Silicon as a Tool to Manage Diaphorina citri and Relation Soil and Leaf Chemistry in Tahiti Lime
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Plant Material
2.2. Experimental Design
2.3. Variables Evaluated
2.4. Statistical Analysis
3. Results
3.1. Bud of Tahiti Lime Plants and Exposure to Controlled and Natural Infestation by D. citri
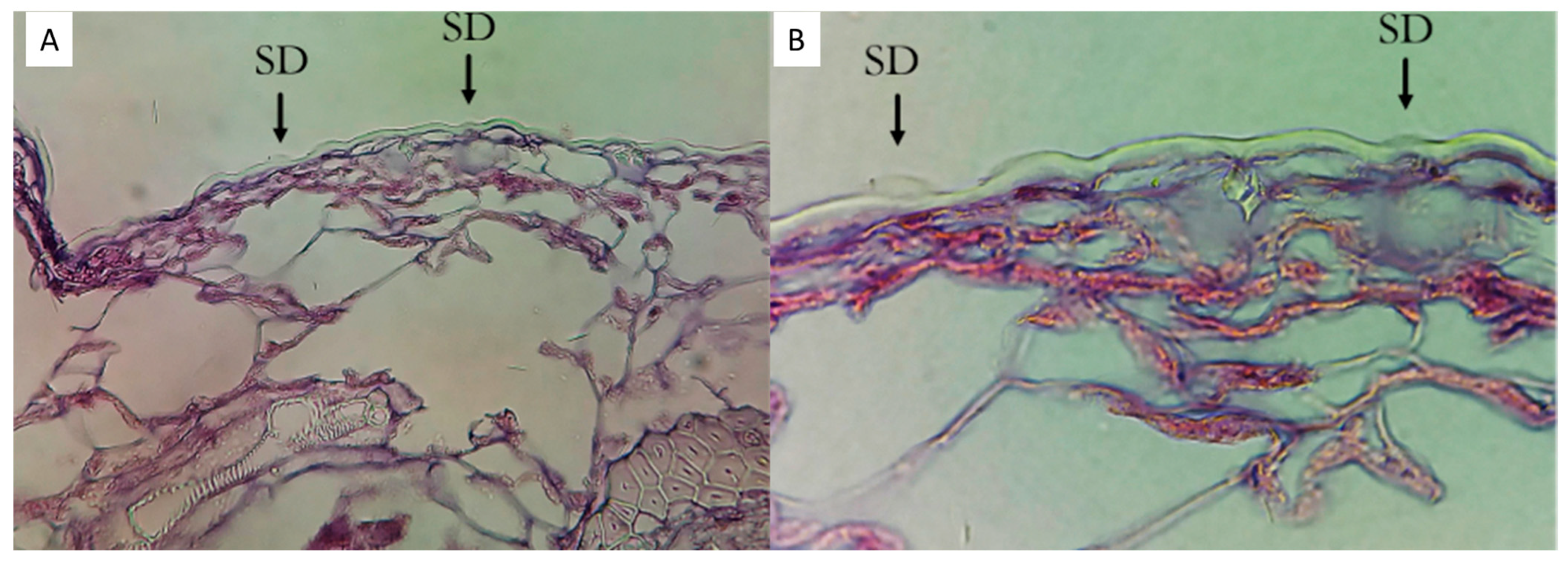
3.2. Deposition of Silicon on the Foliar Surface of Tahiti Lime Leaves
3.3. The Relationship Between Soil and Leaf Nutrient Content and Silicon Utilization
3.4. Impact of Silicon on the Physical Quality of Tahiti Lime Fruit
4. Discussion
4.1. Effects of Silicon on Shoot Emergence and the Dynamics of the Vector D. citri
4.2. Histological Accumulation of Silicon in Tahiti Lime Leaves
4.3. Interactions Between Silicon, Nutrients, and Soil–Foliar Dynamics
4.4. Impact of Silicon on the Physical Quality of the Fruit
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tipu, M.H.; Rahman, M.; Islam, M.; Elhai, F.E.; Jahan, R.; Islam, R. Citrus greening disease (HLB) on Citrus reticulata (Mandarin) caused by Candidatus Liberibacter asiaticus in Bangladesh. Physiol. Mol. Plant Pathol. 2020, 112, 101558. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, M.; Beattie, G.A.C.; Xia, Y.; Ouyang, G.; Xiong, J. Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: A status report for China. Int. J. Pest Manag. 2006, 52, 343–352. [Google Scholar] [CrossRef]
- Santivañez, T.; Mora, G.; Diaz, G.; López, J.I.; Hurtado, P. Citrus. Marco Estratégico Para la Gestión Regional del Huanglongbing en América Latina y el Caribe, 1st ed.; Food and Agriculture Organization (FAO): Rome, Italy, 2013; pp. 15–54. Available online: http://www.fao.org/3/a-i3319s.pdf (accessed on 6 December 2024).
- Stelinski, L.L. Ecological aspects of the vector-borne bacterial disease, citrus greening (Huanglongbing): Dispersal and host use by Asian Citrus Psyllid, Diaphorina citri Kuwayama. Insects 2019, 10, 208. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Duan, Y.; Singerman, A.; Guan, Z. Citrus greening: Management strategies and their economic impact. HortScience 2020, 55, 604–612. [Google Scholar] [CrossRef]
- Costa, G.V.; Neves, C.S.V.J.; Bassanezi, R.B.; Leite Junior, R.P.; Telles, T.S. Economic impact of Huanglongbing on orange production. Rev. Bras. Frutic. 2021, 43, e-472. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- García-Méndez, V.H.; Ortega-Arenas, L.D.; Villanueva-Jiménez, J.A.; Sánchez-Arroyo, H. Susceptibility of Diaphorina citri Kuwayama (hemiptera: Liviidae) to insecticides, in Veracruz, Mexico. Agrociencia 2016, 50, 319–330. [Google Scholar]
- Liu, T.Y.; Zhou, Y.X.; Yuan, C.Y.; Gao, Y.F.; Fan, J.Y.; Yuan, G.R.; Wang, J.J.; Dou, W. Genetic mutations and insecticide resistance in Diaphorina citri: A comparative study across Chinese citrus regions. Pest. Manag. Sci. 2025, 81, 4831–4838. [Google Scholar] [CrossRef]
- Mamani, A.; Filippone, M.P. Bioinsumos: Componentes claves de una agricultura sostenible. Bio-products: Key components of sustainable agricultura. Rev. Agron. Noroeste Argent. 2018, 38, 9–21. [Google Scholar]
- Marzaini, B.; Mohd, A. Plant growth-promoting microorganisms isolated from plants as potential antimicrobial producers: A review. Pertanika J. Trop. Agric. Sci. 2021, 44, 255–273. [Google Scholar] [CrossRef]
- Chen, X.D.; Stockton, D.; Gossett, H.; Qureshi, J.A.; Ibanez, F.; Pelz-Stelinski, K.S.; Stelinski, L.L. Comparisons of economic thresholds for Asian citrus psyllid management suggest a revised approach to reduce management costs and improve yield. Front. Sustain. Food Syst. 2022, 6, 948278. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; Chapter 8; pp. 257–261. [Google Scholar]
- Suganthy, M.; Sowmiya, A.; Yuvaraj, M.; Anitha, R. Silicon—A potential alternative in insect pest management for sustainable agriculture. Silicon 2024, 16, 1857–1880. [Google Scholar] [CrossRef]
- Deshmukh, R.; Belanger, R.R. The functional role of silicon in plant biology, Molecular evolution of aquaporins and silicon influx in plants. Funct. Ecol. 2016, 30, 1277–1285. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Carballo-Méndez, F.J.; Rodríguez-Salinas, P.A.; Olivares-Sáenz, E.; Rodríguez-Ortiz, J.C. Salinidad y silicio en el crecimiento vegetativo y la concentración de pigmentos en cuatro cultivares de higuera. (Ficus carica L.). ITEA-Inf. Tec. Econ. Agrar. 2022, 118, 19–35. [Google Scholar] [CrossRef]
- Antúnez-Ocampo, O.M.; Sabino-López, J.E.; Hernández-Galeno, C.; Espinosa-Rodríguez, M. Maize (Zea mays L.) yield in response to soil fertilization with nitrogen, phosphorus and silicon. Terra Latinoam. 2023, 41, e1682. [Google Scholar] [CrossRef]
- Kovács, S.; Kutasy, E.; Csajbók, J. The multiple role of silicon nutrition in alleviating environmental stresses in sustainable crop production. Plants 2022, 11, 1223. [Google Scholar] [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Hartley, S.; Singh, I.K. Silicon: Its ameliorative effect on plant defense against herbivory. J. Exp. Bot. 2020, 71, 6730–6743. [Google Scholar] [CrossRef]
- Ramírez-Godoy, A.; Puentes-Pérez, G.; Restrepo-Díaz, H. An evaluation of the use of calcium, potassium and silicon for the management of Diaphorina citri populations in Tahiti lime trees. Not. Bot. Horti Agrobot. 2018, 46, 546–552. [Google Scholar] [CrossRef]
- DeBolt, D.C. A high sample volume procedure for the colorimetric determination of soil organic matter. Commun. Soil Sci. Plant Anal. 1974, 5, 131–137. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Gillman, G.P.; Bruce, R.C.; Davey, B.G.; Kimble, J.M.; Searle, P.L.; Skjemstad, J.O. A comparison of methods used for determination of cation exchange capacity. Commun. Soil Sci. Plant Anal. 1983, 14, 1005–1014. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Lopez-Valdivia, L.M.; Novillo, J.; Obrador, A.; Rico, M.I. Comparison of EDTA and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 2006, 132, 450–463. [Google Scholar] [CrossRef]
- John, K.M.; Chuah, H.H.; Neufeld, J.H. Application of improved Azomethine-H method to the determination of boron in soils and plants. Anal. Lett. 1975, 8, 559–568. [Google Scholar] [CrossRef]
- Johansen, W.A. Plant Micro Techniques; McGraw Hill Book. Co.: New York, NY, USA, 1940. [Google Scholar]
- Samal, I.; Bhoi, T.K.; Mahanta, D.K.; Komal, J. Establishing the role of silicon (Si) in plant resistance to insects: A bibliometric approach. Silicon 2023, 16, 2119–2128. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.M.; Kannan, M.; Prakash, N.B. Chapter 16—Silicon as a natural plant guard against insect pests. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Hassan, E., Abdullah, H., Al, S., Hassan, E., Masayuki, F., Pessarakli, M., Anwar, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 219–227. [Google Scholar] [CrossRef]
- Saw, G.; Nagdev, P.; Jeer, M.; Murali-Baskaran, R.K. Silica nanoparticles mediated insect pest management. Pestic. Biochem. Physiol. 2023, 194, 105524. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, I.; Idrees, A.; Abbasi, A.; Ali, S.; Asad, M.; Li, C.; Liu, C.Z.; Zhang, K.X.; Yasin, M.; Asghar, M.A.; et al. Silicon accumulation in maize and its effects on demographical traits of fall armyworm [Spodoptera frugiperda (J. E. Smith)]. Silicon 2023, 15, 3269–3281. [Google Scholar] [CrossRef]
- Peixoto, M.L.; Moraes, J.C.; Silva, A.A.; Assis, F.A. Effect of silicon on the oviposition preference of Bemisia tabaci Biotype B (GENN.) (Hemiptera: Aleyrodidae) on bean (Phaseolus vulgaris L.) plants. Ciência Agrotecnol. 2011, 35, 478–481. [Google Scholar] [CrossRef]
- Correa, R.S.; Moraes, J.C.; Auad, A.M.; Carvalho, G.A. Silicon and acibenzolar-S-methyl as resistance inducers in cucumber, against the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype B. Neotrop. Entomol. 2005, 34, 429–433. [Google Scholar] [CrossRef]
- Shah, M.A.; Sharma, S.; Sharma, J. Bio-efficacy of potassium silicate against aphids and whitefly in potato. Potato J. 2019, 46, 132–137. [Google Scholar]
- Teixeira, N.C.; Valim, J.O.S.; Oliveira, M.G.A.; Campos, W.G. Combined effects of soil silicon and drought stress on host plant chemical and ultrastructural quality for leaf-chewing and sap-sucking insects. J. Agron. Crop Sci. 2019, 206, 677–686. [Google Scholar] [CrossRef]
- Korndörfer, A.P.; Grisoto, E.; Vendramim, J.D. Induction of insect plant resistance to the spittlebug Mahanarva fimbriolata Stål (Hemiptera: Cercopidae) in sugarcane by silicon application. Neotrop. Entomol. 2011, 40, 387–392. [Google Scholar]
- Shameen, F.; Gulzar, I.; Wani, A.H.; Malik, M.; Rashid, I.; Ahmad, T. Silicon supplementation mitigates the effects of infestation of corn leaf aphid, Rhopalosiphum maidis Fitch (Hemiptera: Aphididae). Phytoparasitica 2025, 53, 53. [Google Scholar] [CrossRef]
- Ghafar, M.A.; Feng, Q.; Ul Haq, I.; Hyder, M.; Sufian, M.; Abbas, D.; Haider, K.; Lubaid, M.; Akhtar, M.; Wang, L.; et al. Impact of silicon-based treatments on the demographic traits of Spodoptera frugiperda in maize. J. Crop Health 2025, 77, 119. [Google Scholar] [CrossRef]
- Mvondo-She, M.A.; Marais, D. The investigation of silicon localization and accumulation in citrus. Plants 2019, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Rotmuenwai, N.; Aryuyo, K.; Kruethaworn, N.; Wattananit, W.; Yookongkaew, N. Exploring silica accumulation in bamboo leaves: A study on phytolith morphology and epidermal patterning in the tropical giant bamboo Dendrocalamus copelandii. Ann. Bot. 2025, 135, 757–768. [Google Scholar] [CrossRef]
- Kajino, H.; Kitajima, K. Lamina-specific localization of silicon accumulation in two broadleaf tree species. J. Plant Res. 2023, 136, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Kajino, H.; Kitajima, K. Leaf silicon accumulation rates in relation to light environment and shoot growth rates in paper mulberry (Broussonetia papyrifera, Moraceae). J. Plant Res. 2021, 134, 1013–1020. [Google Scholar] [CrossRef]
- Szulc, W.; Rutkowska, B.; Hoch, M.; Spychaj-Fabisiak, E.; Murawska, B. Exchangeable silicon content of soil in a long-term fertilization experiment. Plant Soil Environ. 2015, 61, 458–461. [Google Scholar] [CrossRef]
- Tubaña, B.S.; Heckman, J.R. Silicon in soils and plants. In Silicon and Plant Diseases; Rodrigues, F., Datnoff, L., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Zanão Júnior, L.A.; Blanco, I.B.; Pereira, N.; Schneider, J.A. Acidity neutralization and silicon availability using calcium silicate in soil cultivated with wheat (Triticum aestivum L.). Aust. J. Crop Sci. 2017, 11, 1354–1357. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Kulikova, A.K.; Uromova, I.P. Mobility of silicates, fertility indicators of sod-podzolic soil, silicon bioaccumulation and productivity of agricultural crops under zeolite application. Agrobiology 2021, 56, 183–195. [Google Scholar] [CrossRef]
- Prakash, N.B.; Anitha, M.S.; Sandhya, K. Behaviour of different levels and grades of diatomite as silicon source in acidic and alkaline soils. Silicon 2015, 8, 629–636. [Google Scholar] [CrossRef]
- Mehta, S.C.; Grewal, K.S.; Sangwan, P.S.; Dahiya, S.S. Effect of pH on cation exchange capacity of soils from semi-arid and humid regions. Ann. Biol. 2003, 19, 165–168. [Google Scholar]
- Pooniyan, S.; Kour, S.; Yadav, K.K.; Gora, R.; Chadha, D.; Choudhary, S. Silicon status in soils and their benefits in crop production. Commun. Soil Sci. Plant Anal. 2023, 54, 1887–1895. [Google Scholar] [CrossRef]
- Brackhage, C.; Schaller, J.; Bäucker, E.; Dudel, E. Silicon availability affects the stoichiometry and content of calcium and micronutrients in the leaves of common reed. Silicon 2013, 5, 199–204. [Google Scholar] [CrossRef]
- Wadas, W. Effect of foliar silicon application on nutrient content in early crop potato tubers. Agronomy 2022, 12, 2706. [Google Scholar] [CrossRef]
- Tabatabaei, S.J. Interactive effects of Si and NaCl on growth, yield, photosynthesis, and ions content in strawberry (Fragaria × ananassa var Camarosa). J. Plant Nutr. 2016, 39, 1524–1535. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Kaniganti, S.; Hima Kumari, P.; Sudhakar Reddy, P.; Suravajhala, P.; Suprasanna, P. Functional and biotechnological cues of potassium homeostasis for stress tolerance and plant development. Biotechnol. Genet. Eng. Rev. 2022, 40, 3527–3570. [Google Scholar] [CrossRef]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Rizwan, M.; Ali, S. Effects of exogenous taurine on growth, photosynthesis, oxidative stress, antioxidant enzymes and nutrient accumulation by Trifolium alexandrinum plants under manganese stress. Chemosphere 2022, 308 Pt 3, 136523. [Google Scholar] [CrossRef]
- Minden, V.; Schaller, J.; Olde Venterink, H. Plants increase silicon content as a response to nitrogen or phosphorus limitation: A case study with Holcus lanatus. Plant Soil 2021, 462, 95–108. [Google Scholar] [CrossRef]
- Kostic, I.; Nikolic, N.; Milanovic, S.; Milenkovic, I.; Pavlovic, J.; Paravinja, A.; Nikolic, M. Silicon modifies leaf nutriome and improves growth of oak seedlings exposed to phosphorus deficiency and Phytophthora plurivora infection. Front. Plant Sci. 2023, 14, 1265782. [Google Scholar] [CrossRef]
- Soltani, M.; Kafi, M.; Nezami, A.; Taghiyari, H.R. Effects of silicon application at nano and micro scales on the growth and nutrient uptake of potato minitubers (Solanum tuberosum var. Agria) in Greenhouse Conditions. BioNanoSci 2018, 8, 218–228. [Google Scholar] [CrossRef]
- Bokhtiar, S.M.; Huang, H.R.; Li, Y.R. Response of sugarcane to calcium silicate on yield, gas exchange characteristics, leaf nutrient concentrations, and soil properties in two different soils. Commun. Soil Sci. Plant Anal. 2012, 43, 1363–1381. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef]
- Xu, D.; Gao, T.; Fang, X.; Bu, H.; Li, Q.; Wang, X.; Zhang, R. Silicon addition improves plant productivity and soil nutrient availability without changing the grass: Legume ratio response to N fertilization. Sci. Rep. 2020, 10, 10295. [Google Scholar] [CrossRef]
- Rea, R.S.; Islam, M.R.; Rahman, M.M.; Nath, B.; Mix, K. Growth, nutrient accumulation, and drought tTolerance in crop plants with silicon application: A review. Sustainability 2022, 14, 4525. [Google Scholar] [CrossRef]
- Bosnic, P.; Bosnic, D.; Jasnic, J.; Nikolic, M. Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environ. Exp. Bot. 2018, 155, 681–687. [Google Scholar] [CrossRef]
- Bin, Z.; Zhang, R.; Zhang, W.; Xu, D. Effects of nitrogen, phosphorus and silicon addition on leaf carbon, nitrogen, and phosphorus concentration of Elymus nutans of alpine meadow on Qinghai-Tibetan Plateau, China. Acta Ecol. Sin. 2015, 35, 4699–4706. [Google Scholar] [CrossRef]
- Dutta, A.; Dracatos, P.M.; Khan, G.A. Balancing act: The dynamic relationship between nutrient availability and plant defence. Plant J. 2024, 120, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Masullo, L.S.; Derisso, V.D.; Manarim, G.R.; Ferraz, A.V.; Rocha, J.H.T.; de Ávila, P.A.; Florentino, A.L.; de Aguiar, C.L.; Lavres, J.; Gonçalves, J.L. Modulation of structural carbohydrates, phenol compounds and lignin content in Eucalyptus urophylla cuttings grown under boron, copper and zinc induced-deficiency. New For. 2022, 53, 337–352. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Karagöl, A.; Akınoğlu, G.; Korkmaz, H. The effects of silicon on nutrient levels and yields of tomatoes under saline stress in artificial medium culture. J. Plant Nutr. 2018, 41, 123–135. [Google Scholar] [CrossRef]
- de Mesquita Alves, J.; de Lima, A.S.; de Figueredo, L.F.; de Oliveira Mesquita, F.; de Mesquita, E.F.; Bezerra, F.T.C.; da Silva Sousa, C.; da Silva, F.L.; Suassuna, C. Nitrogen and silicon application can increase nutrient uptake and fruit quality of Cucurbita pepo L. Water Air Soil Pollut. 2022, 233, 40. [Google Scholar] [CrossRef]
- Torres, E.; Carrasco-Cuello, F. Foliar- and root-applied silicon-based biostimulant increases the nutritional efficiency, fruit firmness and storage potential of nectarines. Acta Hortic. 2022, 1333, 269–274. [Google Scholar] [CrossRef]
- ICA. Instituto Colombiano Agropecuario, Resolution No. 1668 of 2019. Available online: https://www.ica.gov.co/getattachment/877b5bbb-0f20-4253-9f7d-7b20f03e2629/2019R01668.aspx (accessed on 1 September 2025).


| Treatment | Silicon Source | Application Method | Dose |
|---|---|---|---|
| Foliar-applied organic silicon | Organic | Foliar | 2% p/v |
| Soil-applied organic silicon | Organic | Soil | 2% p/v |
| Combined-applied organic silicon | Organic | Foliar + Soil | 2% p/v |
| Foliar-applied inorganic silicon | Inorganic | Foliar | 2 mL∙L−1 |
| Soil-applied inorganic silicon | Inorganic | Soil | 5 mL∙L−1 |
| Combined applied inorganic silicon | Inorganic | Foliar + Soil | 2 mL∙L−1/5 mL∙L−1 |
| Control | — | No application | — |
| Ca2+ | Mg2+ | K+ | pH | OM | CEC | EC | N, NO3− | P | S | Fe | Mn | Cu | Zn | B | Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | |||||||||||||||
| Mg2+ | 0.981 * | 1 | ||||||||||||||
| K+ | 0.767 | 0.874 | 1 | |||||||||||||
| pH | 0.623 | 0.505 | 0.200 | 1 | ||||||||||||
| OM | 0.429 | 0.499 | 0.683 | 0.494 | 1 | |||||||||||
| CEC | 0.983 * | 1.000 ** | 0.871 | 0.517 | 0.505 | 1 | ||||||||||
| EC | 0.667 | 0.568 | 0.157 | 0.327 | −0.378 | 0.567 | 1 | |||||||||
| N, NO3− | −0.841 | −0.729 | −0.358 | −0.917 | −0.333 | −0.737 | −0.652 | 1 | ||||||||
| P | 0.256 | 0.063 | −0.411 | 0.700 | −0.269 | 0.073 | 0.590 | −0.699 | 1 | |||||||
| S | −0.968 * | −0.913 | −0.604 | −0.609 | −0.201 | −0.915 | −0.830 | 0.868 | −0.424 | 1 | ||||||
| Fe | −0.751 | −0.634 | −0.287 | −0.978 * | −0.420 | −0.644 | −0.505 | 0.980 * | −0.714 | 0.759 | 1 | |||||
| Mn | 0.799 | 0.684 | 0.324 | 0.952 * | 0.379 | 0.693 | 0.580 | −0.995 ** | 0.710 | −0.817 | −0.995 ** | 1 | ||||
| Cu | −0.714 | −0.596 | −0.261 | −0.990 * | −0.445 | −0.606 | −0.451 | 0.965 * | −0.713 | 0.715 | 0.998 ** | −0.986 * | 1 | |||
| Zn | −0.645 | −0.527 | −0.214 | −0.999 ** | −0.483 | −0.539 | −0.356 | 0.930 | −0.704 | 0.635 | 0.984 * | −0.962 * | 0.994 ** | 1 | ||
| B | −0.807 | −0.693 | −0.330 | −0.947 | −0.371 | −0.702 | −0.594 | 0.997 ** | −0.708 | 0.827 | 0.993 ** | −1.000 ** | 0.983 * | 0.957 * | 1 | |
| Si | −0.792 | −0.684 | −0.350 | −0.967 * | −0.448 | −0.693 | −0.517 | 0.986 * | −0.674 | 0.791 | 0.998 ** | −0.997 ** | 0.993 ** | 0.975 * | 0.996 ** | 1 |
| Ca2+ | Mg2+ | K+ | pH | OM | CEC | EC | N, NO3− | P | S | Fe | Mn | Cu | Zn | B | Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | |||||||||||||||
| Mg2+ | 0.996 ** | 1 | ||||||||||||||
| K+ | 0.422 | 0.471 | 1 | |||||||||||||
| pH | 0.980 * | 0.982 * | 0.584 | 1 | ||||||||||||
| OM | 0.730 | 0.787 | 0.820 | 0.798 | 1 | |||||||||||
| CEC | 0.992 ** | 0.997 ** | 0.532 | 0.994 ** | 0.807 | 1 | ||||||||||
| EC | 0.312 | 0.375 | 0.978 * | 0.470 | 0.822 | 0.432 | 1 | |||||||||
| N, NO3− | −0.648 | −0.634 | −0.751 | −0.768 | −0.559 | −0.692 | −0.597 | 1 | ||||||||
| P | 0.601 | 0.557 | 0.490 | 0.685 | 0.295 | 0.606 | 0.297 | −0.942 | 1 | |||||||
| S | −0.812 | −0.821 | −0.008 | −0.698 | −0.559 | −0.773 | 0.012 | 0.083 | −0.050 | 1 | ||||||
| Fe | −0.964 * | −0.984 * | −0.598 | −0.974 * | −0.885 | −0.987 * | −0.525 | 0.646 | −0.511 | 0.779 | 1 | |||||
| Mn | 0.913 | 0.947 | 0.599 | 0.919 | 0.928 | 0.945 | 0.563 | −0.533 | 0.359 | −0.805 | −0.984 * | 1 | ||||
| Cu | −0.969 * | −0.986 * | −0.604 | −0.983 * | −0.876 | −0.992 ** | −0.525 | 0.674 | −0.546 | 0.762 | 0.999 ** | −0.976 * | 1 | |||
| Zn | −0.979 * | −0.988 * | −0.596 | −0.997 ** | −0.837 | −0.997 ** | −0.497 | 0.731 | −0.627 | 0.729 | 0.990 * | −0.948 | 0.995 ** | 1 | ||
| B | −0.950 | −0.974 * | −0.527 | −0.940 | −0.880 | −0.968 * | −0.475 | 0.535 | −0.396 | 0.846 | 0.991 ** | −0.993 ** | 0.984 * | 0.964 * | 1 | |
| Si | −0.866 | −0.896 | −0.812 | −0.936 | −0.944 | −0.923 | −0.745 | 0.772 | −0.582 | 0.561 | 0.953 * | −0.936 | 0.956 * | 0.949 | 0.916 | 1 |
| N | P | K | S | Ca | Mg | Fe | Mn | Cu | B | Zn | Na | Chloride | Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 1 | |||||||||||||
| P | 0.908 | 1 | ||||||||||||
| K | 0.696 | 0.792 | 1 | |||||||||||
| S | 0.055 | 0.184 | −0.414 | 1 | ||||||||||
| Ca | −0.180 | −0.381 | −0.832 | 0.612 | 1 | |||||||||
| Mg | 0.907 | 0.802 | 0.357 | 0.428 | 0.212 | 1 | ||||||||
| Fe | 0.753 | 0.425 | 0.424 | −0.369 | 0.000 | 0.622 | 1 | |||||||
| Mn | −0.240 | 0.135 | −0.136 | 0.710 | 0.002 | −0.063 | −0.811 | 1 | ||||||
| Cu | −0.711 | −0.506 | −0.742 | 0.652 | 0.467 | −0.406 | −0.882 | 0.750 | 1 | |||||
| B | −0.573 | −0.861 | −0.767 | −0.203 | 0.607 | −0.437 | 0.052 | −0.505 | 0.192 | 1 | ||||
| Zn | 0.956 * | 0.960 * | 0.629 | 0.308 | −0.122 | 0.938 | 0.540 | 0.049 | −0.485 | −0.707 | 1 | |||
| Na | 0.822 | 0.979 * | 0.708 | 0.342 | −0.333 | 0.769 | 0.246 | 0.332 | −0.320 | −0.911 | 0.932 | 1 | ||
| Chloride | −0.025 | 0.281 | −0.157 | 0.881 | 0.198 | 0.226 | −0.622 | 0.948 | 0.686 | −0.512 | 0.270 | 0.470 | 1 | |
| Si | −0.189 | −0.500 | −0.969 | 0.397 | 0.978 | 0.240 | 0.992 | −0.500 | 0.866 | 0.756 | −0.189 | −0.470 | −0.156 | 1 |
| N | P | K | S | Ca | Mg | Fe | Mn | Cu | B | Zn | Na | Chloride | Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 1 | |||||||||||||
| P | 0.067 | 1 | ||||||||||||
| K | 0.324 | 0.948 | 1 | |||||||||||
| S | 0.605 | −0.616 | −0.332 | 1 | ||||||||||
| Ca | −0.904 | −0.408 | −0.661 | −0.430 | 1 | |||||||||
| Mg | 0.585 | 0.103 | 0.397 | 0.674 | −0.752 | 1 | ||||||||
| Fe | 0.451 | 0.892 | 0.989 * | −0.195 | −0.764 | 0.501 | 1 | |||||||
| Mn | −0.154 | −0.601 | −0.745 | −0.059 | 0.543 | −0.768 | −0.759 | 1 | ||||||
| Cu | −0.190 | −0.911 | −0.969 * | 0.302 | 0.583 | −0.485 | −0.952 * | 0.872 | 1 | |||||
| B | −0.386 | −0.750 | −0.903 | −0.011 | 0.744 | −0.733 | −0.929 | 0.940 | 0.950 | 1 | ||||
| Zn | 0.794 | 0.610 | 0.731 | 0.005 | −0.852 | 0.306 | 0.786 | −0.263 | −0.560 | −0.575 | 1 | |||
| Na | 0.222 | −0.092 | −0.206 | −0.230 | 0.078 | −0.661 | −0.210 | 0.796 | 0.433 | 0.544 | 0.350 | 1 | ||
| Chloride | −0.097 | −0.955 * | −0.968 * | 0.434 | 0.492 | −0.356 | −0.931 | 0.811 | 0.990 * | 0.895 | −0.536 | 0.367 | 1 | |
| Si | −0.004 | 0.897 | 0.992 | −0.703 | 0.109 | −0.854 | 0.711 | 0.967 | −0.254 | 0.912 | 0.580 | 0.913 | −0.556 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restrepo-García, A.M.; Hurtado-Salazar, A.; Soto-Giraldo, A. Silicon as a Tool to Manage Diaphorina citri and Relation Soil and Leaf Chemistry in Tahiti Lime. Agriculture 2025, 15, 1961. https://doi.org/10.3390/agriculture15181961
Restrepo-García AM, Hurtado-Salazar A, Soto-Giraldo A. Silicon as a Tool to Manage Diaphorina citri and Relation Soil and Leaf Chemistry in Tahiti Lime. Agriculture. 2025; 15(18):1961. https://doi.org/10.3390/agriculture15181961
Chicago/Turabian StyleRestrepo-García, Ana Maria, Alejandro Hurtado-Salazar, and Alberto Soto-Giraldo. 2025. "Silicon as a Tool to Manage Diaphorina citri and Relation Soil and Leaf Chemistry in Tahiti Lime" Agriculture 15, no. 18: 1961. https://doi.org/10.3390/agriculture15181961
APA StyleRestrepo-García, A. M., Hurtado-Salazar, A., & Soto-Giraldo, A. (2025). Silicon as a Tool to Manage Diaphorina citri and Relation Soil and Leaf Chemistry in Tahiti Lime. Agriculture, 15(18), 1961. https://doi.org/10.3390/agriculture15181961








