Linking the Changes of Soil Organic Carbon with Rare Bacterial Diversity in Sagebrush Desert Grassland Under Grazing Exclusion
Abstract
1. Introduction
2. Materials and Methods
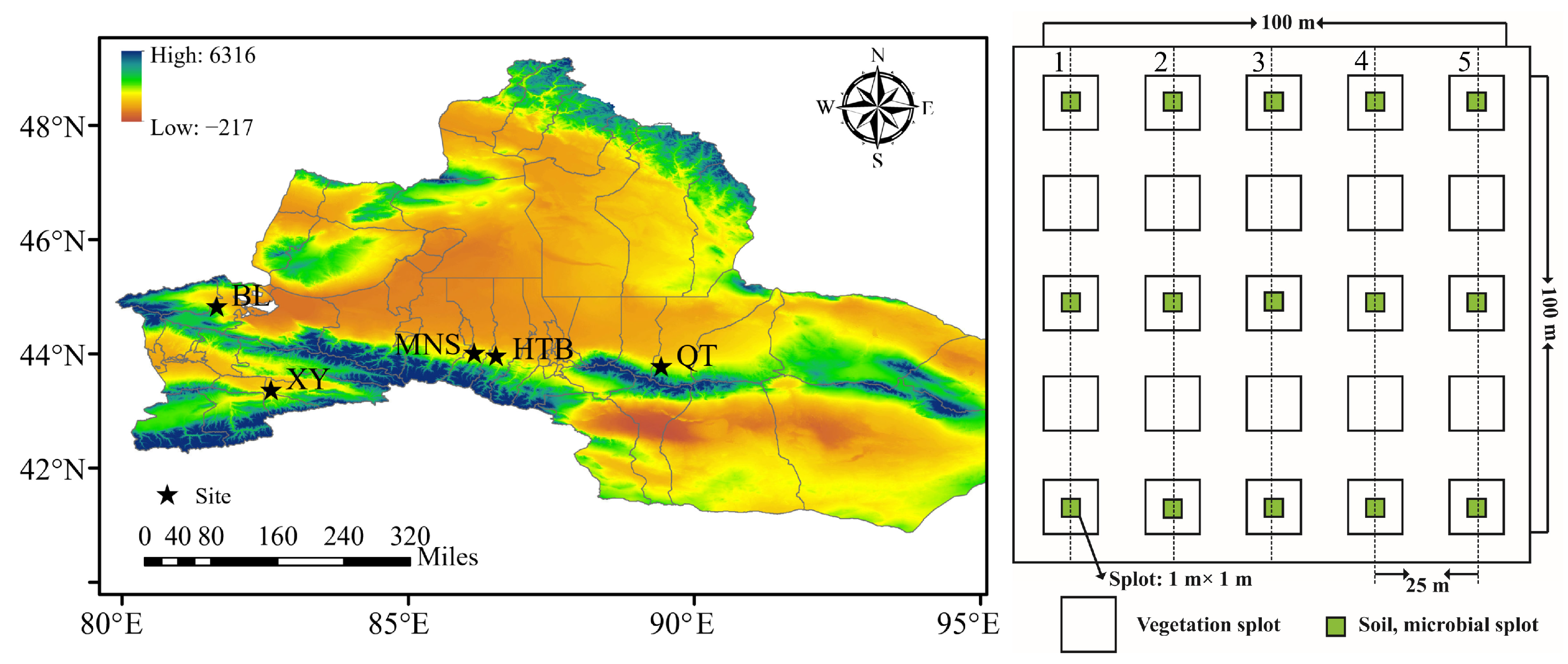
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. DNA Extraction and Sequencing
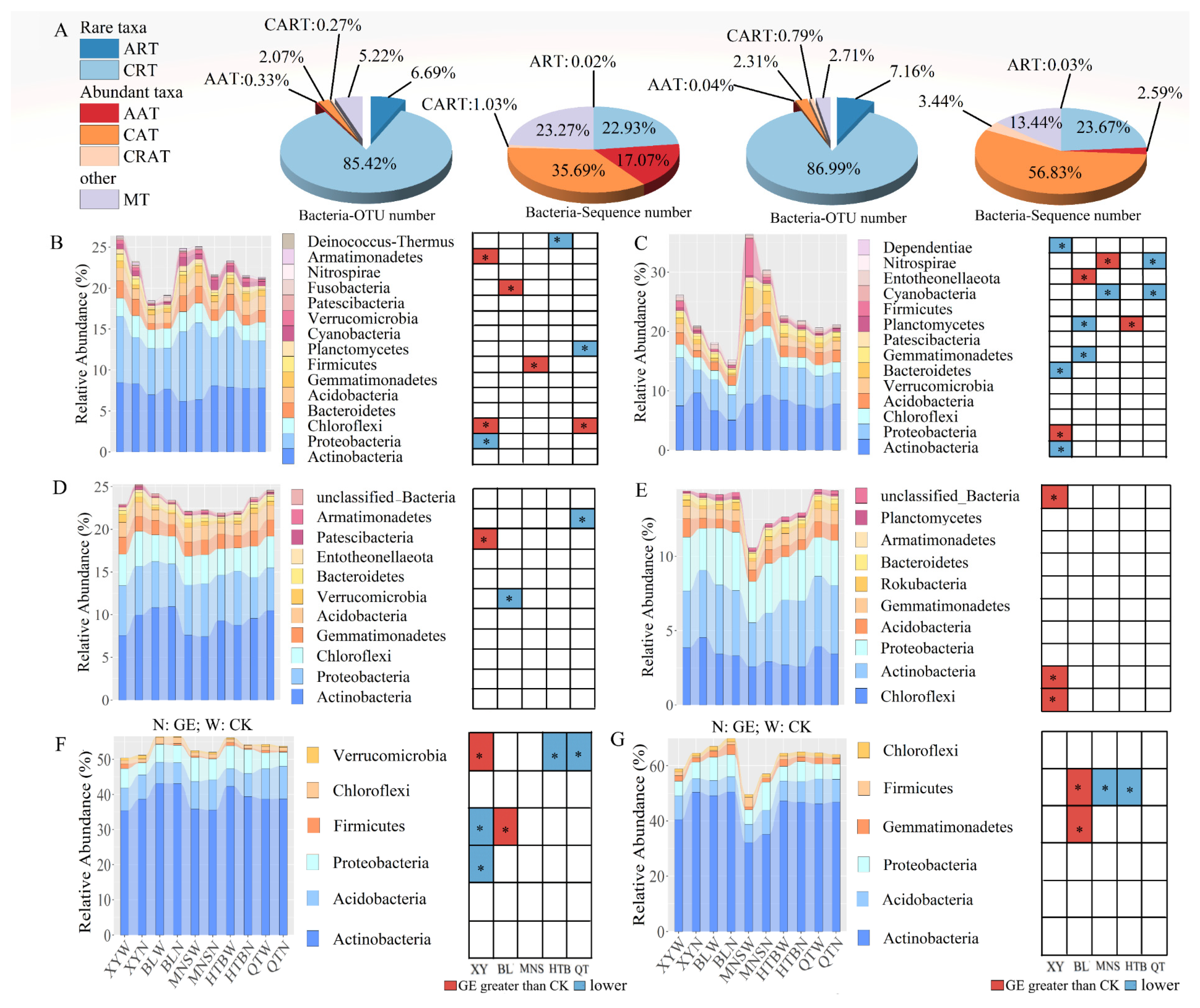
2.4. Definition of the Six Bacterial Taxa
2.5. Statistical Analysis
3. Results
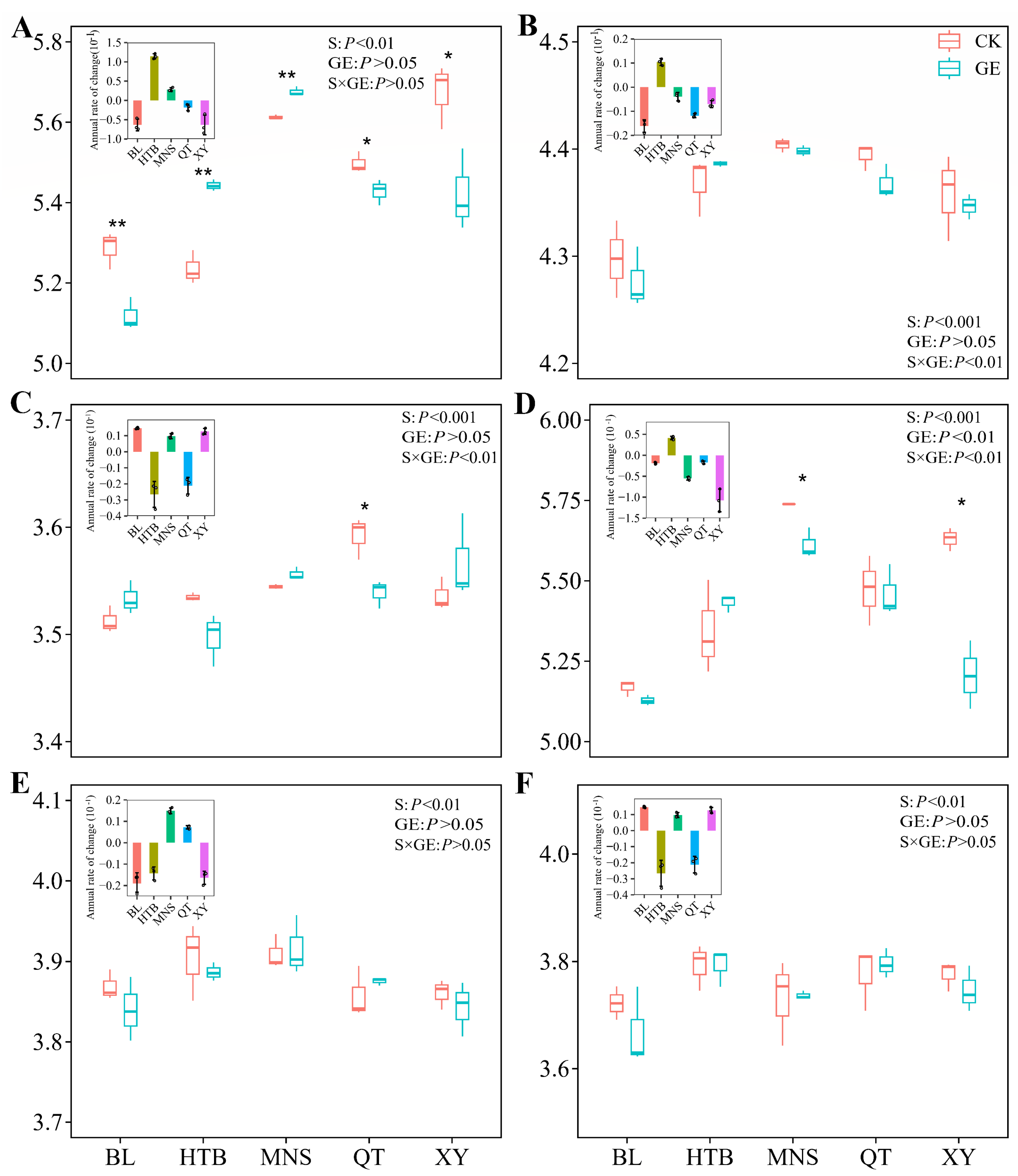
3.1. Responses of Soil Physicochemical Properties and Different Bacterial Taxa to GE
3.2. Diversity of the Different Bacterial Taxa in Response to GE
3.3. Changes in Bacterial Network Co-Occurrence Under GE
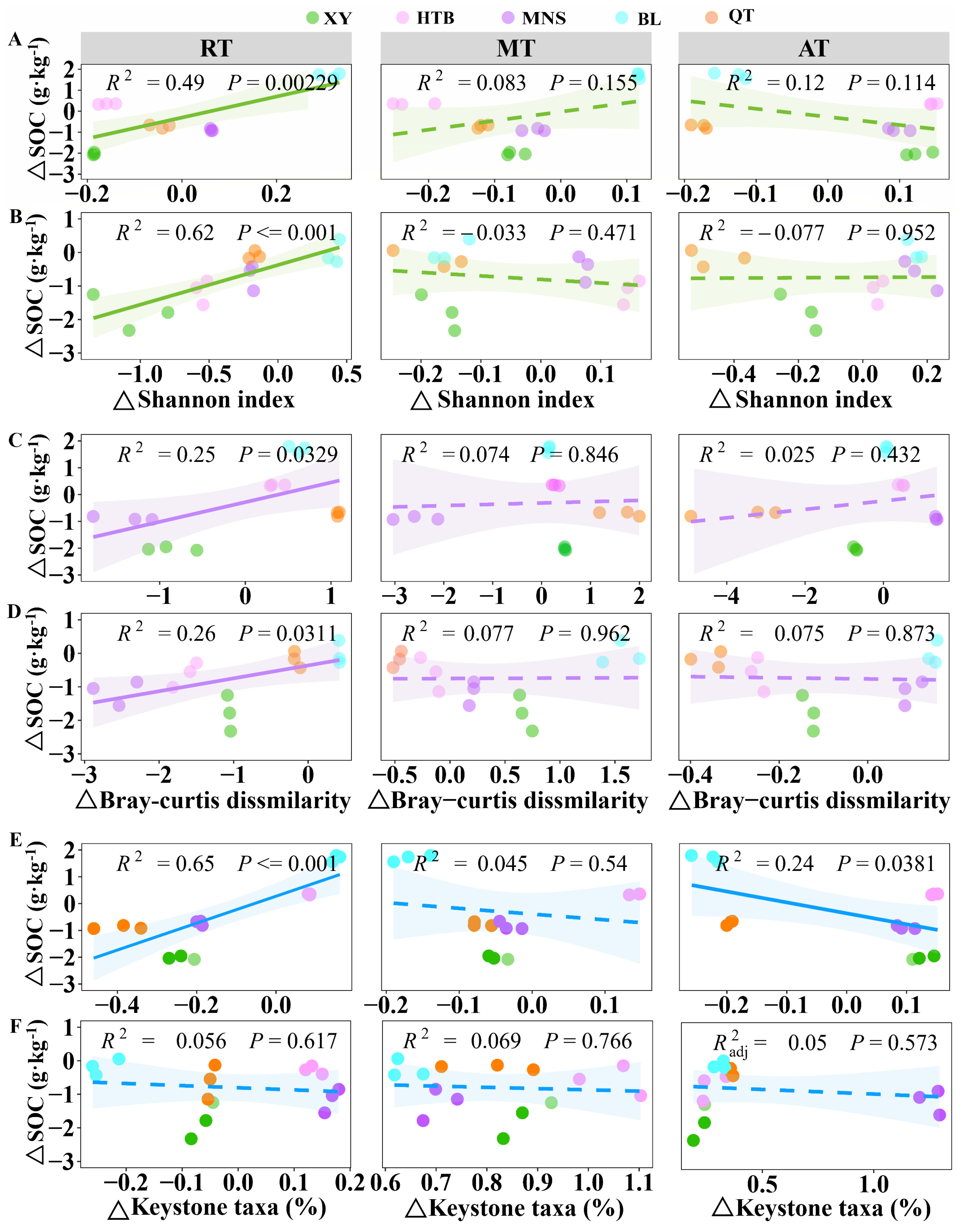
3.4. Relationship Between Soil Bacterial Taxa Characteristics and SOC Changes
4. Discussion
4.1. Response of Different Bacterial Taxa to GE
4.2. Response of Bacterial Network to GE
4.3. Rare Bacterial Taxa Are More Likely to Drive SOC Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| GE | Grazing exclusion |
| XY | Xinyuan |
| BL | Bole |
| MNS | Manasi |
| HTB | Hutubi |
| QT | Qitai |
| SOC | Soil organic carbon |
| SOM | Soil organic matter |
| RF | Random Forest |
| AT | Abundant taxa |
| RT | Rare taxa |
| MT | Moderate taxa |
| AAT | Always abundant taxa |
| ART | Always rare taxa |
| CRT | Conditionally rare taxa |
| CAT | Conditionally abundant taxa |
| CART | Conditionally rare and abundant taxa |
| SEM | Structural equation model |
| LMM | Linear mixed-effects model |
| NMDS | Non-metric multidimensional scaling |
| PERMANOVA | Permutation multivariate analysis of variance |
| OTU | Operational taxonomic unit |
| NC | Negative cohesion |
| PC | Positive cohesion |
| EC | Electrical conductivity |
| TN | Total nitrogen |
| TP | Total phosphorus |
| SWC | Soil water content |
| BD | Bulk density |
| Δ | (GE-CK)/GE years |
References
- Dlamini, P.; Chivenge, P.; Chaplot, V. Overgrazing decreases soil organic carbon stocks the most under dry climates and low soil pH: A meta-analysis shows. Agric. Ecosyst. Environ. 2016, 221, 258–269. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, F. Land-use conversion from open field to greenhouse cultivation differently affected the diversities and assembly processes of soil abundant and rare fungal communities. Sci. Total. Environ. 2021, 788, 147751. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.P.; Wu, G.L.; Chang, X.-F. Effects of grazing exclusion on carbon sequestration in China’s grassland. Earth-Sci. Rev. 2017, 173, 84–95. [Google Scholar] [CrossRef]
- Xiong, D.; Shi, P.; Zhang, X.; Zou, C.B. Effects of grazing exclusion on carbon sequestration and plant diversity in grasslands of China: A meta-analysis. Ecol. Eng. 2016, 94, 647–655. [Google Scholar] [CrossRef]
- Deng, L.; Sweeney, S.; Shangguan, Z. Long-term effects of natural enclosure: Carbon stocks, sequestration rates and potential for grassland ecosystems in the Loess Plateau. Clean 2014, 42, 617–625. [Google Scholar] [CrossRef]
- Listopad, C.M.; Köbel, M.; Príncipe, A.; Gonçalves, P.; Branquinho, C. The effect of grazing exclusion over time on structure, biodiversity, and regeneration of high nature value farmland ecosystems in Europe. Sci. Total. Environ. 2018, 610–611, 926–936. [Google Scholar] [CrossRef]
- Cao, J.; Jiao, Y.; Che, R.; Holden, N.M.; Zhang, X.; Biswas, A.; Feng, Q. The effects of grazer exclosure duration on soil microbial communities on the Qinghai-Tibetan Plateau. Sci. Total. Environ. 2022, 839, 156238. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Adamowski, J.F.; Biswas, A.; Alizadeh, M.R.; Feng, Q. A context-dependent response of soil carbon and nitrogen to grazing exclusion: Evidence from a global meta-analysis. J. Clean. Prod. 2024, 434, 139792. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, R.; Wei, S.; Feng, Y.; Zhang, J.; Lin, X. Response and feedback of C mineralization to P availability driven by soil microorganisms. Soil Biol. Biochem. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Maron, P.A.; Mougel, C.; Ranjard, L. Soil microbial diversity: Methodological strategy, spatial overview and functional interest. Comptes Rendus Biol. 2011, 334, 403–411. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Zang, Z.; Li, Y.; Wang, Y.; Zhang, Y.; Deng, S.; Guo, X.; Yang, K.; Zhao, W. Contrasting roles of plant, bacterial, and fungal diversity in soil organic carbon accrual during ecosystem restoration: A meta-analysis. Sci. Total Environ. 2024, 930, 172767. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Kong, W.; Wang, F.; Yue, L.; Li, X. Fencing decreases microbial diversity but increases abundance in grassland soils on the Tibetan Plateau. Land Degrad. Dev. 2020, 31, 2577–2590. [Google Scholar] [CrossRef]
- Cheng, J.; Jing, G.; Wei, L.; Jing, Z. Long-term grazing exclusion effects on vegetation characteristics, soil properties and bacterial communities in the semi-arid grasslands of China. Ecol. Eng. 2016, 97, 170–178. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Ji, B.; Struik, P.C.; Jin, K.; Tang, S. Coupling between the responses of plants, soil, and microorganisms following grazing exclusion in an overgrazed grassland. Front. Plant Sci. 2021, 12, 640789. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reith, F.; Dennis, P.G.; Hamonts, K.; Powell, J.R.; Young, A.; Singh, B.K.; Bissett, A. Ecological drivers of soil microbial diversity and soil biological networks in the Southern Hemisphere. Ecology 2018, 99, 583–596. [Google Scholar] [CrossRef]
- Lynch, M.D.; Neufeld, J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015, 13, 217–229. [Google Scholar] [CrossRef]
- Yuan, X.; Cui, J.; Wu, L.; Liu, C.-C.; Zhang, Q.; Zeng, Q.; Zhou, J.; Lin, K.; Wu, Y.; Lin, H.; et al. Relationship between soil bacterial communities and dissolved organic matter in a subtropical Pinus taiwanensis forest after short-term nitrogen addition. For. Ecol. Manag. 2022, 512, 120165. [Google Scholar] [CrossRef]
- Liu, B.; Arlotti, D.; Huyghebaert, B.; Tebbe, C.C. Disentangling the impact of contrasting agricultural management practices on soil microbial communities—Importance of rare bacterial community members. Soil Biol. Biochem. 2022, 166, 108573. [Google Scholar] [CrossRef]
- Philippot, L.; Spor, A.; Hénault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Sarr, A.; Maron, P.A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef]
- Pedrós-Alió, C. Marine microbial diversity: Can it be determined? Trends Microbiol. 2006, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Ma, Y.T.; Feng, T.Y.; Kong, X.; Wang, Z.H.; Zheng, W.; Zhai, B.N. Assembly processes of abundant and rare microbial communities in orchard soil under a cover crop at different periods. Geoderma 2022, 406, 115543. [Google Scholar] [CrossRef]
- Fan, J.L.; Zhang, C.H.; Jin, H.; Zhang, J.; Han, G.D. Grazing accelerates labile and recalcitrant soil carbon loss driving by rare microbial taxa in a desert steppe. Land Degrad. Dev. 2021, 32, 4241–4253. [Google Scholar] [CrossRef]
- Shu, D.T.; Guo, Y.Q.; Zhang, B.G.; Zhang, C.F.; Van Nostrand, J.D.; Lin, Y.B.; Zhou, J.Z.; Wei, G.H. Rare prokaryotic sub-communities dominate the complexity of ecological networks and soil multinutrient cycling during long-term secondary succession in China’s Loess Plateau. Sci. Total Environ. 2021, 774, 145737. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Kusel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Wan, W.J.; Grossart, H.P.; He, D.L.; Yuan, W.K.; Yang, Y.Y. Stronger environmental adaptation of rare rather than abundant bacterioplankton in response to dredging in eutrophic Lake Nanhu (Wuhan, China). Water Res. 2021, 190, 116751. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gao, H.; Peng, Z.; Chen, B.; Chen, S.; Liu, Y.; Gu, J.; Wei, X.; Chen, W.; Wei, G.; et al. Aridity threshold induces abrupt change of soil abundant and rare bacterial biogeography in dryland ecosystems. mSystems 2022, 7, e0130921. [Google Scholar] [CrossRef]
- Xue, M.; Guo, Z.; Gu, X.; Gao, H.; Weng, S.; Zhou, J.; Gu, D.; Lu, H.; Zhou, X. Rare rather than abundant microbial communities drive the effects of long-term greenhouse cultivation on ecosystem functions in subtropical agricultural soils. Sci. Total Environ. 2020, 706, 136004. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Casamayor, E.O. A phylogenetic perspective on species diversity, β-diversity and biogeography for the microbial world. Mol. Ecol. 2014, 23, 5868–5876. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Yang, H.L.; Sun, Z.J.; An, S.Z. Difference of soil carbon density under different grazing exclusion duration in seriphidium transiliense desert with distinct degrees of degradation. Arid. Land Res. Manag. 2021, 35, 198–212. [Google Scholar] [CrossRef]
- Cui, Y.X.; Dong, Y.Q.; Liu, H.X.; Sun, Z.J. Short-term grazing exclusions reduced soil organic carbon but not bacterial diversity in the sagebrush desert, Northwest China. Glob. Ecol. Conserv. 2021, 31, e01872. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2; Page, A.L., Miller, D.R., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 230–257. [Google Scholar] [CrossRef]
- Liang, Y.T.; Xiao, X.; Nuccio, E.E.; Yuan, M.T.; Zhang, N.; Xue, K.; Cohan, F.M.; Zhou, J.Z.; Sun, B. Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 2020, 22, 1327–1340. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Cohesion: A method for quantifying the connectivity of microbial communities. ISME J. 2017, 11, 2426–2438. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Dong, K.; Yu, Z.; Kerfahi, D.; Lee, S.S.; Li, N.; Yang, T.; Adams, J.M. Soil microbial co-occurrence networks become less connected with soil development in a high arctic glacier foreland succession. Sci. Total Environ. 2022, 813, 152565. [Google Scholar] [CrossRef]
- Hu, J.P.; Zhang, M.X.; Lü, Z.L.; He, Y.Y.; Yang, X.X.; Khan, A.; Xiong, Y.C.; Fang, X.L.; Dong, Q.M.; Zhang, J.L. Grazing practices affect phyllosphere and rhizosphere bacterial communities of Kobresia humilis by altering their network stability. Sci. Total Environ. 2023, 900, 165814. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Wu, Y.; Wang, J.; Zhao, Z.; Li, Y.; Qiao, L.; Chen, K.; Liu, G.; Ritsema, C.; et al. Long-term warming impacts grassland ecosystem function: Role of diversity loss in conditionally rare bacterial taxa. Sci. Total Environ. 2023, 892, 164722. [Google Scholar] [CrossRef]
- Li, W.; Shi, F.; Yi, S.; Feng, T.; Wang, C.; Li, Z.; Zheng, W.; Zhai, B. Soil multifunctionality predicted by bacterial network complexity explains differences in wheat productivity induced by fertilization management. Eur. J. Agron. 2024, 153, 127058. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, Y.; Qiu, C.; Zheng, D.; Liu, Y. Wildfire drives the transition from deterministic- to stochastic-dominated community assembly of abundant bacterial in forest soils. Catena 2022, 215, 106290. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Wang, C.; Wang, X.; Miao, L.; Liu, S.; Yuan, Q.; Sun, S. Distinct assembly mechanisms underlie similar biogeographic patterns of rare and abundant bacterioplankton in cascade reservoirs of a large river. Front. Microbiol. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Lu, Y. Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef]
- Xu, M.; Huang, Q.Y.; Xiong, Z.Q.; Liao, H.G.; Lv, Z.; Chen, W.L.; Luo, X.S.; Hao, X.L. Distinct responses of rare and abundant microbial taxa to in situ chemical stabilization of cadmium-contaminated soil. mSystems 2021, 6, e0104021. [Google Scholar] [CrossRef]
- Du, S.C.; Dini-Andreote, F.; Zhang, N.; Liang, C.L.; Yao, Z.Y.; Zhang, H.J.; Zhang, D.M. Divergent co-occurrence patterns and assembly processes structure the abundant and rare bacterial communities in a salt marsh ecosystem. Appl. Environ. Microbiol. 2020, 86, e00322-20. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Cheng, H.; Chen, Z.; Wang, Y. Bacterial abundant taxa exhibit stronger environmental adaption than rare taxa in the Arctic Ocean sediments. Mar. Environ. Res. 2024, 199, 106624. [Google Scholar] [CrossRef] [PubMed]
- Santolini, M.; Barabasi, A.L. Predicting perturbation patterns from the topology of biological networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6375–6383. [Google Scholar] [CrossRef]
- Menéndez-Serra, M.; Ontiveros, V.J.; Barberán, A.; Casamayor, E.O. Absence of stress-promoted facilitation coupled with a competition decrease in the microbiome of ephemeral saline lakes. Ecology 2022, 103, e3834. [Google Scholar] [CrossRef]
- Qiu, L.P.; Zhang, Q.; Zhu, H.S.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Liu, D.; Bhople, P.; Keiblinger, K.M.; Wang, B.; An, S.; Yang, N.; Chater, C.C.C.; Yu, F. Soil rehabilitation promotes resilient microbiome with enriched keystone taxa than agricultural infestation in barren soils on the Loess Plateau. Biology 2021, 10, 1261. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Singh, B.R.; Sitaula, B.K.; Lal, R.; Bajracharya, R.M. Soil aggregate- and particle-associated organic carbon under different land uses in nepal. Soil Sci. Soc. Am. J. 2007, 71, 1194–1203. [Google Scholar] [CrossRef]
- Ju, F.; Xia, Y.; Guo, F.; Wang, Z.P.; Zhang, T. Taxonomic relatedness shapes bacterial assembly in activated sludge of globally distributed wastewater treatment plants. Environ. Microbiol. 2014, 16, 2421–2432. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xu, C.; Wu, F.; Hu, H. Microbial co-occurrence networks driven by low-abundance microbial taxa during composting dominate lignocellulose degradation. Sci. Total Environ. 2022, 845, 157197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ge, X.; Wang, L.; You, Y.; Cheng, Y.; Ma, J.; Chen, F. Biochar rebuilds the network complexity of rare and abundant microbial taxa in reclaimed soil of mining areas to cooperatively avert cadmium stress. Front. Microbiol. 2022, 13, 972300. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Tang, S.; Ma, Q.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.; Jones, D.L. Microbial community succession in soil is mainly driven by carbon and nitrogen contents rather than phosphorus and sulphur contents. Soil Biol. Biochem. 2023, 180, 109019. [Google Scholar] [CrossRef]
- Lin, L.; Jing, X.; Lucas-Borja, M.E.; Shen, C.; Wang, Y.; Feng, W. Rare taxa drive the response of soil fungal guilds to soil salinization in the Taklamakan Desert. Front. Microbiol. 2022, 13, 862245. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, X.; Sun, T.; Lin, L.; Wu, L.; Zhang, T.; Gong, Y.; Li, Y.; Wu, H.; Xiong, J.; et al. Rare taxa mediate microbial carbon and nutrient limitation in the rhizosphere and bulk soil under sugarcane–peanut intercropping systems. Front. Microbiol. 2024, 15, 1403338. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Jones, S.E.; Caporaso, J.G.; Handelsman, J.; Knight, R.; Fierer, N.; Gilbert, J.A. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 2014, 5, e01371-14. [Google Scholar] [CrossRef] [PubMed]



| Network Metrics | 0–5 cm Soil Layer | 5–10 cm Soil Layer | ||
|---|---|---|---|---|
| GE | CK | GE | CK | |
| Number of edges/number of nodes | 3.993 | 3.081 | 4.670 | 3.223 |
| Proportions of positive correlations (%) | 55.34 | 61.45 | 60.27 | 73.44 |
| Proportions of negative correlations (%) | 44.67 | 38.55 | 39.73 | 26.56 |
| Average clustering coefficient | 0.273 | 0.276 | 0.310 | 0.281 |
| Average path length | 4.029 | 3.983 | 3.828 | 4.346 |
| Connectance | 0.030 | 0.029 | 0.038 | 0.025 |
| Diameter | 10.120 | 10.891 | 12.043 | 13.610 |
| Density | 0.014 | 0.013 | 0.018 | 0.012 |
| Average degree | 8.057 | 7.535 | 9.340 | 6.446 |
| Modularity | 0.543 | 0.565 | 0.493 | 0.613 |
| Centralization betweenness | 0.011 | 0.012 | 0.015 | 0.022 |
| Centralization degree | 0.079 | 0.063 | 0.107 | 0.079 |
| Natural connectivity | 1.117 | 1.119 | 4.046 | 1.494 |
| Robustness | 0.534 | 0.895 | 1.103 | 1.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Sun, Z.; Cui, Y.; Liu, H. Linking the Changes of Soil Organic Carbon with Rare Bacterial Diversity in Sagebrush Desert Grassland Under Grazing Exclusion. Agriculture 2025, 15, 1959. https://doi.org/10.3390/agriculture15181959
Yu B, Sun Z, Cui Y, Liu H. Linking the Changes of Soil Organic Carbon with Rare Bacterial Diversity in Sagebrush Desert Grassland Under Grazing Exclusion. Agriculture. 2025; 15(18):1959. https://doi.org/10.3390/agriculture15181959
Chicago/Turabian StyleYu, Bingjie, Zongjiu Sun, Yuxuan Cui, and Huixia Liu. 2025. "Linking the Changes of Soil Organic Carbon with Rare Bacterial Diversity in Sagebrush Desert Grassland Under Grazing Exclusion" Agriculture 15, no. 18: 1959. https://doi.org/10.3390/agriculture15181959
APA StyleYu, B., Sun, Z., Cui, Y., & Liu, H. (2025). Linking the Changes of Soil Organic Carbon with Rare Bacterial Diversity in Sagebrush Desert Grassland Under Grazing Exclusion. Agriculture, 15(18), 1959. https://doi.org/10.3390/agriculture15181959





