Abstract
Mismanagement of rural wastewater can lead to environmental contamination with the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D). Fungi with bioremediating potential constitute a sustainable alternative to decontaminate such wastewater before its reuse. This study evaluated the ability of Aspergillus oryzae pellets to remove 2,4-D from natural and sterile rural wastewater (i.e., with/without native microbiota). The pellets were produced by incubating conidial solutions of A. oryzae strains RCA2, RCA4, RCA5, and RCA10 in synthetic wastewater for 21 days at 25 °C. The wastewater samples were characterized physicochemically and microbiologically upon arrival at the laboratory. Afterwards, they were supplemented with 1, 2.5, or 5 mmol L−1 of 2,4-D and inoculated with the pellets. Physicochemical characterization was repeated throughout the experiment. Herbicide removal and the presence of 2,4-D degradation intermediate, 2,4-dichlorophenol (2,4-DCP), were assessed through high-pressure liquid chromatography with UV/Vis detection (HPLC-UV) and mass spectrometry. At the beginning of the assay, the macro- and micronutrient content in the samples were suitable to sustain fungal growth. By the end, pH had increased and sodium and nitrate levels decreased in comparison with the control. RCA2, RCA4, and RCA10 removed over 80% of 2,4-D after 7 days of incubation, at the three herbicide concentrations tested. Moreover, wet fungal biomass had increased by the end of the assay. These findings demonstrate that RCA2, RCA4, and RCA10 can grow, form pellets, and remove 2,4-D in natural rural wastewater, which makes them potential candidates for bioremediation strategies aimed at improving the quality of water set to be reused.
1. Introduction
As a consequence of modern industrialization and urbanization, natural ecosystems around the world have become contaminated with hazardous pollutants like pesticides, heavy metals, petroleum hydrocarbons, and organic dyes [1,2,3]. Some of these pollutants are chlorinated aromatics, which are used as intermediates or solvents to manufacture a vast array of products, including flame retardants, dyes, textile additives, pharmaceuticals, polymers and resins, air fresheners, drain cleaners, paint thinners, and condenser liquids [4,5]. In the context of agriculture, chlorinated aromatic herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) is one of the most widely used herbicides for large crops in Argentina and around the world. Intensive application of 2,4-D at higher-than-recommended concentrations leads to its accumulation and persistence in the environment [6]. Its main degradation product, 2,4-dichlorophenol (2,4-DCP), is also an intermediate in the degradation of compounds such as triclosan, dichlorprop, and 2,4-dichlorophenoxybutanoic acid [4]. This chlorinated phenol is just as toxic as 2,4-D, if not more so. It has restricted volatility, low biodegradability, and a particular aptness for persistence, thanks to its structure featuring a hydroxyl group, two chlorine atoms, and a benzene ring [7,8].
One of the ways in which 2,4-D ends up in the environment is through contaminated liquid waste generated by intensive animal farming. Disposing of these effluents safely is problematic [9]. National and international regulations set limits to the amount of pollutants permitted in wastewater before its release or reuse. In Argentina, wastewater meant to be reused for crop irrigation must conform to state-mandated physicochemical standards. For instance, the total pesticide concentration must not exceed 0.005 mg L−1. However, mismanagement of rural wastewater often means that these standards are not complied with [10,11].
Wastewater can be made safer for discharge/reuse through traditional physical and chemical methods aimed at removing the contaminants within it. Some of these techniques are water washing, heat application, and hydrolysis [12,13]. Cleaning solutions containing acids, salts, and surfactants are also used for enhanced pesticide residue removal [14,15]. Oxidizing agents, e.g., chlorine, ozone, and chlorine dioxide, are able to degrade pesticides through chemical reactions [16,17,18,19]. Ozone, in particular, may do so either by direct oxidation or by forming free radicals, and has shown promise for the removal of pesticide residues in food and water [20,21,22]. Similarly, sulfate radical-based processes use oxidants such as peroxymonosulfate and persulfate to generate free radicals that degrade pesticides [20]. Another emerging tool is electrolyzed water, which has gained attention as a way to remove pesticides from water and disinfect food [18,19].
Although sometimes effective, conventional physicochemical remediation has several drawbacks. It can have high operating costs and involve complicated technology. Moreover, in the process of removing contaminants from wastewater, it can itself generate secondary pollution in the form of chemical residues with environmental risks. Biological remediation, in contrast, is a less costly and more sustainable alternative [23]. The many techniques grouped under this umbrella term can target pollutants with great specificity. For instance, stabilization ponds are a low-cost strategy that harnesses the remediating ability of bacteria and algae to degrade organic and inorganic components in wastewater. Nevertheless, they have a significant disadvantage: degradation rates are slow; so, good results require large ponds and long periods of time [23]. A new biological technique, which may bypass these limitations and is increasingly being investigated, is the use of fungal pellets, also known as mycelial pellets.
Compared to free-living microbial cells, fungal hyphae aggregate in the form of pellets are more tolerant to environmental stressors, have better sedimentation qualities, and are more efficient in removing pollutants [24,25,26,27]. They can act as biological carriers that provide a suitable environment for the immobilization of bacteria, microalgae, and other remediating microorganisms. Furthermore, they can be combined with biochar, nanomaterials, or other substances to create composite materials with enhanced capabilities for wastewater treatment [28]. They have been successfully used to remove heavy metals and organic contaminants [26], and recent research has demonstrated their potential for pesticide cleanup [25,27,29,30,31,32]. Their incorporation into bioremediation systems reduces secondary pollution and can be scaled up at feasible costs in both aquatic and terrestrial ecosystems [25,31,32]. As biotechnological products with the capacity to convert waste into a resource, reduce pollution, and generate commercial fungal biomass, fungal pellets fit right into models of circular economy [33,34,35]. Finally, they can make wastewater suitable to be reused for crop irrigation [36].
Filamentous fungi are highly appropriate candidates for the formation of these pellets. They can colonize a variety of substrates, even heavily salted and pesticide-polluted ones, thanks to their enzymatic machinery, which breaks down harmful substances as sources of carbon, phosphorus, or nitrogen. Moreover, they proliferate quickly and have brief acclimation periods in the presence of xenobiotic chemicals [28,37]. The mechanism through which they remove 2,4-D has not been fully elucidated. Nevertheless, 2,4-DCP is known to be one of the most significant intermediates in the process, which means degradation is likely involved.
Our previous studies explored the potential of certain filamentous strains to remove 2,4-D and 2,4-DCP from natural and synthetic wastewater [32,38,39]. Fungal pellets of Penicillum crustosum RCP4 and RCP13 achieved removal efficiencies of 54 to 75% at an herbicide concentration of 5 mmol L−1 in synthetic media simulating natural wastewater [32]. The same strains tolerated and removed 2,4-D after 21 days in rural wastewater containing 1, 2.5, and 5 mmol L−1 of 2,4-D. Removal rates were between 20 and 50% for RCP4 and between 10 and 70% for RCP13 [39]. In yet another study, Aspergillus oryzae strains were selected according to their capacity to develop in synthetic wastewater and eliminate 2,4-D from it [38]. On the basis of those results, we hypothesized that A. oryzae pellets could efficiently remove 2,4-D from natural rural wastewater and that their performance might be enhanced by synergistic interactions with the native microbiota. Thus, the present study evaluated the ability of pellets formed with four A. oryzae strains to remove the herbicide from natural rural wastewater, under both natural and sterile conditions. It also assessed the development of fungal biomass and changes in key wastewater parameters throughout treatment. The findings aim to contribute to the design of sustainable and scalable biotechnological strategies to remediate herbicide-contaminated wastewater before its reuse in agricultural ecosystems.
2. Materials and Methods
2.1. Collection and Characterization of Wastewater Samples
Sampling was carried out on a farm in the south of the province of Córdoba, Argentina (33°02′49.4″ S, 64°48′24.0″ W). There, wastewater generated by the cleaning of farrowing pens is discharged once a week into an aeration lagoon, where it is treated mechanically. More precisely, machinery in the lagoon stirs the water and supplies it with oxygen to promote the biological degradation of organic matter by aerobic microorganisms. Then, solids are removed by sedimentation, and the wastewater proceeds to a second, stabilization-only lagoon, in which it undergoes additional decantation/sedimentation for 7 to 10 days. Having thus only received primary treatment (i.e., basic treatment to remove solid particles), the wastewater is finally released into the field to irrigate crops. The samples for our study were collected about 30 to 50 cm below the surface in the second lagoon, on day 10, just before the water was released into the field, so that it would be as stabilized as possible. Figure 1 shows the location of the rural establishment where sampling took place and both lagoons.
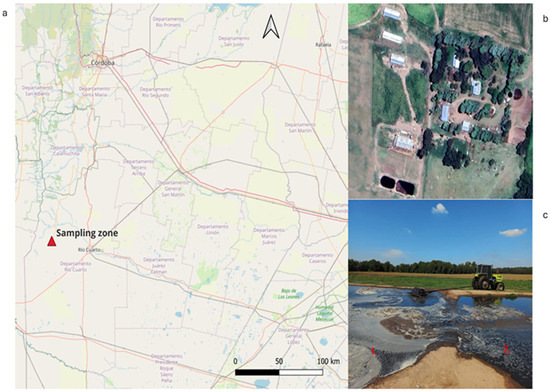
Figure 1.
(a) Geographic location of the sampling site in the province of Córdoba, Argentina. The red triangle indicates the coordinates 33°02′49.4″ S, 64°48′24.0″ W (datum WGS84). The map was generated using QGIS 3.34 with OpenStreetMap base layer. (b) Satellite view of the wastewater treatment lagoons, which are between 1.5 and 2 m deep and 30 m long, and can hold a volume of approximately 1,000,000 L. (c) Photo showing both lagoons (labeled in red 1 and 2) and mechanical machinery aerating the wastewater in the first.
Upon their arrival at the laboratory, the samples were characterized physicochemically and microbiologically [40]. Physicochemical characterization was then repeated every 14 days throughout the 2,4-D removal assays (described further below). The following parameters were determined: macronutrient levels (carbon indirectly measured as total solids, nitrate, phosphorus); micronutrient levels (magnesium, calcium, potassium, sodium); pH; electrical conductivity (EC); and total solids (TS). Microbiological characterization was performed by mixing 10 mL of wastewater with 90 mL of a 0.1% peptone water solution. After homogenization for 30 min, serial dilutions were inoculated (0.1 mL) on plates in triplicate by the surface sowing method. Dichloran Rose Bengal Chloramphenicol (DRBC) plates, used for fungi, were incubated at 25 °C for 7 days; nutritive agar (NA) plates, used for bacteria, were incubated at 37 °C for 48 h. At the end of the incubations, fungal and bacterial counts were determined and expressed as colony-forming units per milliliter (CFU mL−1).
2.2. Fungal Strains and Production of Fungal Pellets
The fungal strains chosen for pellet formation were Aspergillus oryzae RCA2 (OP032101), RCA4 (OP032102), RCA5 (OP032104), and RCA10 (OP032103), which had been originally isolated from soils with a history of pesticide contamination. Earlier studies found that they were tolerant to 2,4-D and non-toxigenic, and that they achieved high removal efficiency in synthetic wastewater [38]. Conidial suspensions were prepared by gently scraping 7-day-old colonies grown on Malt Extract Agar (MEA) using distilled water. The suspensions were decanted and quantified with a hemocytometer (Boeco, Hamburg, Germany) [41]. After that, 2 mL of each suspension (108 spores mL−1) was inoculated into Erlenmeyer flasks containing 100 mL of synthetic wastewater [39] and incubated at 25 °C for 3–5 days with agitation (150 rpm), to maintain adequate oxygenation. The resulting fungal pellets were recovered by filtration, rinsed with sterile distilled water, and directly inoculated into the rural wastewater samples [42].
2.3. Inoculation and Incubation of Rural Wastewater
The wastewater samples were either kept in their natural state or sterilized by autoclaving at 121 °C and 1 atm for 15 min. The aim of sterilization was to inactivate native microbiota, so as to subsequently evaluate the activity of the inoculated fungal pellets on their own. On the other hand, a 1 mol L−1 stock solution of 2,4-D was prepared, as described by Magnoli et al. (2023) [32]. The herbicide used was the commercial formulation 2,4-D amine (2,4-dichlorophenoxyacetic acid, Herbifen, Atanor®, Buenos Aires, Argentina). Erlenmeyer flasks were then prepared with 150 mL of the wastewater samples, to which appropriate volumes of the stock herbicide solution were added to obtain final concentrations of 1, 2.5, and 5 mmol L−1. These concentrations represent the range reported for rural/agricultural wastewater at the time of its release into the environment [43].
Each flask was inoculated with 4 g of the fungal pellets produced, as outlined in Section 2.2. The controls consisted of natural and sterile wastewater in which the pellets were inoculated, but the herbicide was not added, and vice versa. Additional controls were prepared in flasks containing the herbicide and inoculated with dead fungal biomass, i.e., inactive mycelium of the four A. oryzae strains. All the flasks were incubated at 25 °C for 42 days with agitation (150 rpm). Each treatment was performed in triplicate, and the entire experiment was repeated twice.
2.4. Measurement of Residual 2,4-D and 2,4-DCP by High-Pressure Liquid Chromatography with UV/Vis Detection (HPLC-UV)
The concentrations of 2,4-D and 2,4-DCP were measured by HPLC-UV in the natural wastewater upon its arrival at the laboratory and throughout the experiment. On days 0, 7, 14, 21, and 42, 1 mL aliquots were taken from each treatment and from the controls and stored at 4 °C until analysis. Acidification or fixation was not performed, since the compounds are stable under refrigeration. Before the detection study, the samples were centrifuged and filtered (Whatman 0.45 µm, GE Healthcare Life Sciences- Cytiva, Marlborough, Massachusetts, USA).
For 2,4-D, the modified protocol by Sanchis et al. (2014) was performed [32,44] in a Waters Alliance e2695 system equipped with a separation module and a fluorescence/UV-PDA detector (Waters Corporation, Milford, MA, USA). A 25 cm long reverse-phase C18 analytical column was used (Luna, Phenomenex, Torrance, CA, USA), with an internal diameter of 4.6 mm and a particle size of 5 μm. For protection, it was coupled to a SecurityGuard pre-column (KJO-4282; 20 mm × 4.6 mm, 5 μm particle size, also from Phenomenex). The samples were separated under gradient elution, with acetonitrile and deionized water as the mobile phase. The gradient program was set as follows: acetonitrile:H2O from 80:20 to 65:35 between minutes 0 and 15, and from 65:35 to 25:75 from minutes 15 to 30. The flow rate was maintained at 0.80 mL min−1 throughout the run. Detection was performed at 280 nm (UVA). Quantification was based on the retention time of standard 2,4-D solutions (Sigma-Aldrich, Buenos Aires, Argentina), which appeared after 5 min. Compound removal was estimated by considering a calibration curve that plotted the peak area against known concentrations of 2,4-D (from 0.2 to 5 × 106 µg L−1), according to the linear regression model expressed in Equation (1):
where y represents the peak area, and x corresponds to the 2,4-D concentration. The limit of detection (LOD) was 0.02 µg L−1, and the limit of quantification (LOQ) was 0.2 µg L−1.
y = 7313.2x − 516,274
For 2,4-DCP, HPLC-UV was performed as in Gutiérrez-Zapata et al. (2017) [39,45], with modifications. Elution was carried out with acetonitrile and water as solvents under the following gradient schedule: 0–1 min: 64% acetonitrile/36% water; 2.5–6 min: 74% acetonitrile/26% water; and 7 min: 74% acetonitrile/36% water. Detection was performed at 227 nm. Quantification was based on the retention time of certified 2,4-DCP standards, which appeared approximately after 5 min, and considered a calibration curve generated by plotting the peak area against known 2,4-DCP concentrations (from 2 × 104 to 1.6 × 105 µg L−1), as in:
where y represents the peak area, and x corresponds to the 2,4-DCP concentration. The LOD was 0.06 µg L−1, and the LOQ was 0.2 µg L−1.
y = 20717x − 131,068
A recovery assay was also performed in HPLC-UV for both analytes. The 10 mL samples were spiked with decreasing concentrations (from 2 × 105 to 2 × 104 μg L−1) of 2,4-D and 2,4-DCP stock solutions (2 × 105 μg L−1). Each spiked sample was prepared in triplicate, and one blank sample was analyzed.
The results obtained with this method for 2,4-D were validated by subjecting the samples to high-sensitivity mass spectrometry (UHPLC-ESI-QqQ-MS/MS, Acquity, Waters Corporation, Milford, MA, USA) [46]. Prior to this analysis, the liquid extracts were gently evaporated to dryness under a nitrogen stream at 40 °C and reconstituted with 1 mL of a 1:1 methanol:water solution. Each reconstituted sample was filtered through a 0.22 μm nylon membrane (Microclar, Buenos Aires, Argentina) and transferred to a 1.5 mL autosampler vial. An injection volume of 20 μL was loaded into an Acquity UHPLC BEH C18 column (100 × 2.1 mm, 1.7 μm), preceded by a VanGuard C18 pre-column (5 × 2.1 mm, 1.7 μm) (Acquity, Waters Corporation, Milford, MA, USA). To calculate removal percentages, a standard curve was constructed with the same concentration range used for HPLC-UV. The LOD was 0.005 µg L−1. Compounds were identified based on their retention time compared with that of certified standards, as well as by considering the detection of two fragment ions derived from a precursor ion. The most abundant product ion (Q) was used for quantification, while the second, less intense fragment (q) served as confirmation. The Q/q ratio had to fall between 0.8 and 1.2 for identification to be valid. The data were acquired and processed on MassLynx NT (version 4.1, Waters). Isotopic patterns, particularly from chlorine atoms (i.e., 35Cl and 37Cl), were also used to strengthen analyte identification by selecting characteristic ion transitions. The precursor ion to detect 2,4-D was m/z 218.8. The formula below was used to estimate the percentage of 2,4-D reduction in the samples:
where %D is the degradation percentage, and Ci and Cf are the initial and final concentrations of 2,4-D, respectively.
%D = [(Ci − Cf)/Ci] × 100
2.5. Viability and Measurement of Fungal Biomass
To assess the viability of the fungal pellets at the end of the experiment, representative pellets were taken from the wastewater treatments on day 42 of incubation. They were transferred to MEA dishes and incubated at 25 °C for 7 days [47]. Viability was then ascertained through observation. On the same day, the pellets in each flask were filtered out, and their wet biomass was weighed. These measurements were compared to the weight of the initial inocula, to assess fungal growth at different 2,4-D concentrations.
2.6. Statistical Analyses
The data for physicochemical parameters, herbicide removal, and fungal biomass were analyzed using ANOVA. Means were compared through a linear mixed model and Fisher’s protected least significant difference (LSD) test. For variance homogeneity, removal percentages and herbicide concentrations were log-transformed (log10 (x + 1)). Fisher’s LSD was also used to identify significant differences between the 2,4-D removal percentages corresponding to different fungal strains. The linear relationship between the results obtained through HPLC-UV and mass spectrometry was verified with Pearson’s correlation coefficient. All the data were statistically analyzed on InfoStat Professional (v2017) [48].
3. Results
3.1. Physicochemical and Microbiological Characterization of the Wastewater Samples
Table 1 shows the physicochemical characterization of the wastewater samples, performed both at the beginning of assay (control) and throughout the 42 days that the removal assay lasted. The results shown correspond to those samples inoculated with RCA2 or RCA10, since they were very similar to those obtained in the samples inoculated with RCA4 or RCA5, respectively.

Table 1.
Physicochemical characterization of rural wastewater at the beginning of the assay (day 0, control) and after 14, 28, and 42 days of incubation with pellets of A. oryzae RCA2 and RCA10.
On day 0, the macro- and micronutrient levels in the wastewater were adequate to support fungal growth and metabolic activity. In other words, the conditions were appropriate to study herbicide removal by the strains since they would be able to grow and survive in the substrate. The microbiological characterization conducted on the same day revealed a mesophilic bacterial count between 3.0 and 3.52 log10 CFU mL−1 and a total mycota count between 3.5 and 3.7 log10 CFU mL−1.
The physicochemical measurements differed significantly (p < 0.001) throughout the incubation (Table 1). The behavior of RCA 4 and RCA5 is, respectively, similar to that of RCA2 and RCA10. For this reason, two representative strains were selected to present the results. In wastewater inoculated with RCA2, electrical conductivity decreased by 16.3%, total solids by 2.3%, potassium by 23.5%, nitrate by 13%, and phosphorus by 46.13%. On the other hand, wastewater inoculated with RCA10 underwent a reduction of 21.5% in electrical conductivity, 0.54% in total solids, 25% in sodium, 13% in potassium, 20% in nitrate, and 43% in phosphorus. Both strains achieved noteworthy reductions in calcium and magnesium levels: 75 and 66.6%, respectively. As for pH, it increased by 13% in RCA2-treated samples and by 14% in RCA10-treated ones.
3.2. Removal of 2,4-D by the A. oryzae Pellets in Natural and Sterile Rural Wastewater
The average recovery rates of HPLC-UV were determined for both analytes in this study (2,4-D and 2,4-DCP). Across all concentrations of 2,4-D, they ranged between 88 and 95%, i.e., they were within acceptable analytical limits. The highest recovery (95 ± 3%) was observed at 5 × 106 µg L−1, while the lowest (88 ± 5%) corresponded to the lowest spiking level (0.2 × 106 µg L−1) but remained above the LOD of the method (0.02 µg L−1). 2,4-D was not detected in the blank sample. On the other hand, the recovery rates for 2,4-DCP were consistently above 85% for concentrations ≥2 × 104 μg L−1, with a mean recovery of 92 ± 4% at the highest spiking level. These results confirm the efficiency and reliability of HPLC-UV to quantify 2,4-D and 2,4-DCP in a complex aqueous matrix like wastewater.
Analysis of the natural wastewater samples upon their arrival at the laboratory showed no evidence of 2,4-DCP, whereas residual 2,4-D levels were 0.05 ± 0.02 µg L−1. This is above the LOD but below the LOQ of the technique.
In the uninoculated natural wastewater controls containing different concentrations of 2,4-D, herbicide removal varied from 20 to 30% throughout the incubation. Similarly, 10–26% and 5–7% of the herbicide, respectively, were removed in natural and sterile wastewater inoculated with the inactive biomass of the four strains, at all the 2,4-D concentrations tested.
Figure 2 shows the removal percentages registered in sterile and natural wastewater inoculated with the different fungal pellets. In samples inoculated with RCA2 (Figure 2a), rising herbicide concentrations were associated with increasing removal percentages for the duration of the incubation. By day 7, removal was over 80% under all the conditions assayed. In natural and sterile wastewater supplemented with 2.5 and 5 mmol L−1 of 2,4-D, removal was 100 and 99% after 14 and 21 days of incubation (p < 0.001). Natural wastewater containing 1 mmol L−1 of the herbicide also exhibited complete removal after 21 days.
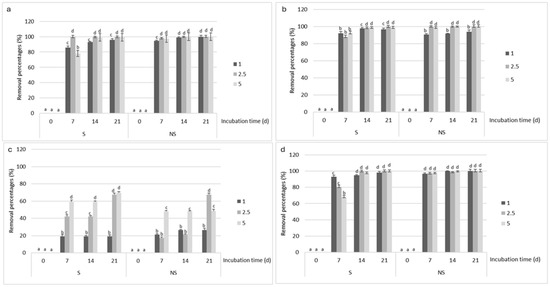
Figure 2.
2,4-D removal percentages at different initial herbicide concentrations (1, 2.5, and 5 mmol L−1) in sterile (S) and natural (N) rural wastewater. The measurements were made over 21 days of incubation at 25 °C after inoculation with (a) A. oryzae RCA2; (b) A. oryzae RCA4; (c) A. oryzae RCA5; and (d) A. oryzae RCA10. a–d Mean values are based on triplicate data. Means in a row with a letter in common are not significantly different according to the LSD test (p < 0.01). LOD = 0.02 µg L−1.
In the case of RCA4 (Figure 2b) in sterile wastewater, removal was greater than 90% after seven days of incubation at all 2,4-D concentrations evaluated. In the same wastewater supplemented with 2.5 and 5 mmol L−1 of 2,4-D, it respectively reached 99% and 98% after 21 days of incubation. In natural wastewater, the strain achieved 100% removal after 7 and 14 days at the two highest 2,4-D concentrations.
The highest removal percentages for RCA5 (Figure 2c) were 68 and 69% in sterile wastewater containing 2.5 and 5 mmol L−1 of 2,4-D, respectively. In natural wastewater, the percentages were 67% at 2.5 mmol L−1 and 48% with 5 mmol L−1 (p < 0.001). At the lowest concentration (1 mmol L−1), the strain removed 19% of the herbicide in the sterile samples and 26% in the natural ones.
Lastly, removal in sterile wastewater inoculated with RCA10 (Figure 2d) was, respectively, 68, 80, and 97% at 5, 2.5, and 1 mmol L−1. In natural wastewater, it exceeded 96% after 7 days of incubation, independently of 2,4-D concentration, and was complete at 2.5 and 5 mmol L−1 after 21 days.
In all cases, 2,4-DCP levels remained below the LOQ of HPLC-UV (less than 200 µg L−1) throughout the incubation.
When considering these results, RCA2, RCA4, and RCA10 emerge as the best-performing strains out of the four that were assessed, both in sterile and natural wastewater. For these three strains, the optimal combination of variables for efficient herbicide removal was 2.5 mmol L−1 of 2,4-D and 7 days of incubation.
As seen in Table 2, the F-values determined through an analysis of variance (ANOVA) revealed that all the variables intervening in our study were statistically significant (p < 0.01) for the results obtained. These variables are fungal strain, 2,4-D concentration, days of incubation, and wastewater condition (natural or sterile). With an F-value of 26,770.91, herbicide concentration was the most influential and is thus likely to account for the largest proportion of the variability observed. Additionally, all two-, three-, and four-way interactions between variables were statistically significant, which highlights the complex interdependencies between these factors. Strong synergy was especially recorded for interactions such as strain × concentration (F = 1541.00 *), strain × wastewater condition (F = 1117.75 *), and concentrations × wastewater conditions (F = 869.43 *). The fact that a significant value (F = 130.44 *) was obtained even for the highest-order interaction, i.e., the one involving all four variables, is evidence of the multifactorial nature of the system. On the other hand, the minimal residual variance (mean square error = 0.91) confirmed that the experimental design was robust and successfully captured the main sources of variation. Furthermore, a positive correlation (r = 0.96; p < 0.01) was found between the results obtained through HPLC-UV and mass spectrometry.

Table 2.
Analysis of variance of the relative influence of strain (S), 2,4-D concentration (C), days of incubation (D), wastewater condition (Co), and their interactions on herbicide removal by A. oryzae strains.
3.3. Fungal Biomass Viability and Growth
The fungal pellets taken from the different treatments after 42 days of incubation showed good development on MEA plates. As for fungal biomass, it remained constant with respect to the initial weight (4 g) in all the controls.. In contrast, the biomass of RCA2 (Figure 3a) registered significant increases with respect to the initial inoculum, the highest of which was 530% with 1 mmol L−1 of 2,4-D in sterile wastewater (p < 0.001). In natural wastewater, it increased by 94% at 2.5 mmol L−1 and by 108% at 5 mmol L−1 of 2,4-D (p< 0.001). RCA4 generally developed better at both higher and lower concentrations of the herbicide in natural than in sterile wastewater. Nevertheless, its biomass increased significantly under all the conditions assayed (Figure 3b), with the exception of 5 mmol L−1 in sterile samples. The highest percentages for this strain were 260% in sterile wastewater and 285% in natural wastewater, at 2.5 mmol L−1 of 2,4-D in both cases (p < 0.001). This last percentage was the highest increase recorded for any of the strains in natural wastewater. In the case of RCA5 (Figure 3c), biomass increased significantly, by 155, 188, and 251% in sterile wastewater with 1, 2.5, and 5 mmol L−1 of 2,4-D, respectively (p < 0.001). In natural wastewater, the percentages at each concentration were, respectively, 41, 99, and 82% at 1, 2.5, and 5 mmol L−1. Lastly, the fungal biomass of RCA10 (Figure 3d) underwent its most considerable increase (134%) at the highest 2,4-D concentration in natural wastewater.
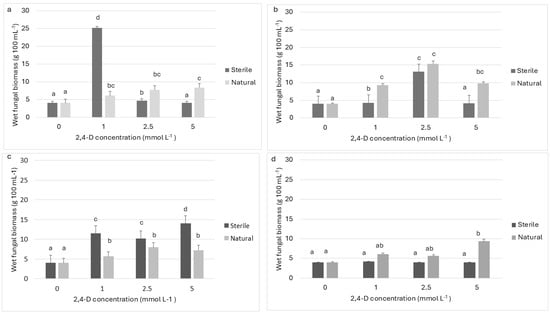
Figure 3.
Wet fungal biomass in sterile and natural rural wastewater at 25 °C, supplemented with different 2,4-D concentrations (1, 2.5, and 5 mmol L−1) and inoculated with (a) A. oryzae RCA2; (b) A. oryzae RCA4; (c) A. oryzae RCA5; and (d) A. oryzae RCA10. Measurements were made at the end of the removal assay (42 days). Mean values are based on triplicate data. a–d: Means in a row with a letter in common are not significantly different according to the LSD test (p < 0.01).
To illustrate the growth observed for the strains, RCA4 is used as an example in Figure 4. Figure 4a shows the uniformly sized and star-shaped flocs (around 1–2 cm2) formed by the strain after 5 days at 25 °C in synthetic wastewater. Figure 4b shows the growth of the strain after 42 days in rural wastewater during the 2,4-D removal assay.
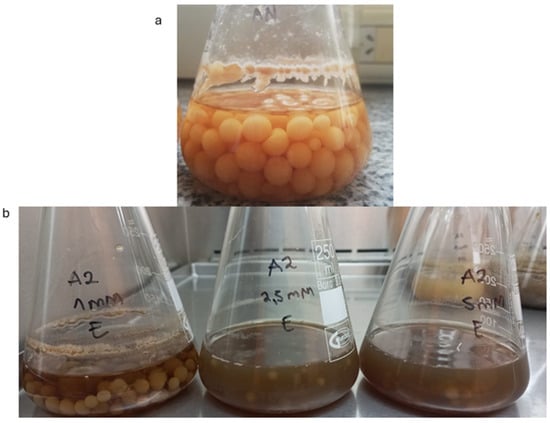
Figure 4.
(a) Fungal pellets of A. oryzae RCA4 formed after 5 days of incubation in synthetic wastewater. These pellets were directly inoculated into natural and sterile wastewater for the 2,4-D removal assay. (b) A. oryzae RCA4 fungal pellets after 42 days of incubation in the removal assay.
4. Discussion
This study explored the ability of pellets formed with four different strains of A. oryzae (RCA2, RCA4, RCA5, and RCA10) to remove 2,4-D from rural wastewater. A physicochemical evaluation showed that several parameters in the wastewater had undergone significant changes after treatment with the pellets. Sodium levels, total nitrates, and pH had been modified in such a way that they complied with limitations established by national and provincial legislation for the reuse of wastewater to irrigate crops [11]. The increase observed in pH might have been due to the fungi consuming organic acids and releasing ammonium as part of their metabolic activity [49,50]. The decrease in nitrates and phosphorus indicates active nutrient assimilation by the fungi and the potential involvement of enzymatic pathways related to the metabolism of nitrogen and phosphate [50]. In this respect, RCA2 was the most efficient at removing phosphorus, possibly due to strain-specific metabolic capabilities. Electrical conductivity and total solids also decreased over time, probably because of the fungi uptaking or transforming dissolved ions and particulate matter. This is consistent with the substantial reductions recorded in magnesium, sodium, and potassium levels, which can be attributed to bioaccumulation and ion exchange processes within the mycelial biomass.
When 2,4-D removal was measured, there were differences in the percentages obtained with each strain even though they all belong to the same species, possibly due to specific physiological variations that allow each one to adapt to the presence of the herbicide. Nevertheless, they were all able to remove the herbicide efficiently. Other fungal species have likewise been reported to reduce contaminant levels in wastewater. Aspergillus terreus and Penicillium expansum strains isolated from surface seawater at a fishing harbor in the Mediterranean Sea degraded 82% of the petroleum hydrocarbons found in refinery wastewater [51]. Trametes versicolor ATCC 42530, from the American Type Culture Collection, was immobilized on wood chips and used to treat agricultural wastewater from a drainage canal in Catalonia, Spain. It reduced diuron (58%) and chemical oxygen demand levels (>50%) and eliminated color within 225 days [52].
Out of the four strains tested in our study, A. oryzae RCA2, RCA4, and RCA10 performed best at removing 2,4-D from both natural and sterile rural wastewater samples. Removal percentages had generally surpassed 80% by day 7 of incubation. In sterile wastewater, these percentages were higher than 90% after 14 and 21 days of incubation at all 2,4-D concentrations tested. Removal reached 100% in natural wastewater after 14 days, also at the three chosen herbicide concentrations. In terms of growth measured as wet biomass, these three strains generally developed better in natural rather than in sterile wastewater, although as mentioned before, each one had distinct physiological responses. RCA4 seems to have adapted best to the physicochemical conditions in the wastewater, since it achieved the highest percentage recorded in natural wastewater (285%) supplemented with 2.5 mmol L−1 of 2,4-D. Thus, it may be the most promising candidate for rapid colonization of a wastewater ecosystem contaminated with the herbicide. Unlike the other three strains, RCA5 had the best removal and growth behavior in sterile rather than natural wastewater. This might be due to it competing for the herbicide with native microbiota, whereas in the case of the other three strains, interaction with the native microbiota seems to have positively impacted both growth and removal. Such synergy might also have sped up the strains’ removal rates, in such a way that the herbicide was entirely removed well before the end of the incubation. Moreover, native microbiota may have helped improve the physicochemical parameters in the wastewater, so that they were progressively more adequate for fungal performance and development.
Positive interactions between different microorganisms participating in contaminant removal have been posited by other researchers. Khan et al. (2023) [53] observed this between Fusarium equiseti and Sphingobium sp., a fungus and bacterium isolated from the same hexachlorocyclohexane (HCH)-contaminated soil. On Minimal Salt Medium (MSM) spiked with HCH and incubated at 25 °C for 10 days, removal of the pollutant was higher using a mixed culture made up of the two microorganisms than with monocultures of each [53] Similarly, Yu et al. (2020) [54] described a positive interaction between A. niger Y3 and Arthrobacter sp. ZXY-2 for the biodegradation of atrazine in MSM after 8 h of incubation. More specifically, the porous structure of the pellets formed by the fungal strain and the polysaccharides composing it allowed the bacterium to adsorb onto their surface. The biomixture thus created was able to immobilize the herbicide and enabled its subsequent biodegradation.
Interestingly, a remarkable increase in fungal biomass was observed in our assay after 42 days of incubation, i.e., long after complete removal of 2,4-D had been achieved between days 14 and 21. This might mean that after the herbicide had been eliminated, fungal development continued to be supported by the high concentration of organic matter in the wastewater [25]. In turn, this opens up the possibility of 2,4-D removal being the result of cometabolism: the herbicide might have been removed thanks to fungal enzymes, which were in fact stimulated by the consumption of other organic molecules in the wastewater. Once the herbicide ran out, this organic matter may have continued to fuel fungal growth [55].
Another important observation was that the pellets preserved their viability and compactness until the end of the assay, which may indicate an optimal carbon:nitrogen ratio (C:N) in the wastewater. The opposite, i.e., an imbalance in the C:N ratio, has been linked to the disintegration of fungal flocs in long-term cultures [56]. The optimal C:N ratio for fungal biomass production typically ranges from 20:1 to 40:1, depending on the fungal species and substrate composition [26,27]. Thus, the degradation of a substrate and its consequent use as a nutrient source seems to depend on this parameter. A 30:1 ratio would allow a fungus to incorporate into its biomass one-third of the carbon and all of the nitrogen in the substrate; the remaining two-thirds could be used for energy production and respiration and be subsequently released in the form of CO2 [57]. The exact C:N ratio in our wastewater samples was not determined, so further elucidation is needed regarding this. In addition, the morphology of the pellets is likely to have been greatly favored by the oxygen in the wastewater, itself a result of aeration [26] at the treatment lagoon on the farm where the wastewater was sampled.
Regarding the process through which the A. oryzae pellets may have removed 2,4-D, several mechanisms have been proposed by other authors for other fungi [55,58]. The herbicide may be degraded as the main source of carbon and energy, with both extra- and intracellular enzymes playing an important role. Another alternative is cometabolism, as explained above, i.e., fungal enzymes stimulated by the degradation of other organic compounds may break down the herbicide as a second substrate. The fact that 2,4-DCP, an intermediate of 2,4-D degradation, was detected in our wastewater samples suggests that the A. oryzae strains indeed removed 2,4-D through a degradation route. Nevertheless, it is encouraging that detection of the metabolite was below the quantification limit of the technique, since 2,4-DCP tends to persist in the environment and is highly toxic to animals and humans [59,60]. In addition to biodegradation, biosorption was posited by Pereira et al. (2021) [56] as one of the mechanisms through which fungal treatment reduces the low levels of organochlorine compounds detected in water resources. In our study, the biomass of RCA10 did not increase with respect to the initial inoculum in sterile wastewater supplemented with 2,4-D, so adsorption onto the fungal mycelium is a possible explanation for the herbicide removal observed in that treatment.
Fungal pellets, moreover, have been studied as platforms for the immobilization and application of other remediating microorganisms in wastewater. For example, Zheng et al. [27] immobilized Pseudomonas stutzeri sp. GF3 on fungal pellets and successfully removed pesticides, heavy metals, and other organic contaminants from wastewater. However, there are no reports specifically on the ability of Aspergillus spp., whether in the form of pellets or otherwise, to remove 2,4-D from rural wastewater. The genus has been studied in connection with the removal of other aromatic organochlorinated compounds, both in culture media and in environmental matrices like wastewater and soils [61,62,63,64,65]. For instance, agar plugs of active A. niger DSMZ 11167, a strain isolated from industrial wastewater with high concentrations of chlorpyrifos, removed 80% of the pesticide in soil extract medium over 5 days. The authors ascribed the elimination to a mechanism of degradation [61]. For their part, Marinho et al. (2017) [62] reported that a spore suspension of A. niger AN400 degraded 72% of the atrazine in synthetic wastewater over 8 days of incubation.
The findings described here show that, thanks to their capacity to form pellets, grow in rural wastewater, and efficiently remove 2,4-D from it, A. oryzae strains could be promising candidates to treat rural wastewater for future reuse. At a more general level, fungal inoculation holds great potential as a safe and environmentally friendly biotechnology to improve wastewater quality and reintegrate it into the water cycle within agricultural systems, in alignment with global goals for sustainability and the long-term management of water as a precious resource.
5. Conclusions
In this study, pellets formed by A. oryzae strains were effective in removing the herbicide 2,4-D from rural wastewater. Strains RCA2, RCA4, and RCA10 removed over 80% of the herbicide within 7 days post-inoculation, even at the highest 2,4-D concentration tested (5 mmol L−1), in both natural and sterile wastewater. The fact that 2,4-DCP was detected at the end of the assay suggests the mechanism of removal might have been biodegradation; but the levels detected were very low, which is encouraging, given the toxicity of this metabolite. Additionally, wet fungal biomass generally increased throughout the experiment, which demonstrates that the fungi were able to proliferate and maintain metabolic activity under the nutritional and environmental conditions in the wastewater. The ease with which the strains formed flocculent pellets that preserved their viability and compactness until the end of the assay is a major advantage, since it could make bioaugmentation with these pellets scalable at sensible costs. Furthermore, the rapid rate at which they removed the herbicide indicates that their incorporation into rural wastewater stabilization lagoons could significantly shorten treatment times. Overall, the results support the potential application of A. oryzae pellets as a sustainable biotechnological tool to improve contaminated rural wastewater quality and make it fit for reuse.
Several related aspects could be explored in future studies. For instance, the mechanism through which the strains removed 2,4-D requires further elucidation. The strains’ ability to degrade other toxic compounds in rural wastewater could also be investigated. The toxicity of the wastewater itself could be assessed further through biological indicators measured before, during, and after treatment with A. oryzae pellets. This would enable a more comprehensive understanding of the degree of sanitization achieved, as well as of the strains’ removal ability before the first 7 days of treatment.
Author Contributions
K.M. designed and conducted experiments and analyzed data, with the assistance of M.E.A., and N.B. and K.M. wrote the manuscript, with additional support from supervisors C.E.M. and C.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Agency for Scientific and Technological Promotion (ANPCYT-PICT-02728/20), the Ministry of Science and Technology of the Province of Córdoba (GRFT-2018), and the Secretary of Science and Technology of the National University of Río Cuarto (SECYT-UNRC-540/20).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to Héctor Terraneo, at whose establishment the wastewater samples were collected.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2,4-D | 2,4-dichlorophenoxyacetic Acid |
| 2,4-DCP | 2,4-dichlorophenol |
| DRBC | Dichloran Rose Bengal Chloramphenicol |
| AN | Nutritive Agar |
| MEA | Malt Extract Agar |
| LSD | Fisher’s Protected Least Significant Difference |
| CFU | Colony-Forming Units |
| HCH | Hexachlorocyclohexane |
| MSM | Minimal Salts Medium |
| LOQ | Limit of Quantification |
| LOD | Limit of Detection |
| HPLC-UV | High-Pressure Liquid Chromatography with UV/Vis Detection |
References
- Hu, K.; Barbieri, M.V.; López-García, E.; Postigo, C.; López de Alda, M.; Caminal, G.; Sarrà, M. Fungal degradation of selected medium to highly polar pesticides by Trametes versicolor: Kinetics, biodegradation pathways, and ecotoxicity of treated waters. Anal. Bioanal. Chem. 2022, 414, 439–449. [Google Scholar] [CrossRef]
- Xiang, L.; Li, G.; Wen, L.; Su, C.; Liu, Y.; Tang, H.; Dai, J. Biodegradation of aromatic pollutants meets synthetic biology. Synth. Syst. Biotechnol. 2021, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. A Review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Engesser, K.H. Degradation of 2-Chlorotoluene by Rhodococcus sp. OCT 10. Appl. Microbiol. Biotechnol. 2012, 93, 2205–2214. [Google Scholar] [CrossRef]
- Srivastav, A.L. Chemical Fertilizers and Pesticides: Role in Groundwater Contamination. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–159. [Google Scholar]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Xu, C.; Qiao, C.; Chen, S.; Zhao, C.; Liu, Q.; Zhang, X. Reconstruction of microbiome and functionality accelerated crude oil biodegradation of 2,4-DCP-oil-contaminated soil systems using composite microbial agent B-Cl. J. Hazard. Mater. 2023, 447, 130808. [Google Scholar] [CrossRef]
- Silva-Gálvez, A.L.; López-Sánchez, A.; Camargo-Valero, M.A.; Prosenc, F.; González-López, M.E.; Gradilla-Hernández, M.S. Strategies for livestock wastewater treatment and optimised nutrient recovery using microalgal-based Technologies. J. Environ. Manag. 2024, 354, 120258. [Google Scholar] [CrossRef]
- Blumenthal, U.J.; Mara, D.D.; Peasey, A.; Ruiz-Palacios, G.; Stott, R. Guidelines for the microbiological quality of treated wastewater used in agriculture: Recommendations for revising WHO Guidelines. Bull. World Health Organ. 2000, 79, 1104–1116. [Google Scholar]
- Provincia de Córdoba. Poder Ejecutivo. Decreto N 847/2016. Reglamentación de Estándares y Normas Sobre Vertidos Para La Preservación Del Recurso Hídrico Provincial. Boletín Oficial de La Provincia de Córdoba, 21 July 2016. [Google Scholar]
- Assad, R.; Sofi, I.A.; Bashir, I.; Rafiq, I.; Reshi, Z.A.; Rashid, I. Microbiological aspects of pesticide remediation in freshwater and soil environs. In Pesticide Contamination in Freshwater and Soil Environs; Apple Academic Press: Burlington, ON, USA, 2021; pp. 173–232. [Google Scholar] [CrossRef]
- Wang, J.; Han, R. Removal of Pesticide on Food by Electrolyzed Water. In Electrolyzed Water in Food: Fundamentals and Applications; Springer: Singapore, 2019; pp. 39–65. [Google Scholar] [CrossRef]
- Assad, R.; Bashir, I.; Rafiq, I.; Sofi, I.A.; Mir, S.H.; Reshi, Z.A.; Rashid, I. Global Scenario of Remediation Techniques to Combat Pesticide Pollution. In Agricultural Waste; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–97. [Google Scholar] [CrossRef]
- Munir, S.; Azeem, A.; Sikandar Zaman, M.; Zia Ul Haq, M. From Field to Table: Ensuring Food Safety by Reducing Pesticide Residues in Food. Sci. Total Environ. 2024, 922, 171382. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Z.; Chen, H.; Zhang, X.; El-Mesery, H.S.; Lu, W.; Dai, X.; Xu, R. Technology Empowering to Safeguard Agricultural Products: A review of innovative approaches toward pesticide residue monitoring. Microchem. J. 2025, 213, 113693. [Google Scholar] [CrossRef]
- Yang, J.; Song, L.; Pan, C.; Han, Y.; Kang, L. Removal of ten pesticide residues on/in Kumquat by washing with alkaline electrolysed water. Int. J. Environ. Anal. Chem. 2022, 102, 3638–3651. [Google Scholar] [CrossRef]
- Qi, H.; Huang, Q.; Hung, Y.C. Effectiveness of Electrolyzed Oxidizing Water Treatment in Removing Pesticide Residues and Its Effect on Produce Quality. Food Chem. 2018, 239, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lian, J.; Jiang, Z.; Li, Y.; Wen, C. Thermodynamic analysis on wetting states and wetting state transitions of rough surfaces. Adv. Colloid. Interface Sci. 2020, 278, 102136. [Google Scholar] [CrossRef] [PubMed]
- Calvo, H.; Redondo, D.; Remón, S.; Venturini, M.E.; Arias, E. Efficacy of electrolyzed water, chlorine dioxide and photocatalysis for disinfection and removal of pesticide residues from stone fruit. Postharvest Biol. Technol. 2019, 148, 22–31. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Chia, S.Y.; Dofuor, A.K.; Antwi-Agyakwa, A.K.; Okyere, H. Remediation of pesticide residues using ozone: A comprehensive overview. Sci. Total Environ. 2023, 894, 164933. [Google Scholar] [CrossRef]
- Feng, L.; Yue, X.; Li, J.; Zhao, F.; Yu, X.; Yang, K. Research advances in nanosensor for pesticide detection in agricultural products. Nanomaterials 2025, 15, 1132. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Recent strategies for environmental remediation of organochlorine pesticides. Appl. Sci. 2020, 10, 6286. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future Prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Varjani, S.; Taherzadeh, M.J. A Critical review on the ubiquitous role of filamentous fungi in pollution mitigation. Curr. Pollut. Rep. 2020, 6, 295–309. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Pakshirajan, K.; van Hullebusch, E.D.; Lens, P.N.L. Fungal pelleted reactors in wastewater treatment: Applications and perspectives. Chem. Eng. J. 2016, 283, 553–571. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Huang, T.; Wang, Y.; Zhang, S. Fungal pellets immobilized bacterial bioreactor for efficient nitrate removal at low C/N wastewater. Bioresour. Technol. 2021, 332, 125113. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.; Prabha, D.; Negi, Y.K.; Prasad, R. Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-77385-8. [Google Scholar]
- Ogawa, M.; Moreno-García, J.; Barzee, T.J. Filamentous fungal pellets as versatile platforms for cell immobilization: Developments to date and future perspectives. Microb. Cell Factories 2024, 23, 280. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, Z.; Zhang, R.; Ma, J.; Zhang, H.; Li, S.; Li, J. Aspergillus oryzae, a novel eco-friendly fungal bioflocculant for turbid drinking water treatment. Sep. Purif. Technol. 2021, 279, 119669. [Google Scholar] [CrossRef]
- Li, L.; Liang, T.; Zhao, M.; Lv, Y.; Song, Z.; Sheng, T.; Ma, F. A review on mycelial pellets as biological carriers: Wastewater treatment and recovery for resource and energy. Bioresour. Technol. 2022, 355, 127200. [Google Scholar] [CrossRef]
- Magnoli, K.; Carranza, C.S.; Aluffi, M.E.; Benito, N.; Magnoli, C.E.; Barberis, C.L. Survey of organochlorine-tolerant culturable mycota from contaminated soils and 2,4-D removal ability of Penicillium species in synthetic wastewater. Fungal Biol. 2023, 127, 891–899. [Google Scholar] [CrossRef]
- Soh, E.; Chew, Z.Y.; Saeidi, N.; Javadian, A.; Hebel, D.; Le Ferrand, H. Development of an extrudable paste to build mycelium-bound composites. Mater. Des. 2020, 195, 109058. [Google Scholar] [CrossRef]
- Piercy, E.; Verstraete, W.; Ellis, P.R.; Banks, M.; Rockström, J.; Smith, P.; Witard, O.C.; Hallett, J.; Hogstrand, C.; Knott, G.; et al. A Sustainable waste-to-protein system to maximize waste resource utilization for developing food- and feed-grade protein solutions. Green. Chem. 2023, 25, 808–832. [Google Scholar] [CrossRef]
- Borkertas, S.; Viskelis, J.; Viskelis, P.; Streimikyte, P.; Gasiunaite, U.; Urbonaviciene, D. Fungal biomass fermentation: Valorizing the food Industry’s Waste. Fermentation 2025, 11, 351. [Google Scholar] [CrossRef]
- Leonel, L.P.; Tonetti, A.L. Wastewater Reuse for Crop Irrigation: Crop Yield, Soil and Human Health Implications Based on Giardiasis Epidemiology. Sci. Total Environ. 2021, 775, 145833. [Google Scholar] [CrossRef]
- Gangola, S.; Joshi, S.; Kumar, S.; Pandey, S.C. Comparative Analysis of Fungal and Bacterial Enzymes in Biodegradation of Xenobiotic Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128183076. [Google Scholar]
- Magnoli, K.; Benito, N.; Aluffi, M.; Magnoli, C.; Barberis, C. Ability of non-Aalatoxigenic Aspergillus section Flavi strains to grow in the presence of herbicide 2,4-D and remove It from synthetic wastewater. Mycologia 2025, 117, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, K.; Benito, N.; Carranza, C.; Aluffi, M.; Magnoli, C.E.; Barberis, C.L. Effects of 2,4-D and environmental conditions on growth of P. crustosum strains and herbicide removal from rural-wastewater. Int. J. Environ. Sci. Tech. 2024, 22, 2625–2638. [Google Scholar] [CrossRef]
- Rice, E.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- King, A.D. Methodology for routine mycological examination of food—A collaborative study. In Modern Methods in Food Mycology, Developments in Food Science; Samson, R.A., Hocking, A.D., Pitt, J.I., King, A.D., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 11–20. [Google Scholar]
- Olicón-Hernández, D.R.; Camacho-Morales, R.L.; Pozo, C.; González-López, J.; Aranda, E. Evaluation of diclofenac biodegradation by the Ascomycete fungus Penicillium oxalicum at flask and bench bioreactor scales. Sci. Total Environ. 2019, 662, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Osborne, P.P.; Xu, Z.; Swanson, K.D.; Walker, T.; Farmer, D.K. Dicamba and 2,4-D Residues Following Applicator Cleanout: A Potential Point Source to the Environment and Worker Exposure. J. Air Waste Manage Assoc. 2015, 65, 1153–1158. [Google Scholar] [CrossRef]
- Sanchis, S.; Polo, A.M.; Tobajas, M.; Rodriguez, J.J.; Mohedano, A.F. Strategies to evaluate biodegradability: Application to chlorinated herbicides. Environ. Sci. Pollut. Res. 2014, 21, 9445–9452. [Google Scholar] [CrossRef]
- Gutiérrez-Zapata, H.M.; Rojas, K.L.; Sanabria, J.; Rengifo-Herrera, J.A. 2,4-D abatement from groundwater samples by photo-fenton processes at Circumneutral pH using naturally iron present. Effect of inorganic ions. Environ. Sci. Pollut. Res. 2017, 24, 6213–6221. [Google Scholar] [CrossRef]
- Pérez, D.J.; Okada, E.; De Gerónimo, E.; Menone, M.L.; Aparicio, V.C.; Costa, J.L. Spatial and temporal trends and flow dynamics of glyphosate and other pesticides within an agricultural watershed in Argentina. Environ. Toxicol. Chem. 2017, 36, 3206–3216. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-Assisted Algal Flocculation: Application in Wastewater Treatment and Biofuel Production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, Y.C. InfoStat; versión 2017. Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. Available online: https://www.infostat.com.ar/ (accessed on 14 March 2025).
- Shaheen, M.N.F.; Elmahdy, E.M.; Rizk, N.M.; Abdo, S.M.; Hussein, N.A.; Elshershaby, A.; Shahein, Y.E.; Fawzy, M.E.; El-Liethy, M.A.; Marouf, M.A.; et al. Evaluation of physical, chemical, and microbiological characteristics of waste stabilization ponds, Giza, Egypt. Environ. Sci. Eur. 2024, 36, 170. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sarker, P.; Rahaman, M.S.; Ahmed, F.F.; Shaibur, M.R.; Khabir Uddin, M. Biological treatment of textile wastewater by total aerobic mixed bacteria and comparison with chemical Fenton process. Pollution 2022, 8, 1418–1433. [Google Scholar] [CrossRef]
- El Ayari, T.; Bouhdida, R.; Ouzari, H.I.; El Menif, N.T. Bioremediation of petroleum refinery wastewater by fungal stains isolated from the fishing harbour of Bizerte (Mediterranean Sea). Biodegradation 2024, 35, 755–767. [Google Scholar] [CrossRef]
- Beltrán-Flores, E.; Pla-Ferriol, M.; Martínez-Alonso, M.; Gaju, N.; Blánquez, P.; Sarrà, M. Fungal bioremediation of agricultural wastewater in a long-term treatment: Biomass stabilization by immobilization strategy. J. Hazard. Mater. 2022, 439, 129614. [Google Scholar] [CrossRef]
- Khan, N.; Muge, E.; Mulaa, F.J.; Wamalwa, B.; von Bergen, M.; Jehmlich, N.; Wick, L.Y. Mycelial nutrient transfer promotes bacterial co-metabolic organochlorine pesticide degradation in nutrient-deprived environments. ISME J. 2023, 17, 570–578. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef]
- Magnoli, K.; Carranza, C.; Aluffi, M.; Magnoli, C.; Barberis, C. Fungal Biodegradation of Chlorinated Herbicides: An Overview with an Emphasis on 2,4-D in Argentina. Biodegradation 2023, 34, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.C.V.; Serbent, M.P.; Skoronski, E. Application of immobilized mycelium-based pellets for the removal of organochlorine compounds: A Review. Water Sci. Technol. 2021, 83, 1781–1796. [Google Scholar] [CrossRef] [PubMed]
- Cepero de García, M.C.; Restrepo, S.; Franco, A.E. Biologia de Hongos; Universidad de los Andes, Facultad de Ciencias, Ed.; Uniandes: Bogotá, Colombia, 2012. [Google Scholar]
- Vroumsia, T.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.L. Fungal bioconversion of 2,4-Dichlorophenoxyacetic acid (2,4-D) and 2,4-Dichlorophenol (2,4-DCP). Chemosphere 2005, 60, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Bokade, P.; Purohi, H.; Bajaj, A. Myco-remediation of chlorinated pesticides: Insights into fungal metabolic system. Indian J. Microbiol. 2021, 61, 237–249. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides. Environ. Chem. Let. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Karas, P.A.; Perruchon, C.; Exarhou, K.; Ehaliotis, C.; Karpouzas, D.G. Potential for bioremediation of agro-industrial effluents with high loads of pesticides by selected fungi. Biodegradation 2011, 22, 215–228. [Google Scholar] [CrossRef]
- Marinho, G.; Barbosa, B.C.A.; Rodrigues, K.; Aquino, M.; Pereira, L. Potential of the filamentous fungus Aspergillus niger AN 400 to degrade atrazine in wastewaters. Biocatal. Agric. Biotechnol. 2017, 9, 162–167. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Mohammed, M.K. Influence of fungicide (carbendazim) and herbicides (2, 4-D and metribuzin) on non-target beneficial soil microorganisms of rhizospheric soil of tomato crop. J. Environ. Sci. Toxicol. Food Technol. 2013, 5, 47–50. [Google Scholar] [CrossRef]
- Jahin, H.S.; Gaber, S.E.; Hussain, M.T. Bioremediation of diazinon pesticide from aqueous solution by fungal strains isolated from wastewater. World J. Chem. 2020, 15, 15–23. [Google Scholar] [CrossRef]
- Lizano-Fallas, V.; Masís-Mora, M.; Espinoza-Villalobos, D.; Lizano-Brenes, M.; Rodríguez-Rodríguez, C.E. Removal of pesticides and ecotoxicological changes during the simultaneous treatment of triazines and chlorpyrifos in biomixtures. Chemosphere 2017, 182, 106–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).