Effects of Co-Application of Biochar and Nitrogen Fertilizer on Soil Properties and Microbial Communities in Tea Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sample Collection and Analysis
2.4. High-Throughput Sequencing Analysis
2.4.1. DNA Extraction, Library Construction, and Metagenomic Sequencing
2.4.2. Sequence Quality Control and Genome Assembly
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Characteristics
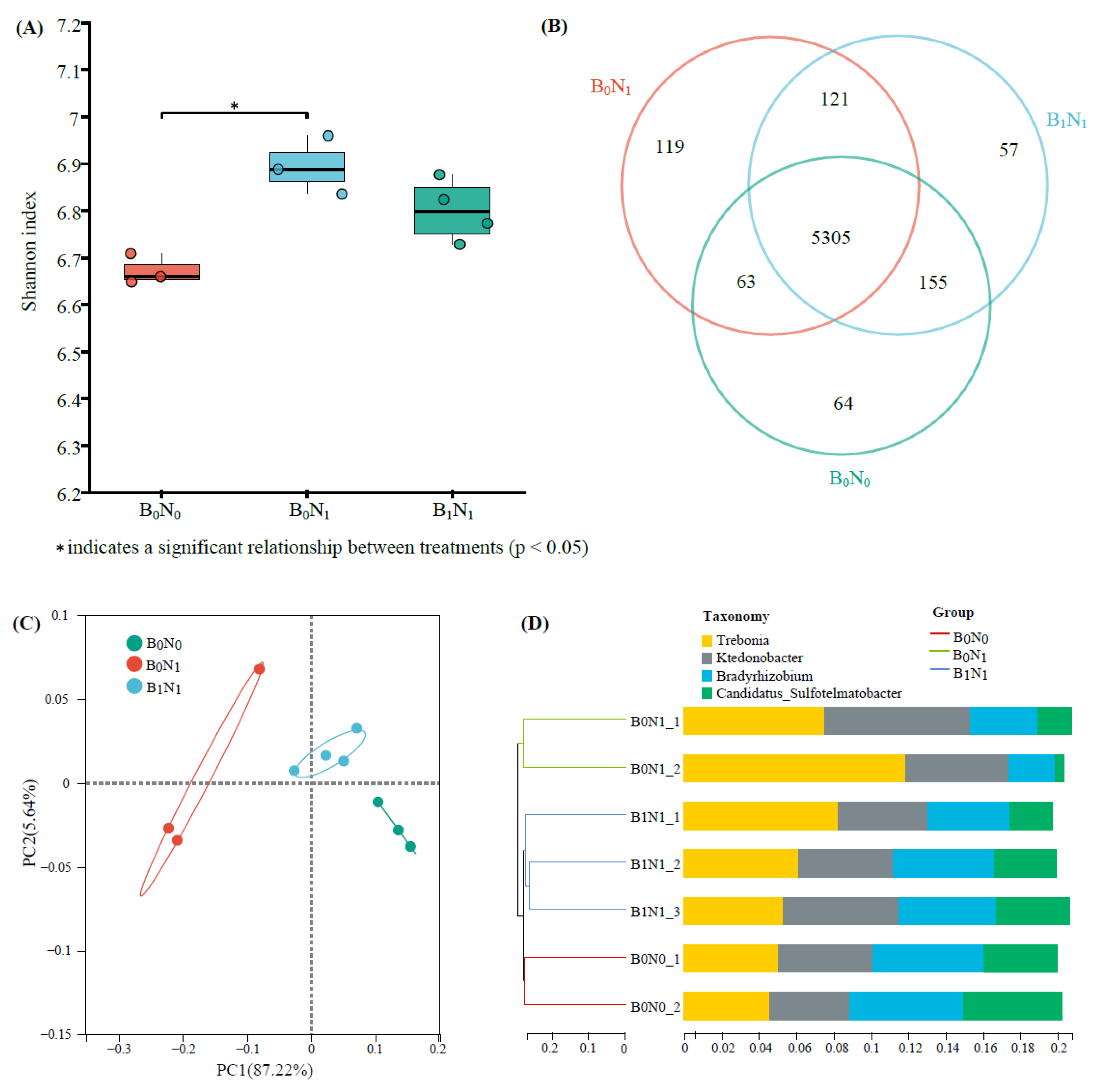
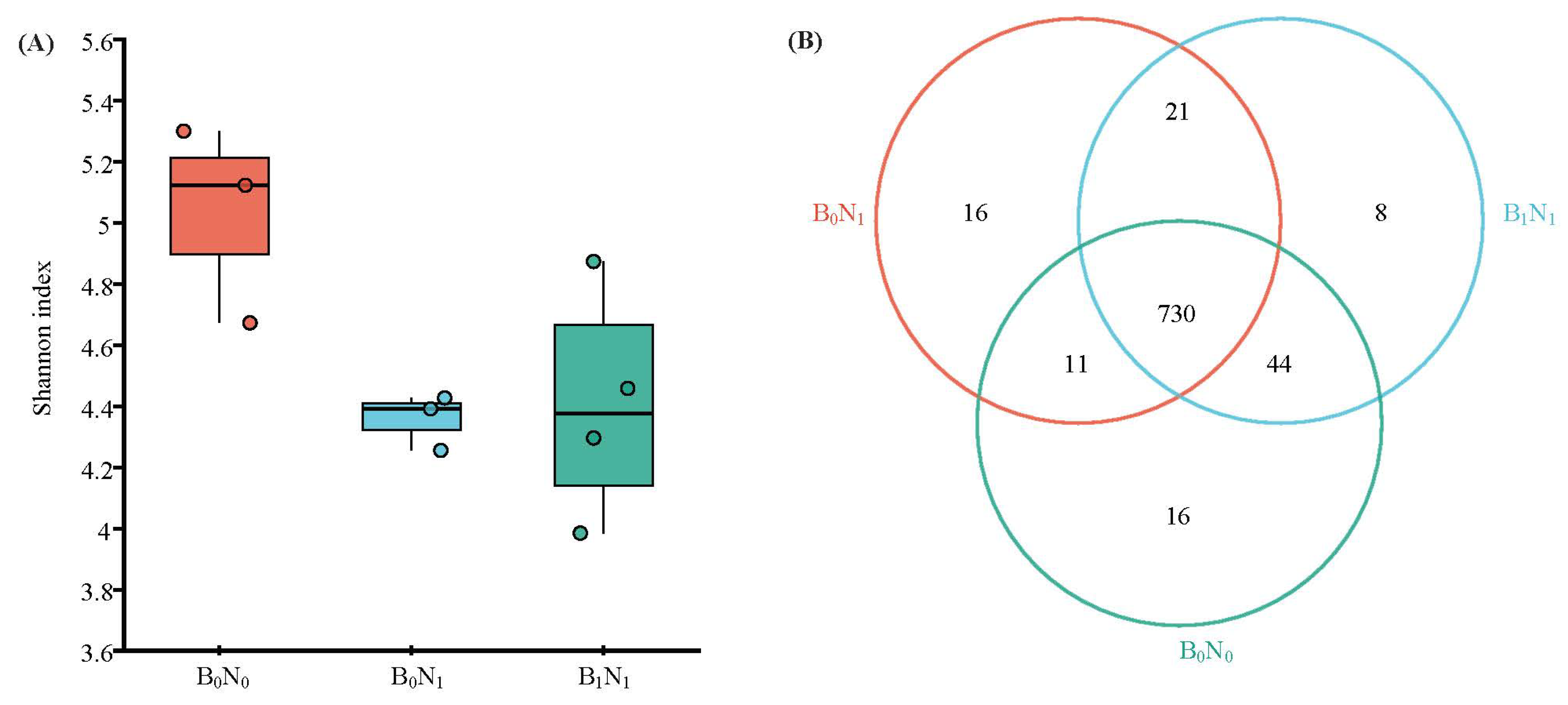
3.2. Soil Microbial Community Diversity and Structure
3.2.1. Soil Bacterial Community Diversity
3.2.2. Soil Fungal Community Diversity
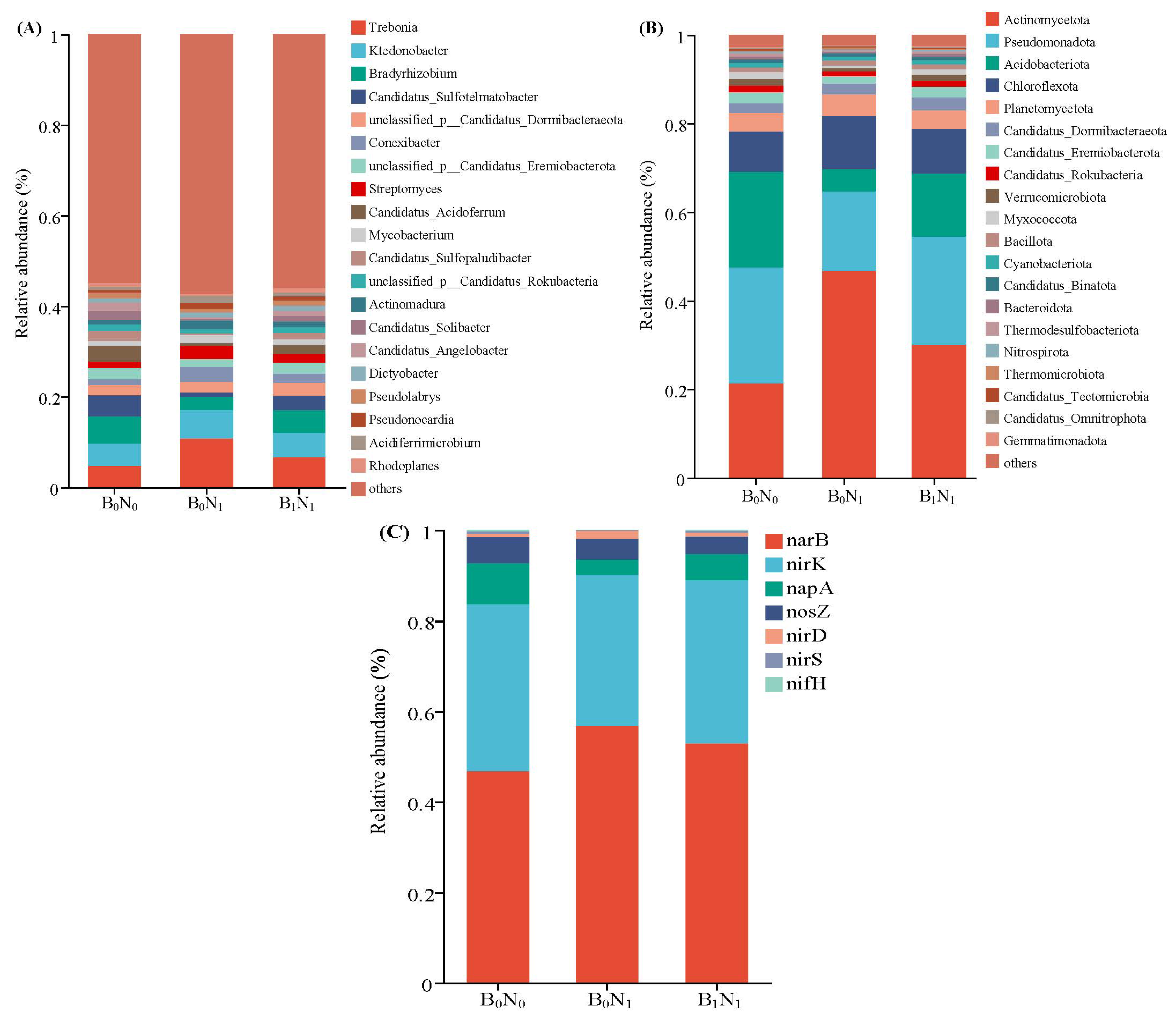
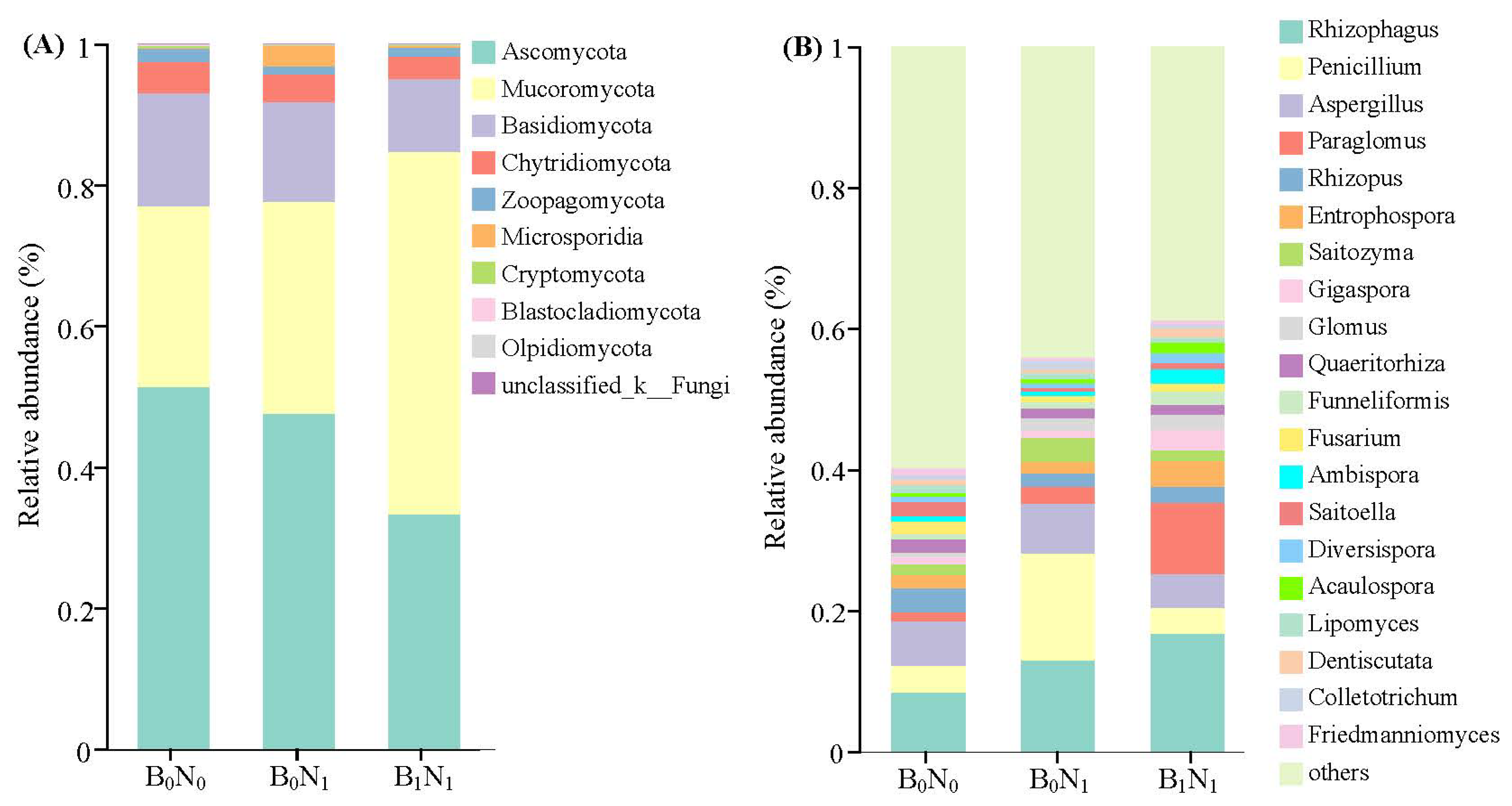
3.2.3. Soil Bacterial Community Composition
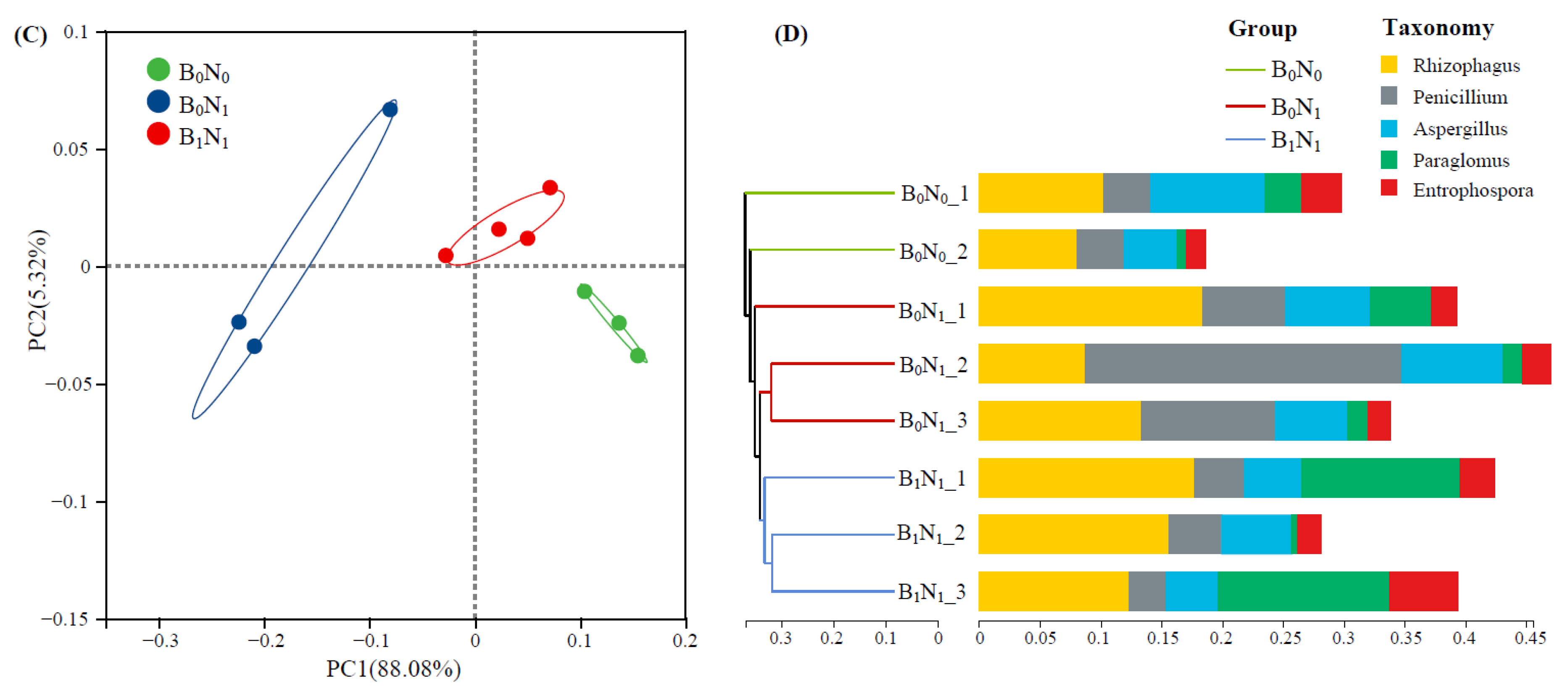
3.2.4. Soil Fungal Community Composition
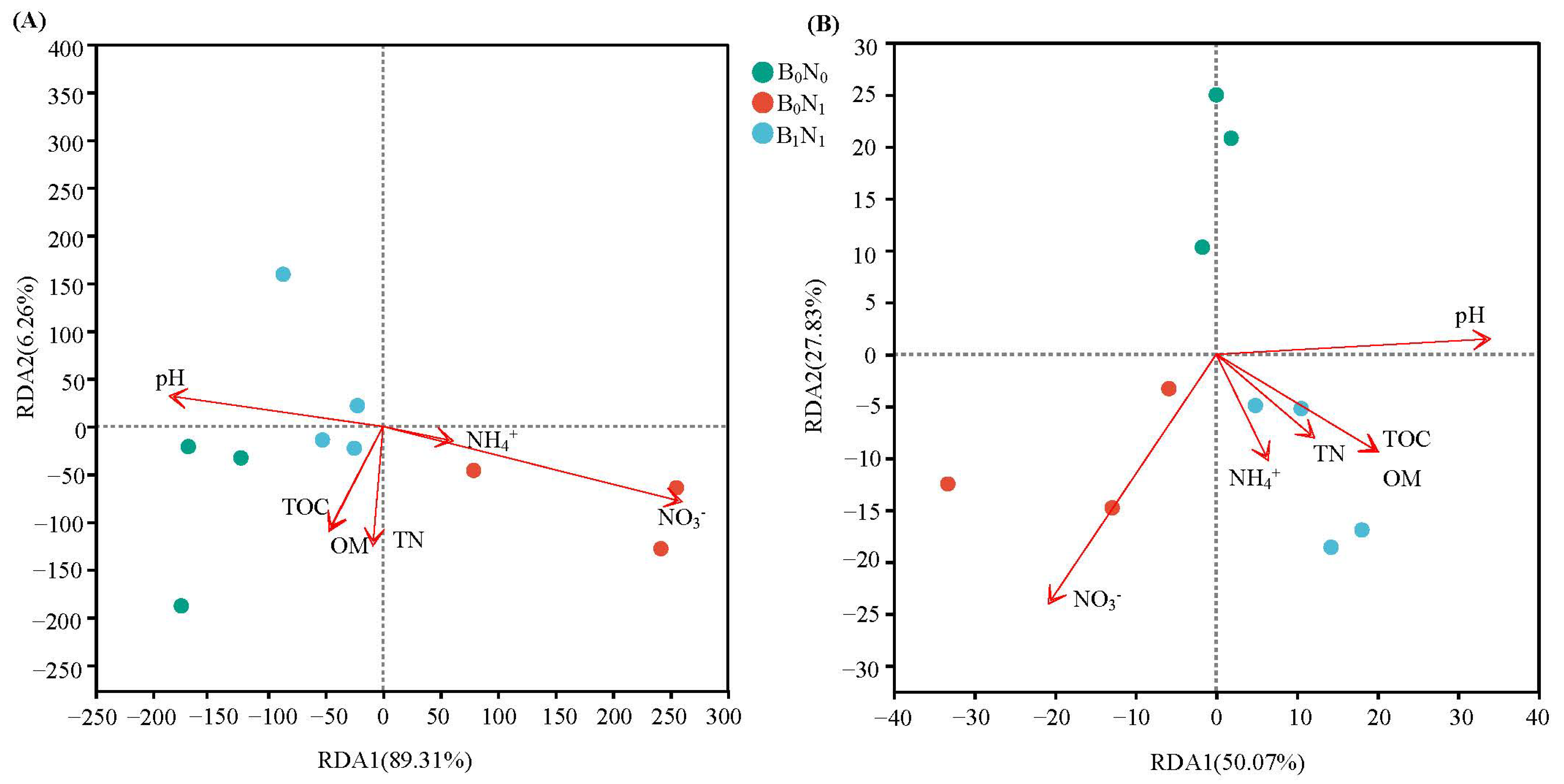
3.3. Environmental Factors Influencing Microbial Community Structure
4. Discussion
4.1. The Effect of Biochar Combined with Fertilizer on Soil Characteristics
4.2. The Effect of Biochar Combined with Fertilizer on Soil Microbial Community Structure and Diversity
4.3. The Effects of Co-Application of Nitrogen Fertilizer and Biochar on the Relationship Between Soil Microorganisms and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.D.; Yang, S.S.; Nie, S.A.; Deng, J.Z.; Chen, C.M. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.Q.; Wang, D.H.; Fu, J.Y.; Shen, C.; Li, X.; Zhang, L.P.; Zhang, L.; Fan, L.C. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.Y.; Ni, N.; Wang, R.H.; Nkoh, J.N.; Pan, X.Y.; Dong, G.; Xu, R.-K.; Cui, X.M.; Li, J.Y. Dissolved biochar fractions and solid biochar particles inhibit soil acidification induced by nitrification through different mechanisms. Sci. Total Environ. 2023, 874, 162464. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Yang, W.; Li, B.; Shang, J. Aggregation kinetics of biochar nanoparticles in aqueous environment: Interplays of anion type and bovine serum albumin. Sci. Total Environ. 2022, 833, 155148. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Wang, L.; Ruan, S.; Chen, J.; Li, H.; Yang, J.; Mechtcherine, V.; Tsang, D.C.W. Biochar-augmented carbon-negative concrete. Chem. Eng. J. 2022, 431, 133946. [Google Scholar] [CrossRef]
- Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K. Biochar-clay, biochar-microorganism and biochar-enzyme composites for environmental remediation: A review. Environ. Chem. Lett. 2023, 21, 1837–1862. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, K.; Loik, M.E.; Sun, W. Differential responses of soil bacteria and fungi to altered precipitation in a meadow steppe. Geoderma 2021, 384, 114812. [Google Scholar] [CrossRef]
- Xiong, R.; He, X.; Gao, N.; Li, Q.; Qiu, Z.; Hou, Y.; Shen, W.; Arias, R.S. Soil pH amendment alters the abundance, diversity, and composition of microbial communities in two contrasting agricultural soils. Microbiol. Spectr. 2024, 12, e04165-23. [Google Scholar] [CrossRef]
- Wu, H.; Wu, H.; Jiao, Y.; Zhang, Z.; Rensing, C.; Lin, W. The combination of biochar and PGPBs stimulates the differentiation in rhizosphere soil microbiome and metabolites to suppress soil-borne pathogens under consecutive mono-culture regimes. GCB Bioenergy 2022, 14, 84–103. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Zhang, J.; Cai, Q.; Lin, J.; Zeng, J.; Liu, X. Biochar promotes the growth of arbuscular mycorrhizal fungi on Taxodium ‘Zhongshanshan’ in coastal saline–alkali soils. Forests 2025, 16, 828. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Z.; Wang, H.; Wang, J.; Yang, S. Assessing soil organic matter content in a coal mining area through spectral variables of different numbers of dimensions. Sensors 2020, 20, 1795. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhou, J.J.; Pan, W.K.; Sun, T.; Liu, M.J.; Tang, R.; Li, Z.J.; Ma, Q.-X.; Wu, L.H. Effects of combined application of nitrogen, phosphorus, and potassium fertilizers on tea (Camellia sinensis) growth and fungal community. Appl. Soil Ecol. 2023, 181, 104661. [Google Scholar] [CrossRef]
- Shifa, S.; Worku, M.; Beyene, A. Co-application of compost and biochar improves soil properties and desho grass growth on acidic soils in a tropical environment of southwestern Ethiopia. Cogent Food Agric. 2024, 10, 2290338. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Zhang, R.; Chen, Y.; Lei, L.; Qiu, C.; Xu, H. Effect of slow-released biomass alkaline amendments oyster shell on microecology in acidic heavy metal contaminated paddy soils. J. Environ. Manag. 2022, 319, 115683. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhu, S.; Jin, C.; Sun, Z.; Wang, L.; Wen, Q.; Ma, F. Sorption mechanisms of antibiotic sulfamethazine (SMT) on magnetite-coated biochar: pH-dependence and redox transformation. Chemosphere 2021, 268, 128805. [Google Scholar] [CrossRef]
- He, D.; Liu, X.; Hu, D.; Lei, P.; Zhang, J.; Dong, Z.; Zhu, B. Density functional theory calculation for understanding the roles of biochar in immobilizing exchangeable Al3+ and enhancing soil quality in acidic soils. Ecotoxicol. Environ. Saf. 2025, 290, 117630. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Li, L.; Tian, R.; Chen, J.; Ning, Q.; Wang, B.; Tang, L.; Zeng, G. Influence of feedstocks and modification methods on biochar’s capacity to activate hydrogen peroxide for tetracycline removal. Bioresour. Technol. 2019, 291, 121840. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, Z.; Song, H.; Li, K.; Lu, H.; Liu, Y.; Cheng, F. Biochar induced changes of soil dissolved organic matter: The release and adsorption of dissolved organic matter by biochar and soil. Sci. Total Environ. 2021, 783, 147091. [Google Scholar] [CrossRef]
- Ayito, E.O.; John, K.; Iren, O.B.; John, N.M.; Mngadi, S.; Moodley, R.; Heung, B. Effect of biochar treatment on soil pH and cucumber fruit: A demonstration of the importance of biochar amendment on the tropical soils of Nigeria. J. Soil Water Conserv. 2024, 79, 202–210. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, X.; Singh, B.P.; Mandal, S.; Guo, J.; Che, L.; Wang, H. Effects of contrasting biochars on the leaching of inorganic nitrogen from soil. J. Soils Sediments 2020, 20, 3017–3026. [Google Scholar] [CrossRef]
- Li, H.; Ren, R.; Zhang, H.; Zhang, G.; He, Q.; Han, Z.; Meng, S.; Zhang, Y.; Zhang, X. Factors regulating interaction among inorganic nitrogen and phosphorus species, plant uptake, and relevant cycling genes in a weakly alkaline soil treated with biochar and inorganic fertilizer. Sci. Total Environ. 2023, 905, 167280. [Google Scholar] [CrossRef]
- Chen, X.; Yang, S.-H.; Jiang, Z.-W.; Ding, J.; Sun, X. Biochar as a tool to reduce environmental impacts of nitrogen loss in water-saving irrigation paddy field. J. Clean. Prod. 2021, 290, 125811. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, Y.; Auwal, M.; Singh, B.P.; Van Zwieten, L.; Xu, J. Biochar accelerates soil organic carbon mineralization via rhizodeposit-activated Actinobacteria. Biol. Fertil. Soils 2022, 58, 565–577. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Niazi, N.K.; Bolan, N.; Zhao, L.; Zhang, S.; Xue, J.; Yao, B.; Li, Y. Biochar addition increased soil bacterial diversity and richness: Large-scale evidence of field experiments. Sci. Total Environ. 2023, 893, 164961. [Google Scholar] [CrossRef]
- Hua, B.; Li, Z.; Gao, W.; Feng, H.; Chen, N.; Li, J.; Ji, X.; Zhang, L.; Wu, Z.; Yan, S.; et al. Soil amendment in plastic greenhouse using modified biochar: Soil bacterial diversity responses and microbial biomass carbon and nitrogen. Biotechnol. Lett. 2021, 43, 655–666. [Google Scholar] [CrossRef]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.-N.; Fu, S. Effects of arbuscular mycorrhizal fungi and biochar on growth, nutrient absorption, and physiological properties of maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef]
- You, Y.; Ma, F.; Zhang, W.; Guo, H.; Liu, L.; Zhang, Y. Modulation by arbuscular mycorrhizal fungi on biochar—Phragmites australis system in P-deficient environment: Cd tolerance and migration. J. Hazard. Mater. 2025, 490, 137747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Deng, M.; Zhang, T.; Yu, J.; Lin, X. An efficient fungi-biochar-based system for advancing sustainable man-agement of combined pollution. Environ. Pollut. 2025, 367, 125649. [Google Scholar] [CrossRef]
- Jiang, X.; Tan, X.; Cheng, J.; Haddix, M.L.; Cotrufo, M.F. Interactions between aged biochar, fresh low molecular weight carbon and soil organic carbon after 3.5 years soil-biochar incubations. Geoderma 2019, 333, 99–107. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
| Treatment | pH | Organic Matter (g kg−1) | Total Nitrogen (g kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) |
|---|---|---|---|---|---|
| B0N0 | 4.5 ± 0.03 b | 32.8 ± 1.4 b | 1.1 ± 0.02 b | 24.7 ± 3.2 c | 11.8 ± 2.1 b |
| B0N1 | 4.8 ± 0.01 b | 35.6 ± 1.5 b | 1.4 ± 0.04 b | 53.9 ± 7.8 b | 36.2 ± 2.8 a |
| B1N1 | 5.3 ± 0.03 a | 40.4 ± 1.7 a | 1.7 ± 0.06 a | 48.1 ± 18.6 a | 31.5 ± 2.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ye, J.; Lin, Y.; Wu, X.; Shu, W.; Wang, Y. Effects of Co-Application of Biochar and Nitrogen Fertilizer on Soil Properties and Microbial Communities in Tea Plantation. Agriculture 2025, 15, 1941. https://doi.org/10.3390/agriculture15181941
Liu C, Ye J, Lin Y, Wu X, Shu W, Wang Y. Effects of Co-Application of Biochar and Nitrogen Fertilizer on Soil Properties and Microbial Communities in Tea Plantation. Agriculture. 2025; 15(18):1941. https://doi.org/10.3390/agriculture15181941
Chicago/Turabian StyleLiu, Cenwei, Jing Ye, Yi Lin, Xiaomei Wu, Weixi Shu, and Yixiang Wang. 2025. "Effects of Co-Application of Biochar and Nitrogen Fertilizer on Soil Properties and Microbial Communities in Tea Plantation" Agriculture 15, no. 18: 1941. https://doi.org/10.3390/agriculture15181941
APA StyleLiu, C., Ye, J., Lin, Y., Wu, X., Shu, W., & Wang, Y. (2025). Effects of Co-Application of Biochar and Nitrogen Fertilizer on Soil Properties and Microbial Communities in Tea Plantation. Agriculture, 15(18), 1941. https://doi.org/10.3390/agriculture15181941






