The Infection of Yellow Lupin (Lupinus luteus L.) with Bean Yellow Mosaic Virus (BYMV) and Cucumber Mosaic Virus (CMV) in Organic Farming in Eastern Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Experiment
2.2. The Detection of Viruses
2.2.1. Serological Tests
2.2.2. Molecular Tests
2.3. Statistical Analysis
3. Results
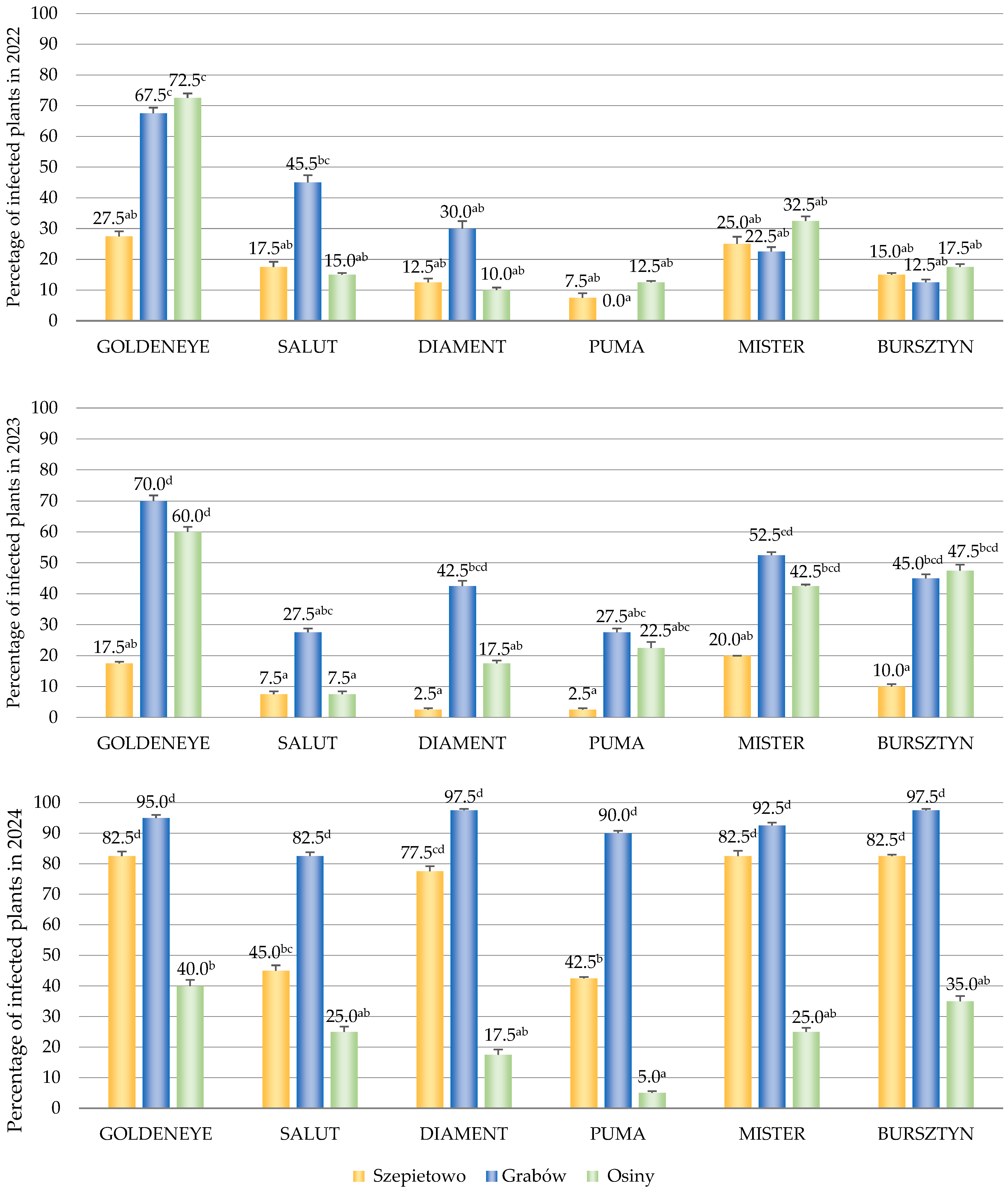
3.1. The Occurrence of BYMV
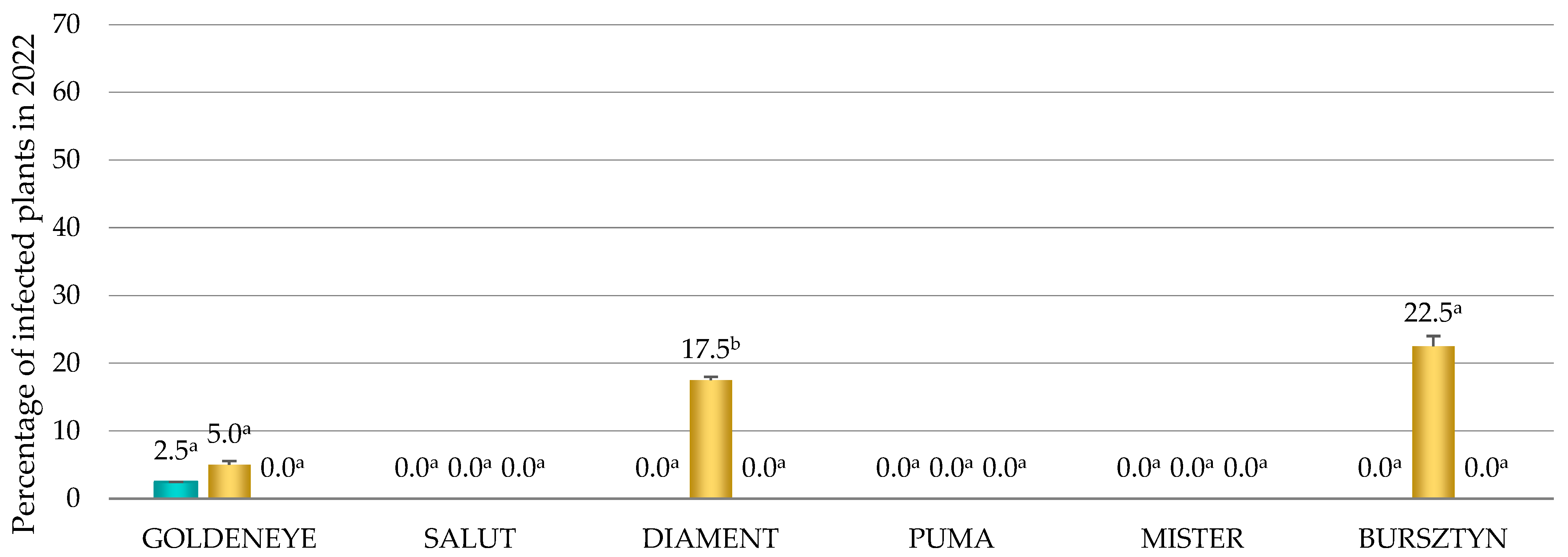
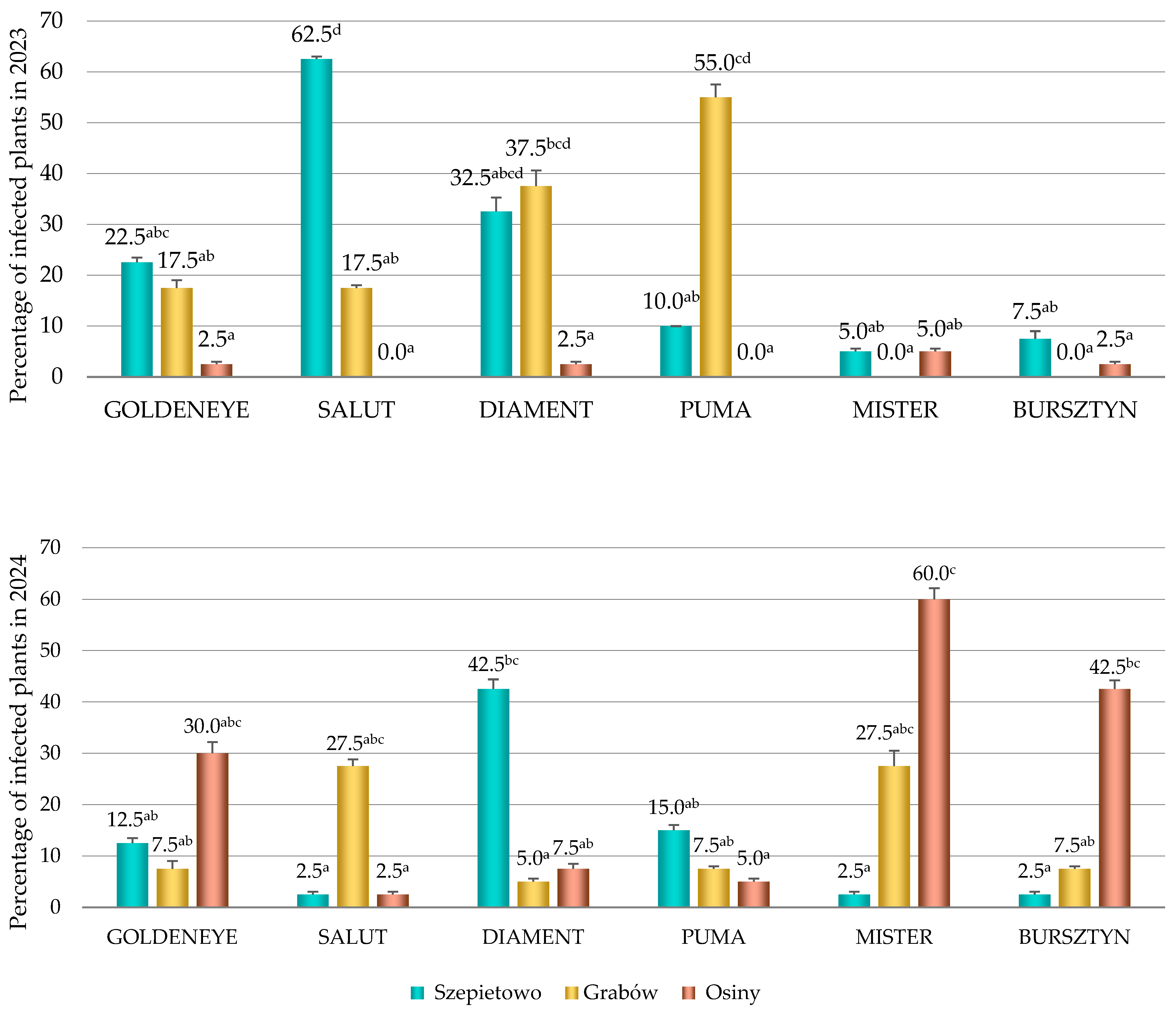
3.2. The Occurrence of CMV
3.3. The Occurrence of Viruses Depending on Cultivars, Locations and Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BYMV | bean yellow mosaic virus |
| CMV | cucumber mosaic virus |
References
- Jones, R.A.C. Host Resistance to Virus Diseases Provides a Key Enabler towards Fast Tracking Gains in Grain Lupin Breeding. Plants 2023, 12, 2521. [Google Scholar] [CrossRef] [PubMed]
- Cebulska, A.; Jankowiak, H.; Weisbauerova, E.; Nevrkla, P. Influence of an increased content of pea and yellow lupin protein in the diet of pigs on meat quality. Porc. Health Manag. 2021, 7, 63. [Google Scholar] [CrossRef]
- Batista, A.; Kesy, J.; Sadowska, K.; Karolewski, Z.; Bocianowski, J.; Wozniak, A.; Morkunas, I. The role of silver nanoparticles in yellow lupine (Lupinus luteus L.) defense response to Fusarium oxysporum f.sp. lupini. Sci. Rep. 2025, 15, 16136. [Google Scholar] [CrossRef]
- Kordan, B.; Gabryś, B.; Dancewicz, K.; Lahuta, L.B.; Piotrowicz-Cieślak, A.; Rowińska, E. European yellow lupine, Lupinus luteus, and narrow-leaf lupine, Lupinus angustifolius, as hosts for the pea aphid, Acyrthosiphon pisum. Entomol. Exp. Et Appl. 2008, 128, 139–146. [Google Scholar] [CrossRef]
- Gołębiewska, B.; Pajewski, T. Changes in the Development Trends of Organic Farming in the World. Ann. Pol. Assoc. Agric. Agribus. Econ. 2025, XXVII, 83–95. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of Agriculture; Statistics Poland: Warszawa, Poland, 2024; p. 92.
- Rybicki, E.P.; Foster, G.D. Chapter 17—Plant diseases caused by viruses. In Agrios’ Plant Pathology, 6th ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 547–606. [Google Scholar] [CrossRef]
- Dell’Olmo, E.; Tiberini, A.; Sigillo, L. Leguminous Seedborne Pathogens: Seed Health and Sustainable Crop Management. Plants 2023, 12, 2040. [Google Scholar] [CrossRef]
- Sastry, K.S.; Mandal, B.; Hammond, J.; Scott, S.W.; Briddon, R.W. Lupinus spp. (Lupin). In Encyclopedia of Plant Viruses and Viroids, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1414–1421. [Google Scholar]
- Hasiów-Jaroszewska, B.; Budzyńska, D.; Rymelska, N.; Korpys, P.; Borodynko-Filas, N. Phylogenetic evidence of natural reassortants in the Cucumber mosaic virus population in Poland. Can. J. Plant Pathol. 2018, 40, 587–593. [Google Scholar] [CrossRef]
- Meena, B.R.; Biswas, K.K. Status of the major aphid-transmitted viruses of pulse crops in India. Indian Phytopathol. 2024, 77, 561–572. [Google Scholar] [CrossRef]
- Rhee, S.J.; Watt, L.G.; Bravo, A.C.; Murphy, A.M.; Carr, J.P. Effects of the cucumber mosaic virus 2a protein on aphid-plant interactions in Arabidopsis thaliana. Mol. Plant Pathol. 2020, 21, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, W.; Luo, C.; Li, Y.; Liu, T. Transmission Comparisons of Cucumber Mosaic Virus Subgroup I and II Isolates by Different Aphid Species. J. Phytopathol. 2012, 160, 299–303. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Congdon, B.S. Seed-Borne Virus Research: 1. Alfalfa and Cucumber Mosaic Viruses and Less Important Viruses. Viruses 2024, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Ohki, S.T. Cucumber mosaic virus: Viral genes as virulence determinants. Mol. Plant Pathol. 2012, 13, 217–225. [Google Scholar] [CrossRef]
- Hasiów-Jaroszewska, B.; Kasprowicz, M.; Borodynko-Filas, N. Rapid detection of Cucumber mosaic virus isolates representing distinct phylogenetic subgroups by reverse transcription, loop-mediated isothermal amplification. J. Plant Dis. Prot. 2017, 125, 227–230. [Google Scholar] [CrossRef]
- Mrkvová, M.; Kemenczeiová, J.; Achs, A.; Alaxin, P.; Predajna, L.; Šoltys, K.; Šubr, Z.; Glasa, M. Molecular Characteristics and Biological Properties of Bean Yellow Mosaic Virus Isolates from Slovakia. Genes 2024, 10, 262. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Coutts, B.A.; Cheng, Y. Yield limiting potential of necrotic and non-necrotic strains of Bean yellow mosaic virus in narrow-leafed lupin (Lupinus angustifolius). Aust. J. Agric. Res. 2003, 54, 849–859. [Google Scholar] [CrossRef]
- Carpenter, D.; Luckett, D. Cucumber mosaic virus in lupins. Puls Point 2003, 18, 1–2. [Google Scholar]
- Hasiów-Jaroszewska, B.; Komorowska, B. Diversity of the Polish isolates of bean yellow mosaic virus (BYMV) isolated from broad bean. Prog. Plant Prot. 2022, 62, 44–50. [Google Scholar] [CrossRef]
- Minicka, J.; Zarzynska-Nowak, A.; Budzynska, D.; Borodynko-Filas, N.; Hasiow-Jaroszewska, B. High-Throughput Sequencing Facilitates Discovery of New Plant Viruses in Poland. Plants 2020, 9, 820. [Google Scholar] [CrossRef]
- Boschin, G.; Resta, D. Alkaloids Derived from Lysine: Quinolizidine (a Focus on Lupin Alkaloids). In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Adhikari, K.N.; Edwards, O.R.; Wang, S.; Ridsdill-Smith, T.J.; Buirchell, B. The role of alkaloids in conferring aphid resistance in yellow lupin (Lupinus luteus L.). Crop. Pasture Sci. 2012, 63, 444. [Google Scholar] [CrossRef]
- Estivi, L.; Brandolini, A.; Gasparini, A.; Hidalgo, A. Lupin as a Source of Bioactive Antioxidant Compounds for Food Products. Molecules 2023, 28, 7529. [Google Scholar] [CrossRef]
- PN-EN ISO 10390:2022-09; Soil, Treated Biowaste and Sludge–Determination of pH. Polish Committee for Standardizations: Warsaw, Poland, 2022.
- Clark, M.F.; Adams, A.N. Characteristics of the Microplate Method of Enzyme-Linked Immunosorbent Assay for the Detection of Plant Viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Katoch, M.; Ram, R.; Zaidi, A.A.; Garg, I.D. Status of bean yellow mosaic virus on Gladiolus. Crop. Prot. 2002, 21, 861–865. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, X.; Willingmann, P.; Adam, G.; Heinze, C. Problems Encountered with the Selection of Cucumber Mosaic Virus (CMV) Isolates for Resistance Breeding Programs. J. Phytopathol. 2011, 159, 621–629. [Google Scholar] [CrossRef]
- van Bruggen, A.H.; Gamliel, A.; Finckh, M.R. Plant disease management in organic farming systems. Pest Manag. Sci. 2016, 72, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Das, R. Organic farming to mitigate biotic stresses under climate change scenario. Bull. Natl. Res. Cent. 2024, 48, 71. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Burchell, G.M. Resistance to Cucumber mosaic virus in Lupinus mutabilis (Pearl lupin). Australas. Plant Pathol. 2004, 33, 591–593. [Google Scholar] [CrossRef]
- Jones, R.A.C. Patterns of spread of two non-persistently aphid-borne viruses in lupin stands under four different infection scenarios. Ann. Appl. Biol. 2005, 146, 337–350. [Google Scholar] [CrossRef]
| Cultivar | Flowering | Maturation | Type | Breeder | Year of Entry in the Polish Register |
|---|---|---|---|---|---|
| Goldeneye | early | medium | indeterminate | PHR | 2019 |
| Salut | late | medium | indeterminate | HR Smolice | 2020 |
| Diament | early | medium | indeterminate | PHR | 2019 |
| Puma | late | late | indeterminate | HR Smolice | 2017 |
| Mister | medium | medium | indeterminate | PHR | 2003 |
| Bursztyn | early | early | indeterminate | PHR | 2014 |
| Virus | Primer Name | Primer Sequence | PCR Conditions | Amplicon Size [bp] | Reference |
|---|---|---|---|---|---|
| BYMV | PI | TTGAATCTGAACTGAAGTATT | 94 °C 5 min; 30 × (94 °C 60 s, 55 °C 90 s, 72 °C 60 s) 72 °C 5 min | 733 | [28] |
| PII | CTCCTTTCTACAAAATGGACA | ||||
| CMV | 5′CP | ATGGACAAATCTGRATCWMCC | 40 × (92 °C 30 s, 60 °C 45 s, 72 °C 60 s) 72 °C 5 min | 760 | [29] |
| 3′CP | CTGGATGGACAACCCGTTC |
| Percentage of Plants Infected with: | ||
|---|---|---|
| BYMV | CMV | |
| Cultivar | ||
| Goldeneye | 59.17 e | 11.11 a |
| Salut | 30.28 ab | 12.50 a |
| Diament | 34.17 bc | 16.11 a |
| Puma | 23.34 a | 10.28 a |
| Mister | 43.89 d | 11.10 a |
| Bursztyn | 40.3 cd | 12.20 a |
| Location | ||
| Szepietowo | 32.08 A | 12.22 AB |
| Grabów | 55.42 B | 14.17 B |
| Osiny | 28.05 A | 8.89 A |
| Year | ||
| 2022 | 24.58 † | 2.64 † |
| 2023 | 28.89 † | 15.56 * |
| 2024 | 61.94 * | 17.08 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubacka, A.; Czarnecka, D.; Księżak, J. The Infection of Yellow Lupin (Lupinus luteus L.) with Bean Yellow Mosaic Virus (BYMV) and Cucumber Mosaic Virus (CMV) in Organic Farming in Eastern Poland. Agriculture 2025, 15, 2003. https://doi.org/10.3390/agriculture15192003
Czubacka A, Czarnecka D, Księżak J. The Infection of Yellow Lupin (Lupinus luteus L.) with Bean Yellow Mosaic Virus (BYMV) and Cucumber Mosaic Virus (CMV) in Organic Farming in Eastern Poland. Agriculture. 2025; 15(19):2003. https://doi.org/10.3390/agriculture15192003
Chicago/Turabian StyleCzubacka, Anna, Diana Czarnecka, and Jerzy Księżak. 2025. "The Infection of Yellow Lupin (Lupinus luteus L.) with Bean Yellow Mosaic Virus (BYMV) and Cucumber Mosaic Virus (CMV) in Organic Farming in Eastern Poland" Agriculture 15, no. 19: 2003. https://doi.org/10.3390/agriculture15192003
APA StyleCzubacka, A., Czarnecka, D., & Księżak, J. (2025). The Infection of Yellow Lupin (Lupinus luteus L.) with Bean Yellow Mosaic Virus (BYMV) and Cucumber Mosaic Virus (CMV) in Organic Farming in Eastern Poland. Agriculture, 15(19), 2003. https://doi.org/10.3390/agriculture15192003






