Abstract
This study examined the effects of 2, 4 and 8% digestate-derived compost (DCP) on the retention/release of the fungicide penconazole (PEN), the herbicide S-metolachlor (S-MET) and the endocrine disruptor bisphenol A (BPA) in two agricultural soils sampled in Valenzano (SOV) and Trani (SOT), in Sothern Italy. DCP alone showed a conspicuous adsorption of the three xenobiotics, followed by their slow and scarce release. Sorption isotherm data of the compounds on unamended and DCP-amended soils were well described by the Freundlich model. Compared to unamended soil, the addition of the highest dose (8%) DCP to SOV increased the distribution coefficient, Kd, values of PEN, S-MET and BPA by 281%, 192% and 176%, respectively, while for SOT, the increases were 972%, 786% and 563%, respectively. Desorption of PEN and S-MET from all treatments was slow and partial (hysteresis), and only slightly reduced or unaffected by the addition of DCP, whereas BPA was almost entirely undesorbed in all treatments. Highly significant correlations between the adsorption coefficients of the three compounds in all soil treatments and the corresponding organic C contents confirm the prominent role of native and anthropogenic OM in the adsorption of contaminants and, consequently, in the control of their transfer into natural waters and/or entry in crop plants.
1. Introduction
From the perspective of the current concept of circular economy, expressed as the “reduce, reuse, recycle” paradigm, researchers are focusing their scientific efforts on the valorisation of organic wastes, trying to convert them into a valuable resource for the energy sector and modern agriculture [1]. Some current technologies allow operators to respond to multiple community needs through a single sustainable process [2]. This is the case of anaerobic digestion (AD), which has gained increasing interest since it combines multiple benefits, such as the disposal of agrozootechnical waste, the production of bioenergy and the contrast to climate change through carbon (C) sequestration in the by-product digestate (DG).
The effectiveness of DG as a soil improver is widely recognized both when used raw and after its conversion into safer and more profitable products such as compost (CP), vermicompost and biochar [3]. On the other hand, it is well known that intensive agricultural use of soil has led to a progressive depletion of the native organic fraction, thus exposing the soil to degradation (erosion and desertification) and resulting in a reduction in the quantity and quality of crops [4]. Soil organic matter (OM) is undoubtedly the protagonist of the overall soil fertility, and, in addition to ensuring good edaphic and trophic conditions for plants, it regulates the level of inorganic and organic contaminants present in pore water, thus controlling their availability to all soil-dwelling organisms. The control action is also exerted on the dynamics of pollutants in the entire soil–plant-water system [5].
In addition to biogas, AD releases a raw semisolid product from which, after a solid–liquid separation, a liquid DG and a solid DG are obtained. The latter can be converted into high-quality biofertilizer, namely DG-derived compost (DCP) and vermicompost (VC), through aerobic treatment lasting several weeks [6,7]. During this time, the lignocellulosic components of DG undergo biodegradation, and a humified fraction is gradually formed until the product is fully mature. Soil application of DCP or VC has multiple beneficial effects in agricultural systems as they provide fairly stable OM that improves soil fertility by stimulating plant growth and microbial activity [8,9]. Therefore, AD and subsequent aerobic treatment constitute a virtuous recycling of biowaste, generating both renewable energy and zero-waste soil improvers. Thanks to DCP properties such as its humic component, loose structure, large specific surface area, and nutrient content, this material is very suitable as a soil amendment. A recent study reports interesting applications of DCP in bioremediation and soil detoxification from industrial xenobiotics [10].
Although the European Green Deal and the Sustainable Development Goals of 2030 Agenda are aimed at significantly reducing pesticide use, the quantities of these products currently employed are still very high and only slowly declining, which causes soil and water pollution in areas where intensive and super-intensive agriculture is practised. In 2022, the global use of such products in agriculture amounted to 3.70 Mt of active substances, with 4% increase compared to the previous year [11]. While the more water-soluble compounds are particularly dangerous to the quality of surface and groundwater, the more hydrophobic ones are the most dangerous to human and animal health, as they can accumulate in vital organs and cause disease and death. Furthermore, hydrophobic compounds are by far the most persistent in the environment as they can bind tightly to OM in soil and sediments and are resistant to biodegradation [12].
Penconazole [1-[2-(2,4-dichlorophenyl)pentyl]-1,2,4-triazole, PEN] is a systemic fungicide belonging to the triazole class. It is worldwide used to protect crops, such as grapes, fruit trees, vegetables and ornamental plants, from a broad spectrum of pathogenic fungi and is particularly effective against powdery mildew [13]. This fungicide inhibits the biosynthesis of ergosterol, an essential component of fungal cell membranes [14]. It is toxic for soil microorganisms and animals and can exert endocrine-disrupting activity [15]. S-Metolachlor [2-chloro-N-(2-ethyl-6-methylphenyl)-N-[(2S)-1-methoxy-2-propanyl]acetamide, S-MET] is a chloroacetamide pre-emergent herbicide widely adopted to control broadleaf weeds in a variety of crops [16]. In Italy, in 2022, S-MET was detected in 240 water samples with a contamination frequency of more than 27% [17]. Its mode of action consists of the inhibition of long-chain fatty acids biosynthesis which disrupts the formation of new cell membrane and consequently cell division during seed germination [18]. S-MET has been reported as a suspected human carcinogen [19] and endocrine disrupting chemical [20].
Intensive human activities result in a continuous discharge of industrial byproducts into aquatic and terrestrial ecosystems. Many of these chemicals, of which bisphenol A [4,4′-(2,2-propandiyl)diphenol, BPA] is a very representative molecule, are endocrine disrupting chemicals with estrogen-like and anti-androgen activity. BPA has been frequently reported in soil and natural waters, especially in highly urbanized and industrialized areas [21]. The global production of BPA is approximately 8 Mt per year and is constantly growing; it is predicted that the volume of BPA produced worldwide will exceed 10 Mt in the next years [22]. As a component of materials such as polycarbonate and epoxy resins, it is present in many commonly used products, including electrical and electronic components, medical devices, food and beverage packaging, and so on [23]. In soil, BPA binds to OM, but, due to its moderate water solubility, it can leach and contaminate groundwater, especially in saturated soil. Various organic amendments are employed in the process of remediating soil and water from BPA contamination [24].
Despite extensive evidence of the potential of soil amendments to retain organic compounds from aqueous media [25,26], very few studies have focused on the role of CP and its humic fraction as a bioadsorbent of soil inorganic and organic pollutants [27,28]. C-rich materials resulting from waste biomass recycling can make a significant contribution to the containment of pollution, especially where soil OM is scarce, and fertility is reduced. Differently from soil amendments obtained through the thermochemical conversion of waste biomass, such as biochar and hydrochar, DCP, which is biologically produced, has the added value of a humic (or humic-like) component of great importance for the overall soil fertility. Similar to native soil OM, this humic fraction interacts with organic xenobiotics and immobilizes them, thereby reducing their concentration in soil pore water and their transfer to natural water [29]. Furthermore, since the ingestate of AD is generally composed of animal waste alone or plant residues alone or their mixture, DCP is less likely to contain hazardous organic compounds or heavy metals compared to CPs prepared from the organic fraction of municipal solid waste or sewage sludge. This is also true when DCP is compared to soil amendments originated from thermochemical processes, which, despite being more efficient as adsorbents, can contain alarming levels of toxic compounds and elements.
The employment of DCP in agriculture as a bioadsorbent represents a valuable way to recover and valorise a by-product (DG) which in turn derives from waste (mainly agro-waste). In this case, the generation of renewable energy (biogas) is virtuously combined with the production of a low-cost multifunctional material (DCP), beneficial for agriculture and the environment, fully respecting the circular economy. Anyway, the addition of DCP to the soil requires a thorough understanding of the modifications it induces in the entire soil–plant system. Most studies on the agricultural use of DCP are limited to their effects on soil physical, chemical, and biological fertility [30]. Furthermore, characterization of DCPs focuses primarily on their content of plant nutrients, the absence of potentially toxic elements, and biological activity [31,32], neglecting their adsorbent properties. Several published studies have focused on adsorption/desorption of organic pollutants on/from amendments alone or soil alone, while investigation on changes following soil amendment has been scarcely explored. Finally, although there is evidence in the literature that soil amendment can attenuate pesticide leaching, systematic data on the adsorption/desorption behaviour of DCP at different application rates and in the coexistence of multiple classes of organic pollutants (fungicides, herbicides, and endocrine disruptors) are still lacking, particularly in soils with low organic matter levels.
In light of all this, the present study aimed to evaluate the role of different DCP dosages on the adsorption and release of three organic xenobiotics, PEN, S-MET and BPA, in two agricultural soils.
2. Materials and Methods
2.1. Chemicals, Compost and Soil
The compounds considered in this study, namely the fungicide PEN with purity ≥ 98%, the herbicide S-MET with purity ≥ 98%, and the endocrine disruptor BPA with purity ≥ 99% were purchased from Merck KGaA, Darmstadt, Germany. Molecular mass, water solubility at 25 °C, and log Kow were, respectively, 284.2 g mol−1, 1.6 and 4.67 for PEN, 283.8, 50.9 and 3.24 for S-MET, and 228.3, 300 and 3.32 for BPA [33]. Single methanolic solutions (HPLC grade) of the compounds were prepared at a concentration of 2000 µg mL−1 and subsequently diluted with double-distilled H2O to obtain the aqueous mixtures used in the experiments.
The DCP was collected from a biogas-producing plant located in Capaccio, South Italy belonging to C&F Energy, Società Agricola S.r.l., Salerno, Italy. The ingestate was a mixture of buffalo manure (80%, w/w), olive mill wastewater (15%, w/w), agrifood industry residues (3%, w/w) and poultry manure (2%, w/w), and was processed under thermophilic conditions (55–60 °C). After the release, the solid DG was subjected to heat treatment at a temperature of 65 °C for 5 days and then exposed to the air under controlled conditions for approximately 2 months. Before use, the DCP was air-dried and thoroughly mixed. The complete characterization of DCP is reported in the recently published article [34]. Some DCP properties are summarized as follows: 17.2% moisture, pH 7.9, EC 1.17 dS m−1 at 25 °C, 10.3% ash, 452 g kg−1 total organic C, 19.7 g kg−1 total N, C/N 23 [34]. Major elements of DCP were Ca (10,802 mg kg−1), K (7765 mg kg−1), P (7180 mg kg−1), S (1940 mg kg−1), Fe (1172 mg kg−1); potentially toxic metals, Cu, Zn, Ni and Pb, were all below the limits fixed by the EU Regulation for organic fertilizers [34].
Two loamy Apulian soils with different OM contents were used. One soil (SOV) was sampled at 0–20 cm depth in an olive grove located in the Martucci experimental station of the University of Bari, in Valenzano. The other soil (SOT) is cultivated with vegetables and was sampled at 0–20 cm depth in a farm located in Trani. After sampling, each soil was air-dried at room temperature (approximately 20 °C), sieved with a 2 mm sieve to remove the skeleton and homogenized.
2.2. Soil Characterization
The properties of SOV were determined using conventional methods described in the work of Colatorti et al. [35] and shown in Table 1. SOT properties were determined according to Italian official methods of soil analysis [36] that are briefly reported as follows. Soil moisture was measured after heating the soil at 105 °C overnight; the pH was potentiometrically measured in a 1:2.5 (w/v) soil-to-H2O suspension; electrical conductivity (EC) was measured at 25 °C in a 1:2 (w/v) soil-to-H2O slurry; organic C and total N were determined using the Walkley-Black method and the Kjeldahl method, respectively. SOT properties are shown in Table 1.

Table 1.
Main properties of the two soils.
2.3. Adsorption and Desorption Experiments
The adsorption study of the three molecules on DCP was conducted at different solution/DCP ratios in batch tests. Quantities of 2, 5, 10 and 20 mg of DCP were interacted with 20 mL of an aqueous mixture of PEN, S-MET and BPA, each at a concentration of 2 µg mL−1, thus obtaining solution/adsorbent ratios of 10,000, 4000, 2000 and 1000. The pH of the aqueous mixture was 6.92. To assess possible matrix effects, blank samples were also prepared by adding 20 mL of double-distilled H2O to DCP. To reach the adsorption equilibrium, the samples were kept under mechanical shaking at 20 °C in the dark for 24 h. Preliminary experiments in which DCP was interacted with the aqueous mixture of the compounds for 4, 8, and 16 h showed that, compared to the 8 h sampling, after 16 h, the variations in adsorbed PEN, S-MET and BPA were 3.9, −0.8 and 3.8%, respectively. Therefore, 24 h was considered a sufficiently long time for all compounds to reach equilibrium. Subsequently, the suspensions were centrifuged at 10,000× g at 10 °C for 10 min. A 10 mL volume of supernatant solution of each sample was filtered through 0.45 µm cellulose acetate filters (Ø 25 mm, Incofar s.r.l., Modena, Italy), and the equilibrium concentration of each molecule was measured by high-performance liquid chromatography (HPLC). All experiments were triplicated. The amount of compound adsorbed after a contact of 24 h per unit mass of DCP (qₜ, µg g−1) was calculated using the following equation:
where C0 (µg mL−1) is the initial concentration of the molecule in solution, Cₜ (µg mL−1) is the concentration of the molecule after 24 h, V (mL) is the volume of the solution, and m (g) is the mass of the adsorbent.
qₜ = (C0 − Cₜ) V/m
Adsorption isotherms were studied to quantify the adsorption of PEN, S-MET, and BPA on unamended soil (SOV and SOT) and DCP-amended soil at doses of 2% (SOV-DCP2 and SOT-DCP2), 4% (SOV-DCP4 and SOT-DCP4) and 8% (w/w) (SOV-DCP8 and SOT-DCP8). After the addition of DCP, the organic C content in SOV-DCP2, SOV-DCP4, SOV-DCP8, SOT-DCP2, SOT-DCP4, SOT-DCP8 were 4.62, 5.45, 7.11, 1.83, 2.71 and 4.48, respectively. An aliquot of 2 g of unamended or amended soil was interacted with 20 mL aqueous mixtures of the compounds at individual concentrations of 0 (blank samples), 0.1, 0.2, 0.4, 0.5, 1, and 2 µg mL−1. For the two pesticides, the concentration range tested was chosen based on the doses commonly applied to soil and in agreement with previous studies concerning PEN [37] and S-MET [38]; identical concentrations were used for BPA for comparison purposes. To reach equilibrium, the suspensions were stirred at 20 ± 1 °C, in the dark, for 48 h. In previous experiments, SOV and SOT were interacted with the aqueous mixture of the compounds for 12, 24 and 48 h; compared to the 24 h sampling, after 48 h, the variations in adsorbed PEN, S-MET and BPA on SOV were −4.2, −0.1 and 1.5%, respectively, while on SOT they were 2.9, −0.6 and 3.2%, respectively. On this basis, an equilibration time of 48 h was considered appropriate for all compounds. Before equilibration, pH values of triplicated suspensions of SOV and SOT (2 g) with 2 µg mL−1 solution (20 mL, pH 6.92) were 7.23 ± 0.09 and 8.16 ± 0.15, respectively; after equilibration (48 h), the pH values, in the same order, were 6.96 ± 0.05 and 7.02 ± 0.01. Subsequently, the samples were processed and analyzed as already described in session 2.3. All experiments were triplicated.
The desorption tests of the three molecules from the unamended and the soil amended with 4 and 8% DCP were started immediately after adsorption using the samples added with the three compounds at concentration of 2 µg mL−1. After each desorption cycle, 10 mL of the supernatant solution was replaced with the same volume of double-distilled H2O; the sample was shaken again for 48 h at 20 ± 1 °C and then centrifuged, filtered and analyzed under the conditions described in the next session. Three desorption cycles were performed in triplicate for each treatment. All experiments conducted in this study were performed from mid-2024 to early 2025.
2.4. Analytical Protocol
Samples were analyzed using a Nexera HPLC system (Shimadzu Corporation, Kyoto, Japan). All instrumental components, including the detectors, were from Shimadzu company. Samples were loaded into a SIL-40C XR autosampler connected to an LC-40D XR pump, a DGU-40S degasser, and a Shim-pack GIST-HP C18 column (150 mm × 3.0 mm × 3 µm) inserted into a CTO-40C thermoregulation system. The mobile phase was water (A) and acetonitrile (B). The gradient elution was set as follows: 0–7 min, 70% B, flow rate 0.5 mL min−1; 7–12 min, 75% B, flow rate 0.7 mL min−1; 12–15 min, 70% B, flow rate 0.5 mL min−1. Under these conditions, the retention times of PEN, S-MET, and BPA were 11.1 min, 10.7 min, and 6.6 min, respectively. PEN and S-MET were detected with an SPD-M40 diode array detector operating at a wavelength of 211 nm, while BPA was detected with an RF-20AXS fluorescence detector operating at wavelengths of 200 and 290 nm for excitation and emission, respectively.
The external standard method was adopted to quantify the molecules. Blank samples were prepared for DCP and soil treatments in duplicate. No interfering peaks from the matrix were observed and no significant changes in the retention times of the compounds were recorded. To quantify the concentration of each compound in solution, calibration curves at five concentration levels (0.1, 0.2, 0.4, 0.5, 1 and 2 µg mL−1) were performed; linearity was expressed by the determination coefficients, R2, which were: 0.99973, 0.99997 and 0.99996 for PEN, S-MET and BPA, respectively.
2.5. Theoretical Models
To find the best suitable model to describe the adsorption process of the compounds, adsorption isotherms data were fitted into the two-parameter nonlinear Freundlich, Langmuir and Temkin equations, and the linear Henry model. The Freundlich isotherm [39] is given by
where qe (µg g−1) is the amount of solute adsorbed at equilibrium per unit of adsorbent, Ce (µg mL−1) is the equilibrium concentration of the compound in solution, 1/n indicates the degree of nonlinearity between solution concentration and amount adsorbed, while the reciprocal, n, expresses the adsorption intensity, and KF (Freundlich adsorption constant, mL g−1) expresses the adsorption capacity of the adsorbent. The Langmuir equation [40] is
where qe and Ce are defined as in the Freundlich equation, b (µg g−1) is the maximum adsorption capacity, i.e., the amount of solute that forms a monolayer on the adsorbent, and KL (L mg−1, Langmuir constant) expresses the energy of adsorption and the affinity of the solute for the adsorbent. The Temkin model [41] is expressed as
where qe and Ce have been already defined for the previous equations, AT (mL g−1) is the Temkin isotherm constant, B = RT/bT, where bT (J mol−1) is the Temkin constant, which expresses the process enthalpy, T is the absolute temperature (K) and R is the universal gas constant (8.314 J mol−1 K−1).
qe = KF Ce1/n
qe = (KLCeb)/(1 + KLCe)
qe = B ln (ATCe)
The parameters of the nonlinear models were calculated by the nonlinear regression method using the solver add-in component of Microsoft Excel. The correlation coefficient, r, was calculated as
where qtm is the theoretical adsorbed concentration of the compound (µg g−1) at time t, qt is the experimental concentration (µg g−1), and is the mean qt.
The linear Henry model:
was used to calculate the distribution coefficient, Kd, from the slope, and the partition coefficient, KOC, as: KOC = (Kd/(% OC)) × 100. The latter parameter expresses the amount of compound adsorbed per organic C unit of the adsorbent.
qe = KdCe
Finally, to estimate the deviation between theoretical and experimental data, two error functions were considered: the correlation coefficient, r, and the sum of squared residuals, SSR.
3. Results and Discussion
3.1. DCP Sample
The concentrations of each molecule adsorbed at equilibrium on DCP at various solution/adsorbent ratios are shown in Figure 1. The ratios adopted are comparable with those used in similar studies on the adsorption of xenobiotics on C-rich matrices. Increasing the solution/DCP ratio, the adsorbed amount of each compound significantly increased at each successive ratio tested (Figure 1). A 10-fold increase in the ratio (from 1000 to 10,000) increased the concentration of absorbed PEN, S-MET and BPA by 2.2, 3.4 and 4.1 times. It can be assumed that at higher ratios, i.e., at smaller amounts of compound for the same solution volume, a greater number of interaction sites are available, including the more internal sites that are generally more difficult to reach for more hydrophobic molecules or more shielded by water molecules for less hydrophobic compounds. At the highest ratio (10,000), the concentration of adsorbed PEN, S-MET and BPA were 2067, 809, and 2356 µg g−1, respectively. Based on these results, the highest ratio was chosen for subsequent adsorption experiments.
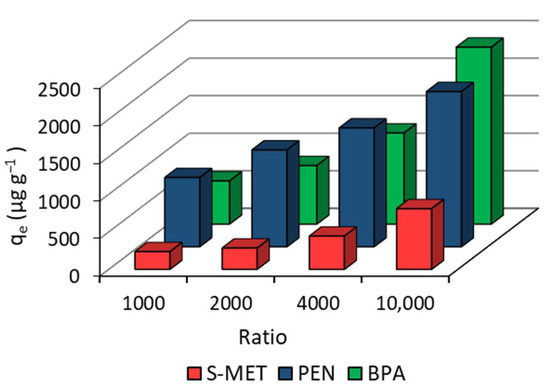
Figure 1.
Effects of different solution/DCP ratio on the equilibrium concentration of the adsorbed compound.
The relatively high adsorption capacity shown by this DCP was quite predictable based on the results of its previous extensive characterization [34]. Structural properties of DCP are briefly summarized here: (i) the BET specific surface area, an essential parameter for adsorbent materials, was approximately 1 m2 g−1; (ii) SEM analysis showed a heterogeneous surface, diffuse irregular ridges, channels, cavities, and low porosity typical of organic matrices originated from biological treatments of biomass; (iii) the FTIR spectrum showed numerous functional groups—such as alcoholic and phenolic OH, C=O of amide groups and conjugated ketones, COO- groups of carboxylic acids, aromatic C=C from quinones of lignin-like bulks, and other absorption peaks typical of polysaccharides or polysaccharide-like moieties possibly associated with silicate impurities, clay minerals and humic acids; (iv) Raman spectroscopy highlighted the presence of CH, CC, CO and CN bonds which confirmed the dual nature of this material, i.e., aliphatic and aromatic. All these features are a clear indication of a remarkable potential of DCP to stably interact with polar and hydrophobic compounds [34].
The desorption study demonstrated a considerable retention of PEN, S-MET and BPA on DCP even after three dilution steps of the liquid phase, when approximately 84, 33 and 55% of PEN, S-MET and BPA, respectively, were still adsorbed (Figure 2). These results demonstrate that DCP, in addition to effectively adsorb the compounds, has a good capacity to retain them when the liquid phase of the soil diluted.
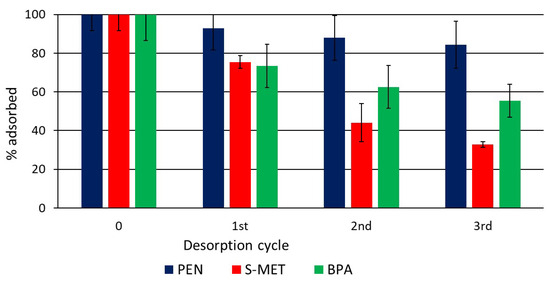
Figure 2.
Percentage of compound that remains adsorbed on DCP after each desorption cycle.
3.2. Soil
Although CP amendment is a widespread agricultural practise, the behaviour of this material as biosorbent of pollutants has certainly been overlooked. The adsorption isotherm describes the relationship between the concentration of a solute on an adsorbent and its concentration in solution at constant temperature. To gain insight into the adsorption mechanisms, experimental isotherm data were described by various theoretical models, namely the two-parameter Freundlich, Langmuir and Temkin models and the linear Henry equation. The Freundlich model (Equation (2)) describes multilayer adsorption on heterogeneous surfaces. Conversely, the Langmuir model (Equation (3)) is more appropriate for homogeneous surfaces with single-layer adsorption. The Temkin isotherm (Equation (4)) is appropriate for intermediate solute concentrations and heterogeneous adsorbents. It is based on the assumption that there is a linear reduction in the heat of adsorption of all solute molecules in a layer with increasing adsorbent surface coverage, and that the process occurs with a uniform distribution of the binding energy [41]. The Henry model (Equation (6)) is appropriate at low solute concentrations and for hydrophobic substances in aqueous solution. The model assumes a linear partitioning of the solute between the adsorbent and the solution over the entire concentration range explored; it is used to calculate the distribution coefficient, Kd, which is a reliable parameter to express the sorption efficiency of a substrate, and the organic C partition coefficient, KOC. The accordance between experimental adsorption data of PEN, S-MET and BPA on unamended and amended soil and the theoretical models was assessed based on the values of the correlation coefficient, r, and the sum of squared residues (SSR).
The adsorption isotherms obtained for the three compounds on unamended and amended SOV and SOT are depicted in Figure 3 and Figure 4, respectively, while Table 2 and Table 3 show the values of the adsorption parameters obtained by fitting the experimental data into the theoretical models. Based on the r and SSR values, with very few exceptions, the experimental sorption data of the three molecules on all treatments were best described by the Freundlich model, although in several cases there was also a good match with one or more of the other models tested. On both soils, the Freundlich parameter 1/n was generally less than or approximately equal to unity. This parameter indicates the type of Giles isotherm, i.e., L-type (1/n < 1), C-type (1/n~1) and S-type (1/n > 1), and is related to the intensity of adsorption and the type of process. When 1/n < 1, the interaction is predominantly physical, while it is predominantly chemical when it is greater than 1, and both processes contribute when it approximates to unity [42].
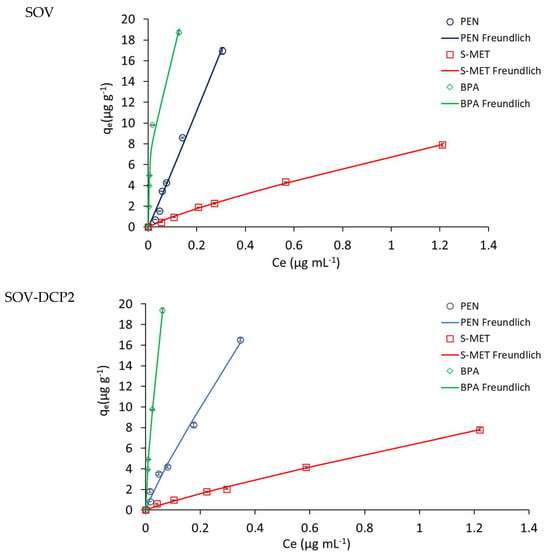
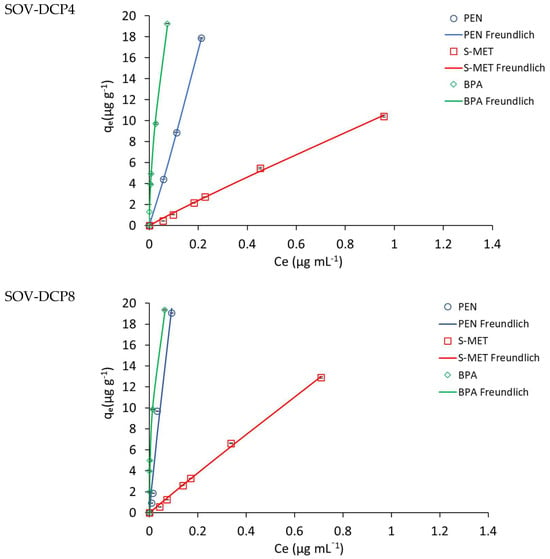
Figure 3.
Adsorption isotherms of the compounds on unamended Valenzano soil (SOV) and SOV amended with 2, 4 and 8% DCP (SOV-DPC2, SOV-DPC4, SOV-DPC8). Standard error is reported as vertical bar on each point (n = 3).
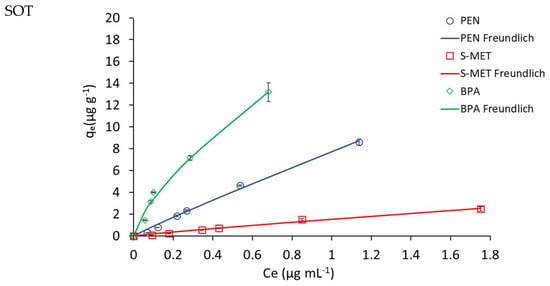
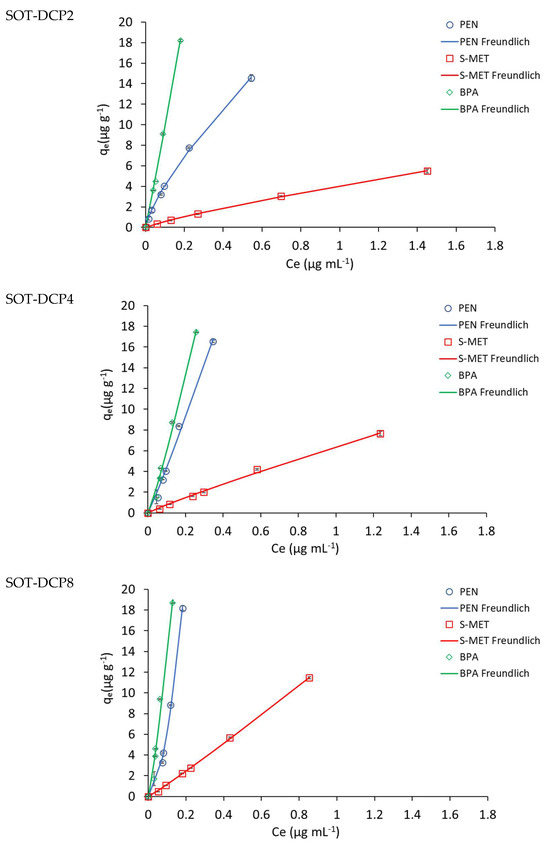
Figure 4.
Adsorption isotherms of the compounds on unamended Trani soil (SOT) and SOT amended with 2, 4 and 8% DCP (SOT-DPC2, SOT-DPC4, SOT-DPC8). Standard error is reported as vertical bar on each point (n = 3).

Table 2.
Adsorption parameters of the compounds on SOV obtained by applying theoretical models via nonlinear regression.

Table 3.
Adsorption parameters of the compounds on SOT obtained by applying theoretical models via nonlinear regression.
The values of the adsorption constants Kd, KF and KOC indicated a general higher adsorption of PEN and BPA, compared to S-MET, on both soils. In particular, in SOV treatments, Kd, KF and KOC values for BPA (119, 46 and 3112 mL g−1, respectively) were all higher than those obtained for PEN (56, 58 and 1476 mL g−1, respectively) and S-MET (6, 7 and 167 mL g−1, respectively) (Table 2). The same trend was observed in SOT treatments, where Kd, KF and KOC values for BPA (21, 17 and 2311 mL g−1, respectively) were all higher than those observed for PEN (8, 8 and 865 mL g−1, respectively) and S-MET (1, 1 and 164 mL g−1, respectively) (Table 3).
As expected, a significant increase in the adsorption constants was observed for both DCP-amended soils, compared to unamended soils (Figure 3 and Figure 4 and Table 2 and Table 3). Based on the Kd values, the adsorption efficiency followed the trend: unamended soil < soil-DCP2 < soil-DCP4 < soil-DCP8. Compared to SOV, the addition of 2, 4 and 8% DCP increased the Kd values of PEN by 22, 56 and 281%, of S-MET by 4, 74 and 192%, and of BPA by 17, 93 and 176%, respectively; while the same additions to SOT increased Kd values of PEN by 266, 510 and 972%, of S-MET by 164, 330 and 786%, and of BPA by 380, 363 and 563%, respectively. Comparing the two soils, it is evident that all SOV treatments present higher values of the adsorption constants, which can be reasonably attributed to the significantly higher organic C content of SOV (37.9%) compared to SOT (9.4%). The KF values obtained in this study for unamended and amended SOV were very similar to those reported by Jiang et al. [37] who added a sugarcane bagasse CP and a chicken manure CP at dosages of 2.5 and 5% in a Chinese soil.
The incorporation of DCP into the soil produced an increase in organic C content, which improved the sorption of each compound. It is known that OM is the main soil component controlling the retention of organic pollutants, especially hydrophobic compounds, although clay minerals can also play a significant role in the process, especially for polar compounds. However, in addition to the quantity of OM present in the soil, its origin and nature are certainly important since they determine its compositional, structural and functional characteristics. Soluble OM could even reduce or have no effects on the sorption of high-water-soluble compounds because of the competition between the two types of sorbates [43]. In soil, stable humic compounds interact with readily decomposable OM of fresh plant and animal residues and/or various organic amendments. The two OM pools have different chemical structure, composition, and accessibility. Their combination, as occurs in the case of anthropogenic OM inputs to the soil, could modify the dynamics of xenobiotics even in unexpected ways. Mitchell and Simpson [44] examined the adsorption affinity of BPA to five soils of varying OM composition and structure, and concluded that polar components of soil OM, such as O-alkyl components, can impair adsorption by blocking high-affinity sorption sites. It is a matter of fact that the adsorption capacity of soil humic substances is far superior to that of many organic amendments, including CP [25].
The possible relationships between Kd values of the compounds and the corresponding organic C percentages of all soil treatments (unamended and DCP-amended SOV and SOT) were explored using linear regressions (Figure 5). The results indicated that for each compound, the correlation was positive and highly significant (Figure 5), confirming the prominent role of soil OM in the retention of the compounds. This is consistent with the findings of other researchers who demonstrated that the adsorption of xenobiotics in soil is mainly controlled by the level of OM [45]. Ibrahim and Shalaby [46] monitored pesticide residues, including PEN, in many agricultural soils and reported positive correlation between OM content and pesticide retention in soil.
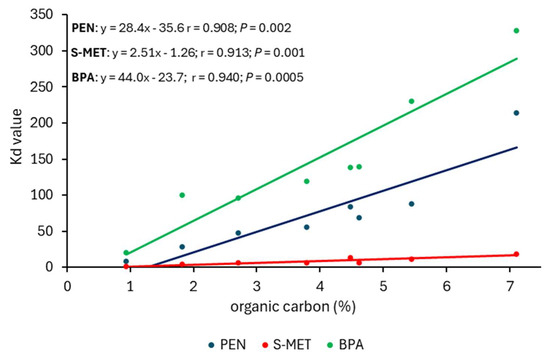
Figure 5.
Relationship between distribution coefficients (Kd) of the compounds and organic carbon percentages of all soil treatments (unamended and amended SOV and SOT).
Desorption experiments started immediately after adsorption. After the three desorption cycles, slow and limited desorption was observed, indicating the occurrence of a hysteresis process (Figure 6 and Figure 7). This phenomenon is related to the binding modes of the sorbate onto the adsorbent that, in turn, depend on the chemical groups of the adsorbent involved in the interaction. Complex macromolecules present in soil, such as humic and fulvic acids, can form both weak and strong bonds with xenobiotics. Weak binding will favour rapid release of the compounds, whereas strong binding will determine hysteretic conditions. The significant hysteresis observed in this study for all soil treatments and all molecules, especially BPA, can be attributed to the interaction of the compounds with the large number of hydrophobic and hydrophilic sites and chemically reactive functional groups—carboxylic and phenolic OH, alcoholic OH, carbonyl (quinonoid and ketonic) C=O, amino groups and so on—of native soil OM, especially the humic fraction, and of CP.
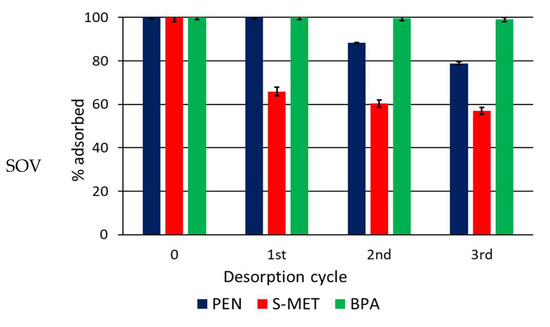
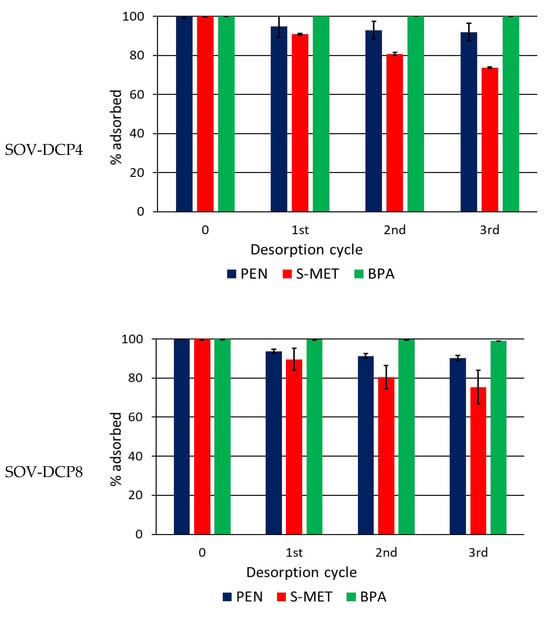
Figure 6.
Percentage of compound that remains adsorbed on Valenzano soil (SOV) and SOV amended with 4 and 8% DCP after each desorption cycle. Standard error is reported as a vertical line on each bar (n = 3).
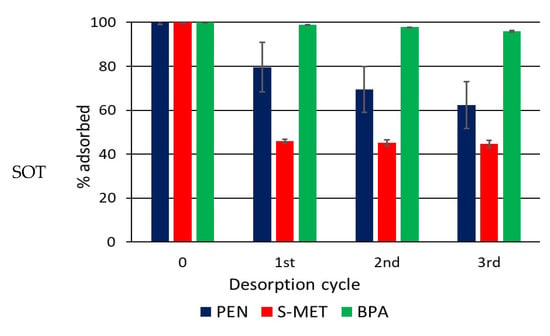
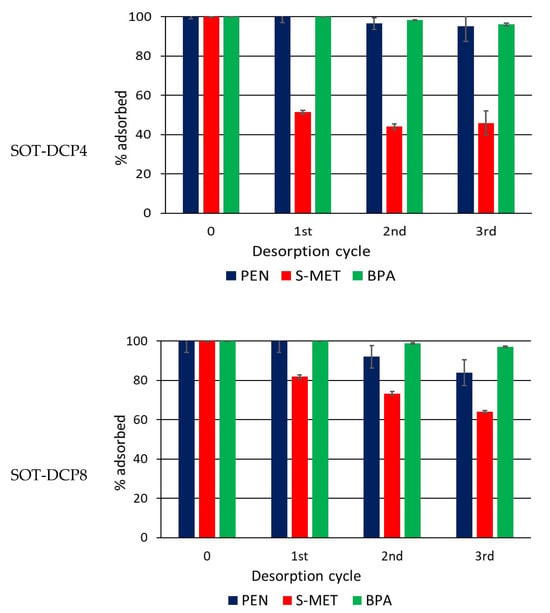
Figure 7.
Percentage of compound that remains adsorbed on Trani soil (SOT) and SOT amended with 4 and 8% DCP after each desorption cycle. Standard error is reported as a vertical line on each bar (n = 3).
While the addition of DCP caused a noticeable increase in each adsorbed compound, the effects were less evident in the desorption patterns, where less evident variations were observed between amended and unamended soil. It is plausible that the strong bonds (chemisorption) formed between the compounds and native soil OM, particularly humic acids, were primarily responsible for the desorption trend, while DCP was less conditioning. The release of PEN and, especially, of S-MET from CP-amended soil appeared generally reduced compared to the unamended soil, whereas only a negligible desorption was measured for BPA in all soil treatments (Figure 6 and Figure 7). At the end of experiments (third dilution) the percentages of PEN, S-MET and BPA still adsorbed on SOV were 79, 57 and 99%, respectively, while they were 92, 74 and 100% on SOV-DCP4 and 90, 75 and 99% on SOV-DCP8 (Figure 6). In the case of SOT, after the third desorption cycle, the percentages of adsorbed PEN, S-MET and BPA on SOT were 62, 45 and 96%, respectively, while they were 95, 46 and 96% on SOT-DCP4 and 84, 64 and 97% on SOT-DCP8 (Figure 7).
In a previous study, Lei et al. [37] observed that the adsorption capacity of a loam soil for PEN greatly increased after the addition of two different CP, whereas the desorption of the compound was drastically reduced. The authors commented that these results were more evident as CP dosage increased. In a recent study, Bushra et al. [47] reported that the poor release of BPA from soil may be due to the entrapment of the compound in the micropores of OM or to the formation of strong bonds with the hydrophobic domain of native OM. The behaviour of BPA, characterized by significant adsorption and negligible release, would suggest a low risk for the environment from the molecule. However, as soil conditions vary, it is possible that at least part of the pollutant load will be leached into deeper soil layers, with potential risks of groundwater contamination. This scenario is particularly real and critical in agricultural areas characterized by intense rainfall or frequent irrigation which could facilitate the transfer of the contaminant into natural waters. Previous studies on the occurrence and bioaccumulation of BPA in the environment highlighted the need to carefully monitor the presence of this contaminant in cultivated soils and suggested the adoption of preventive measures, such as soil organic enrichment, to limit BPA mobility and impact on groundwater resources [48].
Considering the results obtained, it appears clear that CP amendment can significantly influence the retention/release of xenobiotics in soil, whether they are agrochemicals or organic contaminants accidentally present in the soil. The reduced release of xenobiotics in soil amended with CP can be attributed to the increased OM content, which, establishing bonds of varying strength with these compounds, hinders their desorption. This phenomenon is frequently observed in the adsorption of hydrophobic contaminants and is closely related to the affinity between the solute and soil components, especially OM. Hysteresis slows the removal of pesticides, favouring the immobilization of residues in soil even for extended periods. Although this condition can sometimes cause unexpected phytotoxic effects on sensitive plant species included in the crop rotation, it generally ensures prolonged protection for tolerant species. Furthermore, high and long-term pesticide retention in the soil helps limit xenobiotic leaching into groundwater, transport into surface water bodies, and uptake by plants and microorganisms, thus significantly reducing environmental risk. In a very recent work, Bushra et al. [47] reported that the factors influencing BPA adsorption are primarily organic C content, but also soil mineralogy, including clay type and mineral surface area. These factors can influence hydrophobic partitioning and other interactions between BPA and soil. Similar conclusions are reported in the work of Lei et al. [37] regarding the effects of a sugarcane bagasse CP and a chicken manure CP on PEN adsorption/desorption in an amended soil.
4. Conclusions
A CP obtained from aerobic treatment of a mixed biomass digestate, DCP, was evaluated for its contribution to the retention capacity of two different soils for three organic xenobiotics, namely the pesticides PEN and S-MET and the industrial contaminant BPA. DCP alone showed significant capacity to adsorb xenobiotics. The addition of 2, 4, and 8% DCP significantly increased the capacity of each soil to adsorb the compounds. The adsorption constants, calculated from equilibrium adsorption isotherm data and according to various theoretical models, were significantly higher for DCP-amended soils than for soil alone. Overall, the adsorption of each molecule on soil treatments preferentially followed the nonlinear Freundlich model, indicating multilayer adsorption on heterogeneous surfaces. A significant positive correlation was observed between the extent of adsorption of each compound and the organic carbon content of the treatments. The desorption of the three molecules from all soil samples was rather slow and incomplete, indicating a hysteresis phenomenon. Furthermore, the addition of DCP to the soil did not substantially alter the desorption pattern nor the quantitative release of the compounds. The overall results obtained support the use of DCP, which, in addition to its known effects on soil fertility, can be an effective adsorbent for soil contaminants. This role is of fundamental importance in preventing the leaching and transfer of contaminants into natural waters, as well as in limiting their entry into plants, and consequently into the human and animal food chain. Finally, although this study was the first to highlight a generally overlooked aspect of DCP, namely its ability to immobilize organic soil contaminants, it certainly suffers from the limitations of having been conducted solely on a laboratory scale, considering a limited number of contaminants and only two soils, albeit with quite different properties. At the field scale, further evaluation is required to assess the long-term stability of DCP’s adsorption performance under alternating wet-dry cycles and rhizosphere compartment pulses.
Author Contributions
E.L.: conceptualization, methodology, supervision, writing, funding acquisition. E.C.: material preparation, investigation, formal analysis. C.C.: material preparation, investigation, data analysis. N.D.: material preparation, investigation, data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The present work contributes to the project PRIN 2022 PNRR entitled ‘Microbially-enriched biosorbents from waste recycling and soil-resident fungi as novel and sustainable tools to mitigate soil pollution by chemicals of emerging concern and prevent their entry and accumulation in vegetables’, Call 1409, dated 14 September 2022, financially supported by the European Union—NextGenerationEU—PNRR—Mission 4, Component 2, Investment 1.1. CUP: H53D23010520001. This manuscript reflects only the authors’ views and opinions. Neither the European Union nor the European Commission can be considered responsible for them.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank C&F Energy Società Agricola s.r.l., Altavilla Silentina, Italy, for providing the compost sample used in this study. Sincere thanks to the reviewers of the article for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no competing interests.
References
- Global Bioenergy Statistics (GBS). 2024. Available online: https://www.worldbioenergy.org/uploads/241023%20GBS%20Report%20Short%20Version.pdf (accessed on 15 April 2025).
- Singh, L.; Kalia, V.C. Waste Biomass Management—A Holistic Approach; Springer: Cham, Switzerland, 2017; 392p. [Google Scholar] [CrossRef]
- Adnane, I.; Taoumi, H.; Lahrech, K.; Fertahi, S.; Ghodbane, M. From waste to resource: Biogas and digestate valorization strategies for sustainable energy and agriculture. Biomass Bioenergy 2025, 200, 108006. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E. The chemistry of soil organic matter. In Soil Physical Chemistry, 2nd ed.; Sparks, D.L., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 239–370. [Google Scholar] [CrossRef]
- Cesaro, A. The valorization of the anaerobic digestate from the organic fractions of municipal solid waste: Challenges and perspectives. J. Environ. Manag. 2021, 280, 111742. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef]
- Traversa, A.; Loffredo, E.; Gattullo, C.E.; Palazzo, A.; Bashore, T.L.; Senesi, N. Comparative evaluation of compost humic acids and their effects on the germination of switchgrass (Panicum vigatum L.). J. Soils Sediments 2014, 14, 432–440. [Google Scholar] [CrossRef]
- Chen, Y.; Camps-Arbestain, M.; Shen, Q.; Singh, B.; Cayuela, M.L. The long-term role of organic amendments in building soil nutrient fertility: A meta analysis and review. Nutr. Cycl. Agroecosyst. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Dissanayake, P.D.; Igalavithana, A.D.; Tang, R.; Cai, Y.; Chang, S.X. Converting food waste into soil amendments for improving soil sustainability and crop productivity: A review. Sci. Total Environ. 2023, 881, 163311. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Pesticides Use and Trade 1990–2022. Food and Agriculture Organization of the United Nations. 2022. Available online: https://www.fao.org/statistics/highlights-archive/highlights-detail/pesticides-use-and-trade-1990-2022/en (accessed on 20 April 2025).
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zeng, C.; Qin, T.; Lv, T.; Xu, Z.; Xun, Z.; Wang, L.; Chen, X.; Liu, B.; Peng, X. A dual-state-emission chalcone-based supramolecular probe for ratiometric detection of penconazole in environmental samples. Chem. Eng. J. 2023, 468, 143610. [Google Scholar] [CrossRef]
- Mercadante, R.; Polledri, E.; Scurati, S.; Moretto, A.; Fustinoni, S. Identification of Metabolites of the Fungicide Penconazole in HumanUrine. Chem. Res. Toxicol. 2016, 29, 1179–1186. [Google Scholar] [CrossRef]
- Perdichizzi, S.; Mascolo, M.S.; Silingardi, P.; Morandi, E.; Rotondo, F.; Guerrini, A.; Prete, L.; Vaccari, M.; Colacci, A. Cancer-related genes transcriptionally induced by the fungicide penconazole. Toxicol. Vitr. 2014, 28, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kouame, K.B.J.; Savin, M.C.; Willett, C.D.; Bertucci, M.B.; Butts, T.R.; Grantz, E.; Roma-Burgos, N. S-metolachlor persistence in soil as influenced by within-season and inter-annual herbicide use. Environ. Adv. 2022, 9, 100318. [Google Scholar] [CrossRef]
- ISPRA. Rapporto Nazionale Pesticidi Nelle Acque. Istituto Superiore per la Protezione e la Ricerca Ambientale. 2023. Available online: https://www.isprambiente.gov.it/files2022/pubblicazioni/rapporti/rapporto_371_2022.pdf (accessed on 10 April 2025).
- Rangani, G.; Noguera, M.; Salas-Perez, R.; Benedetti, L.; Roma-Burgos, N. Mechanism of Resistance to S-metolachlor in Palmer amaranth. Front. Plant Sci. 2021, 12, 652581. [Google Scholar] [CrossRef]
- European Chemical Agency (ECHA). 2024. Available online: https://echa.europa.eu/search?p_p_id=com_liferay_portal_search_web_portlet_SearchPortlet&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&_com_liferay_portal_search_web_portlet_SearchPortlet_mvcPath=%2Fsearch.jsp&_com_liferay_portal_search_web_portlet_SearchPortlet_redirect=%2Fweb%2Fguest%2Fsearch%3Fp_p_id%3Dcom_liferay_portal_search_web_portlet_SearchPortlet%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview&_com_liferay_portal_search_web_portlet_SearchPortlet_scope=this-site&p_auth= (accessed on 20 August 2025).
- Ou-Yang, K.; Feng, T.; Han, Y.; Li, G.; Li, J.; Ma, H. Bioaccumulation, metabolism and endocrine-reproductive effects of metolachlor and its S-enantiomer in adult zebrafish (Danio rerio). Sci. Total Environ. 2022, 802, 149826. [Google Scholar] [CrossRef]
- Pelch, K.E.; Li, Y.; Perera, L.; Thayer, K.A.; Korach, K.S. Characterization of Estrogenic and Androgenic Activities for Bisphenol A-like Chemicals (BPs): In Vitro Estrogen and Androgen Receptors Transcriptional Activation, Gene Regulation, and Binding Profiles. Toxicol. Sci. 2019, 172, 23–37. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Ranjha, M.M.A.N.; Sameen, A.; Zeng, X.-A.; et al. An insight into bisphenol A, food exposure and its adverse effects on health: A review. Front. Nutr. 2022, 9, 1047827. [Google Scholar] [CrossRef]
- Metcalfe, C.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ Res. 2022, 207, 112658. [Google Scholar] [CrossRef]
- Loffredo, E. Recent advances on innovative materials from biowaste recycling for the removal of environmental estrogens from water and soil. Materials 2022, 15, 1894. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E.; D’Orazio, V.; Brunetti, G.; Miano, T.M.; La Cava, P. Adsorption of pesticides by humic acids from organic amendments and soils. In Humic Substances and Chemical Contaminants; Clapp, C.E., Hayes, M.H.B., Senesi, N., Bloom, P.R., Jardine, P.M., Eds.; ASA, CSSA, SSSA Books: Chichester, UK, 2015; pp. 129–153. [Google Scholar] [CrossRef]
- Fouad, M.R.; El-Aswad, A.F.; Badawy, M.E.I.; Aly, M.I. Effect of soil organic amendments on sorption behavior of two insecticides and two herbicides. Curr. Chem. Lett. 2024, 13, 377–390. [Google Scholar] [CrossRef]
- Gamiz, B.; Pignatello, J.J.; Cox, L.; Hermosín, M.C.; Celis, R. Environmental fate of the fungicide metalaxyl in soil amended with composted olive-mill waste and its biochar: An enantioselective study. Sci. Total Environ. 2016, 54, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Zanin Lima, J.; Monici Raimondi Nauerth, I.; Ferreira da Silva, E.; José Pejon, O.; Guimarães Silvestre Rodrigues, V. Competitive sorption and desorption of cadmium, lead, and zinc onto peat, compost, and biochar. J. Environ. Manag. 2023, 344, 118515. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, E.; Picca, G.; Parlavecchia, M. Single and combined use of Cannabis sativa L. and carbon-rich materials for the removal of pesticides and endocrine-disrupting chemicals from water and soil. Environ. Sci. Pollut. Res. 2021, 28, 3601–3616. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Vitti, A.; Elshafie, H.S.; Logozzo, G.; Marzario, S.; Scopa, A.; Camele, I.; Nuzzaci, M. Physico-chemical characterization and biological activities of a digestate and a more stabilized digestate-derived compost from agro-waste. Plants 2021, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Nogués, I.; Rumpel, C.; Sebilo, M.; Vaury, V.; Moral, R.; Bustamante, M.A. Stable C and N isotope variation during anaerobic digestate composting and in the compost-amended soil-plant system. J. Environ. Manag. 2023, 329, 117063. [Google Scholar] [CrossRef]
- ChemSpider. Available online: https://www.chemspider.com (accessed on 5 April 2025).
- Loffredo, E.; Vona, D.; Porfido, C.; Giangregorio, M.M.; Gelsomino, A. Compositional and structural characterization of bioenergy digestate and its aerobic derivatives compost and vermicompost. J. Sustain. Agric. Environ. 2024, 3, e70002. [Google Scholar] [CrossRef]
- Colatorti, N.; Digregorio, N.V.; Camposeo, S.; Loffredo, E. Solid fraction of digestate from olive pomace modulates abiotic and biotic processes in soil: Retention of agrochemicals and inhibition of fungal pathogens. Sci. Hortic. 2024, 337, 113545. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale della Repubblica Italiana—GU Serie Generale n.248 del 21-10-1999—Suppl. Ordinario n. 185. Available online: https://www.gazzettaufficiale.it/eli/id/1999/10/21/099A8497/sg (accessed on 20 April 2025).
- Jiang, L.; Lin, J.L.; Jia, L.X.; Liu, Y.; Pan, B.; Yang, Y.; Lin, Y. Effects of two different organic amendments addition to soil on sorption-desorption, leaching, bioavailability of penconazole and the growth of wheat (Triticum aestivum L.). J. Environ. Manag. 2016, 167, 130–138. [Google Scholar] [CrossRef]
- Douibi, M.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Sustainable agricultural practices influence s-metolachlor, foramsulfuron and thiencarbazone-methyl degradation and their metabolites formation. Sci. Total. Environ. 2024, 945, 174039. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in losungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Prasannamedha, G.; Senthil Kumar, P.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Cox, L.; Hermosín, M.C.; Cornejo, J. Organic amendments affecting sorption, leaching and dissipation of fungicides in soils. Pest Manag. Sci. 2006, 62, 1207–1215. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Simpson, M.J. High affinity sorption domains in soil are blocked by polar soil organic matter components. Environ. Sci. Technol. 2013, 47, 412–419. [Google Scholar] [CrossRef]
- Parlavecchia, M.; D’orazio, V.; Loffredo, E. Wood biochars and vermicomposts from digestate modulate the extent of adsorption-desorption of the fungicide metalaxyl-m in a silty soil. Environ. Sci. Pollut. Res. 2019, 26, 35924–35934. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Shalaby, S.E.M. Screening and assessing of pesticide residues and their health risks in vegetable field soils from the Eastern Nile Delta, Egypt. Toxicol. Rep. 2022, 9, 1281–1290. [Google Scholar] [CrossRef]
- Bushra, K.; Javaid, I.; Shazia, M.; Muhammad, N.A.; Aitezaz, A.K.; Farwa, J.; Tariq, A.; Saleh, A.A.; Abdulhakeem, S.A.; Majid, A. Sorption and desorption of bisphenol A on agricultural soils and its implications for surface and groundwater contamination. Desalin. Water Treat. 2025, 322, 101180. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).