A Strategic Breeding Approach for Improvement of a Native Greek Chamomile (Matricaria chamomilla L.) Population for High-Yield and Optimized Chemical Profile Under Mediterranean Low-Input Conditions
Abstract
1. Introduction
2. Materials and Methods
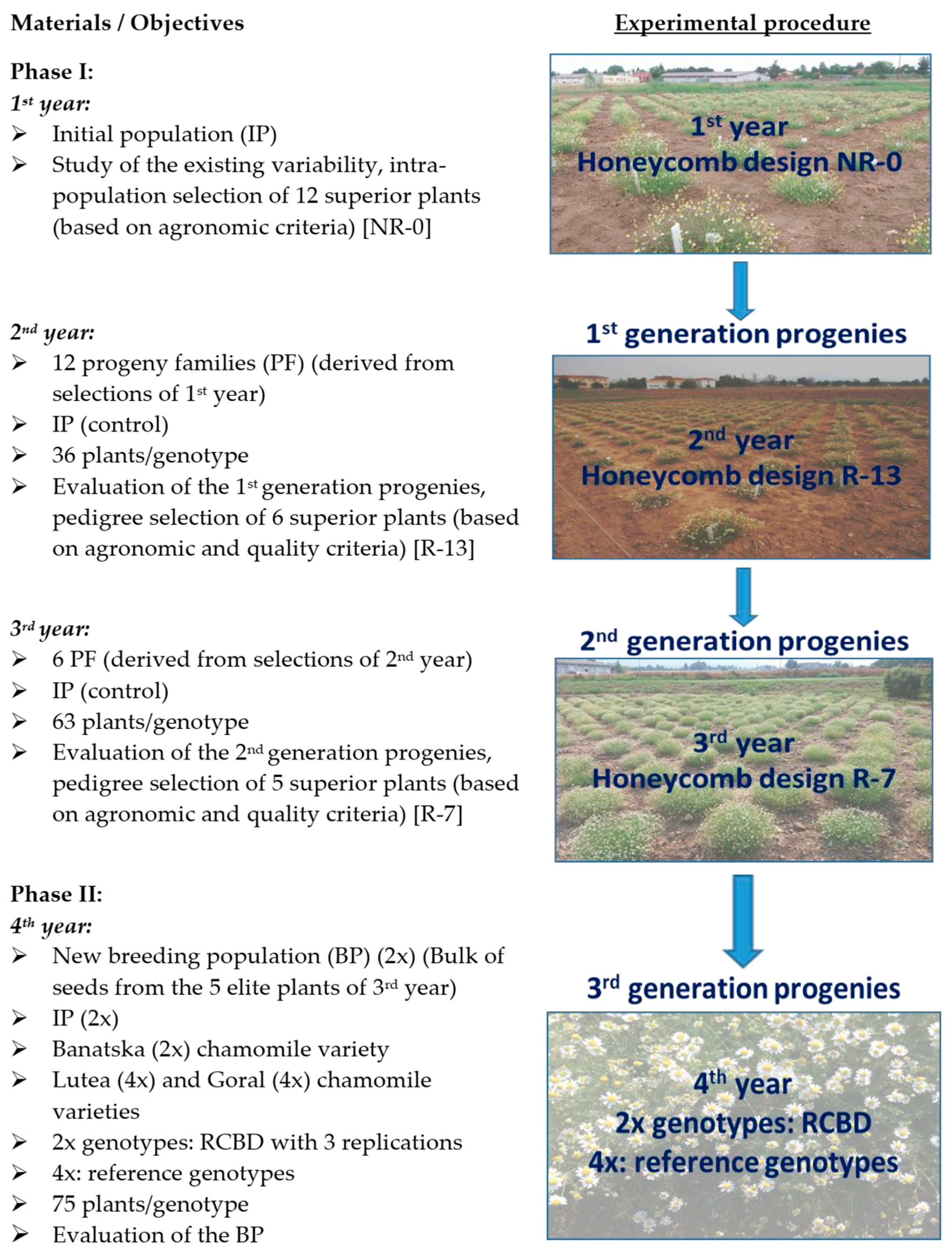
2.1. Plant Material and Experimental Procedure
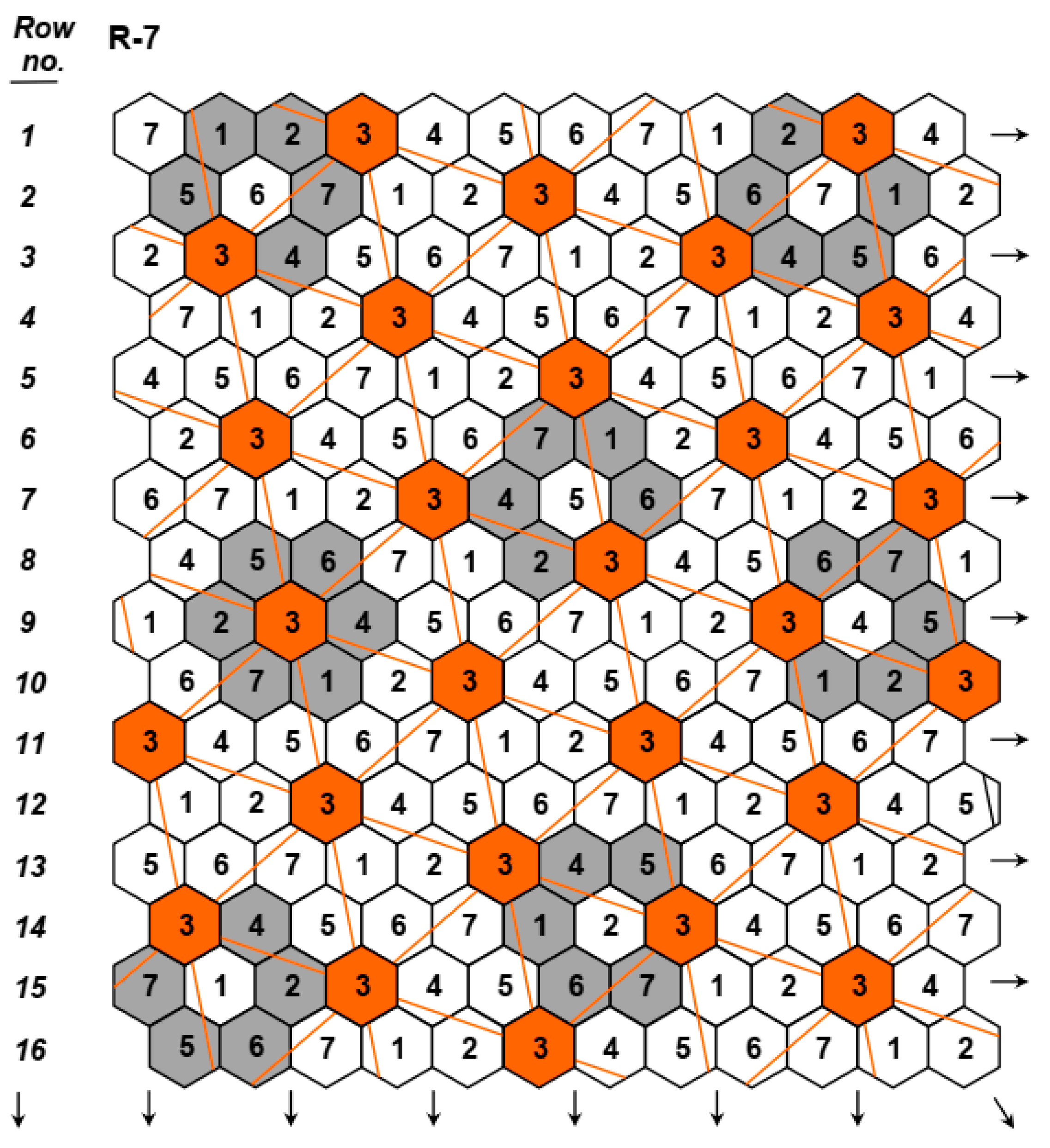
2.1.1. First Phase of Breeding Program (Phase I)
2.1.2. Second Phase of Breeding Program (Phase II)
2.2. Environmental Conditions and Cultivation Treatments
2.3. Agronomic and Morphological Characteristics
2.4. Essential Oil Isolation and Analysis
2.5. Statistical Analysis
3. Results
3.1. First Phase of Breeding Program (Phase I)
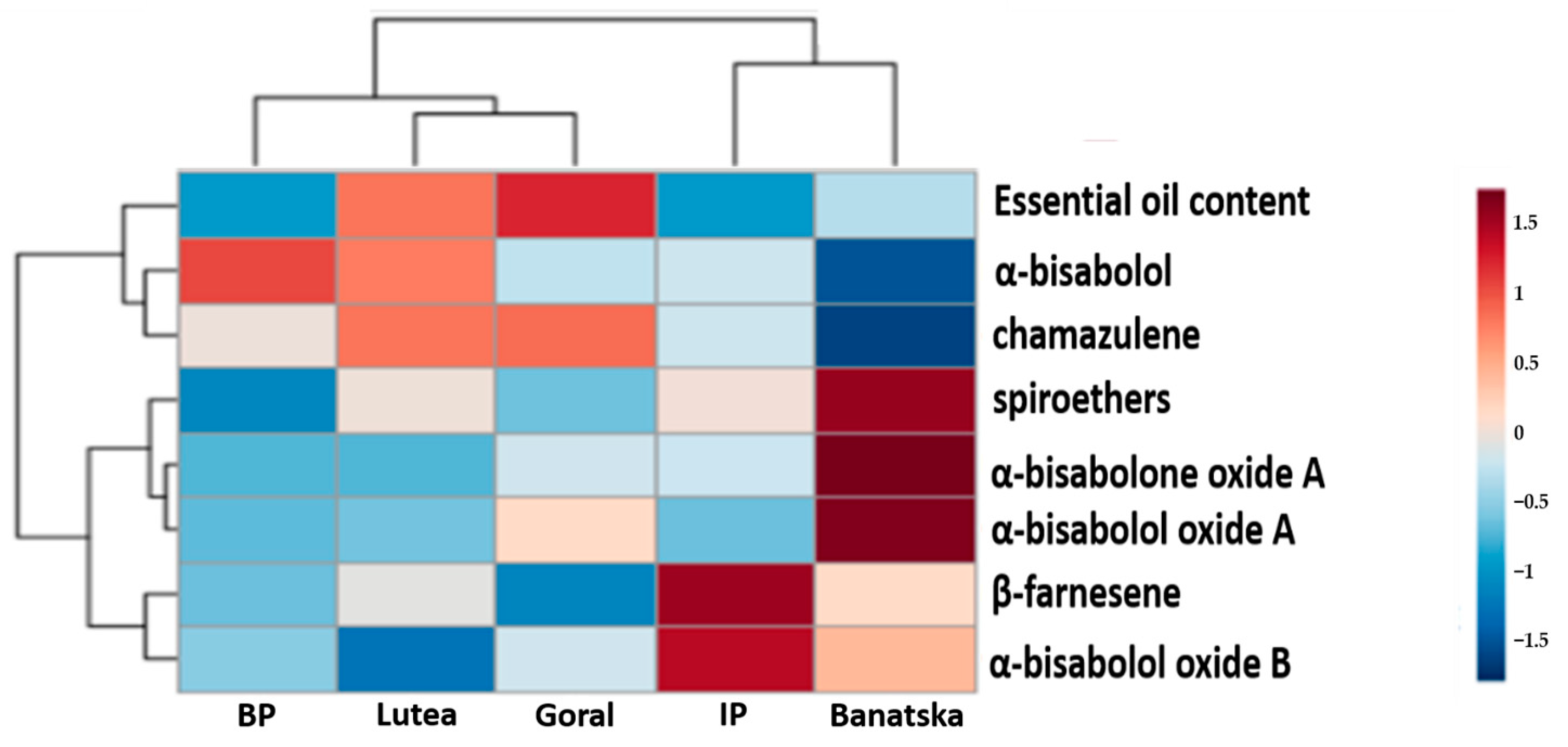
3.2. Second Phase of Breeding Program (Phase II)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuca, H.; Karakaya, S. Matricaria chamomilla L. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence; Gürağaç Dereli, F.T., Ilhan, M., Belwal, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 387–400. ISBN 978-3-031-07753-1. [Google Scholar]
- Parveen, A.; Perveen, S.; Naz, F.; Ahmad, M.; Khalid, M. Chamomile. In Essentials of Medicinal and Aromatic Crops; Zia-Ul-Haq, M., Abdulkreem AL-Huqail, A., Riaz, M., Farooq Gohar, U., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1009–1040. ISBN 978-3-031-35403-8. [Google Scholar]
- Salamon, I.; Ghanavati, M.; Khazaei, H. Chamomile Biodiversity and Essential Oil Qualitative-Quantitative characteristics in Egyptian Production and Iranian Landraces. Emir. J. Food Agric. 2010, 22, 59. [Google Scholar] [CrossRef]
- Das, M. Chamomile: Medicinal, Biochemical, and Agricultural Aspects; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-04-016160-9. [Google Scholar]
- Al-Snafi, A.E.; Hasham, L.F. Bioactive Constituents and Pharmacological Importance of Matricaria chamomilla: A Recent Review. GSC Biol. Pharm. Sci. 2023, 22, 79–98. [Google Scholar] [CrossRef]
- Schilcher, H. Die Kamille: Handbuch für Ärzte, Apotheker und Andere Naturwissenschaftler; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1987. [Google Scholar]
- Wagner, C.; Friedt, W.; Marquard, R.A.; Ordon, F. Molecular Analyses on the Genetic Diversity and Inheritance of (−)-α-Bisabolol and Chamazulene Content in Tetraploid Chamomile (Chamomilla recutita (L.) Rausch.). Plant Sci. 2005, 169, 917–927. [Google Scholar] [CrossRef]
- Wu, H.; Yang, K.; Dong, L.; Ye, J.; Xu, F. Classification, Distribution, Biosynthesis, and Regulation of Secondary Metabolites in Matricaria chamomilla. Horticulturae 2022, 8, 1135. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An Overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-L.; Li, Y.; Wang, Q.; Niu, F.-J.; Li, K.-W.; Wang, Y.-Y.; Wang, J.; Zhou, C.-Z.; Gao, L.-N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2023, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Khazaneha, M.; Zandrahimi, F.; Sadatmoosavi, A.; Salarpour, S.; Karegar-Borzi, H.; Tajedini, O.; Arvan, H.; Raeiszadeh, M.; Raisszadeh, A. An Overview of Scientific Publication of the Chamomile (Matricaria chamomilla) Research: A Bibliometric Analysis. J. Scientometr. Res. 2024, 13, 604–614. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, S.; Kumar, V.; Kumar, A.; Kumari, A.; Rathore, S.; Kumar, R.; Singh, S. A Comprehensive Review on Biology, Genetic Improvement, Agro and Process Technology of German Chamomile (Matricaria chamomilla L.). Plants 2022, 11, 29. [Google Scholar] [CrossRef]
- Liaqat, W.; Barutçular, C.; Farooq, M.; Ahmad, H.; Jan, M.; Ahmad, Z.; Nawaz, H.; Li, M. Climate Change in Relation to Agriculture: A Review. Span. J. Agric. Res. 2022, 20, e03R01. [Google Scholar] [CrossRef]
- Franke, R.; Schilcher, H. Chamomile: Industrial Profiles; CRC Press, Taylor & Francis Books: London, UK, 2005. [Google Scholar]
- Singh, L.B. Utilisation of Saline-Alkali Soils for Agro-Industry without Prior Reclamation. Econ. Bot. 1970, 24, 439–442. [Google Scholar] [CrossRef]
- Patra, D.D.; Singh, D.V. Utilization of salt affected soil and saline/sodic irrigation water for cultivation of medicinal and aromatic plants. Curr. Res. Med. Aromat. Plants 1995, 17, 378–381. [Google Scholar]
- Tomar, O.S.; Minhas, P.S. Performance of winter annual flowering species as affected by different modes of saline and canal water irrigation. Indian. J. Hortic. 2002, 59, 201–206. [Google Scholar]
- Balak, R.; Misra, P.N. Nutrient accumulation and sodicity reclamation potential of German chamomile (Chamomilla recutita) under varying sodicity and fertility levels. J. Med. Aromat. Plant Sci. 2004, 26, 12–16. [Google Scholar]
- Baghalian, K.; Haghiry, A.; Naghavi, M.R.; Mohammadi, A. Effect of Saline Irrigation Water on Agronomical and Phytochemical Characters of Chamomile (Matricaria recutita L.). Sci. Hortic. 2008, 116, 437–441. [Google Scholar] [CrossRef]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of Salinity and Drought Stresses on Growth Parameters and Essential Oil Content of Matricaria chamomila. Int. J. Agric. Biol. 2008, 10, 357–361. [Google Scholar]
- Noori, K.; Omidi, H.; Pirahmadi, L. Morphological Characteristics, Essential Oil, Chamazulene Percentage and Anti-Oxidation Enzymes Activity Changes of Chamomile (Matricaria recutita L.) under the Soil and Water Salinity. J. Fundam. Appl. Sci. 2016, 8, 2293–2310. [Google Scholar]
- Askari-Khorasgani, O.; Mortazaeinezhad, F.; Zeinali, H.; Pessarakli, M. Interactive Effects of Saline Irrigation Water and Genotypes on Nutrient Composition of Chamomile (Matricaria recutita L.). J. Plant Nutr. 2018, 41, 41–49. [Google Scholar] [CrossRef]
- Dagar, J.C.; Yadav, G.; Yadav, R.K. Medicinal chamomile (Matricaria recutita Linn.): A commercial crop for salt-affected conditions in semiarid regions of India. J. Soil Salin. Water Qual. 2020, 12, 34–44. [Google Scholar]
- Shakya, P.; Thakur, R.; Sharan, H.; Yadav, N.; Kumar, M.; Chauhan, R.; Kumar, D.; Kumar, A.; Singh, S.; Singh, S. GGE Biplot and Regression Based Multi-Environment Investigations for Higher Yield and Essential Oil Content in German Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2023, 193, 116145. [Google Scholar] [CrossRef]
- Sharan, H.; Kumar, A.; Singh, S. Newly Developed SSRs Based Genetic Diversity Patterns in Trait Specific Populations of German Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2024, 210, 118152. [Google Scholar] [CrossRef]
- Yadav, N.; Shakya, P.; Kumar, A.; Gautam, R.D.; Chauhan, R.; Kumar, D.; Kumar, A.; Singh, S.; Singh, S. Investigation on Pollination Approaches, Reproductive Biology and Essential Oil Variation during Floral Development in German Chamomile (Matricaria chamomilla L.). Sci. Rep. 2022, 12, 15285. [Google Scholar] [CrossRef]
- Okoń, S.; Surmacz-Magdziak, A.; Paczos-Grzęda, E. Genetic Diversity Among Cultivated and Wild Chamomile Germplasm Based on ISSR Analysis. Acta Sci. Pol. Hortorum Cultus 2013, 12, 43–50. [Google Scholar]
- Otto, L.-G.; Junghanns, W.R.; Plescher, A.; Sonnenschein, M.; Sharbel, T.F. Towards Breeding of Triploid Chamomile (Matricaria recutita L.)—Ploidy Variation within German Chamomile of Various Origins. Plant Breed. 2015, 134, 485–493. [Google Scholar] [CrossRef]
- Salamon, I.; Ibraliu, A.; Kryvtsova, M. Essential Oil Content and Composition of the Chamomile Inflorescences (Matricaria recutita L.) Belonging to Central Albania. Horticulturae 2023, 9, 47. [Google Scholar] [CrossRef]
- Fahmi, T. Chamomile cultivation in Egypt. In Chamomile: Industrial Profiles; Franke, R., Schilcher, H., Eds.; CRC Press: London, UK, 2005; pp. 166–171. [Google Scholar]
- Franke, R.; Bernáth, J.; Fahmi, T.; Fogola, N.R.; Jedinak, D.; Hannig, H.-J.; Holubář, J.; Németh, É.; Oravec, V.; Oravec, V., Jr.; et al. Cultivation. In Chamomile: Industrial Profiles; Franke, R., Schilcher, H., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tsivelika, N.; Sarrou, E.; Gusheva, K.; Pankou, C.; Koutsos, T.; Chatzopoulou, P.; Mavromatis, A. Phenotypic Variation of Wild Chamomile (Matricaria chamomilla L.) Populations and Their Evaluation for Medicinally Important Essential Oil. Biochem. Syst. Ecol. 2018, 80, 21–28. [Google Scholar] [CrossRef]
- Fasoulas, A.C.; Fasoula, V.A. Honeycomb selection designs. Plant Breed. Rev. 1995, 13, 87–139. [Google Scholar]
- Fasoula, V.A.; Fasoula, D.A. Honeycomb breeding: Principles and applications. Plant Breed. Rev. 2000, 18, 177–251. [Google Scholar]
- Missaoui, A.M.; Fasoula, V.A.; Bouton, J.H. The Effect of Low Plant Density on Response to Selection for Biomass Production in Switchgrass. Euphytica 2005, 142, 1–12. [Google Scholar] [CrossRef]
- Fasoula, D.A. Nonstop Selection for High and Stable Crop Yield by Two Prognostic Equations to Reduce Yield Losses. Agriculture 2012, 2, 211–227. [Google Scholar] [CrossRef]
- Czabajska, W. Breeding large-flowered common chamomile (Matricaria chamomilla L.). I. Polyploidization of chamomile. Biul. Inst. Ochr. Rosl. Leczn. 1960, 6, 71–82. [Google Scholar]
- Franz, C.; Hölzl, J.; Máthé, Á.; Winkhofer, A. Recent results on cultivation, harvest time and breeding of chamomile. In Chamomile in Industrial and Pharmaceutical Use; Proceedings; Società italiana di Fitochimica: Trieste, Italy, 1985; pp. 6–17. [Google Scholar]
- Šalamon, I. The Slovak Gene Pool of German Chamomile (Matricaria recutita L.) and Comparison in Its Parameters. Hortic. Sci. 2004, 31, 70–75. [Google Scholar] [CrossRef]
- Azizi, M.; Bos, R.; Woerdenbag, H.J.; Kayser, O. A Comparative Study of Four Chamomile Cultivars Cultivated in Iran. Acta Hortic. 2007, 749, 93–96. [Google Scholar] [CrossRef]
- Otto, L.-G.; Mondal, P.; Brassac, J.; Preiss, S.; Degenhardt, J.; He, S.; Reif, J.C.; Sharbel, T.F. Use of Genotyping-by-Sequencing to Determine the Genetic Structure in the Medicinal Plant Chamomile, and to Identify Flowering Time and Alpha-Bisabolol Associated SNP-Loci by Genome-Wide Association Mapping. BMC Genom. 2017, 18, 599. [Google Scholar] [CrossRef] [PubMed]
- Nastovski, T.L.; Chatzopoulou, P.S.; Radanović, D.S.; Koutsos, T.V. Comparative investigation of organic chamomile production in different agro-ecological regions of Greece and Serbia. In Proceedings of the 4th Conference on Medicinal and Aromatic Plants of South-East European Countries, Iaşi, România, 28–31 May 2006; Book of Contributions. Association for Medicinal and Aromatic Plants of Southeast European Countries (AMAPSEEC): Belgrade, Serbia, 2006; pp. 183–187. [Google Scholar]
- Oravec Sr, V.; Oravec, V., Jr.; Gaia-Mgr. Oravec, V. Breeding of Bisabolol Diploid and Tetraploid Varieties of Chamomile in Slovakia. Acta Hortic. 2007, 749, 115–120. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M.; Maksimović, L.; Adamović, D. Chamomile in Republic of Serbia. Altern. Crops Cultiv. Pract. 2019, 1, 27–31. [Google Scholar]
- Dutta, P.K.; Singh, A. Effect of different spacings on fresh flower and oil yield of Matricaria chamomilla. Indian J. Agron. 1964, 9, 11–12. [Google Scholar]
- Johri, A.K.; Srivastava, L.J.; Singh, J.M.; Rana, R.C. Effect of row spacings and nitrogen levels on flower and essential oil yield in German chamomile. Indian Perfum. 1991, 35, 93–96. [Google Scholar]
- Sood, M.; Singh Rastogi, J.M.; Srivastava, L.J.; Chand, R. Effect of plant densities on German chamomile (Matricaria chamomilla L.). Indian Perfum. 1997, 41, 121–123. [Google Scholar]
- Kanjilal, P.B.; Singh, R.S. Effect of spacing and planting time on chamomile performance. J. Agric. Sci. 2000, 70, 631–637. [Google Scholar]
- Nidagundi, R.; Hegde, L. Cultivation prospects of German chamomile in South India. Nat. Prod. Radiance 2007, 6, 135–137. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Dynamics of Phosphorus and Biostimulants on Agro-Morphology, Yield, and Essential Oil Profile of German Chamomile (Matricaria chamomilla L.) Under Acidic Soil Conditions of the Western Himalaya. Front. Plant Sci. 2022, 13, 917388. [Google Scholar] [CrossRef]
- Franz, C. Content and Composition of the Essential Oil in Flower Heads of Matricaria chamomilla L. During its Ontogenetical Development. Acta Hortic. 1980, 96, 317–322. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; Pereira da Silva, V.M.; Cantão Freitas, L.; Gomes Silva, S.; Nevez Cruz, J.; de Aguiar Andrade, E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers. 2021, 18, e2000982. [Google Scholar] [CrossRef]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite Profiling and Antioxidative Activity of Sage (Salvia fruticosa Mill.) under the Influence of Genotype and Harvesting Period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Kozak, M.; Piepho, H.-P. What’s Normal Anyway? Residual Plots Are More Telling than Significance Tests When Checking ANOVA Assumptions. J. Agron. Crop Sci. 2018, 204, 86–98. [Google Scholar] [CrossRef]
- Batzios, D.P.; Roupakias, D.G. HONEY: A Microcomputer Program for Plant Selection and Analyses of the Honeycomb Designs. Crop Sci. 1997, 37, 744–747. [Google Scholar] [CrossRef]
- Mauromoustakos, A.; Fasoula, V.A.; Thompson, K. Honeycomb Designs Computing and Analysis. In Proceedings of the International Biometric Society: Eastern North American Region, Tampa, FL, USA, 26–29 March 2006. [Google Scholar]
- Koutsika-Sotiriou, M.; Mylonas, I.G.; Ninou, E.; Traka-Mavrona, E. The Cultivation Revival of a Landrace: Pedigree and Analytical Breeding. Euphytica 2010, 176, 15–24. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]
- Massoud, H.; Franz, C. Quantitative Genetical Aspects of Chamomilla recutita (L.) Rauschert. J. Essent. Oil Res. 1990, 2, 15–20. [Google Scholar] [CrossRef]
- Kowalska, J.; Seidler-Lozykowska, K.; Jakubowska, M.; Drozdzynski, D. Does Time of Protective Procedure and Genotype of Chamomile Affect Yield? Herba Pol. 2019, 65, 1–6. [Google Scholar] [CrossRef]
- Trojak-Goluch, A.; Kawka-Lipińska, M.; Wielgusz, K.; Praczyk, M. Polyploidy in Industrial Crops: Applications and Perspectives in Plant Breeding. Agronomy 2021, 11, 2574. [Google Scholar] [CrossRef]
- D’ Andrea, L. Variation of Morphology, Yield and Essential Oil Components in Common Chamomile (Chamomilla recutita (L.) Rauschert) Cultivars Grown in Southern Italy. J. Herbs Spices Med. Plants 2002, 9, 359–365. [Google Scholar] [CrossRef]
- Mežaka, I.; Kronberga, A.; Nakurte, I.; Taškova, I.; Jakovels, D.; Primavera, A. Genetic, Chemical and Morphological Variability of Chamomile (Chamomilla recutita L.) Populations of Latvia. Ind. Crops Prod. 2020, 154, 112614. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Pecetti, L. Yield vs. Morphophysiological Trait-Based Criteria for Selection of Durum Wheat in a Semi-Arid Mediterranean Region (Northern Syria). Field Crops Res. 1998, 59, 163–173. [Google Scholar] [CrossRef]
- Albrecht, S.; Otto, L.-G. Matricaria recutita L.: True Chamomile. In Medicinal, Aromatic and Stimulant Plants; Novak, J., Blüthner, W.-D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 313–331. ISBN 978-3-030-38792-1. [Google Scholar]
- Salamon, I. Effect of the Internal and External Factors on Yield and Qualitative-Quantitative Characteristics of Chamomile Essential Oil. Acta Hortic. 2007, 749, 45–65. [Google Scholar] [CrossRef]
- Sleper, D.A.; Poehlman, J.M. Breeding Field Crops, 5th ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Fasoula, V.A.; Tokatlidis, I.S. Development of Crop Cultivars by Honeycomb Breeding. Agron. Sustain. Dev. 2012, 32, 161–180. [Google Scholar] [CrossRef]
- Pirkhezri, M.; Hassani, M.; Hadian, J. Genetic Diversity in Different Populations of Matricaria chamomilla L. Growing in Southwest of Iran, Based on Morphological. Res. J. Med. Plant 2010, 4, 1–13. [Google Scholar] [CrossRef]
- Franke, R.; Schilcher, H. Relevance and Use of Chamomile (Matricaria recutita L.). Acta Hortic. 2007, 749, 29–43. [Google Scholar] [CrossRef]
- Franz, C. Biochemical Genetics of Essential Oil Compounds. In Proceedings of the 11th International Congress of Essential Oils, Fragrances and Flavours, New Delhi, India, 12–16 November 1990; Volume 3, pp. 17–25. [Google Scholar]
- Rathore, S.; Kumar, R. Agronomic Interventions Affect the Growth, Yield, and Essential Oil Composition of German Chamomile (Matricaria chamomilla L.) in the Western Himalaya. Ind. Crops Prod. 2021, 171, 113873. [Google Scholar] [CrossRef]
- Ghareeb, Y.E.; Soliman, S.S.; Ismail, T.A.; Hassan, M.A.; Abdelkader, M.A.; Abdel Latef, A.A.H.; Al-Khayri, J.M.; ALshamrani, S.M.; Safhi, F.A.; Awad, M.F.; et al. Improvement of German Chamomile (Matricaria recutita L.) for Mechanical Harvesting, High Flower Yield and Essential Oil Content Using Physical and Chemical Mutagenesis. Plants 2022, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Fejer, J.; Salamon, I. Breeding of German Chamomile, Matricaria recutita L., with a High Content of α-Bisabolol. Acta Hortic. 2016, 1125, 287–292. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and Biological Effects of Alpha-Bisabolol: An Updated Review of the Molecular Mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Tai, Y.; Wang, H.; Yao, P.; Sun, J.; Guo, C.; Jin, Y.; Yang, L.; Chen, Y.; Shi, F.; Yu, L.; et al. Biosynthesis of α-Bisabolol by Farnesyl Diphosphate Synthase and α-Bisabolol Synthase and Their Related Transcription Factors in Matricaria recutita L. Int. J. Mol. Sci. 2023, 24, 1730. [Google Scholar] [CrossRef] [PubMed]
- El Joumaa, M.M.; Borjac, J.M. Matricaria chamomilla: A Valuable Insight into Recent Advances in Medicinal Uses and Pharmacological Activities. Phytochem. Rev. 2022, 21, 1913–1940. [Google Scholar] [CrossRef]
- Fiume, M.M. Bisabolol. Int. J. Toxicol. 2017, 36, 24S–25S. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Gabbanini, S.; Neba, J.N.; Matera, R.; Valgimigli, L. Photochemical and Oxidative Degradation of Chamazulene Contained in Artemisia, Matricaria and Achillea Essential Oils and Setup of Protection Strategies. Molecules 2024, 29, 2604. [Google Scholar] [CrossRef]
- Shalaby, A.S.; Hendawy, S.F.; Khalil, M.Y. Evaluation of Some Chamomile Cultivars Introduced and Adapted in Egypt. J. Essent. Oil Bear. Plants 2010, 13, 655–669. [Google Scholar] [CrossRef]
| Agronomic Characteristics | Phase I of the Breeding Program | ||
|---|---|---|---|
| Initiation—1st Year | Completion—3rd Year | ||
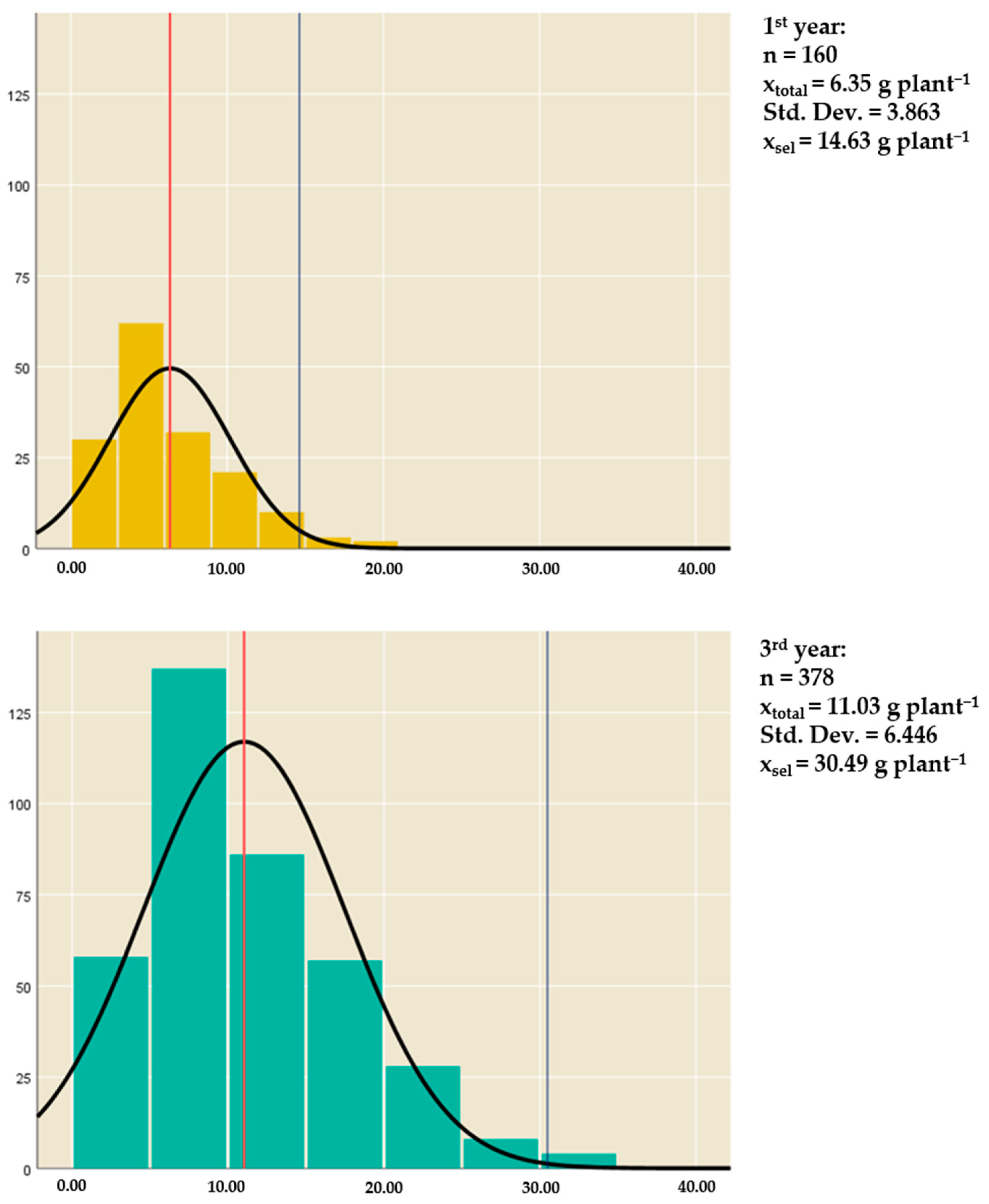
| Inflorescences: dry weight plant−1 (g plant−1) | xtotal * | 6.35 | 11.03 |
| xselected ** | 14.63 | 30.49 | |
| S *** | 8.28 | 19.46 | |
| R *** | 5.70 | ||
| h2 *** | 0.568 | ||
| Quality Characteristics | Phase I of the Breeding Program | ||
|---|---|---|---|
| Initiation—2nd Year | Completion—3rd Year | ||
| Essential oil: α-bisabolol (%) | xtotal * | 24.63 | 31.15 |
| xselected ** | 35.45 | 41.78 | |
| S *** | 10.82 | 10.63 | |
| R *** | 6.52 | ||
| h2 *** | 0.603 | ||
| Essential oil: chamazulene (%) | xtotal | 18.13 | 21.60 |
| xselected | 22.37 | 25.16 | |
| S | 4.24 | 3.56 | |
| R | 3.47 | ||
| h2 | 0.818 | ||
| Genotype * (Ploidy Level) | Inflorescences | Essential Oil Content and Components | |||||
|---|---|---|---|---|---|---|---|
| Dry Weight (g m−2) | Observed Gain (%) | Essential Oil Content (%) | α-Bisabolol (%) | Observed Gain (%) | Chamazulene (%) | Observed Gain (%) | |
| IP (2×) | 56.17 c ** | 12.17 | 0.37 | 27.95 b | 71.45 | 16.14 b | 6.57 |
| BP (2×) | 63.00 b | 0.37 | 47.93 a | 17.20 a | |||
| Banatska (2×) | 68.43 a | 0.45 | 7.26 c | 8.20 c | |||
| Lutea (4×) | 86.37 | 0.61 | 43.47 | 21.83 | |||
| Goral (4×) | 84.49 | 0.67 | 26.80 | 22.03 | |||
| Genotype * (Ploidy Level) | Plant | Inflorescences | ||||
|---|---|---|---|---|---|---|
| Height (cm) | Observed Gain (%) | Head Diameter (mm) | Observed Gain (%) | Disc Diameter (mm) | Observed Gain (%) | |
| IP (2×) | 42.65 c ** | 20.67 | 19.91 b | 16.19 | 6.51 c | 7.32 |
| BP (2×) | 51.47 b | 23.13 a | 6.99 b | |||
| Banatska (2×) | 56.45 a | 21.07 b | 7.44 a | |||
| Lutea (4×) | 55.05 | 27.01 | 10.65 | |||
| Goral (4×) | 53.82 | 26.42 | 10.87 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsivelika, N.; Mylonas, I.; Ninou, E.; Mavromatis, A.; Sarrou, E.; Irakli, M.; Chatzopoulou, P. A Strategic Breeding Approach for Improvement of a Native Greek Chamomile (Matricaria chamomilla L.) Population for High-Yield and Optimized Chemical Profile Under Mediterranean Low-Input Conditions. Agriculture 2025, 15, 1915. https://doi.org/10.3390/agriculture15181915
Tsivelika N, Mylonas I, Ninou E, Mavromatis A, Sarrou E, Irakli M, Chatzopoulou P. A Strategic Breeding Approach for Improvement of a Native Greek Chamomile (Matricaria chamomilla L.) Population for High-Yield and Optimized Chemical Profile Under Mediterranean Low-Input Conditions. Agriculture. 2025; 15(18):1915. https://doi.org/10.3390/agriculture15181915
Chicago/Turabian StyleTsivelika, Nektaria, Ioannis Mylonas, Elissavet Ninou, Athanasios Mavromatis, Eirini Sarrou, Maria Irakli, and Paschalina Chatzopoulou. 2025. "A Strategic Breeding Approach for Improvement of a Native Greek Chamomile (Matricaria chamomilla L.) Population for High-Yield and Optimized Chemical Profile Under Mediterranean Low-Input Conditions" Agriculture 15, no. 18: 1915. https://doi.org/10.3390/agriculture15181915
APA StyleTsivelika, N., Mylonas, I., Ninou, E., Mavromatis, A., Sarrou, E., Irakli, M., & Chatzopoulou, P. (2025). A Strategic Breeding Approach for Improvement of a Native Greek Chamomile (Matricaria chamomilla L.) Population for High-Yield and Optimized Chemical Profile Under Mediterranean Low-Input Conditions. Agriculture, 15(18), 1915. https://doi.org/10.3390/agriculture15181915








