E.A.; Roman, R.; Ozunu, A.; Levei, L. Metal (Pb, Cu, Cd, and Zn) transfer along food chain and health risk assessment through raw milk consumption from free-range cows. Int. J. Environ. Res. Public Health 2019, 16, 4064. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Beltrán, L.; Gavilanes-Terán, I.; Idrovo-Novillo, J.; Valverde, V.H.; Rodríguez-Pinos, A.; Paredes, C.; Signes-Pastor, A.J.; Carbonell-Barrachina, Á.A. Environmental pollution by heavy metals within the area influenced by the Tungurahua volcano eruption– Ecuador. Ecotoxicol. Environ. Saf. 2024, 270, 115919. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Martínez, M. Muestreo Probabilístico y no Probabilístico. Universidad del Istmo, Licenciatura en Ciencias Empresariales, Campus Ixtepec. 2017. Available online: https://www.gestiopolis.com/wp-content/uploads/2017/02/muestreo-probabilistico-no-probabilistico-guadalupe.pdf (accessed on 24 June 2025).
- Hernández-Ávila, C.E.; Escobar, N.A.C. Introducción a los tipos de muestreo. Alerta Rev. Cient. Inst. Nac. Salud 2019, 2, 75–79. [Google Scholar]
- Instituto Ecuatoriano de Normas Técnicas y Certificación (INEN) Leche Cruda. Requisitos; Quinta revisión; INEN: Quito, Ecuador, 2012. [Google Scholar]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mecha-nisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Díaz-Romeu, R.; Hunter, A. Metodologías de Muestreo de Suelos, Análisis Químico de Suelos y Tejido Vegetal y de In-Vestigaciones en Invernadero; Serie Materiales de Enseñanza/CATIE; CATIE: Turrialba, Costa Rica, 1978. [Google Scholar]
- Carrera-Beltrán, L.; Gavilanes-Terán, I.; Idrovo-Novillo, J.; Ramos, C.; Valverde, V.H.; Bravo-Basantes, V.; Ramos-Romero, S.; Paredes, C.; Hernández, F.; Carbonell-Barrachina, Á.A. Soil Amendments, Physicochemical Properties, and Metal Accumulation in Soils and Vegetables of Volcanic and Non-Volcanic Regions in Ecuador. Agronomy 2025, 15, 1166. [Google Scholar] [CrossRef]
- Gupta, I.C.; Yaduvanshi, N.P.S.; K, G.S. Standard Methods for Analysis of Soil, Plant and Water; Scientific Publishers: New Delhi, India, 2012; pp. 20–21. [Google Scholar]
- Jorhem, L.; Engman, J. Determination of Lead, Cadmium, Zinc, Copper, and Iron in Foods by Atomic Absorption Spectrometry After Microwave Digestion: NMKL1 Collaborative Study. J. AOAC Int. 2000, 83, 1189–1203. [Google Scholar] [CrossRef]
- Aras, N.K.; Ataman, O.Y. Trace Element Analysis of Food and Diet; Royal Society of Chemistry: London, UK, 2006; Volume 7. [Google Scholar]
- Mindak, W.R.; Dolan, S.P. Elemental Analysis Manual (EAM) Section 4.4: Inductively Coupled Plasma-Atomic Emission Spectrometric Determination of Elements in Food Using Microwave-Assisted Digestion; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2010; Version 1.1. Available online: https://www.fda.gov/media/95162/download (accessed on 21 May 2025).
- Signes-Pastor, A.J.; Burló, F.; Mitra, K.; Carbonell-Barrachina, A.A. Arsenic Biogeochemistry as Affected by Phosphorus Fertilizer Addition, Redox Potential and pH in a West Bengal (India) Soil. Geoderma 2007, 137, 504–510. [Google Scholar] [CrossRef]
- Paneque Pérez, V.M.; Calaña Naranjo, J.M.; Calderón Valdés, M.; Borges Benítez, Y.; Hernández García, T.C.; Caruncho Contreras, M. Manual de Técnicas Analíticas Para Análisis de Suelo, Foliar, Abonos Orgánicos y Fertilizantes Químicos; Ediciones INCA, Instituto Nacional de Ciencias Agrícolas: San José de las Lajas, Cuba, 2010; ISBN 978-959-7023-51-7. [Google Scholar]
- Signes-Pastor, A.J.; Mitra, K.; Sarkhel, S.; Hobbes, M.; Burló, F.; De Groot, W.T.; Carbonell-Barrachina, A.A. Arsenic Speciation in Food and Estimation of the Dietary Intake of Inorganic Arsenic in a Rural Village of West Bengal, India. J. Agric. Food Chem. 2008, 56, 9469–9474. [Google Scholar] [CrossRef]
- Vyslouzilova, M.; Tlustos, P.; Száková, J.; Pavlíková, D. As, Cd, Pb and Zn uptake by Salix spp. clones grown in soils enriched by high loads of these elements. Plant Soil Environ. 2003, 49, 191–196. [Google Scholar] [CrossRef]
- Tapia, C.E.F.; Cevallos, K.L.F. Pruebas para comprobar la normalidad de datos en procesos productivos: Anderson-Darling, Ryan-Joiner, Shapiro-Wilk y Kolmogórov-Smirnov. Societas 2021, 23, 83–106. Available online: https://revistas.up.ac.pa/index.php/societas/article/view/2302 (accessed on 4 July 2025).
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Patil, I.; Lüdecke, D. Methods and algorithms for correlation analysis in R. J. Open Source Softw. 2020, 5, 2306. [Google Scholar] [CrossRef]
- Secretaria de Medio Ambiente y Recursos Naturales (Semarnat). Norma Oficial Mexicana NOM-021-RECNAT-2000, que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreo y análisis. D. Of. Fed. 2002, 85. Available online: http://legismex.mty.itesm.mx/normas/rn/rn021-02.pdf (accessed on 24 June 2025).
- Nanzyo, M. Unique Properties of Volcanic Ash Soils. Glob. Environ. Res. Engl. Ed. 2002, 6, 99–112. Available online: https://d1wqtxts1xzle7.cloudfront.net/43820929/06_2-11-libre.pdf?1458226389=&response-content-disposition=inline%3B+filename%3DUnique_Properties_of_Volcanic_Ash_Soils.pdf&Expires=1759351566&Signature=K1AmR6pXx5DfCIa4lJHAvJuNA84OMMGkgBSEZoJrlzOqZCCPIzBLt1AsFQEAtzTlFHGnk14zqQc3g-6CoYL-UDrQymprRP0QfRqDMEaEa6RElMck890h87R1LQi36M489ytzQn-w58lKfSj8YpAarBBQiH73KnCrlSC2ZPH7k9vnP6r1DGgE3c3XCM5smOu4eibsuSMQLS5Ft-IsCaNhc8Ra-4AdFpgna8wDtitwHlgo7~lArpsU85QJvBpT5O4zRq4MTy00ddCUBFmCbEaeOrejqd~yrY~A1g1D-CoeqivBGjqtWX00NmyCROLCYgaj6GjUO4BVqvIlvgt2wN1mhg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 24 June 2025).
- Cantú, M.P.; Becker, A.; Bedano, J.C.; Schiavo, H.F. Evaluación de la calidad de suelos mediante el uso de indicadores e índices. Cienc. Suelo 2007, 25, 173–178. [Google Scholar]
- Zúñiga, F.; Buenaño, M.; Risco, D. Caracterización física y química de suelos de origen volcánico con actividad agrícola, próximos al volcán Tungurahua. Rev. Ecuat. Investig. Agropecu. 2016, 1, 5–10. [Google Scholar] [CrossRef]
- Silva, A. La Materia Orgánica del Suelo; Facultad de Agronomía: Montevideo, Uruguay, 1998. [Google Scholar]
- Pérez Leal, F. Fisiología Vegetal i, II, III y IV; Universidad Nacional de Ucayali: Pucallpa, Peru, 2017. [Google Scholar]
- Food and Agriculture Organization of the United Nations Soil Organic Carbon: The Hidden Potential; FAO: Rome, Italy, 2017.
- Alvarado Ochoa, S.P. “Dinámica de la Materia Orgánica en Suelos Agrícolas”. In Memorias del XI Congreso Ecuatori-ano de la Ciencia del Suelo: La Ciencia del Suelo y la Conservación Ambiental; Sociedad Ecuatoriana de la Ciencia del Suelo/Universidad Central del Ecuador: Quito, Ecuador, 2008; pp. 1–10. [Google Scholar]
- Lamz Piedra, A.; González Cepero, M.C. La salinidad como problema en la agricultura: La mejora vegetal una solución inmediata. Cultiv. Trop. 2013, 34, 31–42. [Google Scholar]
- Lovatt, C.J.; Mikkelsen, R.L. Phosphite fertilizers: What are they? Can you use them? What can they do. Better Crops 2006, 90, 11–13. [Google Scholar]
- Espinoza, F.; Argenti, P.; Gil, J.L.; León, L.; Perdomo, E. Evaluación del pasto king grass (Pennisetum purpureun cv. king grass) en asociación con leguminosas forrajeras (Evaluation of King Grass (Pennisetum purpureum cv. King Grass) associated with forages legumes). Zootec. Trop. 2001, 19, 59–71. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principios de Nutrición Vegetal, 4th ed.; International Potash Institute: Basel, Switzerland, 2000; p. 692. ISBN 9783905699066. [Google Scholar]
- FAO. El Manejo del Suelo en la Producción de Hortalizas con Buenas Prácticas Agrícolas; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2013; ISBN 978-92-5-307783-0/978-92-5-307784-7. [Google Scholar]
- Cano, R.M.Y.; Villanueva, M.C. Almacenamiento de carbono en pastos naturales altoandinos. Sci. Agrope-Cuaria 2013, 4, 313–319. [Google Scholar]
- Revilla, A. Tecnología de La Leche: Procesamiento, Manufactura y Análisis, 2nd ed.; IICA Serie de Libros y Materiales Educativos, 53; Instituto Interamericano de Cooperación para la Agricultura: San José, Costa Rica, 1982; p. xiii. ISBN 9789290390381. [Google Scholar]
- Rodríguez Vizcaino, A.Y. Determinación de la Inocuidad y Calidad Fisicoquímica de Leche Cruda en Plantas Procesadoras del Cantón Salcedo. Master’s Thesis, Escuela Politécnica Nacional, Quito, Ecuador, 2016. [Google Scholar]
- Parmar, P.; Lopez-Villalobos, N.; Tobin, J.T.; Murphy, E.; McDonagh, A.; Crowley, S.V.; Kelly, A.L.; Shalloo, L. The effect of compositional changes due to seasonal variation on milk density and the determination of season-based density conversion factors for use in the dairy industry. Foods 2020, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gigli, I. La Buena Leche: Aspectos Biológicos y su Industrialización; Editorial Maipue: Santa Rosa, Argentina, 2017; ISBN 978-9873615139. [Google Scholar]
- Uscanga-Domínguez, L.F.; Orozco-García, I.J.; Vázquez-Frias, R.; Aceves-Tavares, G.R.; Albrecht-Junnghans, R.E.; Amieva-Balmori, M.; Bazaldua-Merino, L.A.; Bernal-Reyes, R.; Camacho-de León, M.E.; Campos-Gutiérrez, J.A. Technical position on milk and its derivatives in adult health and disease from the Asociación Mexicana de Gastro-enterología and the Asociación Mexicana de Gerontología y Geriatría. Rev. Gastroenterol. Méx. Engl. Ed. 2019, 84, 357–371. [Google Scholar] [PubMed]
- Bath, D.L.; Dickinson, F.N.; Tucker, H.A.; Appleman, R.D. Ganado Lechero: Principios, Prácticas, Problemas y Beneficios, 2nd ed.; Editorial Interamericana: Ciudad de Mexico, Mexico, 1982; p. 336. [Google Scholar]
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; van Boekel, M.A.J.S. Dairy Technology: Principles of Milk Properties and Processes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999; 752p, ISBN 978-0-8247-0228-1. [Google Scholar] [CrossRef]
- Ministerio del Ambiente de Ecuador MAE Anexo 2 Del Libro VI Del Texto Unificado de Legislación Secundaria Del Ministerio Del Ambiente: Norma de Calidad Ambiental Del Recurso Suelo y Criterios de Remediación Para Suelos Contaminados. 2015. Available online: https://maeorellana.wordpress.com/wp-content/uploads/2015/11/anexo-2-suelo.pdf (accessed on 1 May 2025).
- Ravichandran, M. Interactions Between Mercury and Dissolved Organic Matter—A Review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef]
- Sanei, H.; Goodarzi, F. Relationship Between Organic Matter and Mercury in Recent Lake Sediment: The Physical–Geochemical Aspects. Appl. Geochem. 2006, 21, 1900–1912. [Google Scholar] [CrossRef]
- Yanai, J.; Mizuhara, S.; Yamada, H. Soluble Selenium Content of Agricultural Soils in Japan and Its Determining Factors with Reference to Soil Type, Land Use and Region. Soil Sci. Plant Nutr. 2015, 61, 312–318. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Jiang, C.; Li, Q.; Liu, Y.; Gu, C.; Shang, L.; Li, P.; Lin, Y.; Larssen, T. Understanding the Paradox of Selenium Contamination in Mercury Mining Areas: High Soil Content and Low Accumulation in Rice. Environ. Pollut. 2014, 188, 27–36. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Zhu, J.; Sapkota, A.; Meng, B.; Yao, H.; Qin, H.; Larssen, T. Selenium in Soil Inhibits Mercury Uptake and Translocation in Rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 10040–10046. [Google Scholar] [CrossRef]
- Tan, Z.; Rong, Q.; Wang, W.; Jiang, H.; Yu, L.; Hu, J.; Chen, J.; Liang, X.; Zhao, X.; Zhou, C. Strategies for Regulating the Bioavailability and Mobility of Se and Cd in Cd-Contaminated Seleniferous Soils: Coupling the Bio-available Se:cd Molar Ratio with Soil Properties. Agronomy 2024, 14, 2941. [Google Scholar] [CrossRef]
- Matamoros Veloza, A. Distribución Espacial de Selenio en Suelos y su Comportamiento Geoquímico Local al Oriente de Los Municipios de Útica y Villeta; Instituto de Investigación Geocientífica, Mineroambiental y Nuclear, Ministerio de Minas y Energía, República de Colombia: Bogotá, Colombia, 2001. [Google Scholar]
- Dai, J.; Becquer, T.; Rouiller, J.H.; Reversat, G.; Bernhard-Reversat, F.; Lavelle, P. Influence of Heavy Metals on c and n Mineralisation and Microbial Biomass in Zn-, Pb-, Cu-, and Cd-Contaminated Soils. Appl. Soil Ecol. 2004, 25, 99–109. [Google Scholar] [CrossRef]
- Xian, X.; Shokohifard, G.I. Effect of pH on Chemical Forms and Plant Availability of Cadmium, Zinc, and Lead in Polluted Soils. Water Air Soil Pollut. 1989, 45, 265–273. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, L.; Hao, S.; Zhou, X. Application of Selenium to Reduce Heavy Metal(loid)s in Plants Based on Meta-Analysis. Chemosphere 2024, 364, 143150. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization. Análisis de Riesgos Relativos a la Inocuidad de los Alimentos: Guía para las Autoridades Nacionales de Inocuidad de los Alimentos; FAO OMS: Roma, Italy, 2007; Available online: https://www.fao.org/4/a0822s/a0822s.pdf (accessed on 1 May 2025).
- Ministerio del Ambiente del Ecuador. Acuerdo Ministerial No. 097 (Registro Oficial Suplemento 387, 4 de noviembre de 2015). Libro VI, Anexo 2 del Texto Unificado de Legislación Secundaria del Ministerio del Ambiente: Normas de Calidad Ambiental del Recurso Suelo y Criterios de Remediación para Suelos Contaminados. Quito, Ecuador; 2015. Publicado en Registro Oficial Suplemento 387 el 4-Nov-2015. Available online: https://www.gob.ec/sites/default/files/regulations/2018-09/Documento_Registro-Oficial-No-387-04-noviembre-2015_0.pdf (accessed on 1 May 2025).
- Misra, S.G.; Mani, D. Soil Pollution; Ashish Publishing House: New Delhi, India, 1991. [Google Scholar]
- Fodor, E.; Szabó-Nagy, A.; Erdei, L. The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J. Plant Physiol. 1995, 147, 87–92. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Ziegler, H. Effect of sublethal concentrations of zinc, cadmium and mercury on the photo-synthetic carbon reduction cycle of Euglena. J. Plant Physiol. 1993, 142, 167–172. [Google Scholar] [CrossRef]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Mukerjee, S. Toxic effects of lead on growth and metabolism of germinating rice (Oryza sativa L.) seeds and on mitosis of onion (A. cepa L.) root tip cells. Ind. J. Exp. Biol. 1976, 14, 519–521. [Google Scholar]
- Kaji, T.; Suzuki, M.; Yamamoto, C.; Mishima, A.; Sakamoto, M.; Kozuka, H. Severe damage of cultured vascular endothelial cell monolayer after simultaneous exposure to cadmium and lead. Arch. Environ. Contam. Toxicol. 1995, 28, 168–172. [Google Scholar] [CrossRef]
- Barrachina, A.C.; Carbonell, F.B.; Beneyto, J.M. Arsenic uptake, distribution, and accumulation in tomato plants: Effect of arsenite on plant growth and yield. J. Plant Nutr. 1995, 18, 1237–1250. [Google Scholar] [CrossRef]
- Cox, M.S.; Bell, P.F.; Kovar, J.L. Differential tolerance of canola to arsenic when grown hydroponically or in soil. J. Plant Nutr. 1996, 19, 1599–1610. [Google Scholar] [CrossRef]
- Marin, A.R.; Pezeshki, S.R.; Masschelen, P.H.; Choi, H.S. Effect of dimethylarsenic acid (DMAA) on growth, tissue arsenic, and photosynthesis of rice plants. J. Plant Nutr. 1993, 16, 865–880. [Google Scholar] [CrossRef]
- Abedin, M.J.; Cotter-Howells, J.; Meharg, A.A. Arsenic uptake and accumulation in rice (Oryza sativa L.) ir-rigated with contaminated water. Plant Soil 2002, 240, 311–319. [Google Scholar] [CrossRef]
- General Standard for Contaminants and Toxins in Food and Feed (Codex Stan 193–1995); Codex Alimentarius Commission: Rome, Italy, 1995.
- Chaney, R.L. Health risks associated with toxic metal in municipal sludge. Sludge-Health Risks Land Appl. 1980, 59–83. [Google Scholar]
- Tulsma, T.U. Norma de Calidad Ambiental y de Descarga de Efluentes: Recurso Agua. Libro VI Anexo1 2015. Available online: https://faolex.fao.org/docs/pdf/ecu112180.pdf (accessed on 1 October 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera-Beltrán, L.; Gavilanes-Terán, I.; Valverde-Orozco, V.H.; Ramos-Romero, S.; Paredes, C.; Carbonell-Barrachina, Á.A.; Signes-Pastor, A.J. Assessment of Heavy Metal Transfer from Soil to Forage and Milk in the Tungurahua Volcano Area, Ecuador. Agriculture 2025, 15, 2072. https://doi.org/10.3390/agriculture15192072
Carrera-Beltrán L, Gavilanes-Terán I, Valverde-Orozco VH, Ramos-Romero S, Paredes C, Carbonell-Barrachina ÁA, Signes-Pastor AJ. Assessment of Heavy Metal Transfer from Soil to Forage and Milk in the Tungurahua Volcano Area, Ecuador. Agriculture. 2025; 15(19):2072. https://doi.org/10.3390/agriculture15192072
Chicago/Turabian StyleCarrera-Beltrán, Lourdes, Irene Gavilanes-Terán, Víctor Hugo Valverde-Orozco, Steven Ramos-Romero, Concepción Paredes, Ángel A. Carbonell-Barrachina, and Antonio J. Signes-Pastor. 2025. "Assessment of Heavy Metal Transfer from Soil to Forage and Milk in the Tungurahua Volcano Area, Ecuador" Agriculture 15, no. 19: 2072. https://doi.org/10.3390/agriculture15192072
APA StyleCarrera-Beltrán, L., Gavilanes-Terán, I., Valverde-Orozco, V. H., Ramos-Romero, S., Paredes, C., Carbonell-Barrachina, Á. A., & Signes-Pastor, A. J. (2025). Assessment of Heavy Metal Transfer from Soil to Forage and Milk in the Tungurahua Volcano Area, Ecuador. Agriculture, 15(19), 2072. https://doi.org/10.3390/agriculture15192072








