Multi-Objective Optimization of Mechanical Properties of Banana Pseudostem Fibers Using Sludge Retting Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. Selection of Experimental Factors
2.2.2. Response Surface Experimental Design
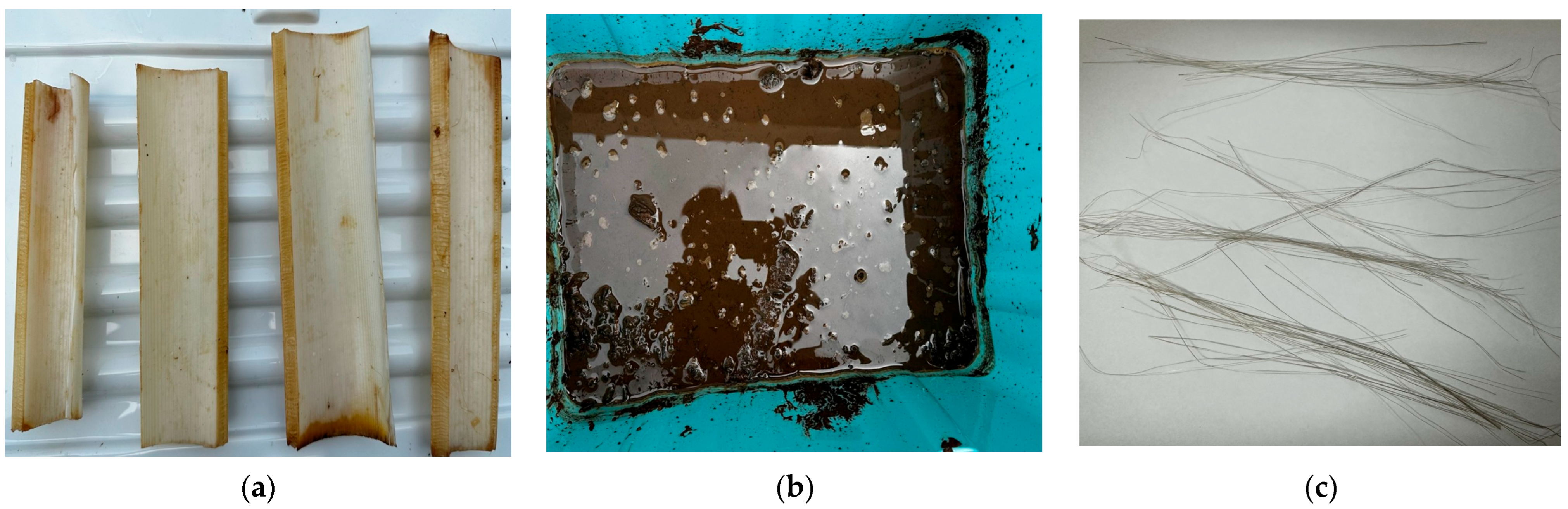
2.2.3. Sludge Retting Pretreatment of Banana Stems
2.3. Testing of Banana Pseudostem Fibers
2.3.1. Tensile Test
2.3.2. Scanning Electron Microscopy
2.3.3. Thermogravimetric Analysis
2.3.4. Fourier Transform Infrared Spectroscopy
2.3.5. X-Ray Diffraction
3. Results and Discussion
3.1. Tensile Test Results and Analysis
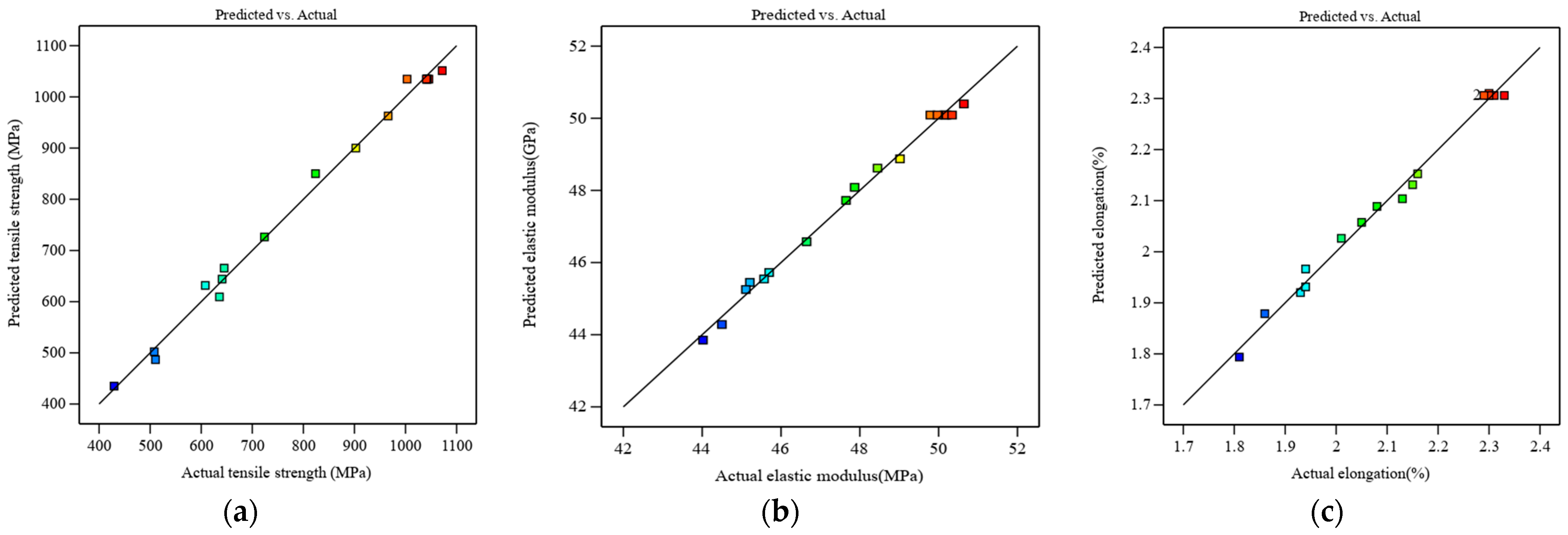
3.1.1. Model Fitting and Analysis of Variance
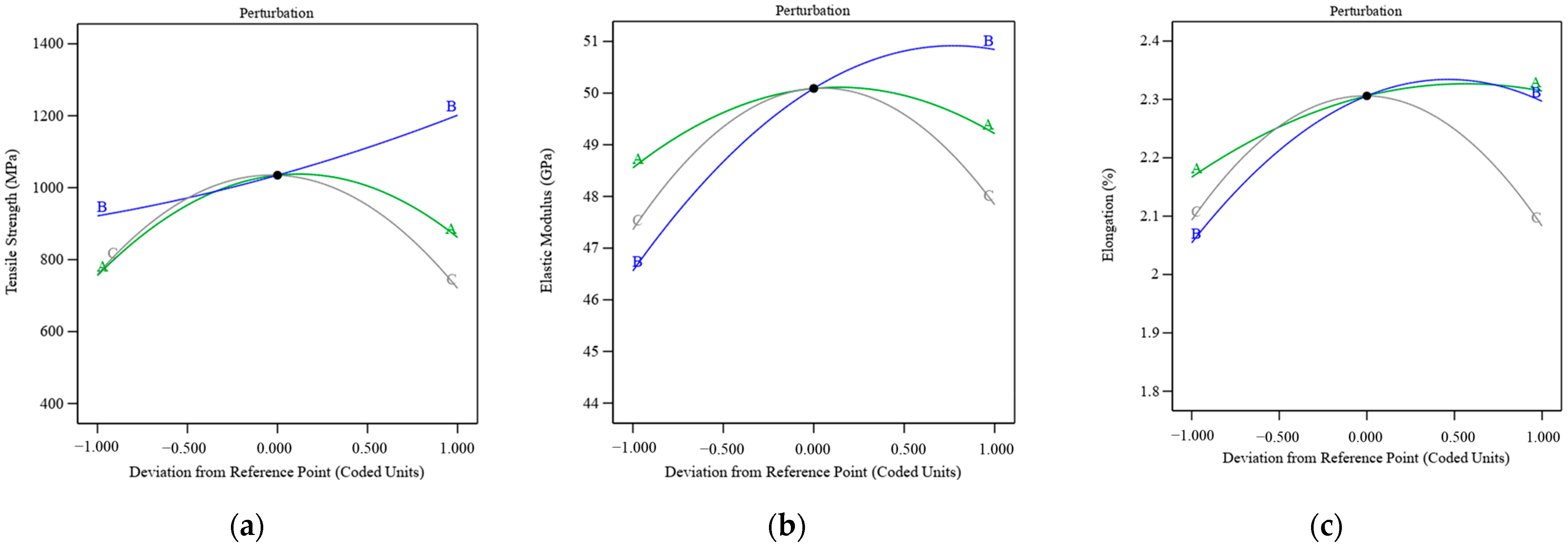
3.1.2. Effects of Interaction Factors on Tensile Strength
3.1.3. Effects of Interaction Factors on Elastic Modulus
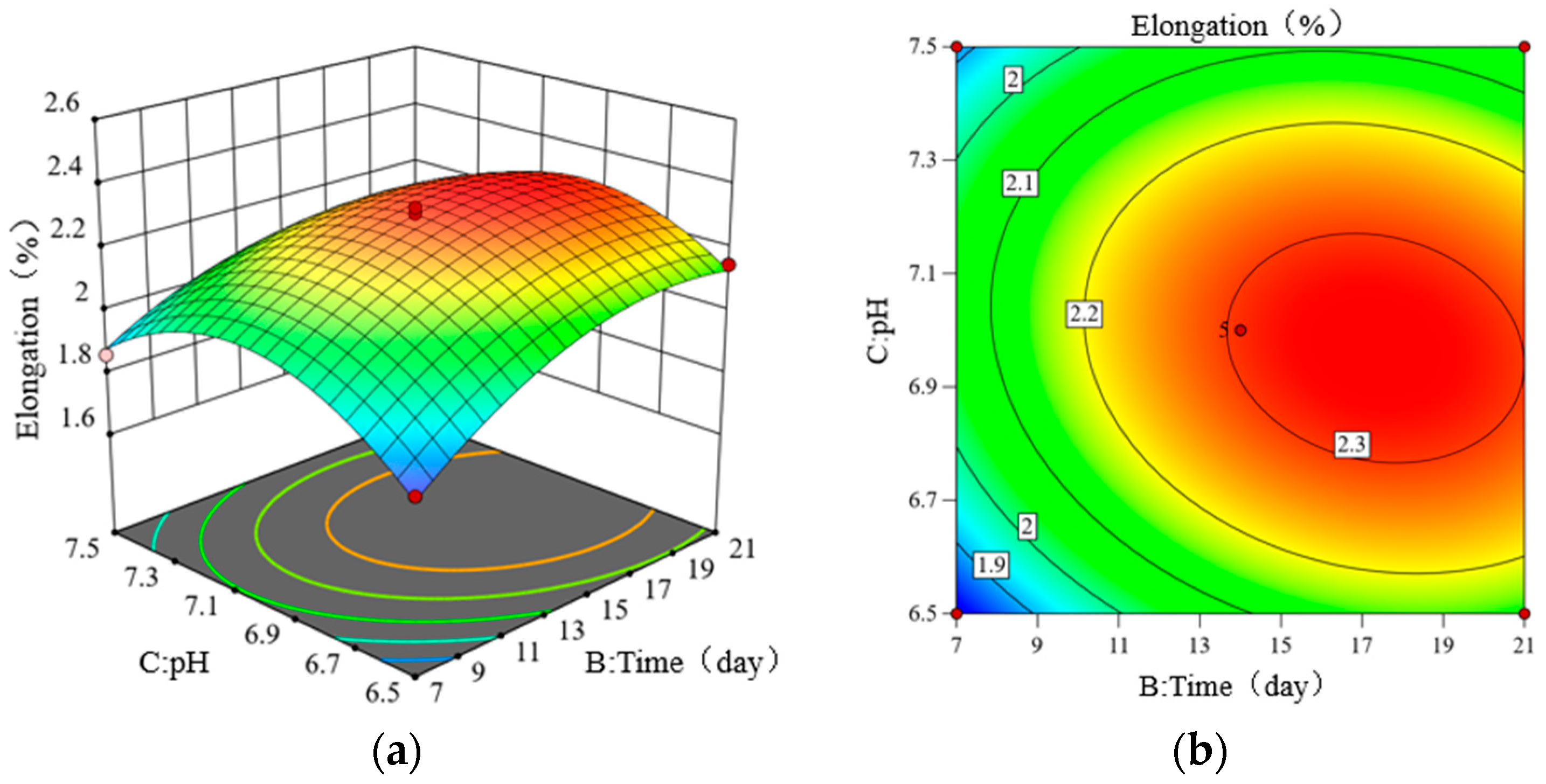
3.1.4. Effects of Interaction Factors on Elongation
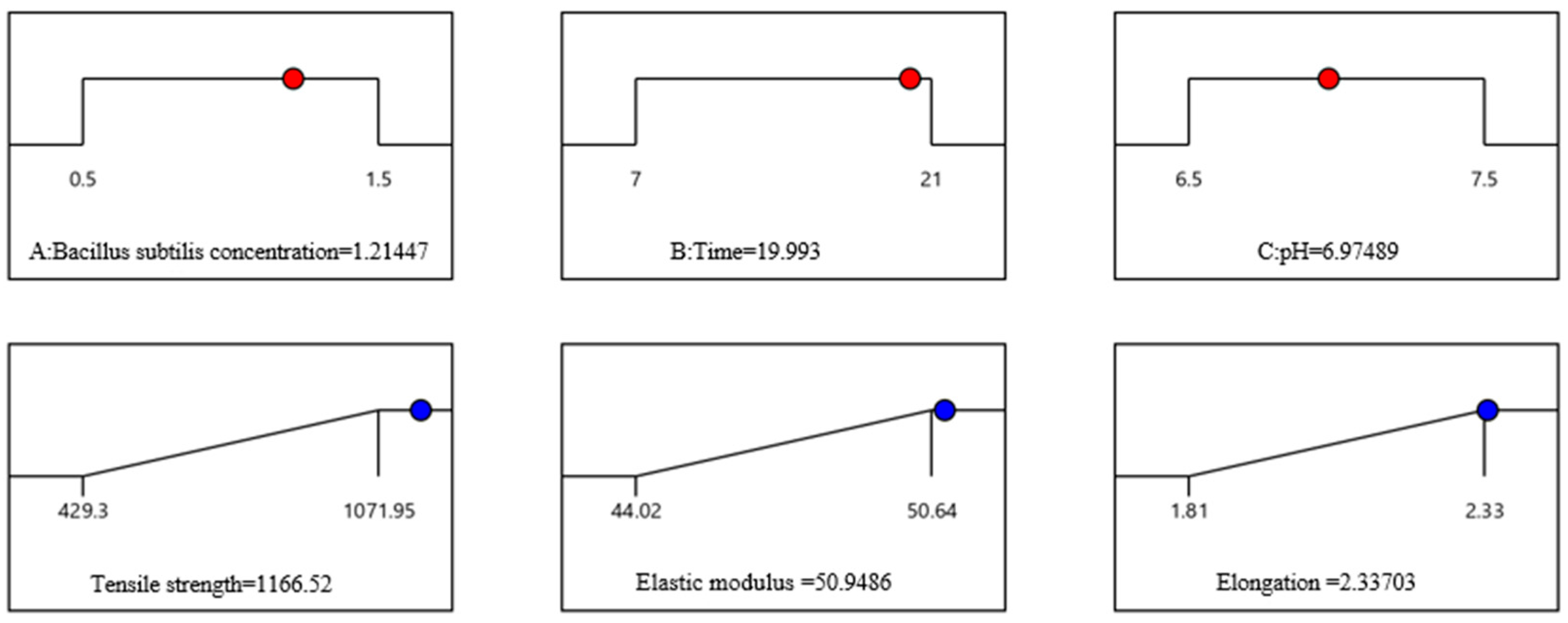
3.1.5. Parameter Optimization of Treatment Conditions
3.1.6. Validation Experiments
3.2. SEM Observation and Analysis
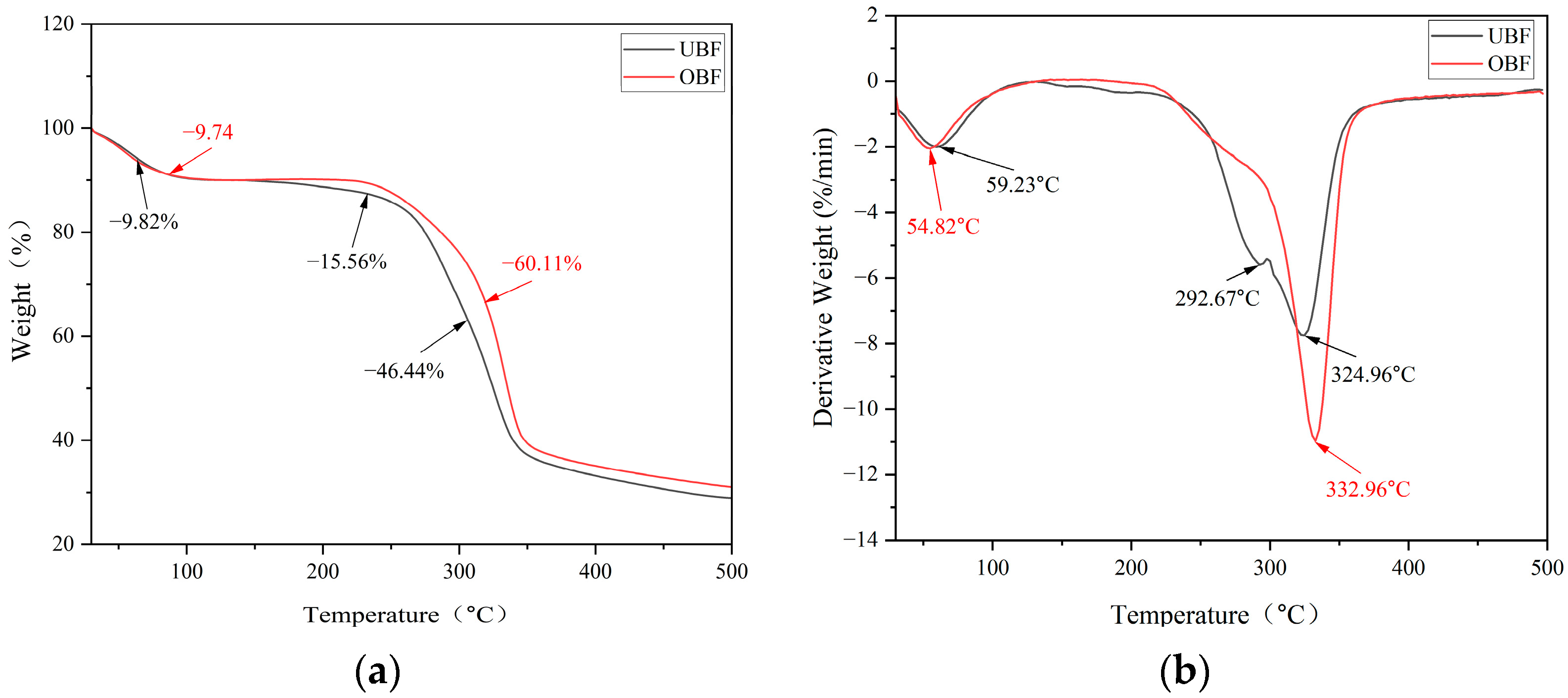
3.3. Thermogravimetric Analysis Results
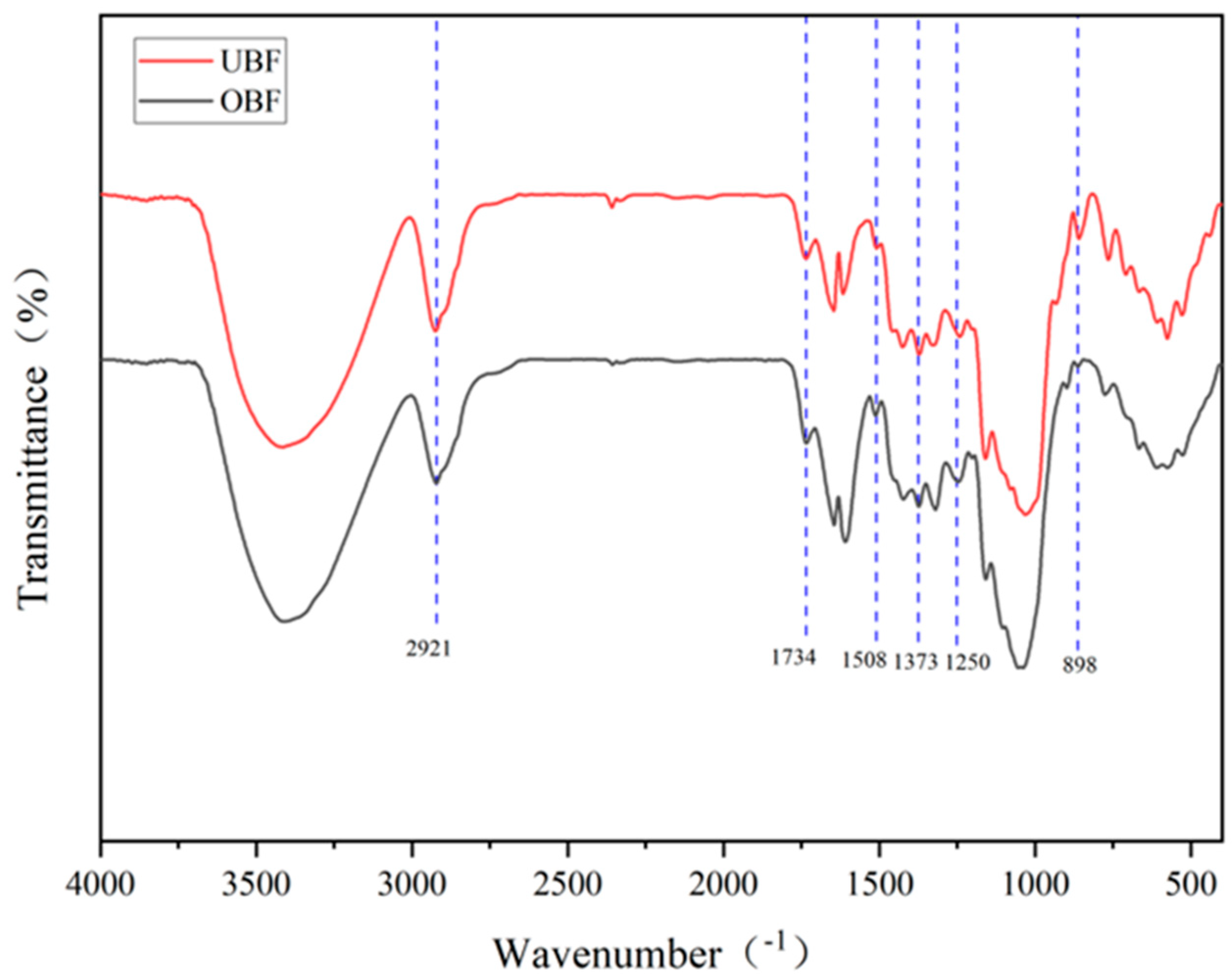
3.4. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
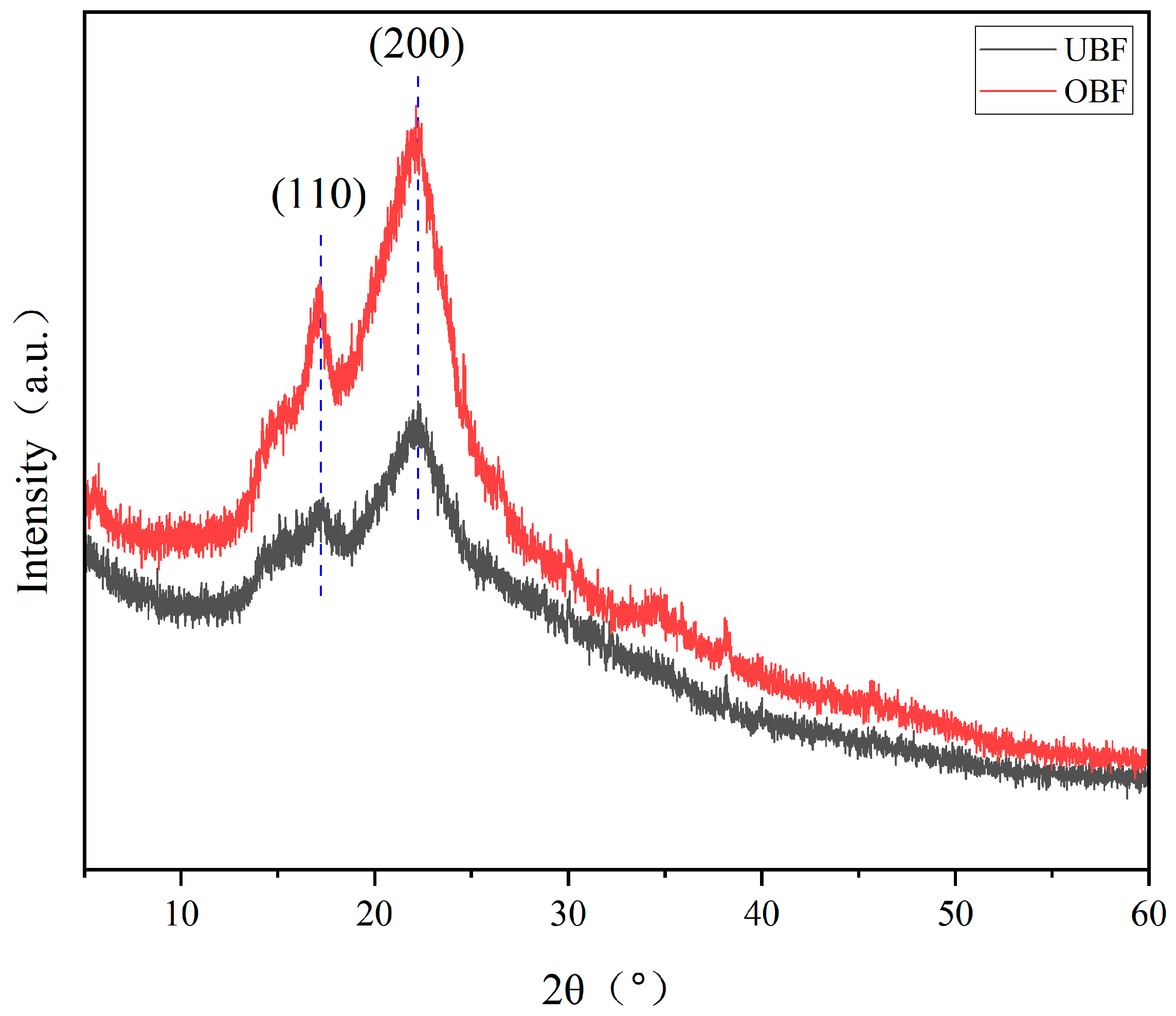
3.5. X-Ray Diffraction Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badanayak, P.; Jose, S.; Bose, G. Banana Pseudostem Fiber: A Critical Review on Fiber Extraction, Characterization, and Surface Modification. J. Nat. Fibers 2023, 20, 2168821. [Google Scholar] [CrossRef]
- Nascimento, R.E.A.; Carvalheira, M.; Crespo, J.G.; Neves, L.A. Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem. Clean Technol. 2023, 5, 1028–1043. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Wickramasinghe, G.D.; Wijayapala, U.S. A Novel Approach for Banana (Musa) Pseudo-Stem Fibre Grading Method: Extracted Fibres from Sri Lankan Banana Cultivars. J. Eng. Fibers Fabr. 2020, 15, 1558925020971766. [Google Scholar] [CrossRef]
- Ruangnarong, C.; Khojitmate, S.; Srivorradatphisan, S.; Panyathikun, N.; Chonsakorn, S. Evaluation of Mechanically Extracted Banana Fibers from Pseudostem Layers: A Sustainable Textile Raw Material. Heliyon 2024, 10, e39880. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Ortega, Z.; Benítez, A.N.; Marrero, M.D.; Carvalheiro, F.; Duarte, L.C.; Matsakas, L.; Krikigianni, E.; Rova, U.; Christakopoulos, P.; et al. Oligosaccharides Production by Enzymatic Hydrolysis of Banana Pseudostem Pulp. Biomass Convers. Biorefin. 2023, 13, 10677–10688. [Google Scholar] [CrossRef]
- Manjusha, W.A.; Josphine, J.S.; Deepa, P.K.; Sujatha, S.; Ahil Raj, S. Biosoftening of Banana Pseudostem Fiber Using Cellulase and Pectinase Enzyme Isolated from Aspergillus Niger for Textile Industry. J. Genet. Eng. Biotechnol. 2023, 21, 170. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of Fungal and Physicochemical Processes for Lignocellulosic Biomass Pretreatment—A Review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Hotor, P.; Hassanin, A.H.; Akatwijuka, O.; Gepreel, M.A.H.; Yamamoto, M.; Saito, Y.; Abdel-Mawgood, A. Evaluating Mechanism of Banana Pseudo-Stem Retting Using Seawater: A Cost-Effective Surface Pre-Treatment Approach. J. Bioresour. Bioprod. 2024, 9, 322–335. [Google Scholar] [CrossRef]
- Yu, X.; Xia, Y.; Liang, D.; Fu, W.; Yin, C. Effect of Warm-Water Retting Pretreatment on the Physical Properties of Banana Stem and Its Fibre. Materials 2022, 15, 8462. [Google Scholar] [CrossRef]
- Harsányi, J.; Poraj-Kobielska, M.; Wedwitschka, H.; Tirsch, M.; Kretzschmar, J. Controlled Anaerobic Water Retting of Flax as Part of an Innovative Biorefinery Process. Biomass Convers. Biorefin. 2025, 15, 16499–16510. [Google Scholar] [CrossRef]
- Ruan, P.; Raghavan, V.; Gariepy, Y.; Du, J. Characterization of Flax Water Retting of Different Durations in Laboratory Condition and Evaluation of Its Fiber Properties. BioResources 2015, 10, 3553–3563. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, Y.; Feng, X.; Xi, G.; Zheng, K.; Peng, Z.; Han, F.; Cheng, L.; Duan, S. Proteomic Insights into citT -Deletion Induced Metabolic Sensitivity in Bio-Degumming of Ramie Fibers by Bacillus Subtilis. J. Nat. Fibers 2024, 21, 2334414. [Google Scholar] [CrossRef]
- Ahmed, Z.; Sarkar, S. Microbial Consortium: A New Approach in Jute Retting of Preserved Dry Ribbons. Int. J. Life Sci. Res. Updates 2022, 4, 126–137. [Google Scholar] [CrossRef]
- Duan, S.; Feng, X.; Cheng, L.; Peng, Y.; Zheng, K.; Liu, Z. Bio-Degumming Technology of Jute Bast by Pectobacterium Sp. DCE-01. AMB Express 2016, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Zhang, C.; Li, J.; Chang, S.; Li, S.; Wang, J.; Zhao, M.; Li, J.; Yu, M.; Chen, X. Optimization of NaOH Pretreatment for Enhancement of Biogas Production of Banana Pseudo-Stem Fiber Using Response Surface Methodology. BioResources 2014, 9, 5073–5087. [Google Scholar] [CrossRef]
- Thi Thuy Van, N.; Gaspillo, P.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Thi Truc Van, N.; Nguyen, T.-T.; Ky Phuong Ha, H. Cellulose from the Banana Stem: Optimization of Extraction by Response Surface Methodology (RSM) and Charaterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef]
- Patel, B.Y.; Patel, H.K. Retting of Banana Pseudostem Fibre Using Bacillus Strains to Get Excellent Mechanical Properties as Biomaterial in Textile & Fiber Industry. Heliyon 2022, 8, e10652. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wadhwa, N. Microbial Retting of Banana Pseudostem. Int. J. Eng. Adv. Technol. 2021, 11, 162–166. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Fangueiro, R.; Shivankar, V. Variability of Tensile Properties of Fibers from Pseudostem of Banana Plant. Text. Res. J. 2009, 79, 387–393. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Sapuan, S.M.; Roslan, S.A.; Aziz, S.A.; Sarip, S. Optimization of Tensile Behavior of Banana Pseudo-Stem (Musa acuminate) Fiber Reinforced Epoxy Composites Using Response Surface Methodology. J. Mater. Res. Technol. 2019, 8, 3517–3528. [Google Scholar] [CrossRef]
- Ru, S.; Zhao, C.; Yang, S. Multi-Objective Optimization and Analysis of Mechanical Properties of Coir Fiber from Coconut Forest Waste. Forests 2022, 13, 2033. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Liu, X.; Wu, L.; Wang, Q.; Ji, X. Biological Pretreatment of Biomass to Decrease Energy Consumption in Mechanical Defiberization Process. BioResources 2020, 15, 9882–9893. [Google Scholar] [CrossRef]
- Wagaye, B.T.; Guo, J. Optimization of Alkali Treatment Conditions of Ramie Fabrics Using Box–Behnken Method. Biopolymers 2024, 115, e23621. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving Enzymatic Hydrolysis of Lignocellulosic Biomass by Bio-Coordinated Physicochemical Pretreatment—A Review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Khelifa, H.; Bezazi, A.; Boumediri, H.; delPino, G.G.; Ellagoune, S.; Reis, P.N.B.; Scarpa, F. Mechanical Properties Optimization Using Response Surface Methodology (RSM) of a Bio-Mortar Manufactured Based on Sisal Fibers. Int. J. Adv. Manuf. Technol. 2025, 138, 2335–2355. [Google Scholar] [CrossRef]
- Siddique, S.H.; Faisal, S.; Ali, M.; Gong, R.H. Optimization of Process Variables for Tensile Properties of Bagasse Fiber-Reinforced Composites Using Response Surface Methodology. Polym. Polym. Compos. 2021, 29, 1304–1312. [Google Scholar] [CrossRef]
- Tesfay, A.G.; Welegebreal, H.G. Optimization of Banana Fiber Treatment Parameters for Improved Textile Performance. Discov. Mater. 2025, 5, 101. [Google Scholar] [CrossRef]
- Ortega, Z.; Morón, M.; Monzón, M.; Badalló, P.; Paz, R. Production of Banana Fiber Yarns for Technical Textile Reinforced Composites. Materials 2016, 9, 370. [Google Scholar] [CrossRef]
- Cheng, L.; Duan, S.; Feng, X.; Zheng, K.; Yang, Q.; Xu, H.; Luo, W.; Peng, Y. Screening and Identification of Pectinolytic Bacteria for Ramie Degumming. Text. Res. J. 2021, 91, 1056–1064. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Q.; Feng, X.; Duan, S.; Yang, Q.; Zheng, K.; Liu, Z.; Liu, Z.; Peng, Y. Screening a Bacterium and Its Effect on the Biological Degumming of Ramie and Kenaf. Sci. Agric. 2018, 75, 375–380. [Google Scholar] [CrossRef]
- Paul, S.A.; Piasta, D.; Spange, S.; Pothan, L.A.; Thomas, S.; Bellmann, C. Solvatochromic and Electrokinetic Studies of Banana Fibrils Prepared from Steam-Exploded Banana Fiber. Biomacromolecules 2008, 9, 1802–1810. [Google Scholar] [CrossRef]
- Jayaprabha, J.S.; Brahmakumar, M.; Manilal, V.B. Banana Pseudostem Characterization and Its Fiber Property Evaluation on Physical and Bioextraction. J. Nat. Fibers 2011, 8, 149–160. [Google Scholar] [CrossRef]
- Subramanya, S.R.; Satyanarayana, K.G.; Pilar, B.S. Evaluation of Structural, Tensile and Thermal Properties of Banana Fibers. J. Nat. Fibers 2016, 15, 478. [Google Scholar]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Ștefan, R. Comparative FT-IR Prospecting for Cellulose in Stems of Some Fiber Plants: Flax, Velvet Leaf, Hemp and Jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Sukmawan, R.; Kusmono, K.; Wildan, M.W. Study of Alkali and Acetylation Treatments on Sisal Fibers Compatibility with Low-Amine/Epoxy Stoichiometric Ratio. Results Eng. 2024, 24, 103127. [Google Scholar] [CrossRef]
- Sharma, S.; Wadhwa, N. Characterization of Banana Fibers Extracted with Pectinase from Staphylococcus sciuri. Curr. Appl. Sci. Technol. 2023, 23, 1–11. [Google Scholar] [CrossRef]
- Das, D.; Hussain, S.; Ghosh, A.K.; Pal, A.K. Studies on Cellulose Nanocrystals Extracted from Musa Sapientum: Structural and Bonding Aspects. Cellul. Chem. Technol. 2018, 52, 729–739. [Google Scholar]
- Pereira, A.L.S.; Nascimento, D.M.; Souza Filho, M.D.S.M.; Cassales, A.R.; Morais, J.P.S.; Paula, R.C.M.; Rosa, M.F.; Feitosa, J.P.A. Banana (Musa sp. cv. Pacovan) Pseudostem Fibers Are Composed of Varying Lignocellulosic Composition throughout the Diameter. BioResources 2014, 9, 7749–7763. [Google Scholar] [CrossRef]
- Madhushani, W.H.; Priyadarshana, R.; Ranawana, S.; Senarathna, K.G.; Kaliyadasa, P.E. Determining the Crystallinity Index of Cellulose in Chemically and Mechanically Extracted Banana Fiber for the Synthesis of Nanocellulose. J. Nat. Fibers 2022, 19, 7973–7981. [Google Scholar] [CrossRef]





| Levels | Factors | ||
|---|---|---|---|
| Bacillus subtilis Concentration /% | Time /day | pH | |
| −1 | 0.5 | 7 | 6.5 |
| 0 | 1 | 14 | 7 |
| 1 | 1.5 | 21 | 7.5 |
| Run | Tensile Strength (MPa) | Elastic Modulus (GPa) | Elongation | |||
|---|---|---|---|---|---|---|
| 1 | 1 | 7 | 6.5 | 635.65 | 44.02 | 1.81 |
| 2 | 1 | 14 | 7 | 1002.94 | 49.97 | 2.3 |
| 3 | 0.5 | 7 | 7 | 644.87 | 45.21 | 1.93 |
| 4 | 1 | 21 | 7.5 | 823.72 | 48.45 | 2.01 |
| 5 | 0.5 | 14 | 7.5 | 510.44 | 45.57 | 1.94 |
| 6 | 0.5 | 21 | 7 | 902.63 | 49.02 | 2.16 |
| 7 | 1.5 | 21 | 7 | 1071.95 | 50.64 | 2.3 |
| 8 | 1.5 | 14 | 7.5 | 507.91 | 47.65 | 2.13 |
| 9 | 1 | 7 | 7.5 | 640.93 | 44.5 | 1.86 |
| 10 | 1 | 14 | 7 | 1041.63 | 49.79 | 2.31 |
| 11 | 0.5 | 14 | 6.5 | 429.3 | 46.65 | 1.94 |
| 12 | 1 | 14 | 7 | 1045.83 | 50.15 | 2.3 |
| 13 | 1 | 14 | 7 | 1042.56 | 50.2 | 2.33 |
| 14 | 1.5 | 7 | 7 | 723.72 | 45.11 | 2.05 |
| 15 | 1 | 21 | 6.5 | 965.93 | 47.87 | 2.15 |
| 16 | 1.5 | 14 | 6.5 | 607.91 | 45.7 | 2.08 |
| 17 | 1 | 14 | 7 | 1040.78 | 50.34 | 2.29 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 810,200 | 9 | 90,026.16 | 132.65 | <0.0001 | ** |
| 22,498.51 | 1 | 22,498.51 | 33.15 | 0.0007 | ** | |
| 156,500 | 1 | 156,500 | 230.65 | <0.0001 | ** | |
| 8202.92 | 1 | 8202.92 | 12.09 | 0.0103 | * | |
| BC | 5438.33 | 1 | 5438.33 | 8.01 | 0.0254 | * |
| 214,700 | 1 | 214,700 | 316.35 | <0.0001 | ** | |
| 3036.82 | 1 | 3036.82 | 4.47 | 0.0722 | * | |
| 366,500 | 1 | 366,500 | 540.07 | <0.0001 | ** | |
| Residual | 4750.79 | 7 | 678.68 | |||
| Lack of Fit | 3471.45 | 3 | 1157.15 | 3.62 | 0.1231 | not significant |
| Pure Error | 1279.33 | 4 | 319.83 | |||
| Cor Total | 815,000 | 16 | <0.0001 | |||
| R2 = 0.9942 | Adjusted R2 = 0.9867 | |||||
| Predicted R2 = 0.9294 | Adequate Precision = 30.8437 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 85.55 | 9 | 0.0541 | 94.77 | <0.0001 | ** |
| 0.8778 | 1 | 0.0435 | 76.24 | <0.0001 | ** | |
| 36.72 | 1 | 0.1176 | 206.08 | <0.0001 | ** | |
| AC | 2.30 | 1 | 2.30 | 31.97 | 0.0008 | ** |
| 6.13 | 1 | 0.0181 | 31.65 | 0.0008 | ** | |
| 8.12 | 1 | 0.0717 | 125.64 | <0.0001 | ** | |
| 26.13 | 1 | 0.2001 | 350.62 | <0.0001 | ** | |
| Residual | 0.5026 | 7 | 0.0006 | |||
| Lack of Fit | 0.32 | 3 | 0.001 | 4.46 | 0.0914 | not significant |
| Pure Error | 0.1826 | 4 | 0.0002 | |||
| Cor Total | 86.05 | 16 | ||||
| R2 = 0.9919 | Adjusted R2 = 0.9814 | |||||
| Predicted R2 = 0.8968 | Adequate Precision = 28.1757 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.4868 | 9 | 9.51 | 132.4 | <0.0001 | ** |
| 0.0435 | 1 | 0.8778 | 12.23 | 0.01 | ** | |
| 0.1176 | 1 | 36.72 | 511.48 | <0.0001 | ** | |
| 0.0090 | 1 | 0.0090 | 15.81 | 0.0053 | ** | |
| 0.0181 | 1 | 6.13 | 85.33 | <0.0001 | ** | |
| 0.0717 | 1 | 8.12 | 113.1 | <0.0001 | ** | |
| 0.2001 | 1 | 26.13 | 363.97 | <0.0001 | ** | |
| Residual | 0.004 | 7 | 0.0718 | |||
| Lack of Fit | 0.0031 | 3 | 0.1067 | 2.34 | 0.2151 | not significant |
| Pure Error | 0.0009 | 4 | 0.0457 | |||
| Cor Total | 0.4908 | 16 | ||||
| R2 = 0.9942 | Adjusted R2 = 0.9867 | |||||
| Predicted R2 = 0.9372 | Adequate Precision = 31.8602 | |||||
| Contrast Items | Tensile Strength (MPa) | Elastic Modulus (GPa) | Elongation (%) |
|---|---|---|---|
| Measured value | 1161.63 | 50.68 | 2.32 |
| Predicted value | 1166.52 | 50.95 | 2.34 |
| Error (%) | 0.42 | 0.53 | 0.85 |
| Wavenumbers (cm−1) | Functional Groups | Source | |
|---|---|---|---|
| UBF | OBF | ||
| 2921 | 2921 | C-H | Cellulose |
| 1734 | 1734 | C=O | Hemicellulose |
| 1508 | 1508 | C=C | Lignin characteristic peak |
| 1373 | 1373 | C-H | Cellulose and hemicellulose |
| 1250 | 1250 | C-O-C | Hemicellulose |
| 898 | 898 | C-H | Cellulose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Yang, Z.; Fu, W.; Shen, Y.; Yu, S.; Zeng, W.; Liu, J. Multi-Objective Optimization of Mechanical Properties of Banana Pseudostem Fibers Using Sludge Retting Pretreatment. Agriculture 2025, 15, 2057. https://doi.org/10.3390/agriculture15192057
Liang D, Yang Z, Fu W, Shen Y, Yu S, Zeng W, Liu J. Multi-Objective Optimization of Mechanical Properties of Banana Pseudostem Fibers Using Sludge Retting Pretreatment. Agriculture. 2025; 15(19):2057. https://doi.org/10.3390/agriculture15192057
Chicago/Turabian StyleLiang, Dong, Zeqin Yang, Wei Fu, Yijun Shen, Shaojie Yu, Wei Zeng, and Ji Liu. 2025. "Multi-Objective Optimization of Mechanical Properties of Banana Pseudostem Fibers Using Sludge Retting Pretreatment" Agriculture 15, no. 19: 2057. https://doi.org/10.3390/agriculture15192057
APA StyleLiang, D., Yang, Z., Fu, W., Shen, Y., Yu, S., Zeng, W., & Liu, J. (2025). Multi-Objective Optimization of Mechanical Properties of Banana Pseudostem Fibers Using Sludge Retting Pretreatment. Agriculture, 15(19), 2057. https://doi.org/10.3390/agriculture15192057





