Methods of Phytic Acid Reduction in Bitter Lupine Seeds and Their Effects on the Microbiota of Calves
Abstract
1. Introduction
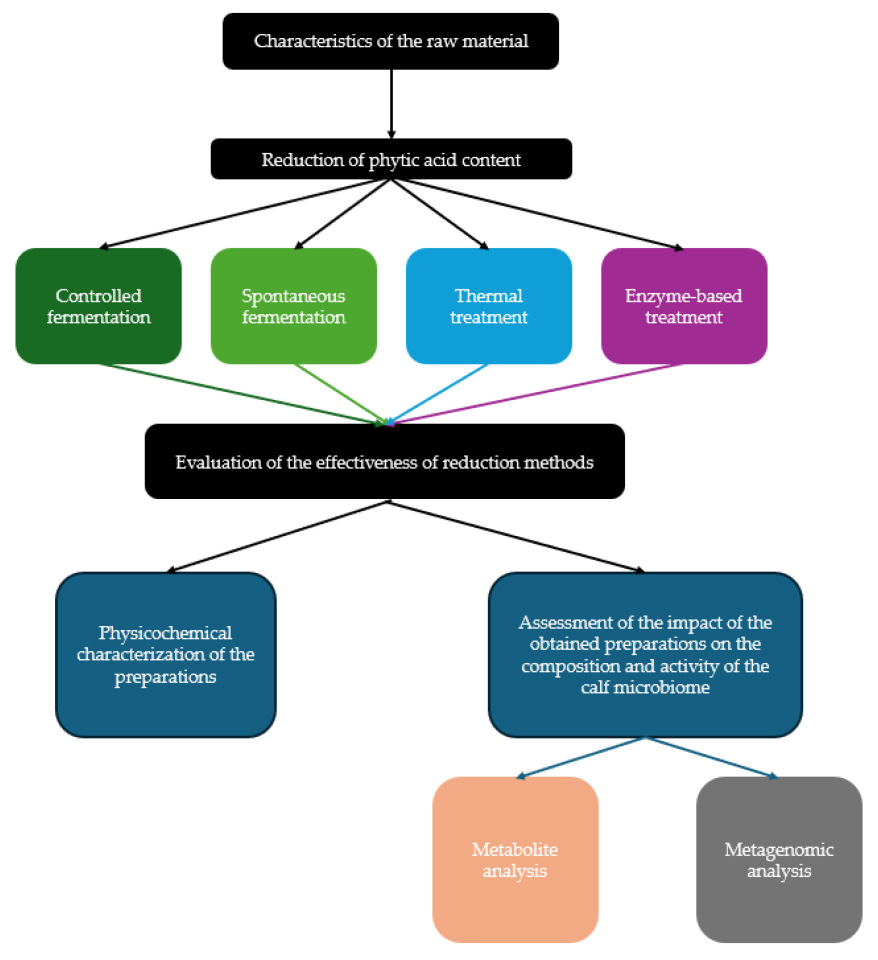
2. Experimental Design
2.1. Research Design
2.2. Materials and Methods
2.2.1. Biological Materials
- Lupine biomass: The biomass source was yellow lupine grains Lupinus angustifolius KARO with a dry matter content of 85 ± 0.021, total protein content of 33.78 ± 2.542, and crude fiber content of 15.06 ± 0.652%. Lupine grains were obtained from GRANUM Sp. J. in Łowicz (Poland).
- Bacterial strains: The bacterial strains used for the study were isolated from fermented corn and lupine. Bacterial strains were isolated from spontaneous maize and lupine silages using MRS medium (de Man, Rogosa and Sharpe), which supports the growth of lactic acid bacteria. After spontaneous fermentation, silage samples were diluted and cultured under anaerobic conditions. Colonies showing typical LAB morphology and positive Gram staining were selected and transferred to liquid MRS medium for further analysis. The following consortiums of strains deposited in the Polish Collection of Microorganisms were used: BPK1 (Lentilactobacillus buchneri, Pediococcus acidilactici), BPK4 (L. buchneri, P. acidilactici, Lentilactobacillus parakefiri); BPKK2 (L. buchneri, P. acidilactici), and BPL2 (Lactobacillus diolivorans, P. acidilactici, Furfurilactobacillus kisonensis). The detailed compositions and functional properties of the consortia were disclosed in a filing to the Polish Patent Office under the following application numbers: BPK1–P. 452894, BPK4–P. 452900, BPKK2–P. 452895, and BPL2–P. 452896.
2.2.2. Reagents and Growth Media
- Culture media and growth conditions: MRS broth (Merck, Darmstadt, Germany), selective for lactic acid bacteria, was used for the cultivation of bacteria (Merck, Darmstadt, Germany). The bacteria were cultured at a temperature of 30 ± 1 °C for 48 h under anaerobic conditions using an AnaeroGen Sachet (Oxoid™ AnaeroGen™ 2.5L Sachet, Thermo Scientific™).
- Digestive system model medium: The basic model medium was prepared by mixing and sterilizing the following ingredients (in grams per liter): arabinogalactan (1.0 g), pectin (2.0 g), xylan (1.0 g), starch (4.0 g), glucose (0.4 g), yeast extract (3.0 g), peptone (3.0 g), mucin (1.0 g), cysteine (0.5 g), and distilled water.
- Pancreatic juice: The composition of pancreatic juice included (in grams per liter): NaHCO3 (12.5 g), bile salts (6.0 g), and pancreatin (0.9 g).
2.2.3. Research Methods
- Phytic Acid Reduction
- 1.
- Controlled fermentation of lupine: 5% of the inoculum was added to crushed lupine biomass with tap water, in such a way as to ensure the even distribution of bacteria in the material. The dry mass of the biomass was previously measured, in order to determine the volume of inoculum that should be introduced into the biomass to obtain the final silage. After thorough mixing, the fermentation material was placed in tight, vacuum-sealed bags, which allowed for the creation of anaerobic conditions conducive to fermentation. The silages were fermented at 20 ± 1 °C for 8 weeks.
- 2.
- Spontaneous fermentation of lupine: Spontaneous fermentation was carried out by adding tap water to crushed lupine seeds and sealing them in tight, vacuum-sealed bags, which allowed for the creation of anaerobic conditions conducive to fermentation. The silages were fermented at 20 ± 1 °C for 8 weeks.
- 3.
- Thermal treatment: The lupine seeds were mixed with tap water in a ratio of 1:2 and cooked at 100 °C until softened, for 4 h.
- 4.
- Enzyme-based processing of lupine: The lupine seeds were soaked in tap water in a ratio of 1:2 and then blended using a food processor. Enzyme phytase of bacterial origin Axtra® PHY 5000G Premixture was added to the biomass in the proportion of 0.4 g per 1 kg of feed and mixed well for 20 min in 20 ± 1 °C (NOACK Polen Sp. z o.o., Poland).
- Dynamic in vitro digestion system: A system was used consisting of six consecutive glass fermenters (Prodigest, Ghent, Belgium) simulating the digestion process, with a total retention time of 72 h. Each fermenter was maintained at 37 °C using a thermostatic water bath, continuously stirred to simulate peristalsis, and maintained under anaerobic conditions by flushing with nitrogen every 24 h [9]. The fermenters were inoculated with a fecal suspension prepared from samples collected from a 9-week-old calf. To prepare the inoculum, 200 g of feces was homogenized in 1 L of sterile model medium. The suspension obtained in this way was added successively to fermenters simulating the large intestine: ascending (AC), transverse (TC), and descending (DC). The experiment consisted of two main stages. The first stage was a stabilization period during which the microbiota adapted to in vitro conditions. During this time, basal medium was added to the fermenters three times every 8 h to support microbial growth. The second stage consisted of replacing the model medium with a lupine feed component.
2.2.4. Analytical Methods
- Determination of dry matter content: Mass was measured on six weighing dishes. About 1 gram of each sample was added to each dish. The samples were placed in an oven (muff oven 12L PRO, Adverti) with the temperature set at 130 °C for 3 h.
- Determination of protein content: Protein content was determined using the Kjeldahl method, according to the methodology developed by Dygas and Berłowska [10].
- Determination of crude fiber content: The crude fiber content was determined according to the method developed by Dygas and Berłowska [10].
- Analysis of the mineral composition of lupine after selected methods of phytic acid reduction: total ammonium nitrogen (TAN, method no. 8038), orthophosphates (method no. 8000), iron (method no. 8112), manganese (method no. 8034), zinc (method no. 8009), and boron (test no. LCK307) were determined using a DR6000 spectrophotometer (HACH-LANGE, Loveland, CO, USA) in samples after mineralization. The tests were performed according to the manufacturer’s instructions.
- Chromatographic analysis of short-chain fatty acids: Samples collected from the ascending colon and descending colon of the gastrointestinal model were submitted to deproteinization using the Carrez method. Samples were centrifuged (4000 rpm, 10 min), and 3.5 mL of supernatant was mixed with 0.2 mL of Carrez I solution, followed after 2 min by 0.2 mL of Carrez II and 3.1 mL of distilled water. After a second centrifugation under the same conditions, the deproteinized supernatant was filtered through a 0.22 μm PTFE syringe filter. Analysis was performed by HPLC–MS using a Repromer H column (9 μm, Dr. Maish GmbH) with sulfuric acid as the mobile phase (0.5 mL/min, 60 min runtime). The column oven was maintained at 60 °C, and detection was carried out using a photodiode array detector.
- Enzymatic analysis of lactic acid: Lactic acid concentrations were determined using assay kits from Megazyme (Bray, Ireland), following the manufacturer’s protocol. The measurements were performed on 96-well plates using a Thermo Scientific Multiskan GO spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) [11].
- Phytic acid determination: In each sample taken from the methods selected to determine the degree of phytate reduction and from the control sample, the phytic acid content was determined using the Burgos-Luján and Tong method [12]. Phytic acid content was determined by extraction with 0.4 M HCl, followed by complex formation with ferric chloride hexaphosphate and sulfosalicylic acid. Samples were incubated for 24 h at room temperature, heated in a boiling water bath for 15 min, cooled, centrifuged (7500 rpm, 10 min), and filtered through a 0.45 μm PTFE filter. The supernatant was diluted (1:10) with deionized water, and pH was adjusted to 2.5 ± 0.5 using glycine. Titration with EDTA at 70–80 °C was performed until the color changed from purple to yellow. Standard solutions of phytic acid (0–700 mg/L) were analyzed in triplicate to generate a calibration curve for quantification.
- Metagenomic analysis of samples collected from the artificial digestive system: Metagenomic analysis of samples collected from the model digestive system was performed by Genomed S.A. (Warsaw, Poland).
2.3. Statistical Analysis
3. Results and Discussion
3.1. Phytic Acid Reduction
3.2. Physicochemical and Biological Characterization of Lupine
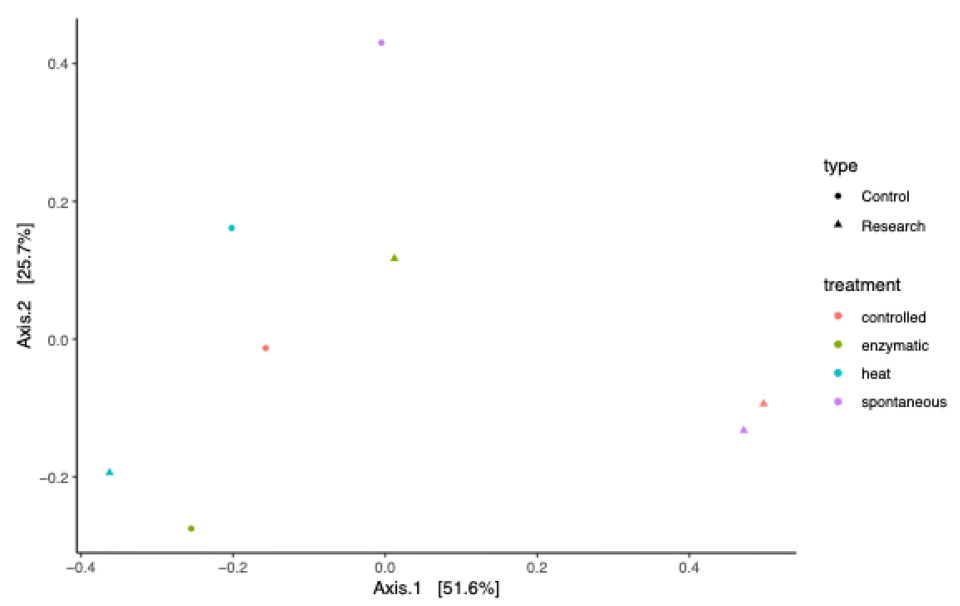
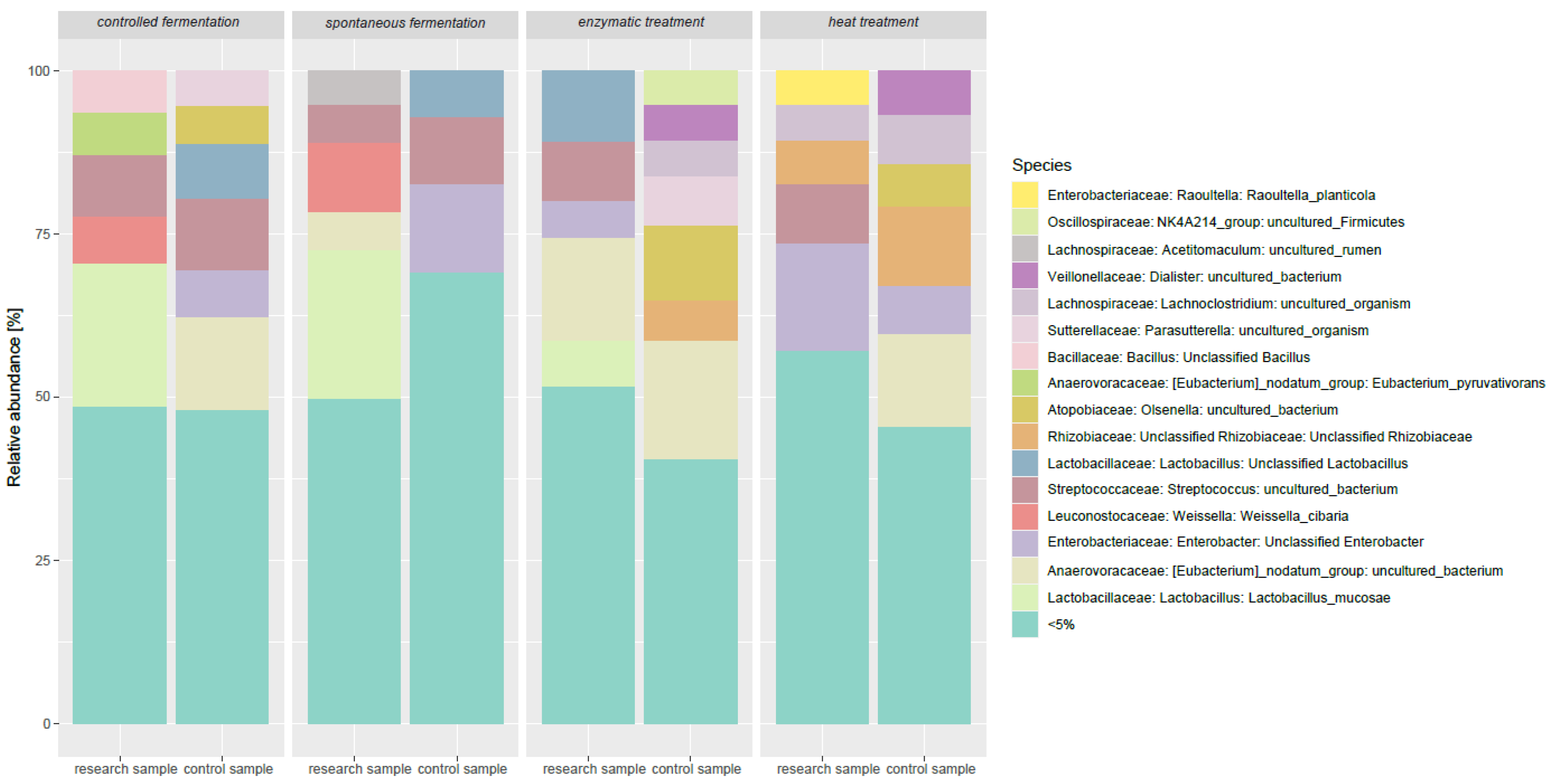
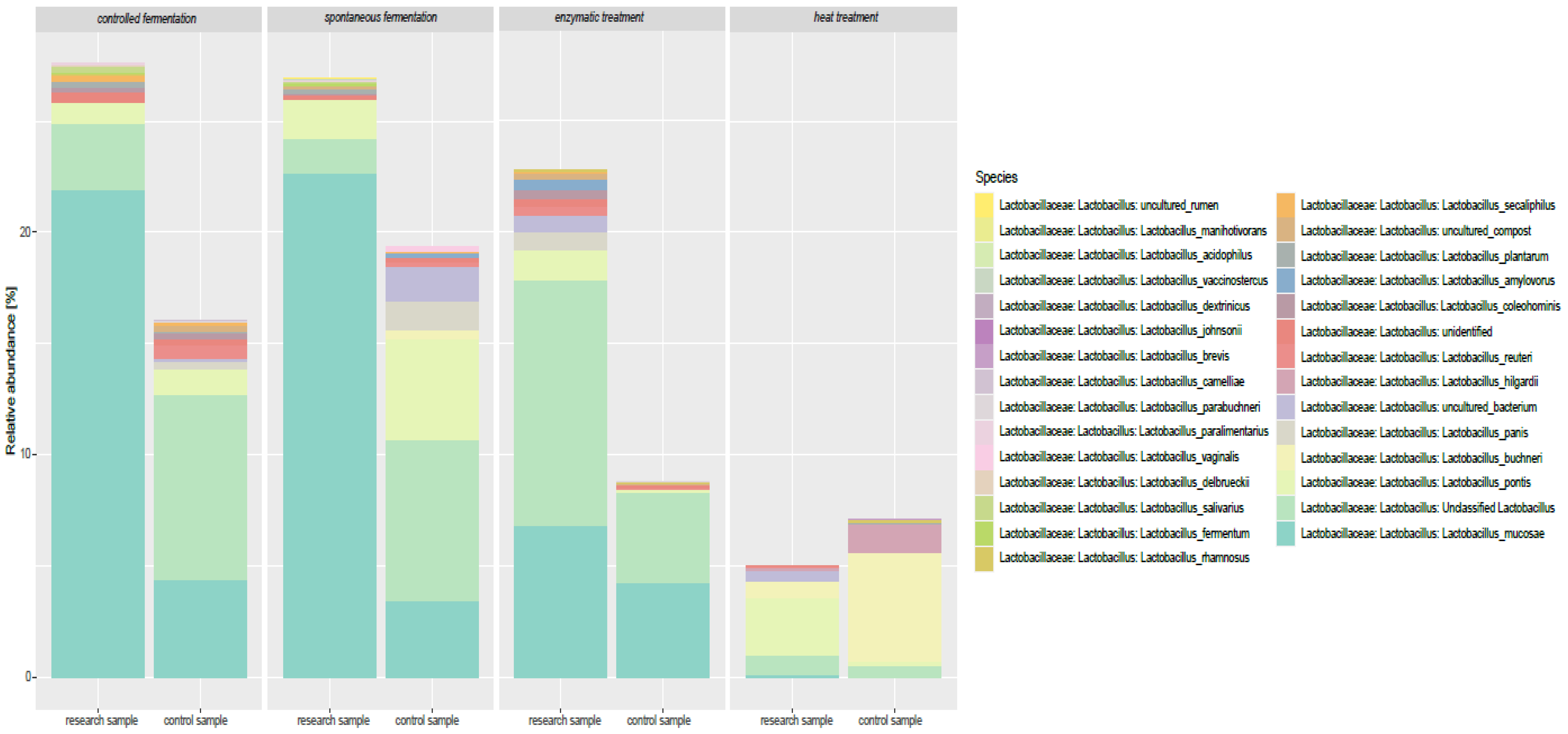
3.3. Impact of Lupine-Based Formulations on the Large Intestine Microbiome and Short-Chain Fatty Acid Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Library Name | Raw Read Pairs | Passing QC | % Passing QC | Pairs Joined | % Pairs Joined | Non-Chimeric Pairs | % Non- Chimeric Reads |
|---|---|---|---|---|---|---|---|
| DC1-1 | 71,834 | 59,390 | 82.68% | 54,330 | 75.63% | 46,095 | 64.17% |
| DC1-2 | 93,156 | 78,579 | 84.35% | 73,412 | 78.81% | 64,019 | 68.72% |
| DC1-3 | 104,329 | 87,053 | 83.44% | 82,164 | 78.75% | 70,897 | 67.96% |
| DC1-4 | 91,885 | 77,809 | 84.68% | 72,940 | 79.38% | 59,287 | 64.52% |
| DC2-1 | 98,736 | 83,822 | 84.90% | 78,833 | 79.84% | 67,424 | 68.29% |
| DC2-2 | 97,920 | 82,654 | 84.41% | 76,815 | 78.45% | 66,320 | 67.73% |
| DC2-3 | 86,606 | 73,336 | 84.68% | 69,328 | 80.05% | 60,737 | 70.13% |
| DC2-4 | 93,910 | 79,758 | 84.93% | 75,370 | 80.26% | 62,057 | 66.08% |
Appendix B
References
- Hyland, J. Majority of European Crops Feeding Animals and Cars, Not People. Greenpeace European Unit 2020. Available online: https://www.greenpeace.org/eu-unit/issues/nature-food/45159/majority-of-european-crops-feeding-animals-and-cars-not-people/ (accessed on 18 December 2023).
- Pepin, I. What’s In Animal Feed? The Animal Feed Industry. The Humane League 2023. Available online: https://thehumaneleague.org/article/animal-feed (accessed on 18 December 2023).
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Von Engelhardt, W.; Rönnau, K.; Rechkemmer, G.; Sakata, T. Absorption of short-chain fatty acids and their role in the hindgut of monogastric animals. Anim. Feed. Sci. Technol. 1989, 23, 43–53. [Google Scholar] [CrossRef]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef]
- Milman, N.T. A Review of Nutrients and Compounds, Which Promote or Inhibit Intestinal Iron Absorption: Making a Platform for Dietary Measures That Can Reduce Iron Uptake in Patients with Genetic Haemochromatosis. J. Nutr. Metab. 2020, 2020, 7373498. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid State Fermentation with Lactic Acid Bacteria to Improve the Nutritional Quality of Lupin and Soya Bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Van de Wiele, T.; Verstraete, W.; Possemiers, S. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef]
- Dygas, D.; Berłowska, J. Sugar beet processing waste as a substrate for yeast protein production for livestock feed. BioResources 2023, 18, 4458–4474. [Google Scholar] [CrossRef]
- Liszkowska, W.; Motyl, I.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Berłowska, J. Mixed Culture of Yeast and Lactic Acid Bacteria for Low-Temperature Fermentation of Wheat Dough. Molecules 2025, 30, 112. [Google Scholar] [CrossRef]
- Burgos-Luján, I.; Tong, A.Z. Determination of Phytic Acid in Juices and Milks by Developing a Quick Complexometric-Titration Method. Food Anal. Methods 2014, 8, 1836–1841. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Soetaert, W. Industrial Biotechnology: Sustainable Growth and Economic Success; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Eeckhout, W.; De Paepe, M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed. Sci. Technol. 1994, 47, 19–29. [Google Scholar] [CrossRef]
- Potocka, M.; Zaworska-Zakrzewska, A.; Gulewicz, P.; Nowak, P. The Effect of Fermentation of High Alkaloid Seeds of Lupinus angustifolius var. Karo by Saccharomyces cerevisiae, Kluyveromyces lactis, and Candida utilis on the Chemical and Microbial Composition of Protein-Xanthophyll Concentrate. 2017. Available online: https://www.researchgate.net/publication/320043626 (accessed on 18 December 2023).
- Zhang, G.; Fang, X.; Feng, G.; Li, Y.; Zhang, Y. Silage Fermentation, Bacterial Community, and Aerobic Stability of Total Mixed Ration Containing Wet Corn Gluten Feed and Corn Stover Prepared with Different Additives. Animals 2020, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Mineral Nutrition of Livestock, 5th ed.; CAB International: Wallingford, UK, 2022. [Google Scholar]
- Parish, J.A.; Karisch, B.B.; Rhinehart, J.D. Mineral and Vitamin Nutrition for Beef Cattle; Mississippi State University Extension Service Publication: Oxford, MS, USA, 2022; p. 2484. Available online: https://extension.msstate.edu/publications/mineral-and-vitamin-nutrition-for-beef-cattle (accessed on 18 December 2023).
- Haji, A.; Teka, T.A.; Bereka, T.Y.; Astatkie, T.; Woldemariam, H.W.; Urugo, M.M. Effect of Processing Methods on the Nutrient, Antinutrient, Functional, and Antioxidant Properties of Pigeon Pea (Cajanus cajan (L.) Millsp.) Flour. J. Agric. Food Res. 2024, 18, 101493. [Google Scholar] [CrossRef]
- Sahu, L.; Panda, S.K.; Paramithiotis, S.; Zdolec, N.; Ray, R.C. Biogenic Amines in Fermented Foods: Overview. In Fermented Foods: Part 1. Biochemistry & Biotechnology; Montet, D., Ray, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Leytem, A.B.; Williams, P.; Zuidema, S.; Martinez, A.; Chong, Y.L.; Vincent, A.; Vincent, A.; Cronan, D.; Kliskey, A.; Wulfhorst, J.D.; et al. Cycling Phosphorus and Nitrogen through Cropping Systems in an Intensive Dairy Production Region. Agronomy 2021, 11, 1005. [Google Scholar] [CrossRef]
- Clark, J.H.; Klusmeyer, T.H.; Cameron, M.R. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef]
- Ørskov, E.R. Protein Nutrition in Ruminants, 2nd ed.; Academic Press: London, UK, 1992; pp. 41–93. [Google Scholar]
- Davidovich, A.; Bartley, E.E.; Milliken, G.A.; Dayton, A.D.; Deyoe, C.W.; Bechtle, R.M. Ammonia toxicity in cattle. IV. Effects of unprocessed or extrusion-cooked mixtures of grain and urea, biuret, or dicyanodiamide and liquid supplements on rumen and blood changes associated with toxicity. J. Anim. Sci. 1977, 45, 1397–1408. [Google Scholar] [CrossRef]
- Braun, U.; Gautschi, A.; Hässig, M. Metabolic and endocrine disorders in calves. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 177–192. [Google Scholar]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Ávila, C.L.S.; Pinto, J.C.; Neri, J.; Schwan, R.F. Microbiological and chemical profile of sugar cane silage fermentation inoculated with wild strains of lactic acid bacteria. Anim. Feed Sci. Technol. 2020, 259, 114369. [Google Scholar] [CrossRef]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A Review of Phytate, Iron, Zinc, and Calcium Concentrations in Plant-Based Complementary Foods Used in Low-Income Countries and Implications for Bioavailability. Food Nutr. Bull. 2010, 31 (Suppl. S2), S134–S146. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, S.; Platel, K.; Srinivasan, K. Influence of Heat Processing on the Bioaccessibility of Zinc and Iron from Cereals and Pulses Consumed in India. J. Trace Elem. Med. Biol. 2007, 21, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 23, 137–156. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Le Sciellour, M.; Labussière, E.; Zemb, O.; Renaudeau, D. Effect of dietary fiber content on nutrient digestibility and fecal microbiota composition in growing-finishing pigs. PLoS ONE 2018, 13, e0206159. [Google Scholar] [CrossRef]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141 (Suppl. S1), S15–S28. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Kim, J.-C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Allen, H.K. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 2012, 3, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Kim, M.; Liu, G.; Zhai, Y.; Liu, T.; Driver, J.D.; Jeong, K.C. The Gut Microbiota of Newborn Calves and Influence of Potential Probiotics on Reducing Diarrheic Disease by Inhibition of Pathogen Colonization. Front. Microbiol. 2021, 12, 772863. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, N.; Nawaz, M.; Yaqub, T.; Mehmood, A.K. Impact of Limosilactobacillus fermentum Probiotic Treatment on Gut Microbiota Composition in Sahiwal Calves with Rotavirus Diarrhea: A 16S Metagenomic Analysis Study. BMC Microbiol. 2024, 24, 114. [Google Scholar] [CrossRef]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ben Ismail, M.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar] [CrossRef]
- Qu, Q.; Li, H.; Bai, L.; Zhang, S.; Sun, J.; Lv, W.; Ye, C.; Liu, C.; Shi, D. Effects of heat stress on gut microbiome in rats. Indian J. Microbiol. 2021, 61, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Tang, H.-Y.; Chiang, M.-L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.E.; Carrender, B.; Roubicek, C.D.; Maurer, R.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Tokach, M.D.; Coble, K.F.; Goodband, R.D. Effects of iron injection timing on suckling and subsequent nursery and growing-finishing performance and hematological criteria. J. Anim. Sci. 2021, 99, skab071. [Google Scholar] [CrossRef]
- Anderson, C.L.; Harris, T.R.; van Kempen, T.A. Plant protein-induced shifts in the calf gut microbiome modulate butyrate production and immune function. Anim. Nutr. 2023, 12, 45–58. [Google Scholar]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35 (Suppl. S1), S35–S38. [Google Scholar] [CrossRef]
- Zhang, H.L.; Chen, Y.; Xu, X.L.; Yang, Y.X. Effects of Branched-chain Amino Acids on In vitro Ruminal Fermentation of Wheat Straw. Asian-Australas. J. Anim. Sci. 2013, 26, 523–528. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef]
- Han, B.; Liang, S.; Sun, J.; Tao, H.; Wang, Z.; Liu, B.; Wang, X.; Liu, J.; Wang, J. The Effect of Lactobacillus plantarum on the Fecal Microbiota, Short Chain Fatty Acids, Odorous Substances, and Blood Biochemical Indices of Cats. Microorganisms 2024, 12, 91. [Google Scholar] [CrossRef] [PubMed]
| Treatment Method | Average Phytic Acid Concentration [mg/mL] | Reduction Compared to the Control Sample [%] |
|---|---|---|
| Control | 2187.0 ± 1.178 * | - |
| Thermal | 433.1 ± 1.178 * | 80.20 |
| Enzymatic | 596.4 ± 2.945 * | 72.73 |
| Spontaneous fermentation | 113.4 ± 1.553 * | 94.81 |
| Controlled fermentation | 79.4 ± 1.773 * | 96.37 |
| Treatment Method | Physicochemical Characterization | Microbiological Characterization | |||
|---|---|---|---|---|---|
| pH | Dry Mass Content [%] | Protein Content [%] | Crude Fiber Content [%] | CFU/mL | |
| Dry biomass | - | 85 ± 0.021 * | 33.78 ± 2.542 | 15.06 ± 0.652 | 2.0 × 105 |
| Control | 6.55 | 35 ± 0.042 * | 25.43 ± 3.435 | 6.21 ± 0.024 * | 3.5 × 104 |
| Thermal | 6.64 | 37 ± 0.043 * | 17.31 ± 0.222 | 5.89 ± 0.295 | 0 |
| Enzymatic | 6.86 | 34 ± 0.022 * | 27.91 ± 0.588 | 5.80 ± 0.065 | 4.2 × 104 |
| Spontaneous fermentation | 4.51 | 36 ± 0.032 * | 17.24 ± 0.068 | 5.04 ± 0.033 * | 1.5 × 107 |
| Controlled fermentation | 5.12 | 40 ± 0.022 * | 20.59 ± 0.045 * | 4.52 ± 0.086 | 1.0 × 109 |
| Treatment Method | |||||
|---|---|---|---|---|---|
| Control | Thermal | Enzymatic | Spontaneous Fermentation | Controlled Fermentation | |
| Ammonia (NH4+) [mgNH4/g d.m.] | 0.47 ± 0.12 | 16.81 ± 0.23 | 4.20 ± 0.00 * | 13.19 ± 3.18 | 5.44 ± 0.65 |
| Total nitrogen [mgN/g d.m.] | 0.39 ± 0.9 | 13.84 ± 0.19 | 3.46 ± 0.00 * | 7.03 ± 4.25 | 4.48 ± 0.53 |
| Treatment Method | |||||
|---|---|---|---|---|---|
| Control | Thermal | Enzymatic | Spontaneous Fermentation | Controlled Fermentation | |
| Phosphates [mgPO43−/g d.m.] | 0.96 ± 0.12 | 1.42 ± 0.13 | 1.20 ± 0.00 * | 1.25 ± 0.00 * | 0.38 ± 0.00 * |
| Treatment Method | |||||
|---|---|---|---|---|---|
| Control | Thermal | Enzymatic | Spontaneous Fermentation | Controlled Fermentation | |
| Boron [mgBor/g d.m.] | 4.42 ± 0.00 * | 2.39 ± 0.00 * | 2.56 ± 0.07 | 2.38 ± 0.13 | 10.08 ± 0.00 * |
| Iron [mgFe/g d.m.] | 9.54 ± 0.42 | 44.42 ± 0.23 | 25.56 ± 0.32 | 26.92 ± 0.31 | 69.92 ± 0.13 |
| Zink [mgZn/g d.m.] | 9.84 ± 0.00 * | 9.46 ± 0.00 * | 7.32 ± 0.00 * | 8.38 ± 0.00 * | 11.19 ± 0.00 * |
| Manganese [mgMn/g d.m.] | 4.00 ± 0.69 | 108.08 ± 1.76 | 2.40 ± 0.00 * | 1.25 ± 0.00 * | 42.37 ± 1.94 |
| SCFA | Week | Thermal Treatment | Enzymatic Treatment | Spontaneous Fermentation | Controlled Fermentation |
|---|---|---|---|---|---|
| Formic Acid [%] | 1 | 16.53 | 13.14 | 1.63 | 1.16 |
| 2 | 0.21 | 4.66 | 0.23 | 2.09 | |
| 3 | 8.90 | 0.00 | 0.70 | 0.47 | |
| Acetic Acid [%] | 1 | 28.15 | 32.17 | 36.55 | 39.15 |
| 2 | 21.45 | 15.34 | 39.33 | 36.18 | |
| 3 | 28.70 | 25.22 | 35.44 | 38.40 | |
| Propionic Acid [%] | 1 | 4.70 | 9.94 | 12.86 | 10.46 |
| 2 | 4.96 | 7.70 | 17.90 | 15.91 | |
| 3 | 3.75 | 12.15 | 19.17 | 16.24 | |
| Butanoic Acid [%] | 1 | 47.49 | 42.46 | 7.70 | 17.10 |
| 2 | 3.22 | 17.10 | 10.45 | 13.21 | |
| 3 | 25.36 | 19.37 | 14.75 | 8.91 | |
| Valeric Acid [%] | 1 | 265.18 | 260.62 | 27.65 | 59.06 |
| 2 | 318.21 | 276.76 | 28.92 | 47.34 | |
| 3 | 84.50 | 435.86 | 15.48 | 34.40 | |
| Lactic acid [%] | 1 | 25.69 | 24.50 | 24.02 | 31.69 |
| 2 | 21.41 | 94.15 | 25.31 | 37.47 | |
| 3 | 33.81 | 37.66 | 42.51 | 26.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płacheta-Kwiatkowska, B.; Brodowicz, O.; Cieciura-Włoch, W.; Wlaźlak, M.; Wilkowska, A.; Motyl, I.; Berłowska, J. Methods of Phytic Acid Reduction in Bitter Lupine Seeds and Their Effects on the Microbiota of Calves. Agriculture 2025, 15, 2061. https://doi.org/10.3390/agriculture15192061
Płacheta-Kwiatkowska B, Brodowicz O, Cieciura-Włoch W, Wlaźlak M, Wilkowska A, Motyl I, Berłowska J. Methods of Phytic Acid Reduction in Bitter Lupine Seeds and Their Effects on the Microbiota of Calves. Agriculture. 2025; 15(19):2061. https://doi.org/10.3390/agriculture15192061
Chicago/Turabian StylePłacheta-Kwiatkowska, Barbara, Oliwia Brodowicz, Weronika Cieciura-Włoch, Małgorzata Wlaźlak, Agnieszka Wilkowska, Ilona Motyl, and Joanna Berłowska. 2025. "Methods of Phytic Acid Reduction in Bitter Lupine Seeds and Their Effects on the Microbiota of Calves" Agriculture 15, no. 19: 2061. https://doi.org/10.3390/agriculture15192061
APA StylePłacheta-Kwiatkowska, B., Brodowicz, O., Cieciura-Włoch, W., Wlaźlak, M., Wilkowska, A., Motyl, I., & Berłowska, J. (2025). Methods of Phytic Acid Reduction in Bitter Lupine Seeds and Their Effects on the Microbiota of Calves. Agriculture, 15(19), 2061. https://doi.org/10.3390/agriculture15192061






