First Report of Heterodera schachtii (Schmidt, 1879) on Camelina sativa (L.) Crantz in Poland and Assessment of Its Host Suitability for This Nematode
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Cyst Nematode in Soil Cultivated with Camelina—Sample Collection
2.2. Evaluation of the Heterodera schachtii Development on Camelina Roots
2.2.1. Heterodera schachtii Growth on Camelina Roots
2.2.2. Susceptibility of Camelina at Various Growth Stages to Heterodera schachtii
2.2.3. Assessment of Changes in the Heterodera schachtii Population Density in the Soil Cultivated with Selected Camelina Cultivars
2.3. Data Analysis
3. Results
3.1. Identification of Cyst Nematode in Soil Cultivated with Camelina
3.2. Evaluation of the Heterodera schachtii Growth on Camelina Roots
3.2.1. Heterodera schachtii Growth on Camelina Roots
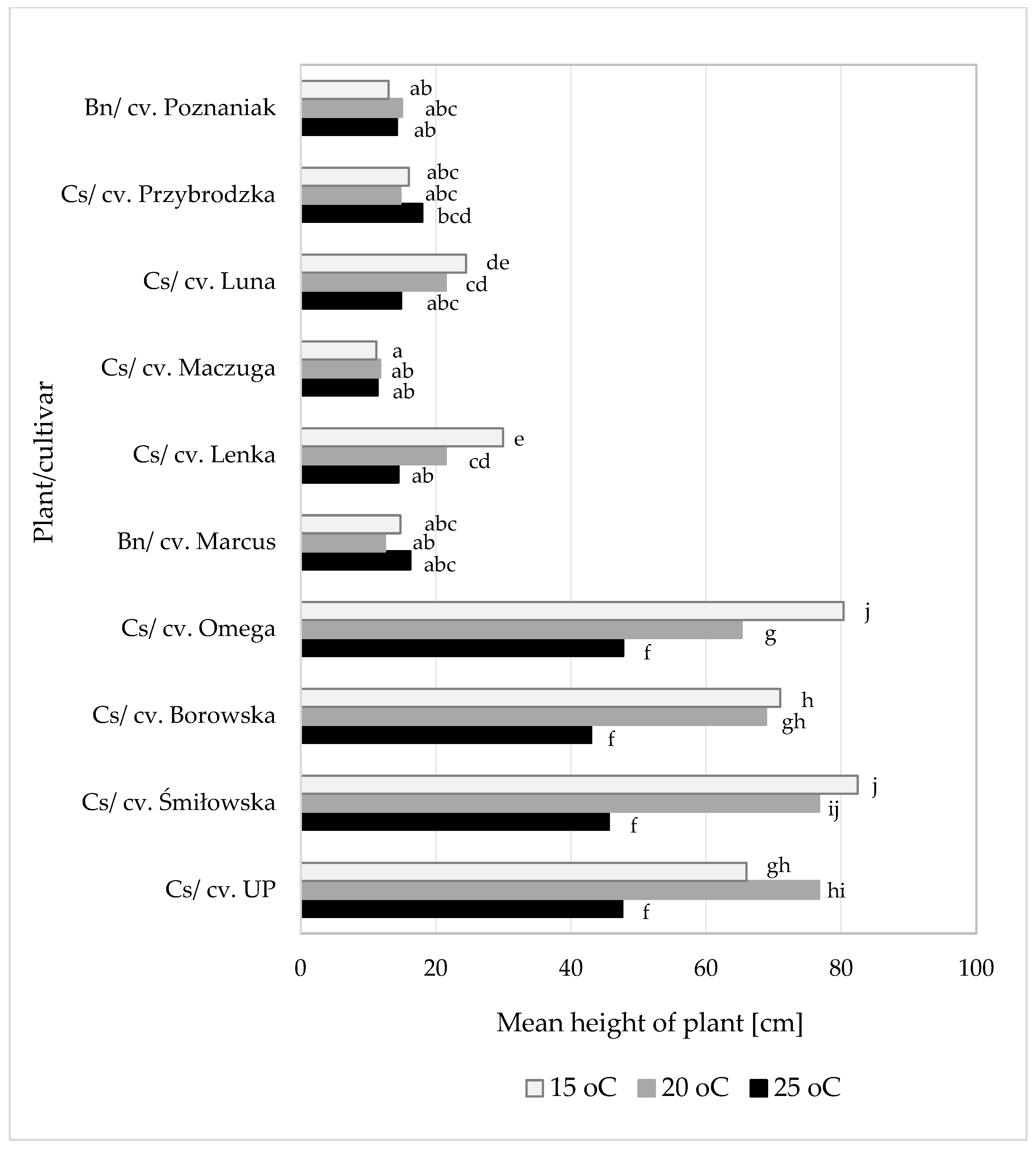
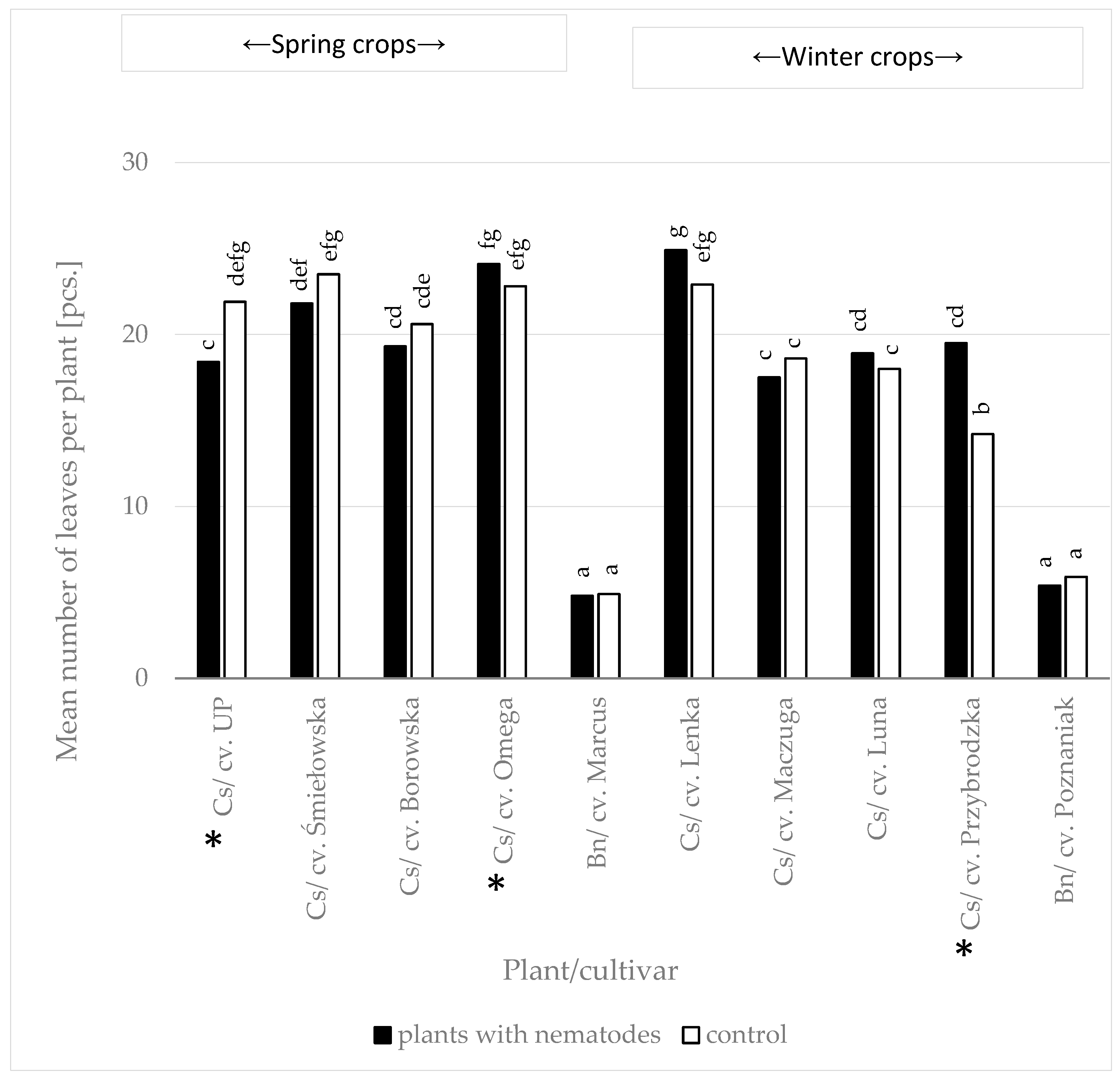
3.2.2. Susceptibility of Camelina at Various Growth Stages to Heterodera schachtii
3.2.3. Assessment of Changes in the Heterodera schachtii Population Density in the Soil Cultivated with Selected Camelina Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guendouz, A.; Hannachi, A.; Benidir, M.; Fellahi, Z.E.A.; Frih, B. Agro-biochemical Characterization of Camelina sativa: A Review. Agric. Rev. 2022, 43, 278–287. [Google Scholar] [CrossRef]
- Zanetti, F.; Peroni, P.; Pagani, E.; von Cossel, M.; Greiner, B.E.; Krzyzaniak, M.; Stolarski, M.J.; Lewandowski, I.; Alexopoulou, E.; Stefanoni, W.; et al. The opportunities and potential of camelina in marginal land in Europe. Ind. Crops Prod. 2024, 211, 118224. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crop Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Masella, P.; Galasso, I. A comparative cradle-to-gate life cycle study of bio-energy feedstock from Camelina sativa, an Italian case study. Sustainability 2020, 12, 9590. [Google Scholar] [CrossRef]
- Ghidoli, M.; Ponzoni, E.; Araniti, F.; Miglio, D.; Pilu, R. Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants 2023, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Carton, N.; Carlsson, G.; Jensen, E.; Corre-Hellou, G. Intercropping of Lupin for Weed Control and Complementary Grain Production in Organic Farming. Available online: https://zenodo.org/records/3741487 (accessed on 12 March 2024).
- Saucke, H.; Ackermann, K. Weed suppression in mixed cropped grain peas and false flax (Camelina sativa). Weed Res. 2006, 46, 453–461. [Google Scholar] [CrossRef]
- Obour, K.A.; Sintim, Y.H.; Obeng, E.; Jeliazkov, D.V. Oilseed camelina (Camelina sativa L. Crantz): Production systems, prospects and challenges in the USA Great Plains. Adv. Plants Agric. Res. 2015, 2, 00043. [Google Scholar] [CrossRef]
- Gruber, M.Y.; Wang, S.; Ethier, S.; Holowachuk, J.; Bonham-Smith, P.C.; Soroka, J.; Lloyd, A. “Hairy Canola”—Arabidopsis GL3 induces a dense covering of trichomes on Brassica napus seedlings. Plant Mol. Biol. 2006, 60, 679–698. [Google Scholar] [CrossRef]
- Onyilagha, J.C.; Gruber, M.Y.; Hallett, R.H.; Holowachuk, J.; Buckner, A.; Soroka, J.J. Constitutive flavonoids deter flea beetle insect feeding in Camelina sativa L. Biochem. Syst. Ecol. 2012, 42, 128–133. [Google Scholar] [CrossRef]
- Rehmanur, H.; Majeed, B.; Farooqi, M.A.; Rasul, A.; Sagheer, M.; Ali, Q.; Akhtar, Z.R. Green synthesis of silver nitrate nanoparticles from Camelina sativa (L.) and its effect to control insect pests of stored grains. Int. J. Trop. Ins. Sci. 2021, 41, 3031–3039. [Google Scholar] [CrossRef]
- Chesnais, Q.; Verzeaux, J.; Couty, A.; Le Roux, V.; Ameline, A. Is the oil seed crop Camelina sativa a potential host for aphid pests? Bioenergy Res. 2015, 8, 91–99. [Google Scholar] [CrossRef]
- Wang, H.L.; Ding, B.J.; Dai, J.Q.; Nazarenus, T.J.; Borges, R.; Mafra-Neto, A.; Cahoon, E.B.; Hofvander, P.; Stymne, S.; Löfstedt, C. Insect pest management with sex pheromone precursors from engineered oilseed plants. Nat. Sustain. 2022, 5, 981–990. [Google Scholar] [CrossRef]
- Zuck, P.C. Evaluation of Alternative Crops for Management of Pratylenchus neglectus in Montana Winter Wheat Production. Master’s Thesis, Montana State University Bozeman, Bozeman, MT, USA, 2010. [Google Scholar]
- Smiley, R.W.; Machado, S.; Gourlie, J.A.; Pritchett, L.C.; Yan, G.P.; Jacobsen, E.E. Effects of crop rotations and tillage on Pratylenchus spp. in the semiarid Pacific Northwest United States. Plant Dis. 2013, 97, 537–546. [Google Scholar] [CrossRef]
- Smiley, R.W.; Yan, G.P.; Gourlie, J.A. Selected Pacific Northwest crops as hosts of Pratylenchus neglectus and P. thornei. Plant Dis. 2014, 98, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- May, D.B.; Johnson, W.A.; Zuck, P.C.; Chen, C.C.; Dyer, A.T. Assessment and management of root lesion nematodes in Montana wheat production. Plant Dis. 2016, 100, 2069–2079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villate, L.; Morin, E.; Demangea, G.; Van Helden, M.; Esmenjaud, D. Control of Xiphinema index populations by fallow plants under greenhouse and field conditions. Phytopathology 2012, 102, 627–634. [Google Scholar] [CrossRef]
- Warnke, S.A.; Chen, S.Y.; Wyse, D.L.; Johnson, G.A.; Porter, P.M. Effect of rotation crops on Heterodera glycines population density in a greenhouse screening study. J. Nematol. 2006, 38, 391–398. [Google Scholar] [PubMed]
- Acharya, K.; Yan, G.; Berti, M. Can winter camelina, crambe, and brown mustard reduce soybean cyst nematode populations? Ind. Crop Prod. 2019, 140, 111637. [Google Scholar] [CrossRef]
- van Zyl, J.; Meyer, A.J.; de Waele, D.; Malan, A.P. Host plant penetration, development and life cycle of a Heterodera schachtii population from the Western Cape province, South Africa. J. Plant Dis. Prot. 2021, 128, 517–525. [Google Scholar] [CrossRef]
- Nuaima, R.H.; Heuer, H. Genetic Variation among Heterodera schachtii Populations Coincided with Differences in Invasion and Propagation in Roots of a Set of Cruciferous Plants. Int. J. Mol. Sci. 2023, 24, 6848. [Google Scholar] [CrossRef]
- Akyol, G.B.; Yuksel, E.; Elci, E.; Bozbuga, R.; Dababat, A.; Imren, M.; Toktay, H. Heterodera schachtii (Nematoda: Heteroderidae) Associated with Cabbage-Cultivation Systems in Anatolia Region of Turkiye. Horticulturae 2024, 10, 635. [Google Scholar] [CrossRef]
- Meinecke, A.; Westphal, A. Quantitative reproductive potential of Heterodera schachtii on weeds typical for late summer fallow in sugar beet rotations. Weed Res. 2014, 54, 624–634. [Google Scholar] [CrossRef]
- Daub, M. The beet cyst nematode (Heterodera schachtii): An ancient threat to sugar beet crops in Central Europe has become an invisible actor. In Integrated Nematode Management: State-of-the-Art and Visions for the Future; Sikora, R.A., Desaegar, J., Molindijk, L., Eds.; CAB: Wallingford, UK, 2022; pp. 394–399. [Google Scholar] [CrossRef]
- Shepherd, A.M. Extraction and estimation of cyst nematodes. In Laboratory Methods for Work with Plant and Soil Nematodes; Southey, J.F., Ed.; Her Majesty’s Stationary Office: London, UK, 1986; pp. 31–49. [Google Scholar]
- Brzeski, M.W. (Ed.) Heteroderidae. In Nematodes of Tylenchida in Poland and Temperate Europe; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 1998; pp. 217–234. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef]
- Nunn, G.B. Nematode Molecular Evolution. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 1992. [Google Scholar]
- Ferris, V.R.; Ferris, J.M.; Faghih, J. Variation in spacer ribosomal DNA in some cyst-forming species of plant parasitic nematodes. Fund. Appl. Nematol. 1993, 16, 177–184. [Google Scholar]
- Vrain, T.C.I. Restriction fragment length polymorphism separates species of the Xiphinema americaum group. J. Nematol. 1993, 25, 361–364. [Google Scholar]
- Martinelli, T.; Galasso, I. Phenological growth stages of Camelina sativa according to the extended BBCH scale. Ann. Appl. Biol. 2011, 158, 87–94. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. 2022. Available online: https://www.xlstat.com (accessed on 30 June 2025).
- Subbotin, S.A.; Mundo-Okampo, M.; Baldwin, J.G. Systematics of Cyst Nematodes (Nematoda: Heteroderidae); Brill: Boston, MA, USA, 2010; pp. 35–43. [Google Scholar]
- Amiri, S.; Subboin, S.; Moens, M. Comparative morphometrics and RAPD studies of Heterodera schachtii and H. betae populations. Russ. J. Nematol. 2003, 11, 91–99. [Google Scholar]
- Ambrogioni, L.; Irdni, T. Identification of Heterodera schachtii group species in Italy by morphometrics and RAPD-PCR. Nematol. Medit. 2001, 29, 159–168. [Google Scholar]
- Jaskulska, I.; Kamieniarz, J.; Jaskulski, D. Yields and the composition of roots of sugar beet varieties tolerant to the beet cyst nematode. J. Elem. 2017, 22, 799–808. [Google Scholar] [CrossRef]
- Łukomski, M. Development of the beet cyst nematode (Heterodera schachtii Schm.) population in the soil and yield of sugar beet following the cultivation of varieties with different tolerance to nematodes. Prog. Plant Prot. 2019, 59, 83–87. [Google Scholar] [CrossRef]
- Ulatowska, A. Influence of sugar beet variety and initial population of sugar beet cyst nematode (Heterodera schachtii Schmidt) in soil on nematode development and sugar beet yield and root quality. Prog. Plant Prot. 2021, 61, 147–153. [Google Scholar] [CrossRef]
- Nowakowski, M.; Franke, K. Struktura plonu i oddziaływanie na populację mątwika ziemniaczanego (Globodera rostochiensis) wybranych odmian gorczycy białej uprawianej w plonie głównym II. Plony biomasy nadziemnej i korzeni oraz zagęszczenie mątwika ziemniaczanego w glebie. Rośliny Oleiste 2013, 34, 85–94. [Google Scholar] [CrossRef]
- Kornobis, S.; Wolny, S. Occurrence of plant parasitic nematodes on weeds in agrobiocenosis in the Wielkopolska region in Poland. Fund. Appl. Nematol. 1996, 20, 627–632. [Google Scholar]
- Dobosz, R.; Flis, Ł.; Kubicz, M.; Krawczyk, R. Wpływ uprawy lnianki siewnej Camelina sativa (L.) Crantz na liczebność populacji mątwika burakowego Heterodera schachtii Schmidt, 1891 w glebie. In Proceedings of the 59th Scientific Session of the Institute of Plant Protection-National Research Institute, Poznan, Poland, 12–14 February 2019. [Google Scholar]
- Fournet, S.; Pellan, L.; Porte, C.; Piriou, C.; Grenier, E.; Montarry, J. Populations of the beet cyst nematode Heterodera schachtii exhibit strong differences in their life-history traits across changing thermal conditions. Front. Microbiol. 2018, 9, 2801. [Google Scholar] [CrossRef]
- Bowen, S.A. Aspects of the Population Biology of the Cyst Nematode Parasites of Oilseed Rape. Ph.D. Thesis, University of Bedfordshire, Luton, UK, 1988. [Google Scholar]
- Kabir, F.M.; Shin, J.H.; Kwon, O.G.; Lee, D.W. Temperature and root extract effect on egg hatching and development of sugar beet cyst nematode, Heterodera schachtii. Korean J. Soil Zool. 2015, 19, 22–27. [Google Scholar]
- Karakaş, M. Temperature and the life cycle of Heterodera schachtii (Nematoda: Heteroderidae). Commun. Fac. Sci. Univ. Ank. Ser. C 2011, 23, 13–20. [Google Scholar]
- Kurasiak-Popowska, D.; Graczyk, M.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Influence of variety and weather conditions on fatty acid composition of winter and spring Camelina sativa varieties in Poland. Eur. Food Res. Technol. 2021, 247, 465–473. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G. Fatty acids, chemical composition and organic matter digestibility of seeds and vegetative parts of false flax (Camelina sativa L.) after different lengths of growth. Anim. Feed. Sci. Technol. 2007, 13, 341–350. [Google Scholar] [CrossRef]
- Tedone, L.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Colonna, M.A. Camelina sativa (L. Crantz) fresh forage productive performance and quality at different vegetative stages: Effects of dietary supplementation in ionica goats on milk quality. Agriculture 2022, 12, 91. [Google Scholar] [CrossRef]
- Bravi, E.; Falcinelli, B.; Mallia, G.; Marconi, O.; Royo-Esnal, A.; Benincasa, P. Effect of sprouting on the phenolic compounds, glucosinolates, and antioxidant activity of five Camelina sativa (L.) Crantz Cultivars. Antioxidants 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- Soroka, J.; Grenkow, L. Susceptibility of Brassicaceous plants to feeding by flea beetles, Phyllotreta spp. (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2013, 106, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Niere, B. On the assessment of resistance of potato varieties to the potato cyst nematodes Globodera pallida and Globodera rostochiensis. Mitteilungen aus der Biologischen Bundesanstalt fur Land- und Forstwirtschaft Berlin-Dahlem. EPPO Glob. Database 2006, 404, 31–39. [Google Scholar]
- Kabir, F.M.; Leeb, J.K.; Jeonga, M.G.; Okkia, M.A.; Choic, Y.H.; Leea, D.W. Effects of varying temperature on the reproduction and damage potential of Heterodera schachtii to Chinese cabbage (Brassica rapa pekinensis). J. Asia. Pac. Entomol. 2018, 21, 69–74. [Google Scholar] [CrossRef]
- Okada, H.; Uehara, T.; Tateishi, Y.; Kitabayashi, S.; Komatsu, K.; Yosano, S. Host range of the sugar beet cyst nematode (Heterodera schachtii) population detected for the first time in Japan. Nematol. Res. 2021, 51, 11–18. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD (Range of Variability) |
|---|---|
| Cysts (n = 25) | |
| Length (µm) | 749 ± 106 (675–900) |
| Width (µm) | 467 ± 66 (375–500) |
| Vulval slit length (µm) | 44 ± 3 (40–50) |
| Fenestral width (µm) | 38 ± 10 (25–52) |
| Vulval bridge length (µm) | 103 ± 14 (85–120) |
| Distance between vulval slit and anus (µm) | 50 ± 6 (42–60) |
| J2 (n = 25) | |
| Stylet length (µm) | 25 ± 0.7 (23–25) |
| Tail length (µm) | 46± 3 43–51) |
| Hyaline length (µm) | 25 ± 3 (20–32) |
| L | 427 ± 26 (375–499) |
| a | 22.9 ± 1.7 (20–27.6) |
| b′ | 2.8 ± 0.2 (2.5–3.1) |
| c | 9.3 ± 0.5 (8.6–10.4) |
| c′ | 3.8 ± 0.4 (3.2–4.4) |
| Plant/Cultivar | Mean Number of Females and Cysts of Heterodera schachtii Per Plant at Temperatures | ||
|---|---|---|---|
| 15 °C | 20 °C | 25 °C | |
| Spring cultivars: Camelina sativa | |||
| cv. UP | 1.25 ab | 7.25 efgh | 0.25 a |
| Control | 0 a | 0 a | 0 a |
| cv. Śmiłowska | 1.0 a | 7.0 efgh | 2.5 abc |
| Control | 0 a | 0 a | 0 a |
| cv. Borowska | 1.25 ab | 6.25 defg | 0.25 a |
| Control | 0 a | 0 a | 0 a |
| cv. Omega | 1.5 ab | 9.25 hi | 0.5 a |
| Control | 0 a | 0 a | 0 a |
| Brassica napus | |||
| cv. Marcus | 6.5 defgh | 20.5 k | 6.0 def |
| Control | 0 a | 0 a | 0 a |
| B. vulgaris cv. Centurion | 9.0 gh | 25.75 l | 1.75 abc |
| Control | 0 a | 0 a | 0 a |
| Beta vulgaris cv. Kujavia | 8.0 fgh | 21.25 k | 12.0 ij |
| Control | 0 a | 0 a | 0 a |
| Winter cultivars: Camelina sativa | |||
| cv. Lenka | 0.25 a | 1.5 ab | 0.25 a |
| Control | 0 a | 0 a | 0 a |
| cv. Maczuga | 0.5 a | 1.5 ab | 0.25 a |
| Control | 0 a | 0 a | 0 a |
| cv. Luna | 0.25 a | 4.5 cde | 0.75 a |
| Control | 0 a | 0 a | 0 a |
| cv. Przybrodzka | 0.25 a | 4.0 bcd | 0.25 a |
| Control | 0 a | 0 a | 0 a |
| Brassica napus | |||
| cv. Poznaniak | 7.25 efgh | 19.5 k | 13.25 j |
| Control | 0 a | 0 a | 0 a |
| Plant/Cultivar | Number of Siliques at Temperature | ||
|---|---|---|---|
| 15 °C | 20 °C | 25 °C | |
| Spring cultivars | |||
| Camelina sativa | |||
| cv. UP | 18.8 e | 10.8 bc | 1.0 a |
| Control | 18.7 e | 20.0 e | 1.8 a |
| cv. Śmiłowska | 18.3 e | 18.0 e | 0.5 a |
| Control | 32.5 f | 18.5 e | 0.3 a |
| cv. Borowska | 16.5 de | 8.5 b | 0.8 a |
| Control | 20.0 e | 19.8 e | 0.3 a |
| cv. Omega | 15.0 cde | 12.5 bcd | 2.3 a |
| Control | 15.5 cde | 15.0 cde | 2.5 a |
| Brassica napus | |||
| cv. Marcus | 0 a | 0 a | 0 a |
| Control | 0 a | 0 a | 0 a |
| Winter cultivars | |||
| Camelina sativa | |||
| cv. Lenka | 0 a | 0 a | 0 a |
| Control | 0 a | 0 a | 0 a |
| cv. Maczuga | 0 a | 0 a | 0 a |
| Control | 0 a | 0 a | 0 a |
| cv. Luna | 0 a | 0 a | 0 a |
| Control | 0 a | 0 a | 0 a |
| cv. Przybrodzka | 0 a | 0 a | 0 a |
| Control | 0 a | 0 a | 0 a |
| B. napus | |||
| cv. Poznaniak | 0 a | 0 a | 0 a |
| Control | 0 a | 0 a | 0 a |
| Infestation effect on slique numbers, irrespective of temperature conditions | |||
| Mean value for infected plants 3.9 a | |||
| Mean value for uninfected plants 5.5 b | |||
| BBCH Code * | Mean Number of Females and Cysts of Heterodera schachtii Per Plant ** |
|---|---|
| 10 | 0.83 ab |
| 12 | 1.58 b |
| 15 | 0.83 ab |
| 19 | 1.42 b |
| 28–32 | 0.00 a |
| 51–61 | 0.00 a |
| Category | Mean |
|---|---|
| Sowing date effect (spring/winter crop) on nematode density in soil | |
| Spring | 633.9 b |
| Winter | 281. 7 a |
| Crop effect on nematode density in a soil | |
| Camelina sativa | 224.4 a |
| Brassica napus | 901.9 b |
| Bare fallow | 247.2 a |
| Effect of crop and its botanical form on nematode density in soil | |
| Spring Camelina sativa | 200.6 a |
| Spring Brassica napus | 1452.5 c |
| Bare fallow for spring sowing date | 248.8 ab |
| Winter Camelina sativa | 248.1 ab |
| Winter Brassica napus | 351.3 b |
| Bare fallow for winter sowing date | 245.6 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobosz, R.; Krawczyk, R.; Flis, Ł. First Report of Heterodera schachtii (Schmidt, 1879) on Camelina sativa (L.) Crantz in Poland and Assessment of Its Host Suitability for This Nematode. Agriculture 2025, 15, 1908. https://doi.org/10.3390/agriculture15181908
Dobosz R, Krawczyk R, Flis Ł. First Report of Heterodera schachtii (Schmidt, 1879) on Camelina sativa (L.) Crantz in Poland and Assessment of Its Host Suitability for This Nematode. Agriculture. 2025; 15(18):1908. https://doi.org/10.3390/agriculture15181908
Chicago/Turabian StyleDobosz, Renata, Roman Krawczyk, and Łukasz Flis. 2025. "First Report of Heterodera schachtii (Schmidt, 1879) on Camelina sativa (L.) Crantz in Poland and Assessment of Its Host Suitability for This Nematode" Agriculture 15, no. 18: 1908. https://doi.org/10.3390/agriculture15181908
APA StyleDobosz, R., Krawczyk, R., & Flis, Ł. (2025). First Report of Heterodera schachtii (Schmidt, 1879) on Camelina sativa (L.) Crantz in Poland and Assessment of Its Host Suitability for This Nematode. Agriculture, 15(18), 1908. https://doi.org/10.3390/agriculture15181908





