Biochar Effectively Reduced N2O Emissions During Heap Composting and NH3 Emissions During Aerobic Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Experimental Design
2.3. Sample Collection and Determination
2.4. Data Processing and Analysis
3. Results and Discussion
3.1. Changes in Physical and Chemical Properties During Composting
3.1.1. Temperature, Moisture, and pH
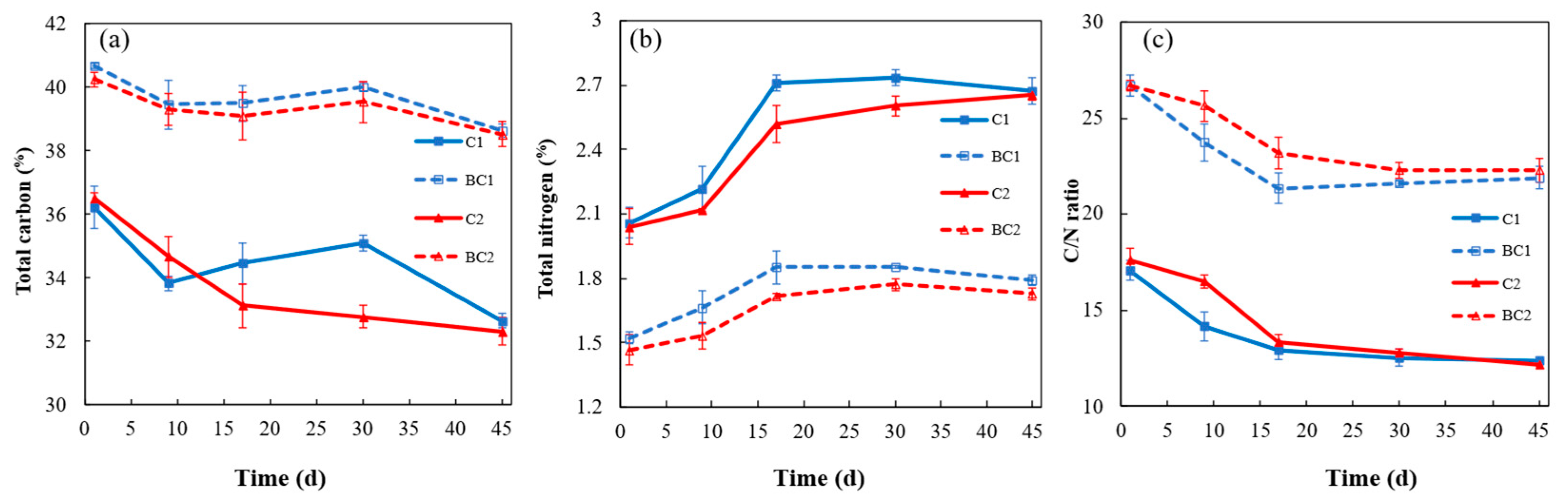
3.1.2. Total Carbon, Total Nitrogen, and C/N Ratio
3.1.3. Changes in NH3 and N2O Emissions During Composting
3.2. Microbiological Changes During Composting
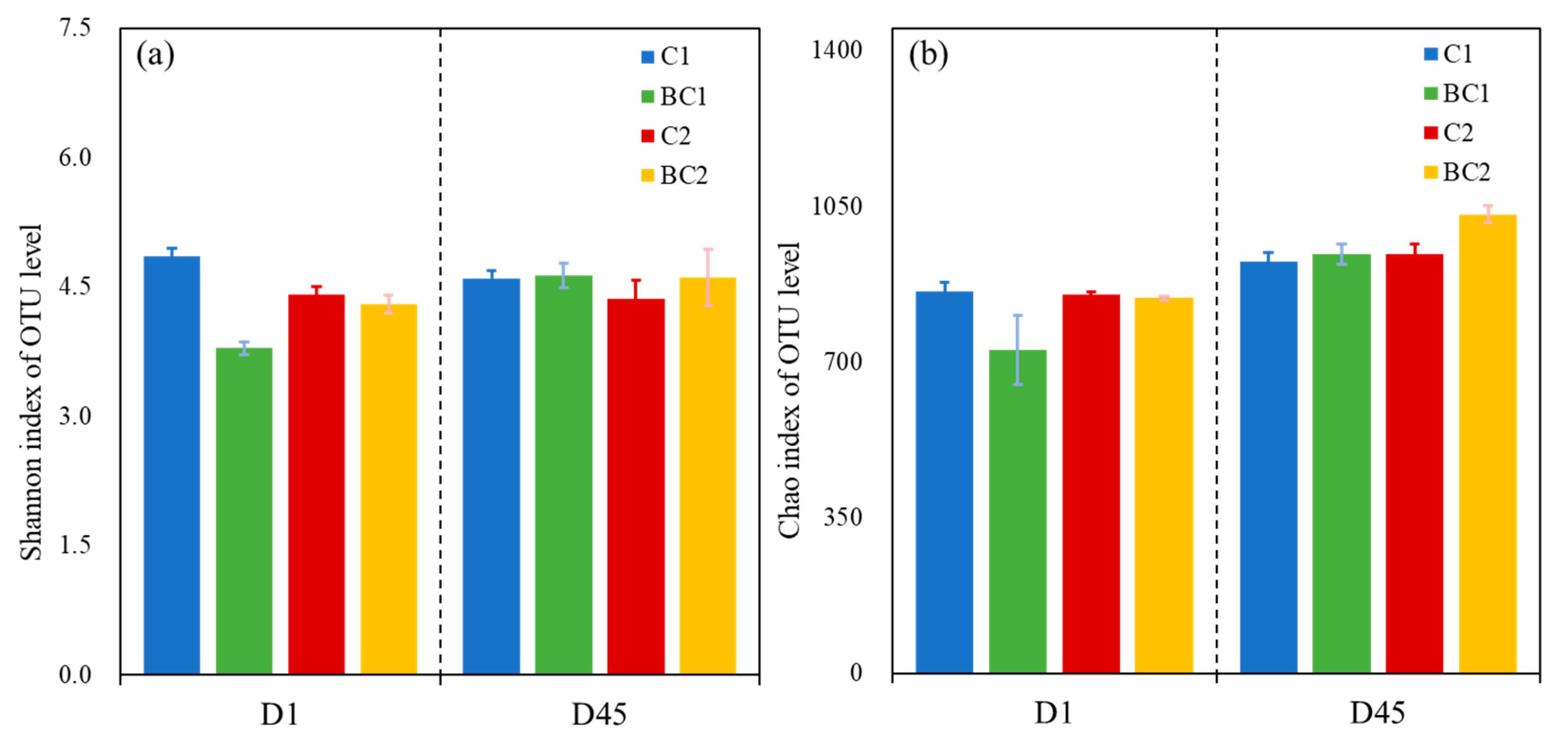
3.2.1. Changes in Shannon and Chao1 Indices of Community Diversity
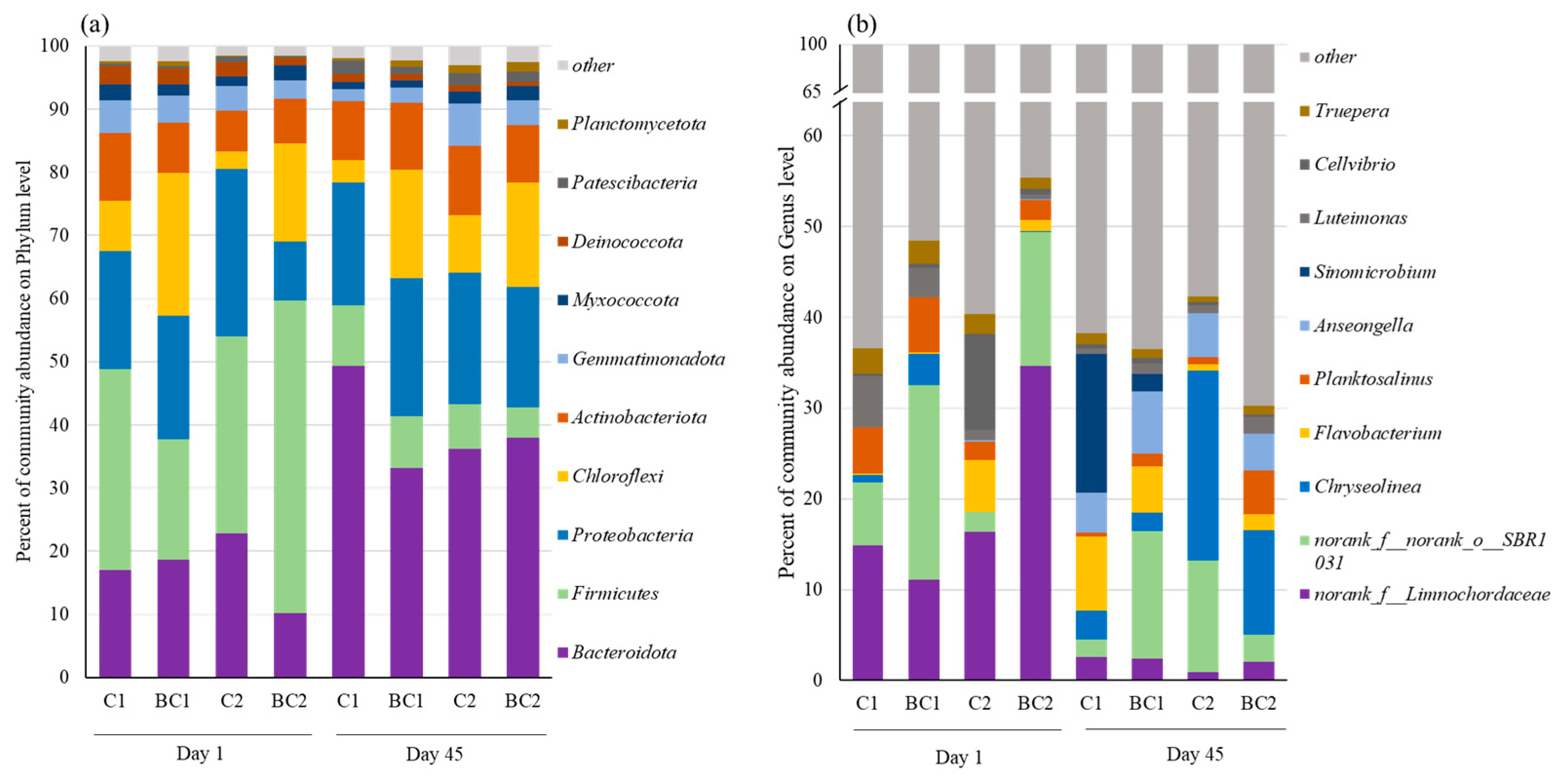
3.2.2. Succession of Bacterial Communities at Phylum and Genus Levels
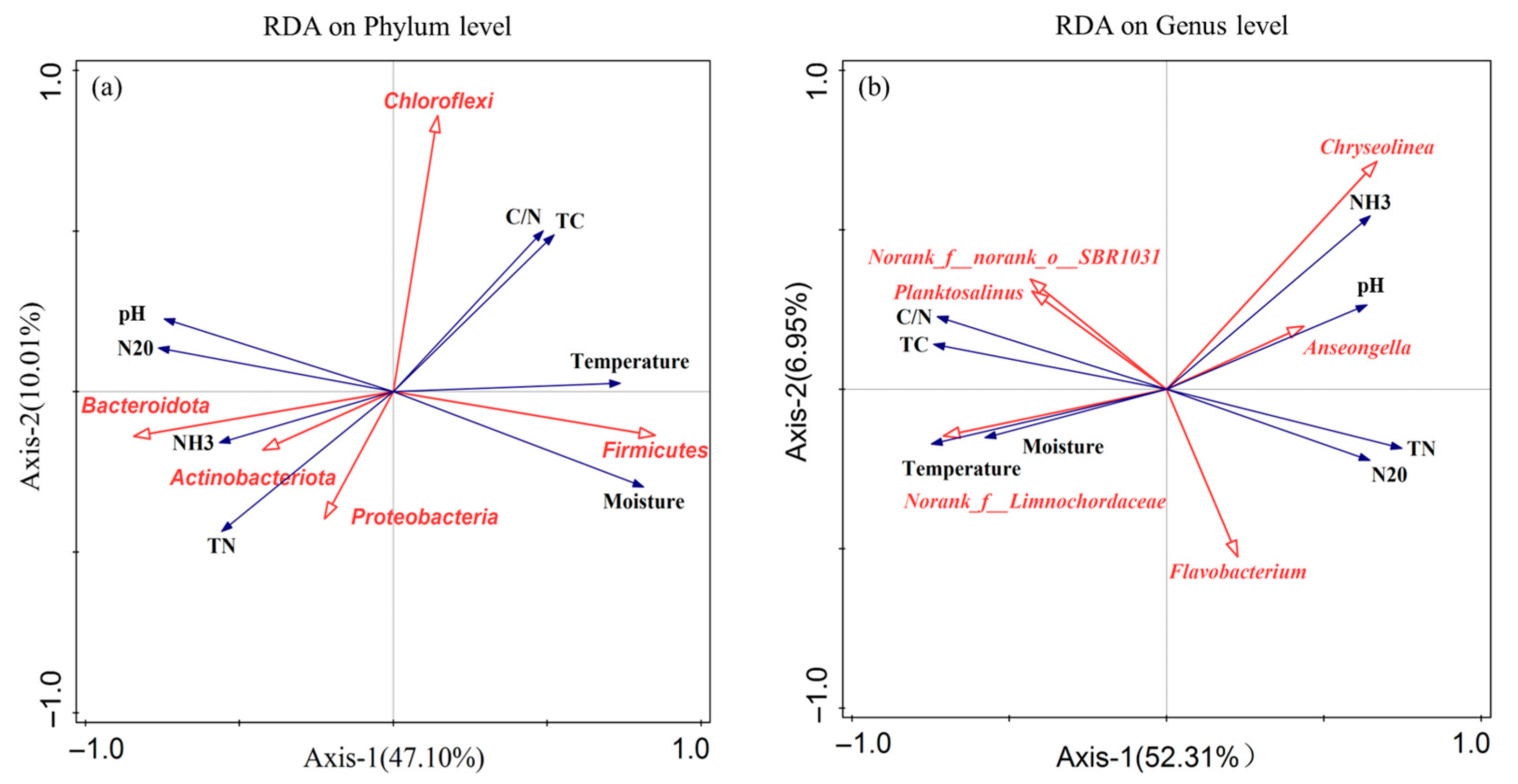
3.3. Correlation of Microbial Community Structure with Environmental Factors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, J.; Shen, X.; Yin, H.; Sun, X.; Dong, H.; Huang, G. Oxygen dynamics, organic matter degradation and main gas emissions during pig manure composting: Effect of intermittent aeration. Bioresour. Technol. 2022, 361, 127697. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X.; Sun, H.; Feng, Y.; Gong, X.; Ma, Y.; Jiang, J.; Xue, L. Effects of biochar and wood vinegar co-application on composting ammonia and nitrous oxide losses and fertility. Bioresour. Technol. 2024, 412, 131388. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Koyama, M.; Nakasaki, K. Effect of oxygen deficiency on organic matter decomposition during the early stage of composting. Waste Manag. 2023, 160, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Tong, B.; Wang, X.; Wang, S.; Ma, L.; Ma, W. Transformation of nitrogen and carbon during composting of manure litter with different methods. Bioresour. Technol. 2019, 293, 122046. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, X.; Cheng, W. Biochar combined with ferrous sulfate reduces nitrogen and carbon losses during agricultural waste composting and enhances microbial diversity. Process Saf. Environ. Prot. 2022, 162, 531–542. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Chen, L.; Meng, L.; Zheng, Z. Effect of enriched thermotolerant nitrifying bacteria inoculation on reducing nitrogen loss during sewage sludge composting. Bioresour. Technol. 2020, 311, 123461. [Google Scholar] [CrossRef]
- Zhou, S.; Wen, X.; Cao, Z.; Cheng, R.; Qian, Y.; Mi, J.; Wang, Y.; Liao, X.; Ma, B.; Zou, Y.; et al. Modified cornstalk biochar can reduce ammonia emissions from compost by increasing the number of ammonia-oxidizing bacteria and decreasing urease activity. Bioresour. Technol. 2021, 319, 124120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, J.; Ma, X.; Li, S.; Chen, X.; Liu, T.; Wang, Q.; Wang, J.J.; Wei, D.; Zhang, Z.; et al. Solid digestate biochar amendment on pig manure composting: Nitrogen cycle and balance. Bioresour. Technol. 2022, 349, 126848. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z. Effect of biochar and bacterial inoculum additions on cow dung composting. Bioresour. Technol. 2020, 297, 122407. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Yin, J.; Cui, Z.; Li, G.; Liu, G.; Jiang, J.; Yuan, J. Risk level and removal performance of antibiotic resistance genes and bacterial pathogens in static composting with different temperatures. Bioresour. Technol. 2024, 412, 131420. [Google Scholar] [CrossRef]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Yu, J.; Wang, J.; Dai, X.; Zhao, W. Effects of phosphogypsum and medical stone on nitrogen transformation, nitrogen functional genes, and bacterial community during aerobic composting. Sci. Total Environ. 2021, 753, 141746. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Zhang, Y.; Fang, Y.; Li, S.; Li, G.; Luo, W. Factors affecting gaseous emissions, maturity, and energy efficiency in composting of livestock manure digestate. Sci. Total Environ. 2020, 731, 139157. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.; Zhai, B.; Wang, Z.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef]
- Wang, P.; Huang, Q.; Xiao, H.; Zhang, Z.; Qiao, Y.; Chen, Y.; Hu, C. The effect of carbonate and biochar on carbon and nitrogen losses during composting. J. Mater. Cycles Waste Manag. 2022, 24, 1485–1493. [Google Scholar] [CrossRef]
- Wang, X.; Cui, H.; Shi, J.; Zhao, X.; Zhao, Y.; Wei, Z. Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour. Technol. 2015, 198, 395–402. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Z.; Liu, G.; Wang, X.; Fang, J. Impacts of adding FeSO4 and biochar on nitrogen loss, bacterial community and related functional genes during cattle manure composting. Bioresour. Technol. 2023, 379, 129029. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-P.; Koyama, M.; Nakasaki, K. Effects of oxygen supply rate on organic matter decomposition and microbial communities during composting in a controlled lab-scale composting system. Waste Manag. 2022, 153, 275–282. [Google Scholar] [CrossRef]
- Vilela, R.N.d.S.; Orrico, A.C.A.; Orrico, M.A.P., Jr.; Aspilcueta Borquis, R.R.; Tomazi, M.; Oliveira, J.D.d.; Ávila, M.R.d.; Santos, F.T.d.; Leite, B.K.V. Effects of aeration and season on the composting of slaughterhouse waste. Environ. Technol. Innov. 2022, 27, 102505. [Google Scholar] [CrossRef]
- Li, M.X.; He, X.S.; Tang, J.; Li, X.; Zhao, R.; Tao, Y.Q.; Wang, C.; Qiu, Z.P. Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 2021, 264, 128549. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Tsang, D.C.W.; Li, G. Effects of external additives: Biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 2020, 248, 125927. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, M.; Alburquerque, J.A.; Sánchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Hu, X.; Zhang, S.; Li, A.; Deng, Y.; Wu, Y.; Li, S.; Che, R.; Cui, X. Downward aeration promotes static composting by affecting mineralization and humification. Bioresour. Technol. 2021, 338, 125592. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Sun, Z.Y.; Wang, S.T.; Tang, Y.Q. Microbial mechanisms of biochar addition on carbon and nitrogen synergistic retention during distilled grain waste composting: Insights from metagenomic analysis. Bioresour. Technol. 2024, 411, 131346. [Google Scholar] [CrossRef]
- Hagemann, N.; Subdiaga, E.; Orsetti, S.; De La Rosa, J.M.; Knicker, H.; Schmidt, H.P.; Kappler, A.; Behrens, S. Effect of biochar amendment on compost organic matter composition following aerobic composting of manure. Sci. Total Environ. 2018, 613–614, 20–29. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L. Addition of mature compost improves the composting of green waste. Bioresour. Technol. 2022, 350, 126927. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, T.P.; Liu, L.L.; Zhang, J.; Yue, B. Process of high-temperature compost of sheep manure with addition of wheat straw. Chin. J. Eco-Agric. 2010, 18, 566–569. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Xie, X.; Mohamed, T.A.; Zhu, L.; Tang, Y.; Chen, Y.; Wei, Z. Role of NH3 recycling on nitrogen fractions during sludge composting. Bioresour. Technol. 2020, 295, 122175. [Google Scholar] [CrossRef]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent developments in biochar utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef]
- Harrison, B.P.; Gao, S.; Thao, T.; Gonzales, M.L.; Williams, K.L.; Scott, N.; Hale, L.; Ghezzehei, T.; Diaz, G.; Ryals, R.A. Methane and nitrous oxide emissions during biochar-composting are driven by biochar application rate and aggregate formation. GCB Bioenergy 2023, 16, e13121. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yin, H.; Han, L.; Cui, R.; Fang, C.; Huang, G. Effects of biochar size and type on gaseous emissions during pig manure/wheat straw aerobic composting: Insights into multivariate-microscale characterization and microbial mechanism. Bioresour. Technol. 2019, 271, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Wang, H.; Hui, X.; Wang, Z.; Qiu, W. Long-term nitrogen fertilization impacts soil fungal and bacterial community structures in a dryland soil of Loess Plateau in China. J. Soils Sediments 2017, 18, 1632–1640. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Chen, X.; Gui, J.; Sun, Y.; Wu, D. Industrial-scale food waste composting: Effects of aeration frequencies on oxygen consumption, enzymatic activities and bacterial community succession. Bioresour. Technol. 2021, 320, 124357. [Google Scholar] [CrossRef]
- Wang, H.; Shao, T.; Zhou, Y.; Long, X.; Rengel, Z. The effect of biochar prepared at different pyrolysis temperatures on microbially driven conversion and retention of nitrogen during composting. Heliyon 2023, 9, e13698. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, X.; Zhang, W.; Men, M.; Xu, B.; Deng, L.; Bello, A.; Jiang, X.; Sheng, S.; Wu, X. Bacterial community succession in dairy manure composting with a static composting technique. Can. J. Microbiol. 2019, 65, 436–449. [Google Scholar] [CrossRef]
- Wells-Bennik, M.H.; Eijlander, R.T.; Den Besten, H.M.; Berendsen, E.M.; Warda, A.K.; Krawczyk, A.O.; Nierop Groot, M.N.; Xiao, Y.; Zwietering, M.H.; Kuipers, O.P.; et al. Bacterial Spores in Food: Survival, Emergence, and Outgrowth. Annu. Rev. Food Sci. Technol. 2016, 7, 457–482. [Google Scholar] [CrossRef]
- Subirats, J.; Sharpe, H.; Topp, E. Fate of Clostridia and other spore-forming Firmicute bacteria during feedstock anaerobic digestion and aerobic composting. J. Environ. Manag. 2022, 309, 114643. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.; Han, Y.; Qian, W.; Li, G.; Luo, W. Performance of mature compost to control gaseous emissions in kitchen waste composting. Sci. Total Environ. 2019, 657, 262–269. [Google Scholar] [CrossRef]
- Wong, J.W.; Fung, S.O.; Selvam, A. Coal fly ash and lime addition enhances the rate and efficiency of decomposition of food waste during composting. Bioresour. Technol. 2009, 100, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, G.; Huda, N.; Zhang, B.; Wang, M.; Luo, W. Effects of moisture and carbon/nitrogen ratio on gaseous emissions and maturity during direct composting of cornstalks used for filtration of anaerobically digested manure centrate. Bioresour. Technol. 2020, 298, 122503. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Zhao, X.; He, X.; Huang, C.; Tan, W.; Gao, R.; Zhang, H.; Li, D. Successions and diversity of humic-reducing microorganisms and their association with physical-chemical parameters during composting. Bioresour. Technol. 2016, 219, 204–211. [Google Scholar] [CrossRef]
- Kim, J.J.; Alkawally, M.; Brady, A.L.; Rijpstra, W.I.C.; Sinninghe Damste, J.S.; Dunfield, P.F. Chryseolinea serpens gen. nov., sp. nov., a member of the phylum Bacteroidetes isolated from soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 654–660. [Google Scholar] [CrossRef]
- Wan, X. Comparative Genome Analyses Reveal the Genomic Traits and Host Plant Adaptations of Flavobacterium akiainvivens IK-1T. Int. J. Mol. Sci. 2019, 20, 4910. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]


| Feedstock | Total Carbon % | Total N % | C/N | pH | Moisture Content % |
|---|---|---|---|---|---|
| Cattle manure | 33.12 | 2.17 | 15.20 | 8.77 | 79.60 |
| Corn Straw | 40.35 | 1.32 | 30.51 | 6.44 | 12.53 |
| Biochar | 44.52 | 0.78 | 57.08 | 9.42 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wu, H.; Zhao, Y.; Wu, Y.; Ming, R.; Liu, D.; Qiao, Y.; Xiao, Z.; Ren, J.; Chen, Y.; et al. Biochar Effectively Reduced N2O Emissions During Heap Composting and NH3 Emissions During Aerobic Composting. Agriculture 2025, 15, 1907. https://doi.org/10.3390/agriculture15181907
Zhang Z, Wu H, Zhao Y, Wu Y, Ming R, Liu D, Qiao Y, Xiao Z, Ren J, Chen Y, et al. Biochar Effectively Reduced N2O Emissions During Heap Composting and NH3 Emissions During Aerobic Composting. Agriculture. 2025; 15(18):1907. https://doi.org/10.3390/agriculture15181907
Chicago/Turabian StyleZhang, Zhi, Haicheng Wu, Yue Zhao, Yupeng Wu, Runting Ming, Donghai Liu, Yan Qiao, Zhuoxi Xiao, Jian Ren, Yunfeng Chen, and et al. 2025. "Biochar Effectively Reduced N2O Emissions During Heap Composting and NH3 Emissions During Aerobic Composting" Agriculture 15, no. 18: 1907. https://doi.org/10.3390/agriculture15181907
APA StyleZhang, Z., Wu, H., Zhao, Y., Wu, Y., Ming, R., Liu, D., Qiao, Y., Xiao, Z., Ren, J., Chen, Y., & Hu, C. (2025). Biochar Effectively Reduced N2O Emissions During Heap Composting and NH3 Emissions During Aerobic Composting. Agriculture, 15(18), 1907. https://doi.org/10.3390/agriculture15181907






