Impact of Nitrogen Fertilisation and Inoculation on Soybean Nodulation, Nitrogen Status, and Yield in a Central European Climate
Abstract
1. Introduction
2. Materials and Methods
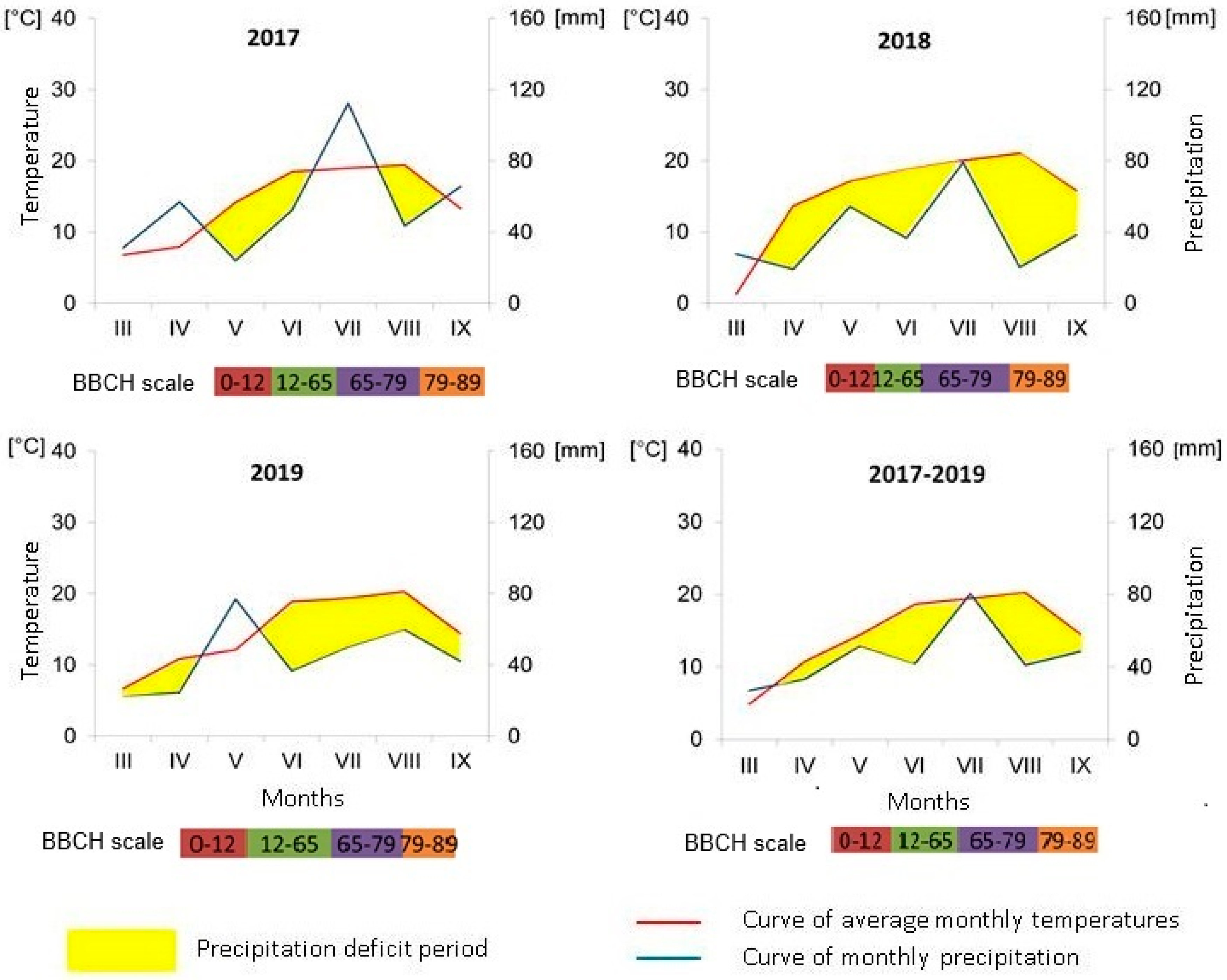
2.1. Site Description, Experimental Design, and Weather and Soil Conditions
2.2. Agronomic Practice
2.3. Chemical Analyses
2.4. Statistical Analysis and Graphs Preparation
3. Results
3.1. Environmental Conditions and Root Nodulation Response
3.2. Nitrogen Content
3.3. Soybean Yield
3.4. Nitrogen Uptake and Allocation Efficiency
4. Discussion
4.1. Environmental Conditions and Root Nodulation Response
4.2. Nitrogen Content
4.3. Soybean Yield
4.4. Nitrogen Uptake and Allocation Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). FAOSTAT Online Database. FAO: Rome, Italy. Available online: http://faostat.fao.org/ (accessed on 12 July 2025).
- Pannecoucque, J.; Goormachtigh, S.; Ceusters, N.; Bode, S.; Boeckx, P.; Roldan-Ruiz, I. Soybean response and profitability upon inoculation and nitrogen fertilisation in Belgium. Eur. J. Agron. 2022, 132, 126390. [Google Scholar] [CrossRef]
- Panasiewicz, K.; Faligowska, A.; Szymańska, G.; Ratajczak, K.; Sulewska, H. Optimizing the Amount of Nitrogen and Seed Inoculation to Improve the Quality and Yield of Soybean Grown in the Southeastern Baltic Region. Agriculture 2023, 13, 798. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen Uptake, Fixation and Response to Fertilizer N in Soybeans: A Review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Pikuła, W.; Jańczak-Pieniążek, M. Effect of Nitrogen Fertilization and Inoculation with Bradyrhizobium japonicum on Nodulation and Yielding of Soybean. Agronomy 2023, 13, 1341. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Bordeleau, L.M.; Prévost, D. Nodulation and nitrogen fixation in extreme environments. Plant Soil 1994, 161, 115–125. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Q.; Zhang, Z.; Ci, D.; Zhang, J.; Xu, Y.; Guo, Q.; Xu, M.; He, K. Effect of Reducing Nitrogen Fertilization and Adding Organic Fertilizer on Net Photosynthetic Rate, Root Nodules and Yield in Peanut. Plants 2023, 12, 2902. [Google Scholar] [CrossRef]
- Cober, E.R.; Stewart, D.W.; Voldeng, H.D. Photoperiod and Temperature Responses in Early-Maturing, Near-Isogenic Soybean Lines. Crop Sci. 2001, 41, 721–727. [Google Scholar] [CrossRef]
- Tamagno, S.; Sadras, V.O.; Haegele, J.W.; Armstrong, P.R.; Ciampitti, I.A. Interplay between nitrogen fertilizer and biological nitrogen fixation in soybean: Implications on seed yield and biomass allocation. Sci. Rep. 2018, 8, 17502. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Stulen, I.; van Keulen, H.; Kuiper, P.J.C. Low concentrations of nitrate and ammonium stimulate nodulation and N2 fixation while inhibiting specific nodulation (nodule DW g−1 root dry weight) and specific N2 fixation (N2 fixed g−1 root dry weight) in soybean. Plant Soil 2004, 258, 281–292. [Google Scholar] [CrossRef]
- Flynn, N.E.; Comas, L.H.; Stewart, C.E.; Fonte, S.J. High N availability decreases N uptake and yield under limited water availability in maize. Sci. Rep. 2023, 13, 14269. [Google Scholar] [CrossRef] [PubMed]
- Albareda, M.; Rodríguez-Navarro, D.N.; Temprano, F.J. Soybean inoculation: Dose, N fertilizer supplementation and rhizobia persistence in soil. Field Crops Res. 2009, 113, 352–356. [Google Scholar] [CrossRef]
- Grossman, J.M.; Schipanski, M.E.; Sooksanguan, T.; Seehaver, S.; Drinkwater, L.E. Diversity of rhizobia in soybean [Glycine max (Vinton)] nodules varies under organic and conventional management. Appl. Soil Ecol. 2011, 50, 14–20. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanisława-Glubiak, E.; Jadczyszyn, T.; Lipiński, W. Nawożenie upraw rolniczych mikroelementami.: Nowe liczby graniczne do oceny zawartości mikroelementów w glebie: Instrukcja upowszechnieniowa. IUNG-POB Puławy 2021, 249, 1–6. [Google Scholar]
- PN-EN ISO 18134-3:2015-11; Biopaliwa Stałe—Oznaczanie Zawartości Wilgoci—Metoda Suszarkowa—Część 3: Wilgoć w Próbce do Analizy Ogólnej. Polski Komitet Normalizacyjny: Warszawa, Poland, 2015. Available online: https://sklep.pkn.pl/pn-en-iso-18134-3-2015-11p.html (accessed on 12 July 2025).
- Łukasiewicz, S. A modification suggestion of the method of drawing the wet humid period in the Walter’s climate diagram. Geogr. Fiz. 2006, 95–99. [Google Scholar]
- Dayoub, E.; Naudin, C.; Piva, G.; Shirtliffe, S.J.; Fustec, J.; Corre-Hellou, G. Traits affecting early season nitrogen uptake in nine legume species. Heliyon 2017, 3, e00244. [Google Scholar] [CrossRef]
- Mohammadi, K. Effective factors on biological nitrogen fixation. Afr. J. Agric. Res. 2012, 7, 1782–1788. [Google Scholar] [CrossRef]
- Dhanushkodi, R.; Matthew, C.; McManus, M.T.; Dijkwel, P.P. Drought-induced senescence of Medicago truncatula nodules involves serpin and ferritin to control proteolytic activity and iron levels. New Phytol. 2018, 220, 196–208. [Google Scholar] [CrossRef]
- Jarecki, W.; Borza, I.M.; Rosan, C.A.; Vicas, S.I.; Domuța, C.G. Soybean Response to Seed Inoculation with Bradyrhizobium japonicum and/or Nitrogen Fertilization. Agriculture 2024, 14, 1025. [Google Scholar] [CrossRef]
- Noh, N.-J.; Son, Y.-W.; Seo, K.-W.; Kim, R.-H.; Koo, J.-W.; Ban, J.-Y.; Kim, J.-G. Root Nodule Biomass of Robinia pseudoacacia and Amorpha fruticosa Seedlings with Fertilization Treatments. J. Ecol. Environ. 2006, 29, 151–155. [Google Scholar] [CrossRef]
- Chaukiyal, S.P.; Mir, R.A.; Pokhriyal, T.C. Effect of nitrogen fertilizer on biomass production and nodulation behavior of Pongamia pinnata Pierre seedlings under nursery conditions. J. For. Res. 2013, 24, 531–538. [Google Scholar] [CrossRef]
- Wysokinski, A.; Wysokińska, A.; Noulas, C.; Wysokińska, A. Optimal Nitrogen Fertilizer Rates for Soybean Cultivation. Agronomy 2024, 14, 1375. [Google Scholar] [CrossRef]
- Bais, J.; Kandel, H.; DeSutter, T.; Deckard, E.; Keene, C. Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense. Agronomy 2023, 13, 2022. [Google Scholar] [CrossRef]
- Wei, W.; Guan, D.; Ma, M.; Jiang, X.; Fan, F.; Meng, F.; Li, L.; Zhao, B.; Zhao, Y.; Cao, F.; et al. Long-term fertilization coupled with rhizobium inoculation promotes soybean yield and alters soil bacterial community composition. Front. Microbiol. 2023, 14, 1161983. [Google Scholar] [CrossRef] [PubMed]
- Trang, K.M.; Giddens, J. Shading and Temperature as Environmental Factors Affecting Growth, Nodulation, and Symbiotic N 2 Fixation by Soybeans 1. Agron. J. 1980, 72, 305–308. [Google Scholar] [CrossRef]
- de Silva, M.; Purcell, L.C.; King, C.A. Soybean Petiole Ureide Response to Water Deficits and Decreased Transpiration. Crop Sci. 1996, 36, 611–616. [Google Scholar] [CrossRef]
- Sadras, V.O.; Lake, L.; Li, Y.; Farquharson, E.A.; Sutton, T. Phenotypic plasticity and its genetic regulation for yield, nitrogen fixation and δ13C in chickpea crops under varying water regimes. J. Exp. Bot. 2016, 67, 4339–4351. [Google Scholar] [CrossRef]
- Lagunas, B.; Richards, L.; Sergaki, C.; Burgess, J.; Pardal, A.J.; Hussain, R.M.F.; Richmond, B.L.; Baxter, L.; Roy, P.; Pakidi, A.; et al. Rhizobial nitrogen fixation efficiency shapes endosphere bacterial communities and Medicago truncatula host growth. Microbiome 2023, 11, 146. [Google Scholar] [CrossRef]
- Gelfand, I.; Philip Robertson, G. A reassessment of the contribution of soybean biological nitrogen fixation to reactive N in the environment. Biogeochemistry 2015, 123, 175–184. [Google Scholar] [CrossRef]
- Rymuza, K.; Radzka, E.; Wysokiński, A. Nitrogen Uptake from Different Sources by Non-GMO Soybean Varieties. Agronomy 2020, 10, 1219. [Google Scholar] [CrossRef]
- Wood, C.W.; Torbert, H.A.; Weaver, D.B. Nitrogen Fertilizer Effects on Soybean Growth, Yield, and Seed Composition. J. Prod. Agric. 1993, 6, 354–360. [Google Scholar] [CrossRef]
- Księżak, J.; Bojarszczuk, J. The Effect of Mineral N Fertilization and Bradyrhizobium japonicum Seed Inoculation on Productivity of Soybean (Glycine max (L.) Merrill). Agriculture 2022, 12, 110. [Google Scholar] [CrossRef]
- Machado, M.V.M.; Maggi, M.F.; Hachisuca, A.M.M.; Mercante, E.; Silva, F.O. Regional Weather Variations and Yields Achieved in Soybean Crops. JAS 2023, 15, 10. [Google Scholar] [CrossRef]
- Kulig, B.; Klimek-Kopyra, A. Sowing Date and Fertilization Level Are Effective Elements Increasing Soybean Productivity in Rainfall Deficit Conditions in Central Europe. Agriculture 2023, 13, 115. [Google Scholar] [CrossRef]
- Ulzen, J.; Abaidoo, R.C.; Ewusi-Mensah, N.; Osei, O.; Masso, C.; Opoku, A. Organic Manure Improves Soybean Response to Rhizobia Inoculant and P-Fertilizer in Northern Ghana. Front. Agron. 2020, 2, 1089. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V. Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.). Agronomy 2020, 10, 826. [Google Scholar] [CrossRef]
- Cordeiro, C.F.D.S.; Echer, F.R. Interactive Effects of Nitrogen-Fixing Bacteria Inoculation and Nitrogen Fertilization on Soybean Yield in Unfavorable Edaphoclimatic Environments. Sci. Rep. 2019, 9, 15606. [Google Scholar] [CrossRef]











| Fertilisation (kg N·ha−1) | Inoculation | Year | Root Nodule per Plant | |
|---|---|---|---|---|
| Number [pcs] | Weight [g] | |||
| 0 | 5.36 ± 0.94a | 60.9 ± 9.0a | ||
| 30 | 4.29 ± 0.90a | 49.2 ± 8.0b | ||
| 60 | 4.58 ± 0.92a | 51.8 ± 10.2ab | ||
| Control | 0.98 ± 0.18c | 14.3 ± 1.9c | ||
| HiStick Soy | 9.46 ± 1.16a | 91.5 ± 11.6a | ||
| Nitragina | 3.79 ± 0.32b | 56.2 ± 5.2b | ||
| 2017 | 6.78 ± 1.17a | 87.5 ± 11.6a | ||
| 2018 | 5.44 ± 0.86a | 54.3 ± 5.4b | ||
| 2019 | 2.00 ± 0.32b | 20.1 ± 4.7c | ||
| Fertilisation (kg N·ha−1) | Inoculation | Year | N Content [g·kg −1] | ||
|---|---|---|---|---|---|
| Seed | Straw | Above-Ground Parts of Plant | |||
| 0 | 52.3 ± 1.0a | 6.61 ± 0.28a | 35.0 ± 2.1a | ||
| 30 | 51.7 ± 1.2a | 6.61 ± 0.27a | 35.8 ± 2.2a | ||
| 60 | 52.8 ± 1.1a | 6.66 ± 0.23a | 36.3 ± 2.0a | ||
| Control | 50.6 ± 1.0b | 6.53 ± 0.24b | 33.6b ± 2.1a | ||
| HiStick Soy | 53.0 ± 1.1a | 6.88 ± 0.31a | 37.9 ± 2.1a | ||
| Nitragina | 53.1 ± 1.1a | 6.47 ± 0.23b | 35.6 ± 2.1b | ||
| 2017 | 46.7 ± 0.7c | 8.13 ± 0.15a | 25.5 ± 0.8c | ||
| 2018 | 51.0 ± 0.6b | 4.78 ± 0.10c | 29.8 ± 0.5b | ||
| 2019 | 58.9 ± 0.7a | 6.97 ± 0.08b | 51.9 ± 0.9a | ||
| Fertilisation (kg N·ha−1) | Inoculation | 2017 | 2018 | 2019 |
|---|---|---|---|---|
| Control | 8.00 ± 0.77abcde | 4.30 ± 0.24h | 6.70 ± 0.21ef | |
| 0 | HiStick Soy | 9.00 ± 0.28ab | 4.60 ± 0.63gh | 7.30 ± 0.41cdef |
| Nitragina | 8.00 ± 0.68abcde | 4.60 ± 0.39gh | 7.00 ± 0.60def | |
| Control | 7.60 ± 0.40abcde | 5.10 ± 0.27gh | 6.80 ± 0.92def | |
| 30 | HiStick Soy | 9.10 ± 0.78a | 4.90 ± 0.47gh | 7.20 ± 0.38cdef |
| Nitragina | 7.50 ± 0.72bcde | 4.10 ± 0.21h | 7.20 ± 0.38cdef | |
| Control | 8.30 ± 0.46abcd | 5.10 ± 0.16gh | 6.90 ± 0.66def | |
| 60 | HiStick Soy | 8.60 ± 1.17abc | 4.40 ± 0.25gh | 6.80 ± 0.38def |
| Nitragina | 7.10 ± 0.96cdef | 5.90 ± 0.19fg | 6.80 ± 0.22def |
| Fertilisation (kg N·ha−1) | Inoculation | Year | Yield [t·ha−1] | ||
|---|---|---|---|---|---|
| Seed | Straw | Total | |||
| 0 | 2.77 ± 0.12b | 2.71 ± 0.08a | 5.48 ± 0.11b | ||
| 30 | 2.89 ± 0.11ab | 2.74 ± 0.09a | 5.63 ± 0.12ab | ||
| 60 | 2.97 ± 0.11a | 2.89 ± 0.09a | 5.86 ± 0.12a | ||
| Control | 2.74 ± 0.12b | 2.72 ± 0.08a | 5.45 ± 0.12a | ||
| HiStick Soy | 3.00 ± 0.10a | 2.74 ± 0.09a | 5.73 ± 0.10a | ||
| Nitragina | 2.89 ± 0.12a | 2.88 ± 0.09a | 5.78 ± 0.13a | ||
| 2017 | 2.26 ± 0.07c | 3.00 ± 0.10a | 5.27 ± 0.14c | ||
| 2018 | 2.68 ± 0.03b | 2.94 ± 0.05a | 5.62 ± 0.07b | ||
| 2019 | 3.69 ± 0.04a | 2.40 ± 0.07a | 6.08 ± 0.10a | ||
| Fertilisation (kg N·ha−1) | Inoculation | Year | NUE (kg Seed per kg N) | NHI (%) | Straw N Share (%) |
|---|---|---|---|---|---|
| 0 | - | 87.9 ± 6.4a | 12.1 ± 6.4a | ||
| 30 | 96.3 ± 22.1a | 88.4 ± 5.2a | 11.6 ± 5.2a | ||
| 60 | 49.5 ± 10.8b | 88.3 ± 5.1a | 11.7 ± 5.1a | ||
| Control | 69.2 ± 27.6b | 87.6 ± 5.9b | 12.4 ± 5.9a | ||
| HiStick Soy | 76.6 ± 30.4a | 88.8 ± 5.1a | 11.2 ± 5.1c | ||
| Nitragina | 72.9 ± 30.2ab | 88.2 ± 5.6ab | 11.8 ± 5.6ab | ||
| 2017 | 59.0 ± 23.1c | 81.1 ± 3.2c | 18.9 ± 3.2a | ||
| 2018 | 67.1 ± 22.0b | 90.7 ± 1.2b | 9.3 ± 1.2b | ||
| 2019 | 92.7 ± 31.1a | 92.9 ± 0.9a | 7.1 ± 0.9c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helios, W.; Serafin-Andrzejewska, M.; Kozak, M.; Lewandowska, S. Impact of Nitrogen Fertilisation and Inoculation on Soybean Nodulation, Nitrogen Status, and Yield in a Central European Climate. Agriculture 2025, 15, 1654. https://doi.org/10.3390/agriculture15151654
Helios W, Serafin-Andrzejewska M, Kozak M, Lewandowska S. Impact of Nitrogen Fertilisation and Inoculation on Soybean Nodulation, Nitrogen Status, and Yield in a Central European Climate. Agriculture. 2025; 15(15):1654. https://doi.org/10.3390/agriculture15151654
Chicago/Turabian StyleHelios, Waldemar, Magdalena Serafin-Andrzejewska, Marcin Kozak, and Sylwia Lewandowska. 2025. "Impact of Nitrogen Fertilisation and Inoculation on Soybean Nodulation, Nitrogen Status, and Yield in a Central European Climate" Agriculture 15, no. 15: 1654. https://doi.org/10.3390/agriculture15151654
APA StyleHelios, W., Serafin-Andrzejewska, M., Kozak, M., & Lewandowska, S. (2025). Impact of Nitrogen Fertilisation and Inoculation on Soybean Nodulation, Nitrogen Status, and Yield in a Central European Climate. Agriculture, 15(15), 1654. https://doi.org/10.3390/agriculture15151654









