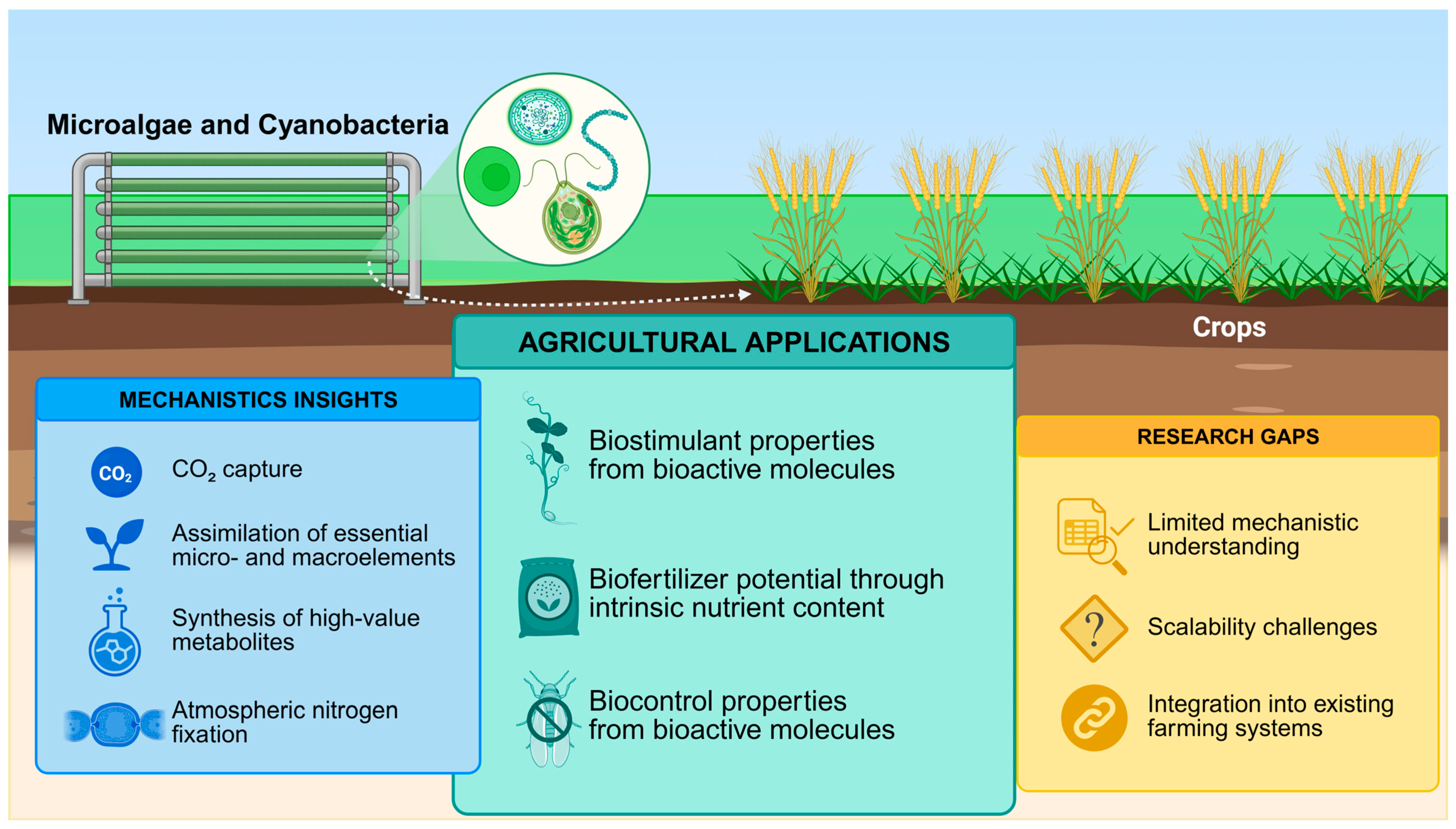
Harnessing Microalgae and Cyanobacteria for Sustainable Agriculture: Mechanistic Insights and Applications as Biostimulants, Biofertilizers and Biocontrol Agents
Abstract
1. Introduction
2. The Use of Microalgae and Cyanobacteria as Biostimulants
2.1. Cyanobacteria and Microalgae Strain Selection
2.2. Modes of Action and Mechanisms of Microalgae- and Cyanobacteria-Derived Biostimulants
- (1)
- Enhancement of Nutrient Uptake and Assimilation
- (2)
- Modulation of Plant Hormonal Balance
- (3)
- Activation of Stress-Response Pathways
- (4)
- Improvement of Soil Microbial Communities
2.3. Cyanobacteria and Microalgae as Biostimulants in Agriculture
2.4. Diatoms as Biostimulants in Agriculture
2.5. Microalgae-Bacteria Consortia
3. Biofertilizers
3.1. Cyanobacteria as Biofertilizers Beyond Nitrogen Fixation
3.2. Microalgae and Cyanoboacteria as Biofertilizers: Nutrient Solubilization
3.3. Microalgae and Cyanobacteria as Biofertilizers: Siderophore-Mediated Growth Promotion
| Species | Group | Biofertilization Mechanism | Plant/System | References |
|---|---|---|---|---|
| Nostoc sp. | Cyanobacteria | Nitrogen fixation, phytohormone production, solubilization of P/K/Zn | Wheat/In vitro Rize/Soil | [94,100] |
| Anabaena vaginicola ISB42 | Cyanobacteria | Phytohormone-linked nutrient uptake, peppermint oil enhancement | Mentha/Greenhouse conditions | [95] |
| Nostoc spongiaeforme var. tenue ISB65 | Cyanobacteria | Phytohormone-linked nutrient uptake, peppermint oil enhancement | Mentha/Greenhouse conditions | [95] |
| Synechococcus mundulus | Cyanobacteria | Siderophore production, enhanced Fe uptake in maize | Maize/In vitro | [126] |
| Arthrospira platensis | Cyanobacteria | Phytohormone production, improved nutrient acquisition in chia | Chia/Soil | [106] |
| Spirulina maxima | Cyanobacteria (marketed as microalgae) | Bioactive compounds enhancing growth and nutrient uptake in rosemary | Rosemary/Soil | [107] |
| Chlorella vulgaris | Microalgae | Nitrogen fixation Phosphorus and potassium solubilization, auxin-like activity | Lettuce/Soil | [45] |
| Scenedesmus obliquus | Microalgae | Nitrogen fixation P/K mobilization, root stimulation under stress | Lettuce/Soil | [45,129] |
| Dunaliella salina | Microalgae | Exopolysaccharides Siderophore-mediated Fe uptake under deficiency | Wheat/In vitro | [128] |
4. Biocontrol Agents
4.1. Phytopathogen Resistance
4.2. Microalgae and Cyanobacteria as Herbicides
| Class of Compound | Characteristics | Microorganism | Underlying Mechanism of Biocontrol | Potential Target Pathogens/Pests | Potential Use in Agriculture | Experimental Evidence | References |
|---|---|---|---|---|---|---|---|
| Alkaloids | Nitrogen-containing heterocyclic compounds | Fischerella sp., Calothrix sp., Desertifilum dzianense | Interfere with DNA replication and protein synthesis in pathogens; disruption of cell walls. | Insects (neurotoxin), fungi (Agroathelia rolfsii), and broad microbial pathogens | Natural bioinsecticides or antimicrobial agents for biocontrol | in vitro bioassays | [147,148] |
| Polyketides | Structurally diverse metabolites derived from carboxylic acid precursors | Gambierdiscus toxicus, Karenia brevis | Inhibition of ion channels, disruption of cell signaling and membrane integrity | Plant-pathogenic fungi, bacteria; brevetoxins also toxic to invertebrates | Broad-spectrum fungicides or bactericides for crops | both in vivo (animal models) and in vitro; in vitro assays (channel agonist) | [149,150] |
| Fatty acids | Extracellular free fatty acids with allelopathic activity | Chlorella vulgaris, Botryococcus braunii | Membrane destabilization; inhibition of seed germination through allelopathy | Competing algae (Pseudokirchneriella subcapitata), weeds | Natural weed growth inhibitors (bioherbicides) | in vitro inhibition assays; in vitro allelopathic tests | [151,152] |
| Peptides | Non-ribosomal peptides biosynthesized by multifunctional enzyme complexes | Anabaena sp. PCC7120, Microcystis sp., Planktothrix sp., Oscillatoria limosa, Synechococcus lividus | Pore formation in membranes; inhibition of protein phosphatases; induction of oxidative stress in pathogens | Other cyanobacteria, aquatic weeds, possible cross-toxicity to pathogenic fungi/bacteria | Plant defense promoters or biostimulants | review (mostly in vitro); in vivo detection in hot springs; in vitro isolation/assays; in situ environmental surveys | [153,154,155,156] |
| Terpenoids | Organic compounds derived from C5 precursors with toxicity to invertebrates | Nostoc commune, Calothrix sp. PCC7507 | Neurotoxic and deterrent effects on insects; oxidative stress induction | Bacteria (Bacillus cerus, S. epidermidis, E. coli), Insect pests (e.g., Lepidoptera larvae), nematodes; general herbivory deterrenc | Natural insecticides or pest repellents | in vitro bioassays; review (includes in vitro and some in vivo reports) | [157,158] |
4.3. Microalgae and Cyanobacteria as Insecticides
5. Commercialized Microalgae- and Cyanobacteria-Based Products
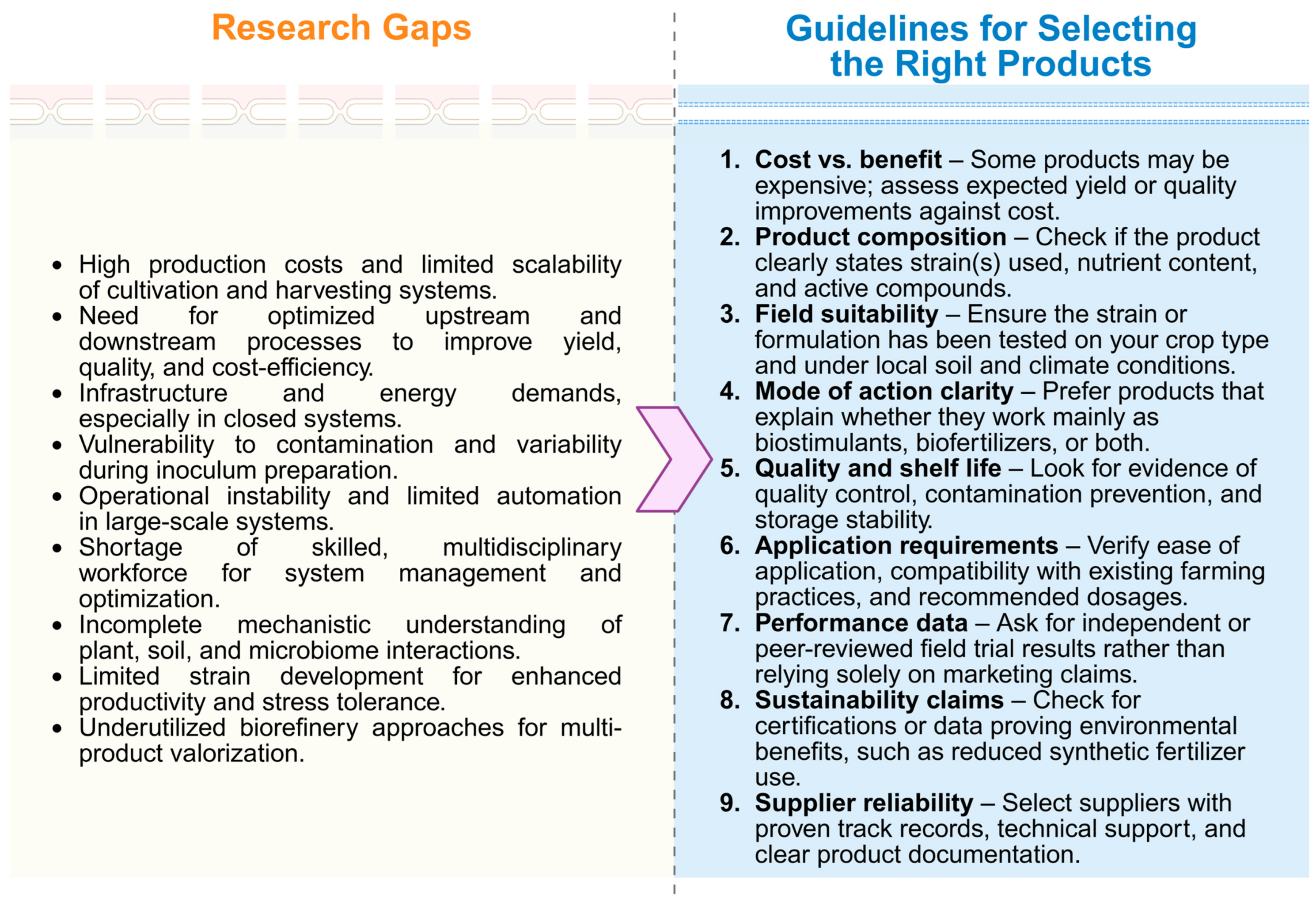
6. Research Bottlenecks and Future Perspectives
7. Conclusions
- Scalable cultivation systems—the development of cost-effective and energy-efficient production platforms, ideally integrating waste streams and renewable resources to minimize costs and environmental impact.
- Formulation and delivery strategies—optimizing stable, field-ready formulations (e.g., encapsulation, consortia-based inoculants, or liquid suspensions) that ensure consistent performance under variable agronomic conditions.
- Mechanistic understanding—advancing molecular and physiological studies to clarify the interactions between plants and microalgae/cyanobacteria, thereby identifying the key pathways responsible for plant growth promotion and stress alleviation.
- Regulatory frameworks and standardization—establishing clear guidelines for safety, efficacy testing, and product approval to accelerate the transition from experimental studies to market-ready solutions.
- Integration into circular economy models—exploring their role in waste valorization, nutrient recovery, and soil regeneration as part of holistic and climate-smart farming practices.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Drivers and Triggers for Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Ren, C.G.; Kong, C.C.; Liu, Z.Y.; Zhong, Z.H.; Yang, J.C.; Wang, X.L.; Qin, S. A Perspective on Developing a Plant ‘Holobiont’ for Future Saline Agriculture. Front. Microbiol. 2022, 13, 763014. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinization Management for Sustainable Development: A Review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef]
- Cuevas, J.; Daliakopoulos, I.N.; Del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Brito-Lopez, C.; Van Der Wielen, N.; Barbosa, M.; Karlova, R. Plant Growth-Promoting Microbes and Microalgae-Based Biostimulants: Sustainable Strategy for Agriculture and Abiotic Stress Resilience. Philos. Trans. R. Soc. B Biol. Sci. 2025, 380, 20240251. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil Microbiomes and One Health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Crystal-Ornelas, R.; Thapa, R.; Tully, K.L. Soil Organic Carbon Is Affected by Organic Amendments, Conservation Tillage, and Cover Cropping in Organic Farming Systems: A Meta-Analysis. Agric. Ecosyst. Environ. 2021, 312, 107356. [Google Scholar] [CrossRef]
- Raihan, A. A Review of Climate Change Mitigation and Agriculture Sustainability through Soil Carbon Sequestration. J. Agric. Sustain. Environ. 2023, 2, 23–56. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, L.M.; de-Bashan, L.E. The Potential of Microalgae–Bacteria Consortia to Restore Degraded Soils. Biology 2023, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Potential of Microalgae and Cyanobacteria to Improve Soil Health and Agricultural Productivity: A Critical View. Environ. Sci. Adv. 2023, 2, 586–611. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A Techno-Economic Analysis of Industrial Production of Marine Microalgae as a Source of EPA and DHA-Rich Raw Material for Aquafeed: Research Challenges and Possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Spinola, M.V.; Díaz-Santos, E. Microalgae Nutraceuticals: The Role of Lutein in Human Health. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020. [Google Scholar]
- León-Bañares, R.; González-Ballester, D.; Galván, A.; Fernández, E. Transgenic Microalgae as Green Cell-Factories. Trends Biotechnol. 2004, 22, 45–52. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Thanaporn, S.; Karapetsi, L.; Nteve, G.M.; Pratsinakis, E.; Stefanidou, E.; Lagiotis, G.; Avramidou, E.; Zorxzobokou, L.; Tsintzou, G.; et al. Diversity of Bioactive Compounds in Microalgae: Key Classes and Functional Applications. Mar. Drugs 2025, 23, 222. [Google Scholar] [CrossRef]
- Maurya, N.; Sharma, A.; Sundaram, S. The Role of PGPB-Microalgae Interaction in Alleviating Salt Stress in Plants. Curr. Microbiol. 2024, 81, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 871, 1782. [Google Scholar] [CrossRef] [PubMed]
- Farda, B.; Djebaili, R.; Sabbi, E.; Pagnani, G.; Cacchio, P.; Pellegrini, M. Isolation and Characterization of Cyanobacteria and Microalgae from a Sulfuric Pond: Plant Growth-Promoting and Soil Bioconsolidation Activities. AIMS Microbiol. 2024, 10, 944–972. [Google Scholar] [CrossRef]
- Farmonaut® Agriculture Biologicals Market: 2025 Growth & Research Guide. Available online: https://farmonaut.com/news/agriculture-biologicals-market-2025-growth-research (accessed on 14 July 2025).
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Vangenechten, B.; De Coninck, B.; Ceusters, J. How to Improve the Potential of Microalgal Biostimulants for Abiotic Stress Mitigation in Plants? Front. Plant Sci. 2025, 16, 1568423. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous Fertilizers: Impact on Environment Sustainability, Mitigation Strategies, and Challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Lorenzi, A.S.; Chia, M.A. Cyanobacteria’s Power Trio: Auxin, Siderophores, and Nitrogen Fixation to Foster Thriving Agriculture. World J. Microbiol. Biotechnol. 2024, 40, 1–18. [Google Scholar] [CrossRef]
- Nawaz, T.; Saud, S.; Gu, L.; Khan, I.; Fahad, S.; Zhou, R. Cyanobacteria: Harnessing the Power of Microorganisms for Plant Growth Promotion, Stress Alleviation, and Phytoremediation in the Era of Sustainable Agriculture. Plant Stress 2024, 11, 100399. [Google Scholar] [CrossRef]
- Mignoni, D.S.B.; Nonato, J.D.S.; Alves, J.S.; Michelon, W.; de Oliveira Nunes, E.; Pandolfi, J.R.; Luchessi, A.D. Agriculture application, comparison, and functional association between macrophytes and microalgae: A review. Discov. Agric. 2025, 3, 1–17. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Fact. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Prasanna, R.; Sood, A.; Ratha, S.K.; Singh, P.K. Cyanobacteria as a “Green” Option for Sustainable Agriculture. In Cyanobacteria: An Economic Perspective; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ferreira, A.; Bastos, C.R.V.; Marques-dos-Santos, C.; Acién-Fernandez, F.G.; Gouveia, L. Algaeculture for Agriculture: From Past to Future. Front. Agron. 2023, 5, 1064041. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Rifna, E.J.; Rajauria, G.; Dwivedi, M.; Tiwari, B.K. Circular Economy Approaches for the Production of High-Value Polysaccharides from Microalgal Biomass Grown on Industrial Fish Processing Wastewater: A Review. Int. J. Biol. Macromol. 2024, 254, 126887. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Ferrer, I.; Díez-Montero, R. Can Microalgae Grown in Wastewater Reduce the Use of Inorganic Fertilizers? J. Environ. Manag. 2022, 323, 116224. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Huang, P.; Yu, J.; Wang, P.; Jiang, H.B.; Jiang, Y.; Deng, S.; Huang, Z.; Yu, J.; Zhu, W. Efficient Wastewater Treatment and Biomass Co-Production Using Energy Microalgae to Fix C, N, and P. RSC Adv. 2025, 15, 14030–14041. [Google Scholar] [CrossRef] [PubMed]
- Asimakis, E.; Shehata, A.A.; Eisenreich, W.; Acheuk, F.; Lasram, S.; Basiouni, S.; Emekci, M.; Ntougias, S.; Taner, G.; May-Simera, H.; et al. Algae and Their Metabolites as Potential Bio-Pesticides. Microorganisms 2022, 10, 307. [Google Scholar] [CrossRef]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and Allelochemicals from Microalgae: An Innovative Source for Bio-Herbicidal Compounds and Biocontrol Research. Algal Res. 2021, 54, 1–15. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Mahreni; Murni, S.W.; Setyoningrum, T.M.; Hadi, F.; Widayati, T.W.; Jaya, D.; Sulistyawati, R.R.E.; Puspitaningrum, D.A.; Dewi, R.N.; et al. Innovative Strategies for Utilizing Microalgae as Dual-Purpose Biofertilizers and Phycoremediators in Agroecosystems. Biotechnol. Rep. 2025, 45, e00870. [Google Scholar]
- Onyeaka, H.; Miri, T.; Obileke, K.C.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing Carbon Footprint via Microalgae as a Biological Capture. Carbon. Capture Sci. Technol. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Chabili, A.; Minaoui, F.; Hakkoum, Z.; Douma, M.; Meddich, A.; Loudiki, M. A Comprehensive Review of Microalgae and Cyanobacteria-Based Biostimulants for Agriculture Uses. Plants 2024, 13, 159. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using Agro-Industrial Wastes for Mixotrophic Growth and Lipids Production by the Green Microalga Chlorella sorokiniana. N. Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Sorokin, B.A.; Mukhambetova, A.N.; Emelianova, A.A.; Kuzmin, V.V.; Belogurova-Ovchinnikova, O.Y.; Kuzmin, D.V. Recent Advances and Fundamentals of Microalgae Cultivation Technology. Biotechnol. J. 2024, 19, e2300725. [Google Scholar] [CrossRef]
- Janssen, M.; Kuijpers, T.C.; Veldhoen, B.; Ternbach, M.B.; Tramper, J.; Mur, L.R.; Wijffels, R.H. Specific Growth Rate of Chlamydomonas reinhardtii and Chlorella sorokiniana Under Medium Duration Light/Dark Cycles: 13–87 s. Prog. Ind. Microbiol. 1999, 35, 323–333. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next Generation Plant Growth Additives: Functions, Applications, Challenges and Circular Bioeconomy Based Solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of Biostimulation of Plants Under Abiotic Stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef]
- Chanda, M.J.; Merghoub, N.; El Arroussi, H. Microalgae Polysaccharides: The New Sustainable Bioactive Products for the Development of Plant Bio-Stimulants? World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ordog, V.; Novák, O.; Rolčík, J.; Strnad, M.; Balint, P.; van Staden, J. Auxin and Cytokinin Relationships in Twenty-Four Microalgae Strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; van Staden, J. Hormone Profiles in Microalgae: Gibberellins and Brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef]
- Nakano, M.; Omae, N.; Tsuda, K. Inter-Organismal Phytohormone Networks in Plant-Microbe Interactions. Curr. Opin. Plant Biol. 2022, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mutale-joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of Microalgae Liquid Extracts for Their Bio Stimulant Properties on Plant Growth, Nutrient Uptake and Metabolite Profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Manjunath, M.; Kanchan, A.; Ranjan, K.; Venkatachalam, S.; Prasanna, R.; Ramakrishnan, B.; Hossain, F.; Nain, L.; Shivay, Y.S.; Rai, A.B.; et al. Beneficial Cyanobacteria and Eubacteria Synergistically Enhance Bioavailability of Soil Nutrients and Yield of Okra. Heliyon 2016, 2, 1–28. [Google Scholar] [CrossRef]
- Yin, W.; Dong, N.; Li, X.; Yang, Y.; Lu, Z.; Zhou, W.; Qian, Q.; Chu, C.; Tong, H. Understanding Brassinosteroid-Centric Phytohormone Interactions for Crop Improvement. J. Integr. Plant Biol. 2025, 67, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Korra, T.; Singh, U.B.; Singh, S.; Bisen, K. Microalgal Based Biostimulants as Alleviator of Biotic and Abiotic Stresses in Crop Plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–216. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, G.; Kwak, Y.S. Role of Chlorella fusca in Promoting Plant Health through Microbiota Regulation in Strawberry. J. Appl. Microbiol. 2025, 136, lxaf060. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant Biostimulants from Cyanobacteria: An Emerging Strategy to Improve Yields and Sustainability in Agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, M.N.; Shukla, P.S.; Kant, P.; Joshi, J.; Critchley, A.T.; Prithiviraj, B. Physiological and Transcriptomics Analyses Reveal That Ascophyllum nodosum Extracts Induce Salinity Tolerance in Arabidopsis by Regulating the Expression of Stress Responsive Genes. J. Plant Growth Regul. 2019, 38, 463–478. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Nishanth, S.; Kokila, V.; Prasanna, R. Metabolite Profiling of Plant Growth Promoting Cyanobacteria—Anabaena laxa and Calothrix elenkinii, Using Untargeted Metabolomics. 3 Biotech 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Sánchez-Quintero, Á.; Fernandes, S.C.M.; Beigbeder, J.B. Overview of Microalgae and Cyanobacteria-Based Biostimulants Produced from Wastewater and CO2 Streams towards Sustainable Agriculture: A Review. Microbiol. Res. 2023, 277, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Huang, Y.; Guo, M.; Song, J.; Zhang, T.; Long, Y.; Wang, B.; Liu, H. A Potential Biofertilizer—Siderophilic Bacteria Isolated From the Rhizosphere of Paris. polyphylla Var. yunnanensis. Front. Microbiol. 2022, 13, 870413. [Google Scholar] [CrossRef]
- Fiorentino, S.; Bellani, L.; Santin, M.; Castagna, A.; Echeverria, M.C.; Giorgetti, L. Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress. Plants 2025, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Park, Y.J.; Choi, Y.B.; Truong, T.Q.; Huynh, P.K.; Kim, Y.B.; Kim, S.M. Physiological Effects and Mechanisms of Chlorella vulgaris as a Biostimulant on the Growth and Drought Tolerance of Arabidopsis thaliana. Plants 2024, 13, 3012. [Google Scholar] [CrossRef]
- Minaoui, F.; Hakkoum, Z.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biostimulant Effect of Green Soil Microalgae Chlorella vulgaris Suspensions on Germination and Growth of Wheat (Triticum aestivum Var. achtar) and Soil Fertility. Algal Res. 2024, 82, 103655. [Google Scholar] [CrossRef]
- La Bella, E.; Baglieri, A.; Rovetto, E.I.; Stevanato, P.; Puglisi, I. Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy 2021, 11, 308. [Google Scholar] [CrossRef]
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Ko, B.G.; Park, J.H.; Hwang, S.G.; Kim, B.H. Effect of Biostimulator Chlorella fusca on Improving Growth and Qualities of Chinese Chives and Spinach in Organic Farm. Plant Pathol. J. 2018, 34, 567–574. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.A.; Mamoon, A.; Abdelghany, M.F.; Abdel-Hamid, E.A.A.; Abdel-Razek, N.; Ali, F.S.; Shady, S.H.H.; Gewida, A.G.A. Dietary Chlorella vulgaris Modulates the Performance, Antioxidant Capacity, Innate Immunity, and Disease Resistance Capability of Nile Tilapia Fingerlings Fed on Plant-Based Diets. Anim. Feed. Sci. Technol. 2022, 283, 115181. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina Biomass Produced from Treatment of Aquaculture Wastewater as Agricultural Fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of Cyanobacterial Inoculation on the Culturable Microbiome and Growth of Rice. Microbiol. Res. 2015, 171, 78–89. [Google Scholar] [CrossRef]
- Fimbres-Olivarria, D.; Carvajal-Millan, E.; Lopez-Elias, J.A.; Martinez-Robinson, K.G.; Miranda-Baeza, A.; Martinez-Cordova, L.R.; Enriquez-Ocaña, F.; Valdez-Holguin, J.E. Chemical Characterization and Antioxidant Activity of Sulfated Polysaccharides from Navicula sp. Food Hydrocoll. 2018, 75, 229–236. [Google Scholar] [CrossRef]
- Piotrowski, K.; Romanowska-Duda, Z.; Messyasz, B. Cultivation of Energy Crops by Ecological Methods Under the Conditions of Global Climate and Environmental Changes with the Use of Diatom Extract as a Natural Source of Chemical Compounds. Acta Physiol. Plant. 2020, 42, 1–13. [Google Scholar] [CrossRef]
- Kiran Marella, T.; Saxena, A.; Tiwari, A. Diatom Mediated Heavy Metal Remediation: A Review. Bioresour. Technol. 2020, 305, 1–11. [Google Scholar] [CrossRef]
- Elhamji, S.; Haydari, I.; Sbihi, K.; Aziz, K.; Elleuch, J.; Kurniawan, T.A.; Chen, Z.; Yap, P.S.; Aziz, F. Uncovering Applicability of Navicula permitis Algae in Removing Phenolic Compounds: A Promising Solution for Olive Mill Wastewater Treatment. J. Water Process Eng. 2023, 56, 1–9. [Google Scholar] [CrossRef]
- Udaypal, U.; Goswami, R.K.; Verma, P. Strategies for Improvement of Bioactive Compounds Production Using Microalgal Consortia: An Emerging Concept for Current and Future Perspective. Algal Res. 2024, 82, 103664. [Google Scholar] [CrossRef]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic Evidence for Algal Auxin Production in Chlamydomonas and Its Role in Algal-Bacterial Mutualism. iScience 2024, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Yang, Y.; Heredia, V.; Horn, S.J.; Keremane, S.R.; Jin, M.M.; Mayfield, S.P. Optimized Production of a Bioactive Human Recombinant Protein from the Microalgae Chlamydomonas reinhardtii Grown at High Density in a Fed-Batch Bioreactor. Algal Res. 2022, 66, 102786. [Google Scholar] [CrossRef]
- Gavilanes, F.Z.; Souza Andrade, D.; Zucareli, C.; Horácio, E.H.; Sarkis Yunes, J.; Barbosa, A.P.; Alves, L.A.R.; Cruzatty, L.G.; Maddela, N.R.; Guimarães, M. de F. Co-Inoculation of Anabaena cylindrica with Azospirillum brasilense Increases Grain Yield of Maize Hybrids. Rhizosphere 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Llamas, A.; Leon-Miranda, E.; Tejada-Jimenez, M. Microalgal and Nitrogen-Fixing Bacterial Consortia: From Interaction to Biotechnological Potential. Plants 2023, 12, 2476. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Nobbe, F.; Hiltner, L. Inoculation of the Soil for Cultivating Leguminous Plants. U.S. Patent US570813A, 3 November 1896. [Google Scholar]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Sadvakasova, A.K.; Bauenova, M.O.; Kossalbayev, B.D.; Zayadan, B.K.; Huang, Z.; Wang, J.; Balouch, H.; Alharby, H.F.; Chang, J.S.; Allakhverdiev, S.I. Synthetic Algocyanobacterial Consortium as an Alternative to Chemical Fertilizers. Environ. Res. 2023, 233, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological Nitrogen Fixation and Prospects for Ecological Intensification in Cereal-Based Cropping Systems. Field Crops Res. 2022, 283, 1–34. [Google Scholar] [CrossRef]
- Álvarez, C.; Jimenez-Ríos, L.; Iniesta-Pallares, M.; Jurado-Flores, A.; Molina-Heredia, F.P.; Ng, C.K.Y.; Mariscal, V. Symbiosis between Cyanobacteria and Plants: From Molecular Studies to Agronomic Applications. J. Exp. Bot. 2023, 74, 6145–6157. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Y.; Han, X.; Li, K.; Zhao, M.; Guo, L.; Li, S.; Wang, K.; Qin, K.; Duan, J.; et al. Microalgae-Based Biofertilizer Improves Fruit Yield and Controls Greenhouse Gas Emissions in a Hawthorn Orchard. PLoS ONE 2024, 19, e0307774. [Google Scholar] [CrossRef]
- Fujita, Y.; Uesaka, K. Nitrogen Fixation in Cyanobacteria. In Cyanobacterial Physiology: From Fundamentals to Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–45. ISBN 9780323961066. [Google Scholar]
- Neilson, A.; Rippka, I.; Kunisawa, R. Heterocyst Formation and Nitrogenase Synthesis in Anabaena sp. A Kinetic Study; Springer-Verlag: Berlin/Heidelberg, Germany, 1971; Volume 76. [Google Scholar]
- Bibi, S.; Saadaoui, I.; Bibi, A.; Al-Ghouti, M.; Abu-Dieyeh, M.H. Applications, Advancements, and Challenges of Cyanobacteria-Based Biofertilizers for Sustainable Agro and Ecosystems in Arid Climates. Bioresour. Technol. Rep. 2024, 25, 1–18. [Google Scholar] [CrossRef]
- Iniesta-Pallarés, M.; Álvarez, C.; Gordillo-Cantón, F.M.; Ramírez-Moncayo, C.; Alves-Martínez, P.; Molina-Heredia, F.P.; Mariscal, V. Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria. Appl. Sci. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Ghotbi-Ravandi, A.A.; Shariatmadari, Z.; Riahi, H.; Hassani, S.B.; Heidari, F.; Nohooji, M.G. Enhancement of Essential Oil Production and Expression of Some Menthol Biosynthesis-Related Genes in Mentha piperita Using Cyanobacteria. Iran. J. Biotechnol. 2023, 21, 96–107. [Google Scholar] [CrossRef]
- Jiménez-Ríos, L.; Torrado, A.; González-Pimentel, J.L.; Iniesta-Pallarés, M.; Molina-Heredia, F.P.; Mariscal, V.; Álvarez, C. Emerging Nitrogen-Fixing Cyanobacteria for Sustainable Cotton Cultivation. Sci. Total Environ. 2024, 924, 1–13. [Google Scholar] [CrossRef]
- Alvarenga, P.; Martins, M.; Ribeiro, H.; Mota, M.; Guerra, I.; Cardoso, H.; Silva, J.L. Evaluation of the Fertilizer Potential of Chlorella vulgaris and Scenedesmus obliquus Grown in Agricultural Drainage Water from Maize Fields. Sci. Total Environ. 2023, 861, 1–10. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular Economy Fertilization: Testing Micro and Macro Algal Species as Soil Improvers and Nutrient Sources for Crop Production in Greenhouse and Field Conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Kollmen, J.; Strieth, D. The Beneficial Effects of Cyanobacterial Co-Culture on Plant Growth. Life 2022, 12, 223. [Google Scholar] [CrossRef]
- Hussain, A.; Shah, S.T.; Rahman, H.; Irshad, M.; Iqbal, A. Effect of IAA on in Vitro Growth and Colonization of Nostoc in Plant Roots. Front. Plant Sci. 2015, 6, 46. [Google Scholar] [CrossRef]
- Toribio, A.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Moreno, J. Prospection of Cyanobacteria Producing Bioactive Substances and Their Application as Potential Phytostimulating Agents. Biotechnol. Rep. 2020, 26, e00449. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Nakano, T. Reconstitution of a Blasia-Nostoc Symbiotic Association Under Axenic Conditions. Nova Hedwig. 1990, 50, 191–200. [Google Scholar] [CrossRef]
- Eily, A.N.; Pryer, K.M.; Li, F.W. A First Glimpse at Genes Important to the Azolla–Nostoc Symbiosis. Symbiosis 2019, 78, 149–162. [Google Scholar] [CrossRef]
- Alvarez, C.; Navarro, J.A.; Molina-Heredia, F.P.; Mariscal, V. Endophytic Colonization of Rice (Oryza sativa L.) by the Symbiotic Strain Nostoc punctiforme PCC 73102. Mol. Plant Microbe Interact. 2020, 33, 1040–1045. [Google Scholar] [CrossRef]
- García-Encinas, J.P.; Ruiz-Cruz, S.; Juárez, J.; Ornelas-Paz, J.D.J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E. Proteins from Microalgae: Nutritional, Functional and Bioactive Properties. Foods 2025, 14, 921. [Google Scholar] [CrossRef]
- Youssef, S.M.; El-Serafy, R.S.; Ghanem, K.Z.; Elhakem, A.; Abdel Aal, A.A. Foliar Spray or Soil Drench: Microalgae Application Impacts on Soil Microbiology, Morpho-Physiological and Biochemical Responses, Oil and Fatty Acid Profiles of Chia Plants Under Alkaline Stress. Biology 2022, 11, 1844. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Hassan, A.; Barakat, K.M.; Ghonam, H.E.B. Improvement of Growth and Biochemical Constituents of Rosmarinus officinalis by Fermented Spirulina maxima Biofertilizer. Plant Physiol. Biochem. 2024, 208, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Gureev, A.P.; Kryukova, V.A.; Eremina, A.A.; Alimova, A.A.; Kirillova, M.S.; Filatova, O.A.; Moskvitina, M.I.; Kozin, S.V.; Lyasota, O.M.; Gureeva, M.V. Plant-Growth Promoting Rhizobacteria Azospirillum Partially Alleviate Pesticide-Induced Growth Retardation and Oxidative Stress in Wheat (Triticum aestivum L.). Plant Growth Regul. 2024, 104, 503–521. [Google Scholar] [CrossRef]
- Gupta, R.; Kumari, A.; Sharma, S.; Alzahrani, O.M.; Noureldeen, A.; Darwish, H. Identification, Characterization and Optimization of Phosphate Solubilizing Rhizobacteria (PSRB) from Rice Rhizosphere. Saudi J. Biol. Sci. 2022, 29, 35–42. [Google Scholar] [CrossRef]
- Sarikhani, M.R.; Khoshru, B.; Greiner, R. Isolation and Identification of Temperature Tolerant Phosphate Solubilizing Bacteria as a Potential Microbial Fertilizer. World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Singh, S.; Benjamin, J.C.; Singh, V.R.; Bisht, D.; Lal, R.K. Plant Growth-Promoting Activities of Serratia marcescens and Pseudomonas fluorescens on Capsicum annuum L. Plants. Ecol. Front. 2024, 44, 654–663. [Google Scholar] [CrossRef]
- Tariq, M.; Tahreem, N.; Zafar, M.; Raza, G.; Shahid, M.; Zunair, M.; Iram, W.; Zahra, S.T. Occurrence of Diverse Plant Growth Promoting Bacteria in Soybean [Glycine max (L.) Merrill] Root Nodules and Their Prospective Role in Enhancing Crop Yield. Biocatal. Agric. Biotechnol. 2024, 57, 1–13. [Google Scholar] [CrossRef]
- Ibrahim, E.; Ahmad, A.A.; Abdo, E.S.; Bakr, M.A.; Khalil, M.A.; Abdallah, Y.; Ogunyemi, S.O.; Mohany, M.; Al-Rejaie, S.S.; Shou, L.; et al. Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles. Nanomaterials 2024, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, H.; Bai, X.; Jiang, B. The Peanut Root Exudate Increases the Transport and Metabolism of Nutrients and Enhances the Plant Growth-Promoting Effects of Burkholderia pyrrocinia Strain P10. BMC Microbiol. 2023, 23, 85. [Google Scholar] [CrossRef]
- Chowaniec, K.; Zubek, S.; Skubała, K. Exopolysaccharides in Biological Soil Crusts Are Important Contributors to Carbon and Nutrient Storage After the Restoration of Inland Sand Dunes. Plant Soil 2025, 1–14. [Google Scholar] [CrossRef]
- Hussain Shah, A.; Naz, I.; Ahmad, H.; Nasreen Khokhar, S.; Khan, K. Impact of Zinc Solubilizing Bacteria on Zinc Contents of Wheat. J. Agric. Environ. Sci. 2016, 16, 449–454. [Google Scholar]
- Sultan, A.A.Y.A.; Gebreel, H.M.; Youssef, H.A.I.A.E. Biofertilizer Effect of Some Zinc Dissolving Bacteria Free and Encapsulated on Zea mays Growth. Arch. Microbiol. 2023, 205, 1–13. [Google Scholar] [CrossRef]
- Hassler, C.S.; Behra, R.; Wilkinson, K.J. Impact of Zinc Acclimation on Bioaccumulation and Homeostasis in Chlorella kesslerii. Aquat. Toxicol. 2005, 74, 139–149. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Ji, Z.; Sun, J.; Liu, X. Distinct Responses of Chlorella vulgaris upon Combined Exposure to Microplastics and Bivalent Zinc. J. Hazard. Mater. 2023, 442, 1–13. [Google Scholar] [CrossRef]
- Wan Maznah, W.O.; Al-Fawwaz, A.T.; Surif, M. Biosorption of Copper and Zinc by Immobilised and Free Algal Biomass, and the Effects of Metal Biosorption on the Growth and Cellular Structure of Chlorella sp. and Chlamydomonas sp. Isolated from Rivers in Penang, Malaysia. J. Environ. Sci. 2012, 24, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron Nutrition, Biomass Production, and Plant Product Quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sindhu, S.S. Iron Sensing, Signalling and Acquisition by Microbes and Plants Under Environmental Stress: Use of Iron-Solubilizing Bacteria in Crop Biofortification for Sustainable Agriculture. Plant Sci. 2025, 356, 1–26. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Ghazanfar, S.; Hussain, A.; Dar, A.; Ahmad, M.; Anwar, H.; Al Farraj, D.A.; Rizwan, M.; Iqbal, R. Prospects of Iron Solubilizing Bacillus Species for Improving Growth and Iron in Maize (Zea mays L.) Under Axenic Conditions. Sci. Rep. 2024, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brick, M.B.; Hussein, M.H.; Mowafy, A.M.; Hamouda, R.A.; Ayyad, A.M.; Refaay, D.A. Significance of Siderophore-Producing Cyanobacteria on Enhancing Iron Uptake Potentiality of Maize Plants Grown Under Iron-Deficiency. Microb. Cell Fact. 2025, 24, 1–18. [Google Scholar] [CrossRef]
- Davidi, L.; Gallaher, S.D.; Ben-David, E.; Purvine, S.O.; Fillmore, T.L.; Nicora, C.D.; Craig, R.J.; Schmollinger, S.; Roje, S.; Blaby-Haas, C.E.; et al. Pumping Iron: A Multi-Omics Analysis of Two Extremophilic Algae Reveals Iron Economy Management. Proc. Natl. Acad. Sci. USA 2023, 120, e2305495120. [Google Scholar] [CrossRef]
- El Arroussi, H.; Elbaouchi, A.; Benhima, R.; Bendaou, N.; Smouni, A.; Wahby, I. Halophilic Microalgae Dunaliella salina Extracts Improve Seed Germination and Seedling Growth of Triticum aestivum L. Under Salt Stress. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science, Florence, Italy, 18 November 2016; Volume 1148, pp. 13–26. [Google Scholar]
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the Potential of Cytokinins for Biomass and Lipid Enhancement in Microalga Acutodesmus obliquus Under Nitrogen Stress. Energy Convers. Manag. 2017, 140, 14–23. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.P.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Xu, H.; Tang, Z.; Yang, D.; Dai, X.; Chen, H. Enhanced Growth and Auto-Flocculation of Scenedesmus quadricauda in Anaerobic Digestate Using High Light Intensity and Nanosilica: A Biomineralization-Inspired Strategy. Water Res. 2023, 235, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ren, Y.; Wang, G.; Li, J.; Zhang, H.; Yang, Y.; Pang, X.; Han, J. Microalgae and Microbial Inoculant as Partial Substitutes for Chemical Fertilizer Enhance Polygala tenuifolia Yield and Quality by Improving Soil Microorganisms. Front. Plant Sci. 2024, 15, 1499966. [Google Scholar] [CrossRef]
- Iovinella, M.; Palmieri, M.; Papa, S.; Auciello, C.; Ventura, R.; Lombardo, F.; Race, M.; Lubritto, C.; di Cicco, M.R.; Davis, S.J.; et al. Biosorption of Rare Earth Elements from Luminophores by G. sulphuraria (Cyanidiophytina, Rhodophyta). Environ. Res. 2023, 239, 117281. [Google Scholar] [CrossRef]
- Shahbaz, A.; Hussain, N.; Saba, S.; Bilal, M. Actinomycetes, Cyanobacteria, and Fungi: A Rich Source of Bioactive Molecules. In Microbial Biomolecules: Emerging Approach in Agriculture, Pharmaceuticals and Environment Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 113–133. ISBN 9780323994767. [Google Scholar]
- Asthana, R.K.; Srivastava, A.; Singh, A.P.; Deepali; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Identification of an Antimicrobial Entity from the Cyanobacterium Fischerella sp. Isolated from Bark of Azadirachta indica (Neem) Tree. J. Appl. Phycol. 2006, 18, 33–39. [Google Scholar] [CrossRef]
- Kajiyama, S.; Kanzakt, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an Antifungal Lipopeptide from the Field-Grown Terrestrial Blue-Green Alga Nostoc commune; Elsevier: Amsterdam, The Netherlands, 1998; Volume 39. [Google Scholar]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A Fatty Acid from the Diatom Phaeodactylum tricornutum Is Antibacterial against Diverse Bacteria Including Multi-Resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Ouaddi, O.; Oukarroum, A.; Bouharroud, R.; Alouani, M.; EL blidi, A.; Qessaoui, R.; Radouane, N.; Errafii, K.; Hijri, M.; Hamadi, F.; et al. A Study of Antibacterial Efficacy of Tetraselmis rubens Extracts against Tomato Phytopathogenic Pseudomonas corrugata. Algal Res. 2025, 86, 1–11. [Google Scholar] [CrossRef]
- Viana, C.; Genevace, M.; Gama, F.; Coelho, L.; Pereira, H.; Varela, J.; Reis, M. Chlorella vulgaris and Tetradesmus obliquus Protect Spinach (Spinacia oleracea L.) against Fusarium oxysporum. Plants 2024, 13, 1697. [Google Scholar] [CrossRef]
- Sandor, R.; Wagh, S.G.; Kelterborn, S.; Großkinsky, D.K.; Novak, O.; Olsen, N.; Paul, B.; Petřík, I.; Wu, S.; Hegemann, P.; et al. Cytokinin-Deficient Chlamydomonas reinhardtii CRISPR-Cas9 Mutants Show Reduced Ability to Prime Resistance of Tobacco against Bacterial Infection. Physiol. Plant 2024, 176, 1–16. [Google Scholar] [CrossRef]
- Schmid, B.; Coelho, L.; Schulze, P.S.C.; Pereira, H.; Santos, T.; Maia, I.B.; Reis, M.; Varela, J. Antifungal Properties of Aqueous Microalgal Extracts. Bioresour. Technol. Rep. 2022, 18, 1–8. [Google Scholar] [CrossRef]
- Hamouda, R.; Hamouda, R.A.; El-Ansary, M.S.M. Potential of Plant-Parasitic Nematode Control in Banana Plants by Microalgae as a New Approach Towards Resistance. Egypt. J. Biol. Pest Contro. 2017, 27, 165–172. [Google Scholar]
- Bang, K.H.; Lee, D.W.; Park, H.M.; Rhee, Y.H. Inhibition of Fungal Cell Wall Synthesizing Enzymes by Trans-Cinnamaldehyde. Biosci. Biotechnol. Biochem. 2000, 64, 1061–1063. [Google Scholar] [CrossRef]
- Berry, J. Marine and Freshwater Microalgae as a Potential Source of Novel Herbicides. In Herbicides and Environment; InTech: Toulon, France, 2011. [Google Scholar][Green Version]
- Berry, J.P. Cyanobacterial Toxins as Allelochemicals with Potential Applications as Algaecides, Herbicides and Insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef]
- Koodkaew, I.; Sunohara, Y.; Matsuyama, S.; Matsumoto, H. Isolation of Ambiguine D Isonitrile from Hapalosiphon sp. and Characterization of Its Phytotoxic Activity. Plant Growth Regul. 2012, 68, 141–150. [Google Scholar] [CrossRef]
- Doan, N.T.; Rickards, R.W.; Rothschild, J.M.; Smith, G.D. Allelopathic Actions of the Alkaloid 12-Epi-Hapalindole E Isonitrile and Calothrixin A from Cyanobacteria of the Genera Fischerella and Calothrix. J. Appl. Phycol. 2000, 12, 409–416. [Google Scholar] [CrossRef]
- Singh, L.; Nayak, A.; Behera, M.; Sethi, G.; Alheswairini, S.S.; Barasarathi, J.; Sayyed, R.; Behera, D.K.; Adarsh, V. Antifungal Potential of Cyanobacterium Desertifilum dzianense against Collar Rot Pathogen in Chickpea. Sci. Rep. 2025, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and Derivative Brevetoxins: Historical Background, Multiplicity, and Effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef]
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some Chemical Properties of Maitotoxin, a Putative Calcium Channel Agonist Isolated from a Marine Dinoflagellate. J. Biochem. 1988, 104, 184–187. [Google Scholar] [CrossRef]
- DellaGreca, M.; Zarrelli, A.; Fergola, P.; Cerasuolo, M.; Pollio, A.; Pinto, G. Fatty Acids Released by Chlorella vulgaris and Their Role in Interference with Pseudokirchneriella subcapitata: Experiments and Modelling. J. Chem. Ecol. 2010, 36, 339–349. [Google Scholar] [CrossRef]
- Chiang, I.Z.; Huang, W.Y.; Wu, J.T. Allelochemicals of Botryococcus braunii (Chlorophyceae). J. Phycol. 2004, 40, 474–480. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The Cyanotoxin-Microcystins: Current Overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Toxic Cyanobacteria and Cyanotoxins in Public Hot Springs in Saudi Arabia. Toxicon 2008, 51, 17–27. [Google Scholar] [CrossRef]
- Christiansen, G.; Yoshida, W.Y.; Blom, J.F.; Portmann, C.; Gademann, K.; Hemscheidt, T.; Kurmayer, R. Isolation and Structure Determination of Two Microcystins and Sequence Comparison of the McyABC Adenylation Domains in Planktothrix Species. J. Nat. Prod. 2008, 71, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Hodoki, Y.; Ohbayashi, K.; Kobayashi, Y.; Okuda, N.; Nakano, S. ichi Detection and Identification of Potentially Toxic Cyanobacteria: Ubiquitous Distribution of Microcystis aeruginosa and Cuspidothrix issatschenkoi in Japanese Lakes. Harmful Algae 2012, 16, 49–57. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel Extracellular Diterpenoids with Biological Activity from the Cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef]
- Casanova, L.M.; Macrae, A.; de Souza, J.E.; Neves Junior, A.; Vermelho, A.B. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants 2023, 12, 1896. [Google Scholar] [CrossRef]
- Rankic, I.; Zelinka, R.; Ridoskova, A.; Gagic, M.; Pelcova, P.; Huska, D. Nano/Microparticles in Conjunction with Microalgae Extract as Novel Insecticides against Mealworm Beetles, Tenebrio molitor. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Hassan, M.E.; Mohafrash, S.M.M.; Fallatah, S.A.; El-Sayed, A.E.K.B.; Mossa, A.T.H. Eco-Friendly Larvicide of Amphora coffeaeformis and Scenedesmus obliquus Microalgae Extracts against Culex pipiens. J. Appl. Phycol. 2021, 33, 2683–2693. [Google Scholar] [CrossRef]
- Becher, P.G.; Jüttner, F. Insecticidal Compounds of the Biofilm-Forming Cyanobacterium Fischerella sp. (ATCC 43239). Environ. Toxicol. 2005, 20, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Keller, S.; Jung, G.; Süssmuth, R.D.; Jüttner, F. Insecticidal Activity of 12-Epi-Hapalindole J Isonitrile. Phytochemistry 2007, 68, 2493–2497. [Google Scholar] [CrossRef]
- Becher, P.G.; Jüttner, F. Insecticidal Activity—A New Bioactive Property of the Cyanobacterium Fischerella. Pol. J. Ecol. 2006, 54, 653–662. [Google Scholar]
- Höckelmann, C.; Becher, P.G.; Von Reuß, S.H.; Jüttner, F. Sesquiterpenes of the Geosmin-Producing Cyanobacterium Calothrix PCC 7507 and Their Toxicity to Invertebrates. Z. Naturforsch. Sect. C-A J. Biosci. 2009, 64, 49–55. [Google Scholar] [CrossRef]
- Delaney, J.M.; Wilkins, R.M. Toxicity of microcystin-LR, isolated from Microcystis aeruginosa, against various insect species. Toxicon 1995, 33, 771–778. [Google Scholar] [CrossRef]
- Aguilar-Marcelino, L.; Pineda-Alegría, J.A.; Salinas-Sánchez, D.O.; Hernández-Velázquez, V.M.; Silva-Aguayo, G.I.; Navarro-Tito, N.; Sotelo-Leyva, C. In Vitro Insecticidal Effect of Commercial Fatty Acids, β-Sitosterol, and Rutin against the Sugarcane Aphid, Melanaphis sacchari Zehntner (Hemiptera: Aphididae). J. Food Prot. 2022, 85, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Jang, M.J.; Kim, J.R.; Kadarkarai, M.; Ahn, Y.J. Larvicidal Activity and Possible Mode of Action of Four Flavonoids and Two Fatty Acids Identified in Millettia pinnata Seed toward Three Mosquito Species. Parasit. Vectors 2015, 8, 1–14. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Effect of Commercial Cyanobacteria Products on the Growth and Antagonistic Ability of Some Bioagents Under Laboratory Conditions. J. Pathog. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.L.; Moreira, Í.T.A. Microalgae Biomass Production from Cultivation in Availability and Limitation of Nutrients: The Technical, Environmental and Economic Performance. J. Clean. Prod. 2022, 370, 133538. [Google Scholar] [CrossRef]

| Biocontrol Mechanisms | Microalgae/Cyanobacteria | Produced Molecules | Mode of Action | Target Pathogens/Organisms | References |
|---|---|---|---|---|---|
| Phytopathogen resistance | Fischerella sp. | Hapalindole T (alkaloid) | Antibacterial activity | Phytopathogenic bacteria | [135,136,137,138] |
| Nostoc commune | Nostofungicin (lipopeptide) | Fungicidal | Phytopathogenic fungi | [135,136,137,138] | |
| Phaeodactylum tricornutum | Eicosapentaenoic acid (EPA, fatty acid) | Antimicrobial | Various pathogens | [135,136,137,138] | |
| Chlorella vulgaris, Tetradesmus obliquus | Cell extracts (phenolics, peptides, not fully identified) | Inhibition of fungal growth | Fusarium oxysporum (spinach) | [139] | |
| Chlamydomonas reinhardtii | Genetic modification | Enhanced bacterial resistance via gene editing | Tobacco plants | [140] | |
| Anabaena HSSASE11 | Phenolic/peptide extracts | Antifungal | Botryodiplodia theobromae | [141] | |
| Oscillatoria nigroviridis HSSASE15 | Phenolic/peptide extracts | Antifungal | Pythium ultimum | [141] | |
| Dunaliella HSSASE13 | Phenolic/peptide extracts | Antifungal | Fusarium solani | [141] | |
| Scenedesmus obliquus | Phenolic extracts | Antifungal | Sclerotium rolfsii | [141] | |
| Scenedesmus obliquus, Chlorella vulgaris, Anabaena oryzae | Phenolics, alkaloids, peptides | Nematicidal; possible inhibition of fungal cell wall biosynthesis | Meloidogyne incognita (banana pathogen) | [142] | |
| Herbicides | Scytonema hofmanni | Cyanobacterin (phenolic) | Inhibition of photosynthetic electron transport | Weeds (phytotoxic effect) | [144,145] |
| Nostoc sp. | Nostocyclamide (peptide) | Inhibition of photosystem II | Weeds | [144,145] | |
| Fischerella sp. | Fischerellins (alkaloids) | Inhibition of photosystem II | Weeds | [144,145] | |
| Microcystis sp. | Microcystins (peptides) | Inhibition of protein phosphatases | Weeds | [144,146] | |
| Various cyanobacteria | Cryptophycins (polyketides) | Blockage of microtubule polymerization | Weeds | [144,146] | |
| Insecticides | Chlamydomonas reinhardtii + ZnO | Cell extracts | Enhanced larvicidal effect when combined with ZnO | Tenebrio molitor | [159] |
| Amphora coffeaeformis, Scenedesmus obliquus | Cell extracts (fatty acids) | Larvicidal activity | Culex pipiens | [160] | |
| Fischerella ATCC 43239 (biofilm) | Biofilm-induced allelochemicals | Increased larval mortality | Chironomus riparius | [161,162,163,164] | |
| Microcystis aeruginosa 205, Anabaena circinalis 86 | Biomass extracts | Toxic | Aedes aegypti | [165] | |
| Various microalgae | Unsaturated fatty acids (linolenic, linoleic acids) | Disruption of octopamine signaling (dual mechanism) | Insect larvae (various species) | [166,167] |
| Product Name | Manufacturer | Microalgae Used | Formulation | Main Effects | URL or Reference |
|---|---|---|---|---|---|
| Algafert | Biorizon Biotech | Spirulina spp. | Dry powder | Provides macro and micronutrients, promotes chlorophyll synthesis. | https://www.biorizon.es/en/products/biostimulants-y-bioenhancers/algafert/ (accessed on 11 August 2025) |
| AgriAlgae | AlgaEnergy | Nannochloropsis spp. | Liquid biostimulant | Enhances photosynthesis, nutrient uptake, and crop vigor. | https://ag.algaenergy.com/es/product-category/agrialgae-premium/?lang=it (accessed on 11 August 2025) |
| Allfertis | Allmicroalgae | Chlorella spp. | Powder | Promote resistance to biotic and abiotic agents; Increases the size and fruit weight. | https://www.allmicroalgae.com/en/agro/#Allfertis (accessed on 11 August 2025) |
| Biofertilizer by MicroAlgaex | Microalgaex | Not specified—described as “microalgae formulations” | Liquid | Enhanced plant growth and yield, improved defense against abiotic stress, and increased nutrient absorption. | https://microalgaex.com/biofertilizer/ (accessed on 11 August 2025) |
| Ecotop | Herogra | Ascophyllum nodosum and blend of other microalgae | Liquid biostimulant | Enhances plant vigor, growth, and resilience to abiotic/biotic stress. | https://herograespeciales.com/en/productos/bioestimulantes/ecotop/ (accessed on 11 August 2025) |
| Kelpak | Kelpak | Ecklonia maxima (macroalga, used in synergy with microalgae) | Liquid biostimulant | Promotes root and shoot development, stress tolerance. | https://www.kelpak.com/kelpakintro.html (accessed on 11 August 2025) |
| AlgaGrow | Plagron | Proprietary blend including cyanobacteria | Liquid biostimulant | Increases nutrient uptake and crop yield. | https://plagron.com/en/hobby/products/alga-grow (accessed on 11 August 2025) |
| Seasol | Seasol International (Australia) | Blend of seaweed and microalgae extracts | Liquid concentrate | Broad-spectrum plant tonic. | https://www.seasol.com.au/products/seasol/ (accessed on 11 August 2025) |
| Weed-Max | Trade S.A.E. Company (Egypt) | Cyanobacteria extract in powder phase | Dry powder | Suppress soil-borne fungi and enhance the antagonistic abilities of other bioagents. | [168] |
| Oligo-X algal | Arabian Group for Agricultural Service | Blue-green algal extracts in liquid phase | Liquid concentrate | Suppress soil-borne fungi. | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado-Flores, A.; Heredia-Martínez, L.G.; Torres-Cortes, G.; Díaz-Santos, E. Harnessing Microalgae and Cyanobacteria for Sustainable Agriculture: Mechanistic Insights and Applications as Biostimulants, Biofertilizers and Biocontrol Agents. Agriculture 2025, 15, 1842. https://doi.org/10.3390/agriculture15171842
Jurado-Flores A, Heredia-Martínez LG, Torres-Cortes G, Díaz-Santos E. Harnessing Microalgae and Cyanobacteria for Sustainable Agriculture: Mechanistic Insights and Applications as Biostimulants, Biofertilizers and Biocontrol Agents. Agriculture. 2025; 15(17):1842. https://doi.org/10.3390/agriculture15171842
Chicago/Turabian StyleJurado-Flores, Ana, Luis G. Heredia-Martínez, Gloria Torres-Cortes, and Encarnación Díaz-Santos. 2025. "Harnessing Microalgae and Cyanobacteria for Sustainable Agriculture: Mechanistic Insights and Applications as Biostimulants, Biofertilizers and Biocontrol Agents" Agriculture 15, no. 17: 1842. https://doi.org/10.3390/agriculture15171842
APA StyleJurado-Flores, A., Heredia-Martínez, L. G., Torres-Cortes, G., & Díaz-Santos, E. (2025). Harnessing Microalgae and Cyanobacteria for Sustainable Agriculture: Mechanistic Insights and Applications as Biostimulants, Biofertilizers and Biocontrol Agents. Agriculture, 15(17), 1842. https://doi.org/10.3390/agriculture15171842








