Ethylene-Triggered Rice Root System Architecture Adaptation Response to Soil Compaction
Abstract
1. Introduction
2. Soil Compaction Reduces Crop Yield by Restricting Root Growth
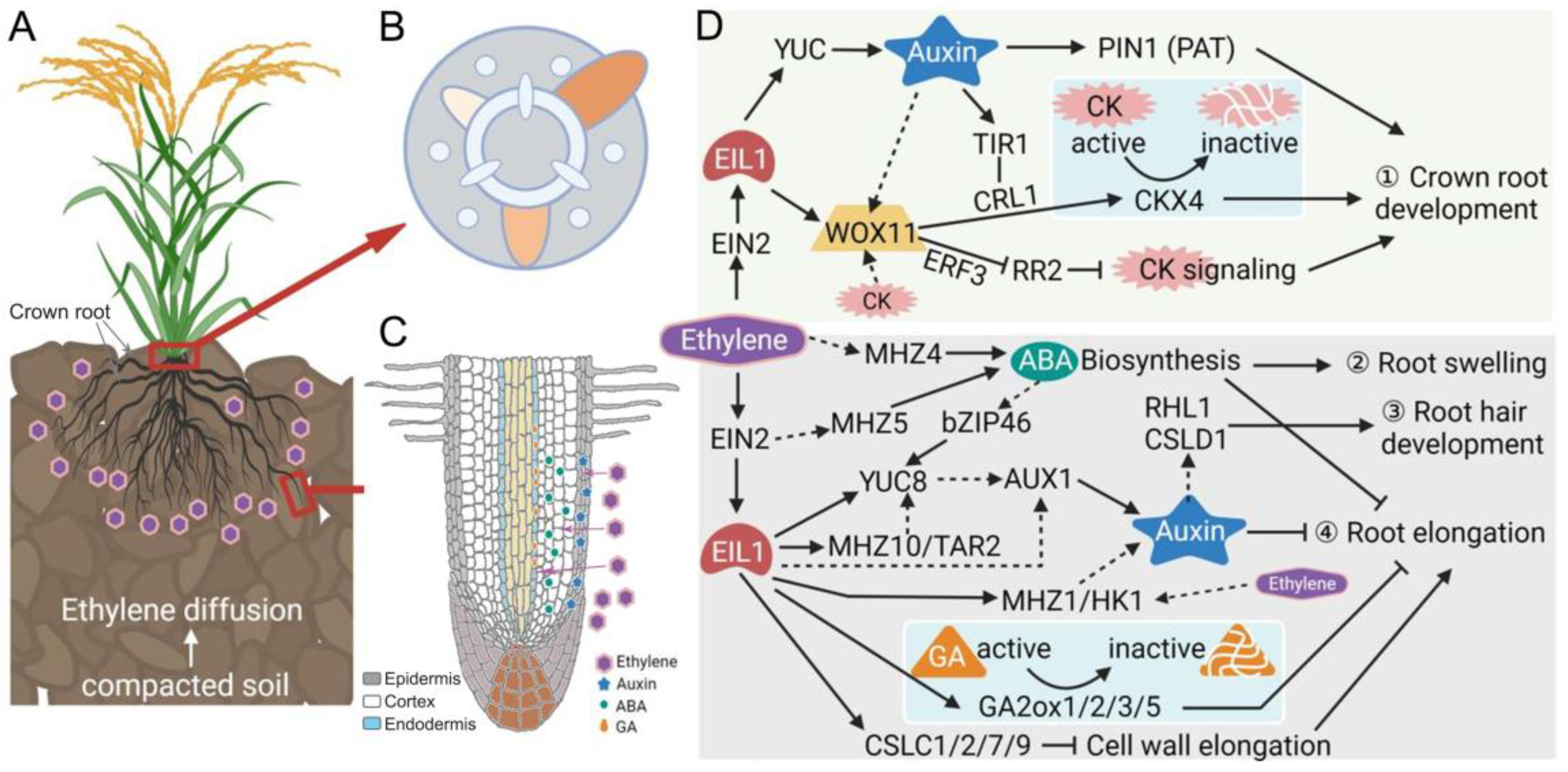
3. Ethylene Functions as a Key Signal for Root Adaption to Compacted Soils
4. Ethylene Interacts with Other Hormones to Modulate Root Architecture Under Soil Compaction
4.1. Interaction of Ethylene and Auxin in Response to Soil Compaction
4.2. Coordination of Ethylene and ABA in Response to Soil Compaction
4.3. Crosstalk of Ethylene and Cytokinin in Response to Soil Compaction
4.4. Integration of Ethylene and GA in Response to Soil Compaction
5. Conclusions and Perspectives
5.1. Bio-Based Practices: Effective Strategy for Soil Compaction Management
5.2. Ethylene: A Central Signal in Root Adaptation to Soil Compaction
5.3. From Lab to Field: Relevant Phenotyping and Functional Validation
5.4. Towards Precision Breeding: Multi-Hormonal Networks and Trait Integration
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and cropproductivity: An overview. Environ. Sci. Pollut. R. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Saqib, M.; Akhtar, J.; Qureshi, R.H. Pot study on wheat growth in saline and waterlogged compacted soil: II. Root growth and leaf ionic relations. Soil Tillage Res. 2004, 77, 179–187. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Janowiak, F.; Szczyrek, P.; Kaczanowska, K.; Ostrowska, A.; Rut, G.; Hura, T.; Rzepka, A.; Grzesiak, S. Impact of soil compaction stress combined with drought or waterlogging on physiological and biochemical markers in two maize hybrids. Acta Physiol. Plant 2016, 38, 109. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Li, C.; McHugh, A.D.; Li, Z.; Wu, C. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in southwestern China. Field Crop Res. 2018, 215, 163–172. [Google Scholar] [CrossRef]
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 2019, 70, 6019–6034. [Google Scholar] [CrossRef] [PubMed]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A Review on the Effect of Soil Compaction and its Management for Sustainable Crop Production. J. Biosyst. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Dörner, J.; Bravo, S.; Stoorvogel, M.; Dec, D.; Valle, S.; Clunes, J.; Horn, R.; Uteau, D.; Wendroth, O.; Lagos, L.; et al. Short-term effects of compaction on soil mechanical properties and pore functions of an Andisol. Soil Tillage Res. 2022, 221, 105396. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Liang, A.; Li, Y.; Song, Q.; Li, X.; Li, D.; Hou, N. Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: Emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef]
- Hiura, T.; Okada, H.; Terada, C.; Nakamura, M.; Kaneko, N. Effects of soil compaction on above- and belowground interactions during the early stage of forest development. Urban For. Urban Green. 2024, 102, 128565. [Google Scholar] [CrossRef]
- Horn, R.; Holthusen, D.; Dörner, J.; Mordhorst, A.; Fleige, H. Scale-dependent soil strengthening processes—What do we need to know and where to head for a sustainable environment? Soil Tillage Res. 2019, 195, 104388. [Google Scholar] [CrossRef]
- Lipiec, J.; Stępniewski, W. Effects of soil compaction and tillage systems on uptake and losses of nutrients. Soil Tillage Res. 1995, 35, 37–52. [Google Scholar] [CrossRef]
- Coudert, Y.; Périn, C.; Courtois, B.; Khong, N.G.; Gantet, P. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010, 15, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.K.; Huang, G.; Bhosale, R.; Hartman, S.; Sturrock, C.J.; Jose, L.; Martin, O.C.; Karady, M.; Voesenek, L.; Ljung, K.; et al. Plant roots sense soil compaction through restricted ethylene diffusion. Science 2021, 371, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Gao, Y.; Pandey, B.K.; Peralta, O.L.; Zhao, Y.; Quan, R.; Zhao, Z.; Jiang, L.; Huang, R.; et al. The OsEIL1-OsWOX11 transcription factor module controls rice crown root development in response to soil compaction. Plant Cell 2024, 36, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.G.R.; Jervis, G.; Xu, J.; Topping, J.F.; Lindsey, K. Root growth responses to mechanical impedance are regulated by a network of ROS, ethylene and auxin signalling in Arabidopsis. New Phytol. 2021, 231, 225–242. [Google Scholar] [CrossRef]
- Huang, G.; Kilic, A.; Karady, M.; Zhang, J.; Mehra, P.; Song, X.; Sturrock, C.J.; Zhu, W.; Qin, H.; Hartman, S.; et al. Ethylene inhibits rice root elongation in compacted soil via ABA- and auxin-mediated mechanisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2093895177. [Google Scholar] [CrossRef]
- Frene, J.P.; Pandey, B.K.; Castrillo, G. Under pressure: Elucidating soil compaction and its effect on soil functions. Plant Soil 2024, 502, 267–278. [Google Scholar] [CrossRef]
- Horn, R.; Domżżał, H.; Słowińska-Jurkiewicz, A.; van Ouwerkerk, C. Soil compaction processes and their effects on the structure of arable soils and the environment. Soil Tillage Res. 1995, 35, 23–36. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Ayantobo, O.O.; Li, Y.; Song, S. Multivariate Drought Frequency Analysis using Four-Variate Symmetric and Asymmetric Archimedean Copula Functions. Water Resour. Manag. 2019, 33, 103–127. [Google Scholar] [CrossRef]
- Khajehali, M.; Safavi, H.R.; Nikoo, M.R.; Najafi, M.R.; Alizadeh-Sh, R. A copula-based multivariate flood frequency analysis under climate change effects. Sci. Rep. 2025, 15, 146. [Google Scholar] [CrossRef]
- Wang, M.; He, D.; Shen, F.; Huang, J.; Zhang, R.; Liu, W.; Zhu, M.; Zhou, L.; Wang, L.; Zhou, Q. Effects of soil compaction on plant growth, nutrient absorption, and root respiration in soybean seedlings. Environ. Sci. Pollut. R. 2019, 26, 22835–22845. [Google Scholar] [CrossRef]
- Pierce, F.J.; Larson, W.E.; Dowdy, R.H.; Graham, W.A.P. Productivity of soils: Assessing long-term changes due to erosion. J. Soil. Water Conserv. 1983, 38, 39–44. [Google Scholar] [CrossRef]
- Pulido-Moncada, M.; Munkholm, L.J.; Schjønning, P. Wheel load, repeated wheeling, and traction effects on subsoil compaction in northern Europe. Soil Tillage Res. 2019, 186, 300–309. [Google Scholar] [CrossRef]
- Ishaq, M.; Hassan, A.; Saeed, M.; Ibrahim, M.; Lal, R. Subsoil compaction effects on crops in Punjab, Pakistan: I. Soil physical properties and crop yield. Soil Tillage Res. 2001, 59, 57–65. [Google Scholar] [CrossRef]
- Chen, G.; Weil, R.R. Root growth and yield of maize as affected by soil compaction and cover crops. Soil Tillage Res. 2011, 117, 17–27. [Google Scholar] [CrossRef]
- Obour, P.B.; Ugarte, C.M. A meta-analysis of the impact of traffic-induced compaction on soil physical properties and grain yield. Soil Tillage Res. 2021, 211, 105019. [Google Scholar] [CrossRef]
- Liu, H.; Colombi, T.; Jäck, O.; Keller, T.; Weih, M. Effects of soil compaction on grain yield of wheat depend on weather conditions. Sci. Total Environ. 2022, 807, 150763. [Google Scholar] [CrossRef]
- Fukai, S.; Cooper, M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crop Res. 1995, 40, 67–86. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Nada, R.M.; Abo-Hegazy, S.E.; Budran, E.G.; Abogadallah, G.M. The interaction of genes controlling root traits is required for the developmental acquisition of deep and thick root traits and improving root architecture in response to low water or nitrogen content in rice (Oryza sativa L.) cultivars. Plant Physiol. Bioch 2019, 141, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.H.; McCouch, S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front. Plant Sci. 2013, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular Mechanisms of Root Development in Rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Monshausen, G.B.; Haswell, E.S. A force of nature: Molecular mechanisms of mechanoperception in plants. J. Exp. Bot. 2013, 64, 4663–4680. [Google Scholar] [CrossRef]
- Bello-Bello, E.; López-Arredondo, D.; Rico-Chambrón, T.Y.; Herrera-Estrella, L. Conquering compacted soils: Uncovering the molecular components of root soil penetration. Trends Plant Sci. 2022, 27, 814–827. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Mooney, S.J. Soil compaction: A review of past and present techniques for investigating effects on root growth. J. Sci. Food Agric. 2011, 91, 1528–1537. [Google Scholar] [CrossRef]
- Fu, M.; Xiong, P.; Zhang, Z.; Peng, X. Responses of root hairs to soil compaction: A review. Plant Sci. 2025, 355, 112461. [Google Scholar] [CrossRef]
- Ma, B.; He, S.J.; Duan, K.X.; Yin, C.C.; Chen, H.; Yang, C.; Xiong, Q.; Song, Q.X.; Lu, X.; Chen, H.W.; et al. Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol. Plant 2013, 6, 1830–1848. [Google Scholar] [CrossRef]
- Yang, C.; Ma, B.; He, S.J.; Xiong, Q.; Duan, K.X.; Yin, C.C.; Chen, H.; Lu, X.; Chen, S.Y.; Zhang, J.S. MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 Regulate Ethylene Response of Roots and Coleoptiles and Negatively Affect Salt Tolerance in Rice. Plant Physiol. 2015, 169, 148–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, S.; Song, Y.; Huang, Y.; Zhou, S.; Liu, X.; Zhou, D.X. The Interaction between Rice ERF3 and WOX11 Promotes Crown Root Development by Regulating Gene Expression Involved in Cytokinin Signaling. Plant Cell 2015, 27, 2469–2483. [Google Scholar] [CrossRef]
- Zhang, T.; Li, R.; Xing, J.; Yan, L.; Wang, R.; Zhao, Y. The YUCCA-Auxin-WOX11 Module Controls Crown Root Development in Rice. Front. Plant Sci. 2018, 9, 523. [Google Scholar] [CrossRef]
- Geng, L.; Li, Q.; Jiao, L.; Xiang, Y.; Deng, Q.; Zhou, D.X.; Zhao, Y. WOX11 and CRL1 act synergistically to promote crown root development by maintaining cytokinin homeostasis in rice. New Phytol. 2023, 237, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yu, S.; Xiong, Y.; Song, X.; Nevescanin-Moreno, L.; Wei, X.; Rao, J.; Zhou, H.; Bennett, M.J.; Pandey, B.K.; et al. Root hairs facilitate rice root penetration into compacted layers. Curr. Biol. 2024, 34, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic acid promotes auxin biosynthesis to inhibit primary root elongation in rice. Plant Physiol. 2023, 191, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yin, C.C.; He, S.J.; Lu, X.; Zhang, W.K.; Lu, T.G.; Chen, S.Y.; Zhang, J.S. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet. 2014, 10, 1004701. [Google Scholar] [CrossRef]
- Yin, C.C.; Ma, B.; Collinge, D.P.; Pogson, B.J.; He, S.J.; Xiong, Q.; Duan, K.X.; Chen, H.; Yang, C.; Lu, X.; et al. Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 2015, 27, 1061–1081. [Google Scholar] [CrossRef]
- Qiu, Y.; Tao, R.; Feng, Y.; Xiao, Z.; Zhang, D.; Peng, Y.; Wen, X.; Wang, Y.; Guo, H. EIN3 and RSL4 interfere with an MYB-bHLH-WD40 complex to mediate ethylene-induced ectopic root hair formation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2110004118. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene signaling in rice and Arabidopsis: New regulators and mechanisms. J. Integr. Plant Biol. 2021, 63, 102–125. [Google Scholar] [CrossRef]
- Benfey, P.N.; Scheres, B. Root development. Curr. Biol. 2000, 10, R813–R815. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Z.; Wang, J.; Chen, X.; Wei, P.; Huang, R. The activation of OsEIL1 on YUC8 transcription and auxin biosynthesis is required for ethylene-inhibited root elongation in rice early seedling development. PLoS Genet. 2017, 13, e1006955. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.H.; Zhang, B.C.; Yang, H.L.; Tian, Y.B.; Huang, Y.H.; Yin, C.C.; Tao, J.J.; Wei, W.; Zhang, W.K.; et al. CELLULOSE SYNTHASE-LIKE C proteins modulate cell wall establishment during ethylene-mediated root growth inhibition in rice. Plant Cell 2024, 36, 3751–3769. [Google Scholar] [CrossRef]
- Qin, H.; Pandey, B.K.; Li, Y.; Huang, G.; Wang, J.; Quan, R.; Zhou, J.; Zhou, Y.; Miao, Y.; Zhang, D.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, B.; Tao, J.; Yin, C.; Hu, Y.; Huang, Y.; Wei, W.; Xin, P.; Chu, J.; Zhang, W.; et al. Rice EIL1 interacts with OsIAAs to regulate auxin biosynthesis mediated by the tryptophan aminotransferase MHZ10/OsTAR2 during root ethylene responses. Plant Cell 2022, 34, 4366–4387. [Google Scholar] [CrossRef]
- Schneider, H.M.; Strock, C.F.; Hanlon, M.T.; Vanhees, D.J.; Perkins, A.C.; Ajmera, I.B.; Sidhu, J.S.; Mooney, S.J.; Brown, K.M.; Lynch, J.P. Multiseriate cortical sclerenchyma enhance root penetration in compacted soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2012087118. [Google Scholar] [CrossRef] [PubMed]
- Leuther, F.; Iseskog, D.; Keller, T.; Larsbo, M.; Pandey, B.K.; Colombi, T. Root Circumnutation Reduces Mechanical Resistance to Soil Penetration. Plant Cell Environ. 2025, 48, 1608–1620. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, K.; Ma, B.; Yin, C.; Hu, Y.; Tao, J.; Huang, Y.; Cao, W.; Chen, H.; Yang, C.; et al. Histidine kinase MHZ1/OsHK1 interacts with ethylene receptors to regulate root growth in rice. Nat. Commun. 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.; Nonomura, K.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Kitano, H.; Nagato, Y. Rice plant development: From zygote to spikelet. Plant Cell Physiol. 2005, 46, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005, 46, 1674–1681. [Google Scholar] [CrossRef]
- Choudhary, P.; Aggarwal, P.R.; Salvi, P.; Muthamilarasan, M. Molecular insight into auxin signaling and associated network modulating stress responses in rice. Plant Physiol. Biochem. 2025, 219, 109452. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Ito, M.; Sumikura, T.; Nakayama, A.; Nishimura, T.; Kitano, H.; Yamaguchi, I.; Koshiba, T.; Hibara, K.; Nagato, Y.; et al. The rice FISH BONE gene encodes a tryptophan aminotransferase, which affects pleiotropic auxin-related processes. Plant J. 2014, 78, 927–936. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Coudert, Y.; Le, V.A.T.; Adam, H.; Bes, M.; Vignols, F.; Jouannic, S.; Guiderdoni, E.; Gantet, P. Identification of CROWN ROOTLESS1-regulated genes in rice reveals specific and conserved elements of postembryonic root formation. New Phytol. 2015, 206, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Exogenous Abscisic Acid Application During Grain Filling in Winter Wheat Improves Cold Tolerance of Offspring’s Seedlings. J. Agron. Crop Sci. 2014, 200, 467–478. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-Epoxycarotenoid Dioxygenase 3 Regulates Plant Growth and Enhances Multi-Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef]
- Shkolnik-Inbar, D.; Bar-Zvi, D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar] [CrossRef]
- Ramachandran, P.; Wang, G.; Augstein, F.; de Vries, J.; Carlsbecker, A. Continuous root xylem formation and vascular acclimation to water deficit involves endodermal ABA signalling via miR165. Development 2018, 145, dev159202. [Google Scholar] [CrossRef]
- Li, C.; Shen, H.; Wang, T.; Wang, X. ABA Regulates Subcellular Redistribution of OsABI-LIKE2, a Negative Regulator in ABA Signaling, to Control Root Architecture and Drought Resistance in Oryza sativa. Plant Cell Physiol. 2015, 56, 2396–2408. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Liao, W.; Ma, Z.; Xu, X.; Wang, M.; Ren, P.; Niu, L.; Jin, X.; Zhu, Y. Hydrogen peroxide is involved in abscisic acid-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Biol. 2016, 59, 536–548. [Google Scholar] [CrossRef]
- Teng, Z.; Lyu, J.; Chen, Y.; Zhang, J.; Ye, N. Effects of stress-induced ABA on root architecture development: Positive and negative actions. Crop J. 2023, 11, 1072–1079. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jiang, W.; Long, F.; Cheng, S.; Yang, W.; Zhao, Y.; Zhou, D. Rice Homeodomain Protein WOX11 Recruits a Histone Acetyltransferase Complex to Establish Programs of Cell Proliferation of Crown Root Meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Tan, M.; Deng, Q.; Wang, Y.; Zhang, T.; Hu, X.; Ye, M.; Lian, X.; Zhou, D.; Zhao, Y. Transcription factors WOX11 and LBD16 function with histone demethylase JMJ706 to control crown root development in rice. Plant Cell 2024, 36, 1777–1790. [Google Scholar] [CrossRef]
- Gao, S.; Chu, C. Gibberellin Metabolism and Signaling: Targets for Improving Agronomic Performance of Crops. Plant Cell Physiol. 2020, 61, 1902–1911. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Lyu, Y.; Dong, X.; Niu, S.; Cao, R.; Shao, G.; Sheng, Z.; Jiao, G.; Xie, L.; Hu, S.; Tang, S.; et al. An orchestrated ethylene-gibberellin signaling cascade contributes to mesocotyl elongation and emergence of rice direct seeding. J. Integr. Plant Biol. 2024, 66, 1427–1439. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Khush, G.S. What it will take to Feed 5.0 Billion Rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Tuong, T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X. Bio-tillage: A new perspective for sustainable agriculture. Soil Till Res. 2021, 206, 104844. [Google Scholar] [CrossRef]
- Domnariu, H.; Trippe, K.M.; Botez, F.; Partal, E.; Postolache, C. Long-term impact of tillage on microbial communities of an Eastern European Chernozem. Sci. Rep. 2025, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; van der Putten, W.H.; Klironomos, J.; Zhang, F.; Zhang, J. Steering plant-soil feedback for sustainable agriculture. Science 2025, 389, eads2506. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Li, J.; Xun, M.; Shi, J.; Chen, B.; Cheng, Y.; Zhang, W.; Yang, H. Root 1-aminocyclopropane-l-carboxylic acid (ACC) and rhizosphere ACC deaminase-producing bacteria affect apple root architecture under soil compaction stress. Plant Soil 2023, 489, 629–643. [Google Scholar] [CrossRef]
- Xu, J.; Long, Z.; Sun, B.; Zhang, F.; Shen, J.; Jin, K. Optimizing Root Phenotypes for Compacted Soils: Enhancing Root-Soil-Microbe Interactions. Plant Cell Environ. 2025, 48, 4656–4667. [Google Scholar] [CrossRef]
- Zhai, M.; Chen, Y.; Pan, X.; Chen, Y.; Zhou, J.; Jiang, X.; Zhang, Z.; Xiao, G.; Zhang, H. OsEIN2-OsEIL1/2 pathway negatively regulates chilling tolerance by attenuating OsICE1 function in rice. Plant Cell Environ. 2024, 47, 2561–2577. [Google Scholar] [CrossRef]
- Chen, G.; Feng, H.; Hu, Q.; Qu, H.; Chen, A.; Yu, L.; Xu, G. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development. Plant Biotechnol. J. 2015, 13, 833–848. [Google Scholar] [CrossRef]
- Pfeifer, J.; Kirchgessner, N.; Colombi, T.; Walter, A. Rapid phenotyping of crop root systems in undisturbed field soils using X-ray computed tomography. Plant Methods 2015, 11, 41. [Google Scholar] [CrossRef]
- Mehra, P.; Banda, J.; Ogorek, L.L.P.; Fusi, R.; Castrillo, G.; Colombi, T.; Pandey, B.K.; Sturrock, C.J.; Wells, D.M.; Bennett, M.J. Root Growth and Development in “Real Life”: Advances and Challenges in Studying Root-Environment Interactions. Annu. Rev. Plant Biol. 2025, 76, 467–492. [Google Scholar] [CrossRef]
- Zhu, M.; Hsu, C.; Peralta Ogorek, L.L.; Taylor, I.W.; La Cavera, S.; Oliveira, D.M.; Verma, L.; Mehra, P.; Mijar, M.; Sadanandom, A.; et al. Single-cell transcriptomics reveal how root tissues adapt to soil stress. Nature 2025, 642, 721–729. [Google Scholar] [CrossRef]
- Wang, G.; Dinneny, J.R. Plant biology: Soil compaction rewires gene expression across root cell types. Curr. Biol. 2025, 35, R715–R718. [Google Scholar] [CrossRef]
- Li, B.; Sun, C.; Li, J.; Gao, C. Targeted genome-modification tools and their advanced applications in crop breeding. Nature 2024, 25, 603–622. [Google Scholar] [CrossRef]
- Tuncel, A.; Pan, C.; Clem, J.S.; Liu, D.; Qi, Y. CRISPR-Cas applications in agriculture and plant research. Nat. Mol. 2025, 26, 419–441. [Google Scholar] [CrossRef]
| Gene Name | Gene Function | Reference |
|---|---|---|
| MHZ7/OsEIN2 (Os07g0155600) | Key component of ethylene signaling pathway, roots of mutants display enhanced ability to penetrate compacted soil. | [13,39] |
| MHZ6/OsEIL1 (Os03g0324300) | Key component of ethylene signaling pathway, roots of mutants display enhanced ability to penetrate compacted soil. | [13,40] |
| OsWOX11 (Os07g0684900) | Key regulator of crown root development, positively regulates ethylene and soil compaction-promoted crown root development | [14,41,42,43] |
| OsAUX1 (Os01g0856500) | Involved in auxin influx, roots of mutant display reduced response to ethylene and enhanced ability to penetrate compacted soil | [16,44] |
| OsYUC8 (Os03g0162000) | Involved in auxin biosynthesis, roots of mutant display reduced response to ethylene and enhanced ability to penetrate compacted soil | [16,44,45] |
| OsbZIP46 (Os06g0211200) | Positively regulates ABA-induced root inhibition and radial expansion; roots of mutant display enhanced ability to penetrate compacted soil. | [45] |
| MHZ4 (Os01g0128300) | Involved in ABA biosynthesis, roots of mutant display reduced response to ethylene and enhanced ability to penetrate compacted soil | [16,46] |
| MHZ5 (Os11g0572700) | Involved in ABA biosynthesis, roots of mutant display reduced response to ethylene and enhanced ability to penetrate compacted soil | [16,47] |
| OsABA1 (Os04g0448900) | Involved in ABA biosynthesis, roots of mutant display reduced response to ethylene and enhanced ability to penetrate compacted soil | [16] |
| OsABA2 (Os03g0810800) | Involved in ABA biosynthesis, roots of mutant display reduced response to ethylene and enhanced ability to penetrate compacted soil | [16] |
| OsRHL1 (Os06g0184000) | Acts downstream of auxin and mediates root hair elongation; roots of mutant display reduced ability to penetrate compacted soil. | [44] |
| OsCSLD1 (Os10g0578200) | Acts downstream of auxin and mediates root hair elongation; roots of mutant display reduced ability to penetrate compacted soil. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ge, B.; Yan, C.; Qi, Z.; Huang, R.; Qin, H. Ethylene-Triggered Rice Root System Architecture Adaptation Response to Soil Compaction. Agriculture 2025, 15, 2071. https://doi.org/10.3390/agriculture15192071
Li Y, Ge B, Yan C, Qi Z, Huang R, Qin H. Ethylene-Triggered Rice Root System Architecture Adaptation Response to Soil Compaction. Agriculture. 2025; 15(19):2071. https://doi.org/10.3390/agriculture15192071
Chicago/Turabian StyleLi, Yuxiang, Bingkun Ge, Chunxia Yan, Zhi Qi, Rongfeng Huang, and Hua Qin. 2025. "Ethylene-Triggered Rice Root System Architecture Adaptation Response to Soil Compaction" Agriculture 15, no. 19: 2071. https://doi.org/10.3390/agriculture15192071
APA StyleLi, Y., Ge, B., Yan, C., Qi, Z., Huang, R., & Qin, H. (2025). Ethylene-Triggered Rice Root System Architecture Adaptation Response to Soil Compaction. Agriculture, 15(19), 2071. https://doi.org/10.3390/agriculture15192071





