Abstract
Phytic acid is an antinutritional factor present in lupine seeds, which limits the bioavailability of essential minerals such as calcium, iron, and zinc. This study evaluated different methods of reducing phytic acid in bitter lupine (Lupinus angustifolius) and investigated the effects of the resulting reduction in phytic acid on the composition of gut microbiota. Bitter lupine is a legume rich in protein and fiber, but its high phytic acid content can limit mineral bioavailability. Four processing methods were compared as follows: thermal treatment, enzymatic hydrolysis with phytase, spontaneous fermentation, and controlled fermentation using lactic acid bacteria. Controlled fermentation resulted in the highest phytic acid reduction (96.37%), significantly improving mineral availability. Simulated digestion revealed that the fermented lupine feed positively influenced gut microbiota, increasing Lactobacillus abundance. Enzymatic and thermal treatments preserved more protein. However, they were less effective at removing phytic acid. These findings highlight controlled fermentation as a promising strategy for improving the nutritional value of lupine-based feed, offering a sustainable alternative to soybean-based livestock diets.
1. Introduction
The livestock industry often supplements low-protein cereals with soybean, raising environmental concerns. Lupine (Lupinus angustifolius) presents a sustainable, high-protein alternative, but its nutritional value is limited by antinutritional factors, notably phytic acid, which chelates essential minerals [1,2].
The addition of probiotics to animal diets can support gastrointestinal health and improve digestion. Feed probiotics often include lactic acid bacteria (e.g., Lactobacillus, Bifidobacterium) and yeasts from the Saccharomyces genus, which are typically species-specific. Probiotics promote gut homeostasis, enhance nutrient absorption, and may increase resistance to external stressors. Prebiotics, which are undigestible compounds, also benefit gut health by stimulating beneficial microflora and reducing food content pH. They are fermented by gut bacteria into short-chain fatty acids (SCFAs) like acetate, butyrate, and propionate. These SCFAs serve as energy sources and exhibit anti-inflammatory properties, promoting intestinal health and cell proliferation [3,4].
Lupine (Lupinus L.), a legume from the Fabaceae family, offers a sustainable alternative to soy as a protein source in animal feed. Cultivated in diverse climates, yellow lupine (Lupinus luteus) and narrowleaf lupine (Lupinus angustifolius) are the most common species in Europe. Lupine seeds are rich in protein (approximately 40% of dry mass) and fiber, with low starch content, which promotes gradual glucose release and supports gut health. Additionally, lupine contains unsaturated fatty acids, essential minerals like calcium and magnesium, and vitamins including niacin and vitamin E. These nutrients make lupine a viable feed source for both monogastric and ruminant animals [5].
Lupine seeds also contain antinutrients such as glucosinolates, which may irritate the gut, tannins, which inhibit iron absorption, and phytic acid (about 1.2 g/100 g), which binds minerals like zinc, magnesium, and calcium, reducing their bioavailability [6,7]. Zinc deficiency from high phytate intake can impair growth and immunity. Processing methods such as soaking, germination, and fermentation—especially lactic fermentation—can reduce phytate levels by up to 90%, improve mineral absorption, and support beneficial gut microbiota, though they may also cause nutrient loss or formation of biogenic amines (e.g., histamine, tyramine) if uncontrolled [6,8]. Lupine’s low alkaloid content in cultivated varieties minimizes toxicity risks, but wild species are rich in quinolizidine alkaloids, which can cause gastrointestinal and neurological issues. Washing seeds helps reduce alkaloid levels through leaching, making sweet lupines preferable for feed. Additionally, lupine lacks protease inhibitors and contains only trace amounts of lectins, unlike other legumes, further enhancing its suitability as animal feed [5].
Overall, lupine is a promising soy alternative in animal feed, combining high protein content, fiber, and bioactive compounds with low antinutrient levels and adaptability to diverse climates. Processing techniques, particularly fermentation, enhance its nutritional value and support gut health. This is especially important for young animals, whose immature digestive systems require carefully balanced protein sources to promote rumen development, reduce digestive disorders, and enable efficient weaning, ultimately lowering reliance on costly milk replacers while maintaining growth rates.
This study evaluated methods to reduce phytic acid in bitter lupine (Lupinus angustifolius) and their effects on mineral bioavailability and gut microbiota. Thermal treatment, phytase hydrolysis, spontaneous and controlled fermentation with lactic acid bacteria were tested to optimize lupine as a sustainable, nutrient-enhanced alternative to soybean-based feed.
The study aimed to compare four methods of reducing phytic acid in bitter lupine seeds—heat treatment, phytase application, spontaneous fermentation, and controlled fermentation with selected isolates—and to evaluate their effects on nutrient composition, microbiological profile, mineral bioavailability, and short-chain fatty acid production. Additionally, metagenomic analyses were performed to assess changes within an artificial digestive system.
2. Experimental Design
2.1. Research Design
The study was conducted in 2024 in four stages (Figure 1). In the first stage, bitter lupine seeds were subjected to four methods of phytic acid reduction: heat treatment, enzymatic degradation with phytase, spontaneous fermentation, and controlled fermentation with bacterial isolates obtained from fermented corn and lupine. The effectiveness of each method was assessed by measuring the phytic acid content. In the second stage, physicochemical and microbiological analyses were performed on the processed feed components, including determination of pH, dry matter, protein, crude fiber, simple sugars, and the content of lactic acid bacteria. The third stage focused on measuring the levels of selected micro- and macroelements before and after phytic acid reduction in order to evaluate the impact of each method on mineral bioavailability. In the final stage, the feed components were tested in an artificial digestive system simulating the gastrointestinal tract of a young calf, with particular attention to the production of short-chain fatty acids, branched-chain fatty acids, and lactic acid. Metagenomic analysis of the microbial community was also performed. The system was inoculated with fecal matter from a 9-week-old calf to replicate rumen conditions with sufficient microbial activity for fiber fermentation.
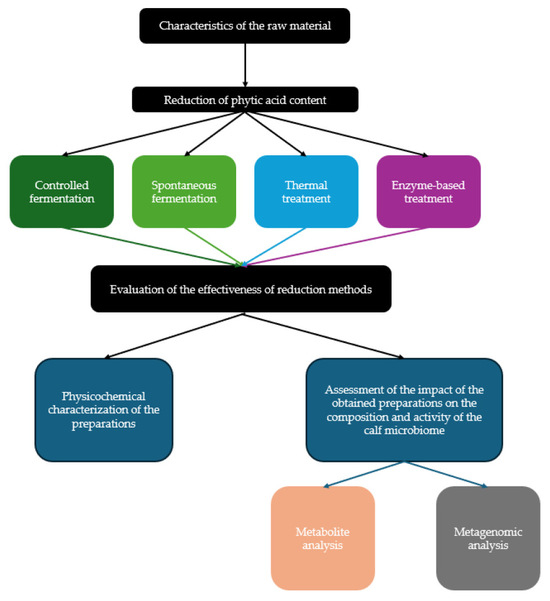
Figure 1.
Flowchart of experimental design.
2.2. Materials and Methods
2.2.1. Biological Materials
- Lupine biomass: The biomass source was yellow lupine grains Lupinus angustifolius KARO with a dry matter content of 85 ± 0.021, total protein content of 33.78 ± 2.542, and crude fiber content of 15.06 ± 0.652%. Lupine grains were obtained from GRANUM Sp. J. in Łowicz (Poland).
- Bacterial strains: The bacterial strains used for the study were isolated from fermented corn and lupine. Bacterial strains were isolated from spontaneous maize and lupine silages using MRS medium (de Man, Rogosa and Sharpe), which supports the growth of lactic acid bacteria. After spontaneous fermentation, silage samples were diluted and cultured under anaerobic conditions. Colonies showing typical LAB morphology and positive Gram staining were selected and transferred to liquid MRS medium for further analysis. The following consortiums of strains deposited in the Polish Collection of Microorganisms were used: BPK1 (Lentilactobacillus buchneri, Pediococcus acidilactici), BPK4 (L. buchneri, P. acidilactici, Lentilactobacillus parakefiri); BPKK2 (L. buchneri, P. acidilactici), and BPL2 (Lactobacillus diolivorans, P. acidilactici, Furfurilactobacillus kisonensis). The detailed compositions and functional properties of the consortia were disclosed in a filing to the Polish Patent Office under the following application numbers: BPK1–P. 452894, BPK4–P. 452900, BPKK2–P. 452895, and BPL2–P. 452896.
2.2.2. Reagents and Growth Media
- Culture media and growth conditions: MRS broth (Merck, Darmstadt, Germany), selective for lactic acid bacteria, was used for the cultivation of bacteria (Merck, Darmstadt, Germany). The bacteria were cultured at a temperature of 30 ± 1 °C for 48 h under anaerobic conditions using an AnaeroGen Sachet (Oxoid™ AnaeroGen™ 2.5L Sachet, Thermo Scientific™).
- Digestive system model medium: The basic model medium was prepared by mixing and sterilizing the following ingredients (in grams per liter): arabinogalactan (1.0 g), pectin (2.0 g), xylan (1.0 g), starch (4.0 g), glucose (0.4 g), yeast extract (3.0 g), peptone (3.0 g), mucin (1.0 g), cysteine (0.5 g), and distilled water.
- Pancreatic juice: The composition of pancreatic juice included (in grams per liter): NaHCO3 (12.5 g), bile salts (6.0 g), and pancreatin (0.9 g).
2.2.3. Research Methods
- Phytic Acid Reduction
- 1.
- Controlled fermentation of lupine: 5% of the inoculum was added to crushed lupine biomass with tap water, in such a way as to ensure the even distribution of bacteria in the material. The dry mass of the biomass was previously measured, in order to determine the volume of inoculum that should be introduced into the biomass to obtain the final silage. After thorough mixing, the fermentation material was placed in tight, vacuum-sealed bags, which allowed for the creation of anaerobic conditions conducive to fermentation. The silages were fermented at 20 ± 1 °C for 8 weeks.
- 2.
- Spontaneous fermentation of lupine: Spontaneous fermentation was carried out by adding tap water to crushed lupine seeds and sealing them in tight, vacuum-sealed bags, which allowed for the creation of anaerobic conditions conducive to fermentation. The silages were fermented at 20 ± 1 °C for 8 weeks.
- 3.
- Thermal treatment: The lupine seeds were mixed with tap water in a ratio of 1:2 and cooked at 100 °C until softened, for 4 h.
- 4.
- Enzyme-based processing of lupine: The lupine seeds were soaked in tap water in a ratio of 1:2 and then blended using a food processor. Enzyme phytase of bacterial origin Axtra® PHY 5000G Premixture was added to the biomass in the proportion of 0.4 g per 1 kg of feed and mixed well for 20 min in 20 ± 1 °C (NOACK Polen Sp. z o.o., Poland).
- Dynamic in vitro digestion system: A system was used consisting of six consecutive glass fermenters (Prodigest, Ghent, Belgium) simulating the digestion process, with a total retention time of 72 h. Each fermenter was maintained at 37 °C using a thermostatic water bath, continuously stirred to simulate peristalsis, and maintained under anaerobic conditions by flushing with nitrogen every 24 h [9]. The fermenters were inoculated with a fecal suspension prepared from samples collected from a 9-week-old calf. To prepare the inoculum, 200 g of feces was homogenized in 1 L of sterile model medium. The suspension obtained in this way was added successively to fermenters simulating the large intestine: ascending (AC), transverse (TC), and descending (DC). The experiment consisted of two main stages. The first stage was a stabilization period during which the microbiota adapted to in vitro conditions. During this time, basal medium was added to the fermenters three times every 8 h to support microbial growth. The second stage consisted of replacing the model medium with a lupine feed component.
2.2.4. Analytical Methods
- Determination of dry matter content: Mass was measured on six weighing dishes. About 1 gram of each sample was added to each dish. The samples were placed in an oven (muff oven 12L PRO, Adverti) with the temperature set at 130 °C for 3 h.
- Determination of protein content: Protein content was determined using the Kjeldahl method, according to the methodology developed by Dygas and Berłowska [10].
- Determination of crude fiber content: The crude fiber content was determined according to the method developed by Dygas and Berłowska [10].
- Analysis of the mineral composition of lupine after selected methods of phytic acid reduction: total ammonium nitrogen (TAN, method no. 8038), orthophosphates (method no. 8000), iron (method no. 8112), manganese (method no. 8034), zinc (method no. 8009), and boron (test no. LCK307) were determined using a DR6000 spectrophotometer (HACH-LANGE, Loveland, CO, USA) in samples after mineralization. The tests were performed according to the manufacturer’s instructions.
- Chromatographic analysis of short-chain fatty acids: Samples collected from the ascending colon and descending colon of the gastrointestinal model were submitted to deproteinization using the Carrez method. Samples were centrifuged (4000 rpm, 10 min), and 3.5 mL of supernatant was mixed with 0.2 mL of Carrez I solution, followed after 2 min by 0.2 mL of Carrez II and 3.1 mL of distilled water. After a second centrifugation under the same conditions, the deproteinized supernatant was filtered through a 0.22 μm PTFE syringe filter. Analysis was performed by HPLC–MS using a Repromer H column (9 μm, Dr. Maish GmbH) with sulfuric acid as the mobile phase (0.5 mL/min, 60 min runtime). The column oven was maintained at 60 °C, and detection was carried out using a photodiode array detector.
- Enzymatic analysis of lactic acid: Lactic acid concentrations were determined using assay kits from Megazyme (Bray, Ireland), following the manufacturer’s protocol. The measurements were performed on 96-well plates using a Thermo Scientific Multiskan GO spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) [11].
- Phytic acid determination: In each sample taken from the methods selected to determine the degree of phytate reduction and from the control sample, the phytic acid content was determined using the Burgos-Luján and Tong method [12]. Phytic acid content was determined by extraction with 0.4 M HCl, followed by complex formation with ferric chloride hexaphosphate and sulfosalicylic acid. Samples were incubated for 24 h at room temperature, heated in a boiling water bath for 15 min, cooled, centrifuged (7500 rpm, 10 min), and filtered through a 0.45 μm PTFE filter. The supernatant was diluted (1:10) with deionized water, and pH was adjusted to 2.5 ± 0.5 using glycine. Titration with EDTA at 70–80 °C was performed until the color changed from purple to yellow. Standard solutions of phytic acid (0–700 mg/L) were analyzed in triplicate to generate a calibration curve for quantification.
- Metagenomic analysis of samples collected from the artificial digestive system: Metagenomic analysis of samples collected from the model digestive system was performed by Genomed S.A. (Warsaw, Poland).
2.3. Statistical Analysis
Statistical analysis (ANOVA, Tukey test, p < 0.05) was performed for each result using Statistica v.14.0.1 (TIBCO Software INC., Palo Alto, CA, USA).
3. Results and Discussion
Over 60% of European cereals are used for livestock feed, but their low protein content is often supplemented with soy, which raises environmental concerns.
This study compared four methods to reduce phytic acid in bitter lupin: heat treatment, phytase, spontaneous fermentation, and controlled fermentation with Lactobacillus strains. Processed seeds were analyzed for nutritional value, then tested in a calf digestive simulator to assess fermentation products and microbiota effects.
3.1. Phytic Acid Reduction
The L. angustifolius seeds were subjected to various types of treatment. First, dry seeds were soaked and blended with water to obtain a dry mass content in the range of 30–40%. Seeds in this way were used as a control sample. Next, the biomass underwent four different treatments to decrease the content of phytates in the lupine seeds. Thermal treatment consisted of cooking the seeds for several hours until a cake-like consistency was obtained. Enzymatic treatment was carried out by supplementing the seeds with enzyme phytase, according to the manufacturer’s instructions. Lupine seeds were also subjected to both spontaneous and controlled fermentation, with an inoculum consisting of lactic acid bacteria. The treatments were compared in terms of their effectiveness at reducing the amount of phytic acid that is naturally present in legumes. For this purpose, the Luján–Tong method was used [12].
The application of heat treatment reduced the content of phytic acid to 433.1 mg/mL, which corresponds to a reduction of 80.20% compared to the control sample. This process, using high temperature, leads to partial degradation of phytic acid and denaturation of enzymatic proteins, which limits its availability (Table 1). According to the literature, heat treatment is effective at reducing antinutritional substances, but its effectiveness depends on the applied temperature and time parameters [13]. The use of the enzyme phytase led to a reduction in phytic acid by 72.73%, reaching an average value of 596.4 mg/mL. Phytase breaks down phytic acid into phosphates and inositol, increasing the bioavailability of minerals. Despite its lower efficiency compared to fermentation, the enzymatic method is valued for its ability to precisely control the process and produce relatively quick results [14].

Table 1.
Phytic acid reduction depending on the method used.
Spontaneous fermentation resulted in a significant reduction in phytic acid concentration, reaching 113.4 mg/mL, which corresponds to a reduction of 94.81%. The high efficiency of this method is due to the action of naturally present microorganisms, which produce phytolytic enzymes and lower pH, promoting phytic acid hydrolysis. Similar results were observed in studies on natural fermentation, which showed that the activity of microorganisms plays a key role in the removal of antinutritional substances. The best results were obtained using controlled fermentation, during which the phytic acid concentration dropped to 79.4 mg/mL, which means a reduction of 96.37%. The efficiency of this process can be maximized by precisely controlling the fermentation parameters, such as temperature, time, and microflora composition. These results are consistent with the literature, which highlights the key role of lactic acid bacteria in the elimination of phytic acid and improving the bioavailability of minerals [15].
In the production of silage for young calves, the choice between spontaneous and controlled fermentation plays a critical role in determining the nutritional quality, safety, and stability of the final feed. Spontaneous fermentation relies on naturally occurring microorganisms on the forage and in the environment, leading to slower acidification and often higher levels of undesirable microbes such as Clostridia, Enterobacteria, yeasts, and molds, which can generate butyric acid, ammonia, and mycotoxins—factors known to compromise feed hygiene and calf health [16]. In contrast, controlled fermentation involves the deliberate inoculation of forage with selected strains of lactic acid bacteria (LAB), such as Lactobacillus plantarum, Pediococcus pentosaceus, or L. buchneri, which rapidly convert water-soluble carbohydrates into lactic acid, lower pH, inhibit spoilage organisms, and reduce proteolysis and butyric acid formation [17]. This controlled approach yields more consistent fermentation outcomes, improved nutrient preservation, and enhanced aerobic stability—a particularly significant advantage when formulating silage for young calves, whose developing digestive systems are especially vulnerable to toxins, inconsistent feed quality, and microbial hazards. By ensuring rapid acidification and hygienic safety, controlled fermentation supports better animal performance, reduces health risks associated with poor-quality silage, and aligns with best practices in feed technology for juvenile ruminants [18]. As shown in Table 1, statistical analysis confirmed significant differences between treatments, with controlled fermentation achieving the highest and most stable reduction of phytic acid.
In summary, both spontaneous and controlled fermentation showed the highest efficiency at reducing phytic acid, which makes them the preferred methods for improving the nutritional value of feed. Controlled fermentation in particular, due to its precision and high efficiency, seems to be the most advantageous solution for use in industrial practice.
3.2. Physicochemical and Biological Characterization of Lupine
All treatments (Table 2) except fermentation significantly reduced dry matter (p < 0.05), with controlled fermentation yielding the highest dry matter (40%). Protein content was highest after enzymatic treatment (27.91%), exceeding spontaneous (17.24%) and controlled fermentation (20.59%), confirming the superior ability of enzymatic methods to preserve protein. Fermentation markedly lowered pH (4.51 for spontaneous, 5.12 for controlled), indicating organic acid production by LAB, whereas enzymatic and thermal treatments showed no significant pH change. Crude fiber decreased across treatments, with the greatest reduction observed in controlled fermentation (4.52%), consistent with microbial breakdown of structural polysaccharides and improved digestibility.

Table 2.
Physicochemical and microbiological characteristics of lupin feed components after the application of selected methods of phytic acid reduction.
The microbial counts (CFU/mL) showed substantial differences depending on treatments. Spontaneous fermentation resulted in a notable increase in LAB populations (1.5 × 107 CFU/mL), whereas controlled fermentation demonstrated the highest microbial count (1.0 × 109 CFU/mL). This difference highlights the efficacy of controlled fermentation at promoting LAB growth under optimized conditions. However, thermal treatment resulted in complete microbial inactivation, as expected due to high temperatures.
These results highlight controlled fermentation as the most effective method for improving the microbiological quality and digestibility of lupine feed. High LAB counts and pH reduction enhanced preservation, while crude fiber degradation improved nutrient availability. Enzymatic treatment best preserved protein but lacked probiotic benefits, and thermal treatment, despite reducing microbial activity, offered limited nutritional improvements. Overall, controlled fermentation provides the most balanced approach, combining microbial proliferation, improved preservation, and enhanced feed quality. These observations align with previous studies indicating that fermentation can modify the chemical composition of lupine seeds, affecting nutrient availability and microbial profiles. For instance, fermentation has been shown to reduce antinutritional factors in lupine seeds, thereby enhancing their nutritional value [13]. Additionally, the increase in lactic acid bacteria during fermentation contributes to the preservation and safety of the feed [15].
Analysis of the chemical composition of lupin biomass with reduced phytic acid content indicated that the different processing techniques affected the distribution of the tested inorganic components. This was primarily due to changes in the structure of phytic acid (Table 3).

Table 3.
Ammonia (NH4+) and total nitrogen content in liquid fractions of lupine samples after different phytic acid reduction treatments.
The effects of different processing methods aimed at reducing phytic acid content on the ammonium ions (NH4+) and total nitrogen levels in lupin samples are presented in Table 3. Compared to the control, all treatments significantly increased the NH4+ concentration, with the highest value observed in the thermally treated sample (16.81 ± 0.23 mg NH4+/g d.m.). Enzymatic treatment resulted in a significantly lower NH4+ level (4.20 ± 0.00 mg NH4+/g d.m., p < 0.05) compared to thermal and spontaneous fermentation treatments. A similar trend was observed for total nitrogen content, where thermal treatment again led to the highest increase (13.84 ± 0.19 mg N/g d.m.), and enzymatic treatment significantly differed from the control (3.46 ± 0.00 mg N/g d.m., p < 0.05). The observed increase in ammonium ions (NH4+) and total nitrogen contents in lupin samples subjected to thermal treatment, enzymatic hydrolysis, and fermentation is consistent with previous findings. Processing methods, particularly heat treatments and fermentation, have been shown to promote protein degradation, leading to the release of free amino groups and ammonium ions [19,20]. The enzymatic treatment, although effective at reducing phytic acid, resulted in a relatively lower increase in NH4+ content in liquid fractions, likely due to the selective action of phytases and minimal protein hydrolysis. In well-balanced diets for growing calves, ammonium ions serve as an important nitrogen source for rumen microorganisms, supporting microbial protein synthesis [21]. This process is particularly valuable as microbial protein constitutes a high-quality protein source for the host animal, providing essential amino acids for growth and development [22]. However, the relationship between ammonium ion concentration and calf performance follows a careful balance. While adequate levels support microbial growth, excessive concentrations may lead to ammonia toxicity and reduced feed efficiency [23]. From a physiological perspective, the rumen of older calves possesses greater capability to metabolize ammonium ions than the rumen of younger calves, due to enhanced microbial colonization and enzymatic activity. This greater capability allows for more efficient incorporation of non-protein nitrogen (NPN) sources like urea into microbial protein. Nevertheless, prudent formulation remains essential, as abrupt increases in ammonium ion load can temporarily disrupt rumen pH and microbial populations, even in older calves [24]. Therefore, lupin after controlled fermentation or enzymatic treatment seems to be the more favorable option for young calves.
Table 4 shows the phosphate (PO43−) concentrations in the differently processed lupin samples. Thermal, enzymatic, and spontaneous fermentation treatments resulted in significantly higher phosphate levels compared to the control (p < 0.05). Increased phosphate concentrations following the treatments further corroborate the effective breakdown of phytic acid, a major storage form of phosphorus in plant seeds. Phytase activity during fermentation liberates phosphate ions, making phosphorus more bioavailable [17]. The concentration of phosphate ions plays a vital role in meeting the nutritional requirements of growing calves. As the animals transition to a more forage-based diet, the phosphorus content becomes increasingly important for supporting their physiological development and metabolic functions [25]. Phosphorus is essential for growth and bone development, making its bioavailability in feed crucial. While inorganic phosphates are readily absorbed, a significant portion of phosphorus in plant-based silage exists as phytate, which older calves can utilize more efficiently than younger animals due to their more developed rumen microbial activity. However, the presence of natural phytases in well-fermented silage may improve phytate phosphorus availability, making proper ensiling techniques crucial for optimizing phosphorus nutrition [26].

Table 4.
Phosphate content in liquid fractions of lupin samples after application of different treatment methods for phytic acid reduction.
The nutritional significance of phosphate ions manifests in several key aspects. As the rumen becomes fully functional, calves gain enhanced capacity to utilize phosphorus for microbial protein synthesis and energy metabolism [21]. The fermentation process itself can affect phosphorus dynamics, with studies indicating that optimal lactic acid fermentation helps maintain phosphorus in forms more accessible to rumen microbes and intestinal absorption. However, during plant biomass fermentation, lactic acid bacteria (LAB) may release phosphates from organic compounds (e.g., phytic acid) via enzymatic activity (e.g., phytases) and simultaneously utilize available inorganic phosphates in the environment [27]. Therefore, the lupine feed obtained via controlled fermentation could be suitable for calves with immature rumens.
The ensiling process is known to significantly influence the availability of trace minerals in plant biomass, via pH changes, microbial enzymatic activity, and the breakdown of organic compounds [22]. Table 5 shows the content of boron, iron, zinc, and manganese in liquid fractions of lupine samples after the application of different treatment methods for phytic acid reduction. The results support literature reports that fermentation can alter the solubility and extractability of trace minerals [18]. Boron levels significantly differed from the control after thermal treatment, controlled fermentation, and enzymatic treatment (p < 0.05), with controlled fermentation causing a marked increase in boron concentration (10.08 ± 0.00 mg B/g d.m.). Iron content increased markedly after processing, particularly following thermal (44.42 ± 0.23 mg Fe/g d.m.) and controlled fermentation treatments (69.92 ± 0.13 mg Fe/g d.m.). Zinc concentrations were significantly lower in all processed samples, except for controlled fermentation, which showed an increase (11.19 ± 0.00 mg Zn/g d.m.) compared to the control (p < 0.05). Manganese levels varied substantially across treatments. Thermal treatment resulted in a notable increase (108.08 ± 1.76 mg Mn/g d.m.), whereas enzymatic and spontaneous fermentation treatments significantly reduced manganese content compared to the control (p < 0.05).

Table 5.
Content of boron, iron, zinc, and manganese in liquid fractions of lupine samples after application of different treatment methods for phytic acid reduction.
Processing affected micronutrient composition of lupine samples. Controlled fermentation increased boron content, likely due to microbial cell wall degradation, whereas thermal and enzymatic treatments reduced it. Iron concentrations rose across all treatments, especially after enzymatic and fermentation processes, likely by preventing insoluble complex formation. Zinc generally decreased but slightly increased after controlled fermentation, consistent with reports linking fermentation to improved zinc bioavailability through phytate degradation [28]. Interestingly, manganese content increased substantially after thermal treatment, possibly due to the release of manganese from protein or polysaccharide complexes under heat [29]. Enzymatic and spontaneous fermentation markedly reduced manganese levels, likely due to leaching or microbial uptake. Overall, processing methods targeting phytic acid reduction influenced not only phosphorus availability but also nitrogen metabolism and trace element profiles. Thermal and enzymatic treatments generally lowered mineral concentrations, whereas fermentation—especially controlled—enhanced mineral content in the liquid fraction, suggesting improved bioavailability and offering strategies to optimize lupine-based feeds for better nutrient utilization.
3.3. Impact of Lupine-Based Formulations on the Large Intestine Microbiome and Short-Chain Fatty Acid Profile
Feed composition strongly affects the gastrointestinal microbiota, shaping animal health and production outcomes. A balanced gut microbiome improves nutrient absorption, immune modulation, and growth performance [30]. Nutritional strategies can optimize microbial populations, enhancing digestion and suppressing pathogens, while reducing reliance on chemical growth promoters and addressing antimicrobial resistance [31]. Microbial activity improves nitrogen utilization and lowers environmental impact through precision feeding [32,33]. Functional additives—probiotics, prebiotics [34], and fermented components [35]—further support microbial diversity and nutrient bioavailability. These approaches achieve three key goals: improved feed efficiency, reduced veterinary interventions, and a lower environmental footprint. Ongoing research into microbiota–host interactions continues to advance precision feeding systems aligned with sustainability objectives [36,37].
In calves, beneficial Lactobacillus species dominate the gut and help limit pathogens such as E. coli and Salmonella. Other Lactobacillus species, such as Lactobacillus acidophilus, L. brevis, L. delbrueckii, and L. fermentum, are also found in the gastrointestinal tracts of calves, although their relative abundance may depend on factors such as diet or probiotic use. The Enterobacteriaceae family, which includes Raoultella planticola, is also present in the calf microbiota, but its overgrowth may be associated with health disorders such as diarrhea. Therefore, it is important to monitor their numbers and promote the growth of beneficial bacteria, such as Lactobacillus, to maintain a healthy balance of gut microbiota. Bacteria from the Veillonellaceae family, including the genus Dialister, have also been identified in the calf digestive tract. Their role in the gut microbiota is not yet fully understood, but they may be involved in the metabolism of short-chain fatty acids, which may influence gut health [38].
Metagenomic analysis of simulated digestion samples revealed clear, processing-dependent shifts in gut microbiota composition (Figure 2 and Figure 3, Table A1). Rarefaction curves confirmed sequencing completeness, and beta-diversity analyses showed distinct clustering between control and treated samples (Figure A1). Spontaneous and controlled fermentation significantly increased the relative abundance of L. mucosae, L. plantarum, and L. reuteri, suggesting potential health-promoting effects. Enzymatic treatment favored L. acidophilus and L. brevis, while heat treatment enriched thermotolerant species such as L. buchneri, L. brevis, and L. hilgardii. Fermentation promoted intensive microbial activity, likely driven by protein hydrolysis and increased production of SCFAs and lactic acid. In contrast, heat treatment reduced most Lactobacillus populations but promoted Enterobacteriaceae (e.g., Raoultella planticola) and butyric/valeric acid producers such as Clostridium, likely due to protein denaturation and subsequent shifts in nutrient availability. Overall, these results demonstrate that processing methods selectively modulate gut microbiota and metabolite profiles, with controlled fermentation providing the most favorable balance for gut health and feed functionality.
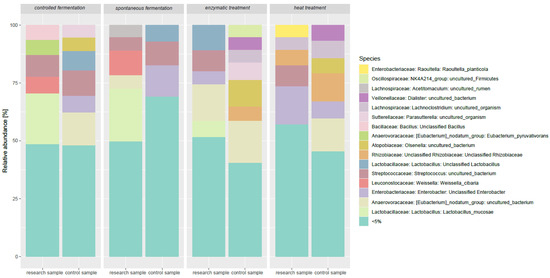
Figure 2.
Influence of phytic acid reduction methods on the profile of the intestinal microflora of calves—relative abundance of dominant bacterial species.
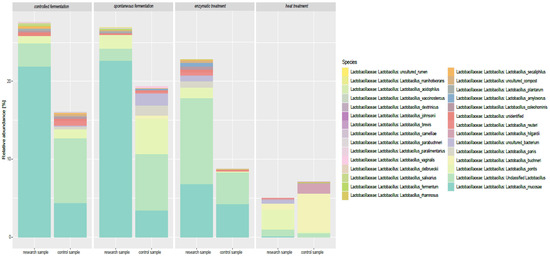
Figure 3.
Profile of Lactobacillus bacteria in the intestinal microflora of calves depending on the method of phytic acid reduction.
The samples subjected to different processing methods also showed significant differences in terms of the relative abundance of L. mucosae. In the control samples, the L. mucosae level was relatively low, whereas after both spontaneous and controlled fermentation, it increased. A particularly high abundance of L. mucosae was observed in samples subjected to controlled fermentation, which may suggest that these conditions are favorable for its proliferation. Studies on different Lactobacillus species indicate they have variable sensitivity to high temperature and the ability to adapt to environmental stresses. Haddaji et al. [39] showed that exposing Lactobacillus casei to high temperature causes changes in the fatty acid composition of the cell membrane, which may affect their survival under heat stress conditions. Moreover, experiments in rats [40] have shown that heat stress can change the structure of the gut microbiome, including the Lactobacillus population, suggesting sensitivity to high temperatures. Studies by Chen et al. [41] on Lactobacillus kefiranofaciens indicate that adaptation to environmental stresses, such as high temperature, acidity, and bile salts, can induce defense mechanisms that increase their tolerance to these and other adverse conditions. In contrast to fermentation, heat and enzymatic treatment led to a decrease in the relative abundance of this species, which may be due to the sensitivity of L. mucosae to high temperature or the specific action of enzymes that break down nutrients necessary for its growth.
In samples after controlled and spontaneous fermentation, an increase in the share of bacteria from the Veillonellaceae family (Dialister spp.) was noted, which may indicate an increased production of fermentation metabolites such as short-chain fatty acids. In the case of controlled fermentation, this was accompanied by high production of SCFA, especially acetic and propionic acid. It can be assumed that the protein component of lupin, as a substrate rich in nitrogen, supported the development of these microorganisms. Zaworska et al. (2021) confirmed that fermentation of lupin seeds increases the number of lactic acid bacteria and improves the digestibility of proteins and amino acids in animals [42]. In the case of spontaneous fermentation, the presence of L. fermentum, L. delbrueckii, and L. salivarius strains was noted, but their numbers were much lower and the production of SCFA was limited. This may be due to the presence of antinutritional substances in lupin, which limit bacterial growth, especially in the absence of appropriate starters. Nevertheless, as the authors of the previously mentioned studies emphasize, fermentation allows for the reduction of these compounds, improving protein availability and supporting the development of the desired microflora.
Another marker for the proper functioning of metabolic processes is the production of a short-chain fatty acid (SCFA). In the calf colon, SCFA profiles are significantly influenced by plant-derived protein sources in the diet, which differentially shape the gut microbial ecosystem and its metabolic output. The fermentation dynamics of plant-derived proteins in the calf colon significantly influence both microbial ecology and short-chain fatty acid (SCFA) production profiles. Research demonstrates that soy protein supplementation increases total SCFA output while elevating branched-chain fatty acid (BCFA) concentrations, reflecting enhanced proteolytic activity by Clostridium clusters XIVa and IV. Comparative studies reveal that wheat gluten promotes higher butyrate proportions relative to other plant proteins. The microbial shifts induced by plant proteins typically involve an increased Firmicutes/Bacteroidetes ratio and expansion of Lachnospiraceae populations, metabolic changes that impact gut barrier function through butyrate availability, and immune modulation via acetate production [43].
The levels of short-chain fatty acids (SCFAs), such as formic, acetic, propionic, butyric, and valeric acids, were quantified for samples incubated with gut microorganisms (Table 6). The formation of SCFAs and lactic acid during the simulated digestion of lupine-based feed was evaluated to determine the effects of the studied processing techniques and control conditions. The reference sample was the value obtained for the model medium. The data are presented as percentage values, clearly indicating the direction of profile changes related to the digestion of the tested component as the only nutritional component.

Table 6.
Analysis of short-chain fatty acids and lactic acid present in the large intestine of the gastrointestinal model.
Analysis of the SCFA profiles revealed significant differences in both the concentration and composition of the fermentation products depending on the applied treatment. Enzymatic treatment led to the highest (2–4-fold higher) overall production of valeric acid. The elevated valeric acid levels following enzymatic treatment suggest that specific enzymatic activities may release or produce more valeric acid from substrates [44]. These findings suggest increased substrate accessibility from enzymatic hydrolysis, likely enhancing microbial fermentation in the large intestine model. Valeric acid, a straight-chain VFA, is mainly produced via microbial fermentation of branched-chain amino acids (BCAAs), particularly isoleucine, with contributions from leucine and valine. Elevated dietary protein increases BCAA availability, promoting valeric acid synthesis, which supports host energy metabolism and modulates the ruminal microbiota. Studies report that BCAA supplementation increases total VFA concentrations and alters fermentation patterns, affecting fiber digestibility and nutrient utilization. As lupine seeds are rich in highly digestible protein and BCAAs, their inclusion in ruminant diets may enhance valeric acid production and improve rumen function [45]. Thermal and enzymatic treatment resulted also in notable SCFA production, especially of butyric acid (up to 47.49 % in relation to the model conditions), indicating that heat processing may break down complex polysaccharides into more fermentable substrates.
Digestion of spontaneous fermented lupine, in contrast, yielded the lowest total SCFA production across most acid types, particularly formic (0.7–1.63%) and valeric acid (15.48–27.65%). This could indicate the low content of precursors or their utilization during lupine treatment. Controlled fermentation improved SCFA yields relative to spontaneous fermentation, particularly acetic (35.18–39.15%) and propionic acid (10.46–16.24%) levels, reflecting the beneficial effect of introducing selected microbial strains capable of efficient fiber degradation. The proper course of metabolic processes requires a balanced composition and appropriate proportions of the protein and carbohydrate source. In the present study, the introduction of a fermented lupine component to feed mixtures ensured the biosynthesis of about 25% of the essential metabolites. This component could therefore be useful for creating new balanced feed mixtures, especially dedicated to young animals.
The high acetic acid levels across all samples—especially in fermented treatments—are consistent with its dominant role as a major fermentation end-product of carbohydrate metabolism. The differences indicate that feed processing methods modulate the availability of fermentable substrates, influencing acetic acid production by gut microbiota. Acetic acid is known to stimulate colonic sodium and fluid absorption, contributing to overall gut health [30]. Propionic and butyric acids also followed this trend, particularly in enzymatically treated samples, underscoring the importance of pre-digestion for enhancing microbial fermentability. The enzymatic hydrolysis of feed components likely enhanced substrate availability, thereby promoting propionate synthesis. Given the role of propionate in lipid metabolism and hepatic gluconeogenesis, its increased production through enzymatic processing may have implications for host energy metabolism. In contrast, fermentation processes yielded lower yet more stable propionic acid levels over time. Propionate has also been shown to influence lipid metabolism and gluconeogenesis in the liver [45].
Lactic acid and SCFA levels varied depending on processing method, with enzymatic treatment producing the highest lactic acid accumulation, likely due to increased simple sugar availability and dominance of lactic acid-producing bacteria. Spontaneous and controlled fermentation also showed elevated lactic acid, consistent with the presence of Lactobacillus spp. Thermal and enzymatic treatments increased acetic, propionic, and butyric acids, likely by enhancing the availability of fermentable substrates through protein and carbohydrate modification. These results highlight the significant influence of processing on SCFA production and gut microbiota activity, underscoring the potential of dietary strategies to modulate SCFA profiles for improved gut health, immune function, and nutrient utilization [46].
Comparison of the different treatments of lupin feeds showed that controlled fermentation was the most effective method in terms of production of SCFA, including key acids such as acetic, propionic, and butyric acids. The high SCFA content in these samples is consistent with previous studies, suggesting that bacterial fermentation using specific strains (Lactobacillus buchneri, Lactobacillus plantarum) promotes the production of beneficial metabolites [47]. Thermal and enzymatic treatments were less effective at generating SCFA, which is due to the limited bacterial activity under these conditions. Nevertheless, these methods may be useful in combination with controlled fermentation, which increases the efficiency of biological processes.
Enzymatic treatment had the strongest effect on lactic acid production, particularly in the second week, indicating higher availability of fermentable substrates, while spontaneous and controlled fermentation showed a gradual increase, reflecting the development of fermentative microflora. Thermal treatment had minimal impact on fermentation dynamics. Metagenomic analysis demonstrated that fermentation and enzymatic treatments promoted beneficial lactic acid bacteria, whereas heat treatment selected more stress-tolerant strains. High lupin protein content further supported microbial growth, with enzymatic and controlled fermentation yielding the most favorable microbiota and metabolite profiles. Fermentation also reduced phytate content, enhancing the bioavailability of minerals such as Ca, Mg, and Fe, which may improve animal health and performance. These results highlight the importance of processing design to optimize microbiota composition, metabolite production, and nutrient utilization in functional lupin-based feeds.
4. Conclusions
This study demonstrated that controlled fermentation using lactic acid bacteria consortia is the most effective method for reducing phytic acid in narrow-leaved lupine, achieving a 96.37% reduction. The research is the starting point to improve the feeding quality of legumes, growing under European conditions.
This study showed that controlled fermentation with lactic acid bacteria is the most effective method for processing narrow-leaved lupine to improve its feeding quality. It resulted in the strongest reduction of phytic acid while also increasing the bioavailability of important minerals such as phosphorus, iron, and boron, and at the same time preserving protein content. In contrast, thermal treatment led to protein denaturation and signs of stronger degradation, while enzymatic processing preserved more protein but was less efficient in breaking down phytic acid.
The microbiome analysis further highlighted the benefits of controlled fermentation, as it supported the growth of favorable bacterial strains and increased the production of short-chain fatty acids known to promote gut health. Other methods were less desirable, with thermal processing linked to bacteria that may negatively affect gut balance, and enzymatic treatment producing compounds that could be harmful in excess.
Overall, controlled fermentation emerged as the most promising strategy for enhancing the nutritional value of lupine used in animal feed. By effectively lowering antinutritional factors, improving mineral absorption, and supporting a healthy gut environment, it provides clear advantages over thermal and enzymatic methods. These findings also emphasize lupine’s potential as a sustainable and nutritious alternative to soybean meal, with further research needed to optimize fermentation conditions and study its long-term effects on animal health and performance.
Author Contributions
Conceptualization, B.P.-K. and J.B.; methodology, B.P.-K., O.B., W.C.-W., A.W., I.M., M.W. and J.B.; software, M.W.; validation, B.P.-K., J.B. and I.M.; formal analysis, B.P.-K.; investigation, B.P.-K.; resources, B.P.-K. and J.B.; data curation, B.P.-K.; writing—original draft preparation, B.P.-K.; writing—review and editing, B.P.-K., J.B. and I.M.; visualization, M.W.; supervision, J.B. and I.M.; project administration, B.P.-K.; funding acquisition, B.P.-K. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
“FU2N–Fund for the Improvement of Skills of Young Scientists” program supporting scientific excellence of the Lodz University of Technology–grant no. 04/2023. The APC was funded by Lodz University of Technology.
Institutional Review Board Statement
Ethical approval was not required as fecal samples were collected non-invasively without direct interaction with animals.
Data Availability Statement
All data supporting the findings of this study are contained within the article.
Acknowledgments
This research was completed while the first author was one of the doctoral candidates in the Interdisciplinary Doctoral School at Lodz University of Technology, Poland. The work was financed under the “FU2N–Fund for the Improvement of Skills of Young Scientists” program supporting scientific excellence of the Lodz University of Technology–grant no. 04/2023.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The sequencing depth for each library (along with other reads statistics generated during analysis) is given in the Table A1. There was one replicate sampled per each treatment.

Table A1.
The sequencing depth for each library (along with other reads statistics generated during analysis).
Table A1.
The sequencing depth for each library (along with other reads statistics generated during analysis).
| Library Name | Raw Read Pairs | Passing QC | % Passing QC | Pairs Joined | % Pairs Joined | Non-Chimeric Pairs | % Non- Chimeric Reads |
|---|---|---|---|---|---|---|---|
| DC1-1 | 71,834 | 59,390 | 82.68% | 54,330 | 75.63% | 46,095 | 64.17% |
| DC1-2 | 93,156 | 78,579 | 84.35% | 73,412 | 78.81% | 64,019 | 68.72% |
| DC1-3 | 104,329 | 87,053 | 83.44% | 82,164 | 78.75% | 70,897 | 67.96% |
| DC1-4 | 91,885 | 77,809 | 84.68% | 72,940 | 79.38% | 59,287 | 64.52% |
| DC2-1 | 98,736 | 83,822 | 84.90% | 78,833 | 79.84% | 67,424 | 68.29% |
| DC2-2 | 97,920 | 82,654 | 84.41% | 76,815 | 78.45% | 66,320 | 67.73% |
| DC2-3 | 86,606 | 73,336 | 84.68% | 69,328 | 80.05% | 60,737 | 70.13% |
| DC2-4 | 93,910 | 79,758 | 84.93% | 75,370 | 80.26% | 62,057 | 66.08% |
Appendix B
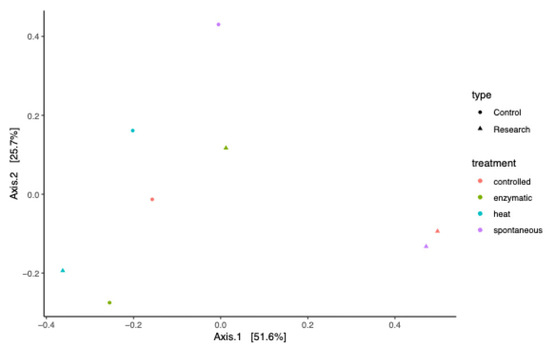
Figure A1.
The beta-diversity plot for samples.
References
- Hyland, J. Majority of European Crops Feeding Animals and Cars, Not People. Greenpeace European Unit 2020. Available online: https://www.greenpeace.org/eu-unit/issues/nature-food/45159/majority-of-european-crops-feeding-animals-and-cars-not-people/ (accessed on 18 December 2023).
- Pepin, I. What’s In Animal Feed? The Animal Feed Industry. The Humane League 2023. Available online: https://thehumaneleague.org/article/animal-feed (accessed on 18 December 2023).
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Von Engelhardt, W.; Rönnau, K.; Rechkemmer, G.; Sakata, T. Absorption of short-chain fatty acids and their role in the hindgut of monogastric animals. Anim. Feed. Sci. Technol. 1989, 23, 43–53. [Google Scholar] [CrossRef]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef]
- Milman, N.T. A Review of Nutrients and Compounds, Which Promote or Inhibit Intestinal Iron Absorption: Making a Platform for Dietary Measures That Can Reduce Iron Uptake in Patients with Genetic Haemochromatosis. J. Nutr. Metab. 2020, 2020, 7373498. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid State Fermentation with Lactic Acid Bacteria to Improve the Nutritional Quality of Lupin and Soya Bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Van de Wiele, T.; Verstraete, W.; Possemiers, S. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef]
- Dygas, D.; Berłowska, J. Sugar beet processing waste as a substrate for yeast protein production for livestock feed. BioResources 2023, 18, 4458–4474. [Google Scholar] [CrossRef]
- Liszkowska, W.; Motyl, I.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Berłowska, J. Mixed Culture of Yeast and Lactic Acid Bacteria for Low-Temperature Fermentation of Wheat Dough. Molecules 2025, 30, 112. [Google Scholar] [CrossRef]
- Burgos-Luján, I.; Tong, A.Z. Determination of Phytic Acid in Juices and Milks by Developing a Quick Complexometric-Titration Method. Food Anal. Methods 2014, 8, 1836–1841. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Soetaert, W. Industrial Biotechnology: Sustainable Growth and Economic Success; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Eeckhout, W.; De Paepe, M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed. Sci. Technol. 1994, 47, 19–29. [Google Scholar] [CrossRef]
- Potocka, M.; Zaworska-Zakrzewska, A.; Gulewicz, P.; Nowak, P. The Effect of Fermentation of High Alkaloid Seeds of Lupinus angustifolius var. Karo by Saccharomyces cerevisiae, Kluyveromyces lactis, and Candida utilis on the Chemical and Microbial Composition of Protein-Xanthophyll Concentrate. 2017. Available online: https://www.researchgate.net/publication/320043626 (accessed on 18 December 2023).
- Zhang, G.; Fang, X.; Feng, G.; Li, Y.; Zhang, Y. Silage Fermentation, Bacterial Community, and Aerobic Stability of Total Mixed Ration Containing Wet Corn Gluten Feed and Corn Stover Prepared with Different Additives. Animals 2020, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Mineral Nutrition of Livestock, 5th ed.; CAB International: Wallingford, UK, 2022. [Google Scholar]
- Parish, J.A.; Karisch, B.B.; Rhinehart, J.D. Mineral and Vitamin Nutrition for Beef Cattle; Mississippi State University Extension Service Publication: Oxford, MS, USA, 2022; p. 2484. Available online: https://extension.msstate.edu/publications/mineral-and-vitamin-nutrition-for-beef-cattle (accessed on 18 December 2023).
- Haji, A.; Teka, T.A.; Bereka, T.Y.; Astatkie, T.; Woldemariam, H.W.; Urugo, M.M. Effect of Processing Methods on the Nutrient, Antinutrient, Functional, and Antioxidant Properties of Pigeon Pea (Cajanus cajan (L.) Millsp.) Flour. J. Agric. Food Res. 2024, 18, 101493. [Google Scholar] [CrossRef]
- Sahu, L.; Panda, S.K.; Paramithiotis, S.; Zdolec, N.; Ray, R.C. Biogenic Amines in Fermented Foods: Overview. In Fermented Foods: Part 1. Biochemistry & Biotechnology; Montet, D., Ray, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Leytem, A.B.; Williams, P.; Zuidema, S.; Martinez, A.; Chong, Y.L.; Vincent, A.; Vincent, A.; Cronan, D.; Kliskey, A.; Wulfhorst, J.D.; et al. Cycling Phosphorus and Nitrogen through Cropping Systems in an Intensive Dairy Production Region. Agronomy 2021, 11, 1005. [Google Scholar] [CrossRef]
- Clark, J.H.; Klusmeyer, T.H.; Cameron, M.R. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef]
- Ørskov, E.R. Protein Nutrition in Ruminants, 2nd ed.; Academic Press: London, UK, 1992; pp. 41–93. [Google Scholar]
- Davidovich, A.; Bartley, E.E.; Milliken, G.A.; Dayton, A.D.; Deyoe, C.W.; Bechtle, R.M. Ammonia toxicity in cattle. IV. Effects of unprocessed or extrusion-cooked mixtures of grain and urea, biuret, or dicyanodiamide and liquid supplements on rumen and blood changes associated with toxicity. J. Anim. Sci. 1977, 45, 1397–1408. [Google Scholar] [CrossRef]
- Braun, U.; Gautschi, A.; Hässig, M. Metabolic and endocrine disorders in calves. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 177–192. [Google Scholar]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Ávila, C.L.S.; Pinto, J.C.; Neri, J.; Schwan, R.F. Microbiological and chemical profile of sugar cane silage fermentation inoculated with wild strains of lactic acid bacteria. Anim. Feed Sci. Technol. 2020, 259, 114369. [Google Scholar] [CrossRef]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A Review of Phytate, Iron, Zinc, and Calcium Concentrations in Plant-Based Complementary Foods Used in Low-Income Countries and Implications for Bioavailability. Food Nutr. Bull. 2010, 31 (Suppl. S2), S134–S146. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, S.; Platel, K.; Srinivasan, K. Influence of Heat Processing on the Bioaccessibility of Zinc and Iron from Cereals and Pulses Consumed in India. J. Trace Elem. Med. Biol. 2007, 21, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 23, 137–156. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Le Sciellour, M.; Labussière, E.; Zemb, O.; Renaudeau, D. Effect of dietary fiber content on nutrient digestibility and fecal microbiota composition in growing-finishing pigs. PLoS ONE 2018, 13, e0206159. [Google Scholar] [CrossRef]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141 (Suppl. S1), S15–S28. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Kim, J.-C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Allen, H.K. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 2012, 3, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Kim, M.; Liu, G.; Zhai, Y.; Liu, T.; Driver, J.D.; Jeong, K.C. The Gut Microbiota of Newborn Calves and Influence of Potential Probiotics on Reducing Diarrheic Disease by Inhibition of Pathogen Colonization. Front. Microbiol. 2021, 12, 772863. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, N.; Nawaz, M.; Yaqub, T.; Mehmood, A.K. Impact of Limosilactobacillus fermentum Probiotic Treatment on Gut Microbiota Composition in Sahiwal Calves with Rotavirus Diarrhea: A 16S Metagenomic Analysis Study. BMC Microbiol. 2024, 24, 114. [Google Scholar] [CrossRef]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ben Ismail, M.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar] [CrossRef]
- Qu, Q.; Li, H.; Bai, L.; Zhang, S.; Sun, J.; Lv, W.; Ye, C.; Liu, C.; Shi, D. Effects of heat stress on gut microbiome in rats. Indian J. Microbiol. 2021, 61, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Tang, H.-Y.; Chiang, M.-L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.E.; Carrender, B.; Roubicek, C.D.; Maurer, R.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Tokach, M.D.; Coble, K.F.; Goodband, R.D. Effects of iron injection timing on suckling and subsequent nursery and growing-finishing performance and hematological criteria. J. Anim. Sci. 2021, 99, skab071. [Google Scholar] [CrossRef]
- Anderson, C.L.; Harris, T.R.; van Kempen, T.A. Plant protein-induced shifts in the calf gut microbiome modulate butyrate production and immune function. Anim. Nutr. 2023, 12, 45–58. [Google Scholar]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35 (Suppl. S1), S35–S38. [Google Scholar] [CrossRef]
- Zhang, H.L.; Chen, Y.; Xu, X.L.; Yang, Y.X. Effects of Branched-chain Amino Acids on In vitro Ruminal Fermentation of Wheat Straw. Asian-Australas. J. Anim. Sci. 2013, 26, 523–528. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef]
- Han, B.; Liang, S.; Sun, J.; Tao, H.; Wang, Z.; Liu, B.; Wang, X.; Liu, J.; Wang, J. The Effect of Lactobacillus plantarum on the Fecal Microbiota, Short Chain Fatty Acids, Odorous Substances, and Blood Biochemical Indices of Cats. Microorganisms 2024, 12, 91. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).