Abstract
Weeds are among the primary factors that adversely affect crop yields. Chlorophyll fluorescence, as a sensitive indicator of photosynthetic activity in green plants, provides direct insight into photosynthetic efficiency and the functional status of the photosynthetic apparatus. This makes it a valuable tool for assessing plant health and stress responses. Active chlorophyll fluorescence technology uses an external light source to excite plant leaves, enabling the rapid acquisition of fluorescence signals for real-time monitoring of vegetation in the field. This technology shows great potential for weed detection, as it allows for accurate discrimination between crops and weeds. Furthermore, since weed-induced stress affects the photosynthetic process of plants, resulting in changes in fluorescence characteristics, chlorophyll fluorescence can also be used to detect herbicide resistance in weeds. This paper reviews the progress in using active chlorophyll fluorescence sensor technology for weed detection. It specifically outlines the principles and structure of active fluorescence sensors and their applications at different stages of field operations, including rapid classification of soil and weeds during the seedling stage, identification of in-row weeds during cultivation, and assessment of herbicide efficacy after application. By monitoring changes in fluorescence parameters, herbicide-resistant weeds can be detected early, providing a scientific basis for precision herbicide application.
1. Introduction
The global population is growing at a rapid rate of around 1.09% per year and is projected to reach nearly 10 billion by 2050 (UN DESA, 2022) [1]. This rapid growth presents new challenges for the future food supply. In modern agricultural production, crop yield losses caused by weeds competing with crops have far exceeded food production losses due to pests and diseases [2]. It is estimated that weed competition reduces yields of the eight most important global crops by 13.2% [3,4]. Therefore, it is imperative that an effective weed management strategy be implemented to mitigate the yield loss caused by weed competition. The most employed weed management techniques can be classified into three principal categories: (1) mechanical weed removal through tillage or hoeing, (2) spraying with chemical herbicides, and (3) high-intensity energy pulse (laser and high-temperature steam) weed removal. The mechanical weed control technique is constrained by the intricate mechanical executor, rendering it applicable solely to inter-row weed control or intra-row weed control of crops with a fixed plant spacing [5,6,7]. In comparison to mechanical weeding, chemical weed control offers the benefits of simplicity and efficiency. Since its inception in 1940, it has been the most widely employed method of weed control throughout the world [8]. However, the conventional uniform and large-scale spraying of chemical herbicides can cause a series of environmental and food security problems, including a decline in biodiversity [9], impacts on aquatic ecosystems [10], and changes to soil microbial communities and soil properties [11,12], which further affect plant growth and crop yields [13]. In response to these challenges, developed nations have implemented stringent pesticide reduction policies, exemplified by the EU’s Farm to Fork Strategy (European Commission, 2020) [14], Japan’s revised Pesticide Control Act (MAFF, 2018) [15], and U.S. FIFRA amendments (US EPA, 2022) [16]. As a regulatory-compliant solution, site-specific weed management (SSWM), which applies herbicides in a spatially targeted manner to minimize ecological disruption, has demonstrated 30–70% chemical input reductions without compromising crop yields [17,18,19]. The general process of SSWM using chemical herbicides and lasers is illustrated in Figure 1. This process typically comprises four main steps: (1) weed detection, employing appropriate sensors to identify weeds within the target area; (2) decision-making, making real-time management decisions based on the detection results; (3) weeding execution, implementing the weed control actions through corresponding actuating mechanisms; and (4) evaluation, assessing the performance and effectiveness of the precise weed control operations. Among the SSWM methods, those based on high-intensity energy pulse techniques, such as laser and high-temperature steam, are more preferable. However, due to the high cost of high-energy pulsed techniques and their limited effectiveness against only small early-emerging weeds, they have not yet fully replaced chemical herbicides. Therefore, this study focuses exclusively on the application of active chlorophyll fluorescence sensors within SSWM scenarios that involve chemical herbicide use [20,21].
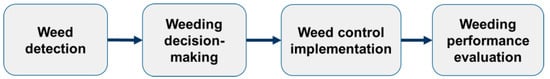
Figure 1.
The general workflow of site-specific weed management (SSWM).
In SSWM, the ability to accurately distinguish between weeds and crops is a prerequisite for subsequent weeding decision-making [22]. The development of sensors that can accurately identify these two types of plants is, therefore, a crucial area of research. A variety of technologies have been employed for the identification of weeds in SSWM, including RGB imaging [23], hyper-/multi-spectral imaging [24], and chlorophyll fluorescence spectroscopy/imaging. A considerable number of scholars have conducted reviews of the applications of hyper-/multi-spectral imaging and digital image processing in SSWM [25,26]. The applications of hyper-/multi-spectral imaging methods are severely restricted due to several factors, including the slow data acquisition speed, fluctuations in reflectance caused by soil and atmospheric factors, changes in solar illumination, and the similar reflectance characteristics of crops and weeds in the early growth stages [27,28]. The efficacy of weed identification through digital image processing is contingent upon the quality of the acquired images, particularly in terms of spatial resolution. In practical applications, the utilization of deep learning techniques has been shown to enhance the accuracy of weed identification [29,30]. However, the available information within RGB images remains limited, with data primarily focusing on the morphology, pigment, and texture of the vegetation. This approach lacks an effective reflection of the physiological state of the plant, which hinders the detection of drug-resistant weeds and the precise evaluation of weed control efficacy following application [31,32]. As a means of investigating photosynthesis in green plants, the intensity and spectral characteristics of chlorophyll fluorescence (ChlF) directly reflect the efficiency of photosynthetic processes and the physiological state of the photosynthetic apparatus, providing critical insights into plant health and stress responses. Given that weeds often exhibit distinct growth patterns and photosynthetic efficiencies compared to crops, ChlF signals offer a reliable method for differentiating weeds from crops [33,34]. Additionally, ChlF can be utilized to detect herbicide resistance in weeds. This capability enables the optimization of herbicide application through weed-sensing technologies and the implementation of variable-rate herbicide strategies, thereby improving resource efficiency and reducing environmental impact [35,36].
ChlF measurements can be classified into two main categories: active and passive methods. Active ChlF detection involves the stimulation of plant chlorophyll to produce fluorescent signals using active light sources, while passive ChlF detection utilizes natural solar radiation as the excitation light source, which is also called solar-induced fluorescence (SIF) [37]. Although passive SIF technology has been widely employed in large-scale ecological environment monitoring, such as the remote sensing assessment of global vegetation photosynthesis [38] and gross primary productivity (GPP) [39,40], it is not suitable for fine detection at small scales due to their significant dependence on ambient lighting conditions and low spatial resolution [41]. By contrast, the active ChlF technology shows considerable promise in small-scale sensing scenarios, including biotic and abiotic stresses [42]. Excitation by an external light source allows the fluorescence signal of plants to be rapidly obtained, thereby enabling real-time detection of green vegetation in the field [43].
In the context of SSWM, active ChlF sensors are primarily employed in three distinct operational scenarios, which are shown in Figure 2: (1) the application of herbicides to eliminate weeds either during pre-sowing fallow periods or within inter-row areas of cultivated fields, where the primary requirement is to distinguish bare soil from weed vegetation; (2) herbicide application during the crop growth stage, where precise discrimination between crop plants and various weed species is required for selective spraying; and (3) detecting weed resistance after herbicide application to assess the effectiveness of weed control strategies.
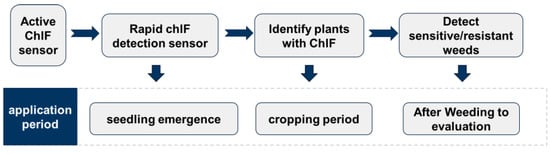
Figure 2.
Active ChlF sensors are primarily employed in three distinct operational scenarios.
The study first outlines the fundamental principles and instrumentation of active ChlF sensing, followed by a critical examination of its applications across different operational stages—ranging from rapid weed detection during crop emergence to intra- and inter-row weed identification during crop growth and post-herbicide efficacy assessment. Furthermore, the influence of environmental factors on sensor performance and their implications for site-specific weed management (SSWM) are discussed. Finally, the paper concludes with a synthesis of key findings and perspectives on future research directions, aiming to bridge current knowledge gaps and highlight opportunities for advancing active ChlF-based weed detection technologies.
2. Principles and Instrumentation of Active ChlF
2.1. Principles of Active ChlF
Chlorophyll fluorescence (ChlF) serves as a key indicator of the photosynthetic process, providing insights into the absorption and subsequent utilization or dissipation of light energy through various pathways. The absorbed light energy by chlorophyll can be redirected or dissipated in three primary ways: (i) participation in the electron transport chain via a photochemical reaction, (ii) dissipation as heat, and (iii) re-emission as fluorescence. During the photosynthetic process, light energy absorbed by chlorophyll molecules is converted and utilized in the carbon assimilation reactions that fix atmospheric carbon dioxide and synthesize essential organic compounds, which are fundamentally crucial for plant growth and development [44]. Under conditions of excessive light intensity or other environmental stresses, when the absorbed light energy exceeds the processing capacity of the photosynthetic machinery, plants activate protective mechanisms to protect their photosynthetic apparatus. These mechanisms involve the dissipation of excess light energy through increased fluorescence and heat dissipation [45]. Typically, fluorescence emission in photosynthetic processes accounts for 2% to 10% of the absorbed light, with a spectral range spanning approximately from 650 to 800 nm.
When leaves are exposed to light following a period of dark adaptation, ChlF initially declines to a minimum level (F0), then rises to a maximum (Fm), and subsequently decreases to a quasi-steady state—a phenomenon known as the “Kautsky effect” or “ChlF transient” (OJIP transient). Quantitative analysis of the rise from F0 to Fm and the subsequent decline enables the assessment of dynamic fluorescence changes over time, providing valuable insights into transient photochemical reactions and the operational status of the photosynthetic apparatus [46]. Furthermore, changes in the ratios of specific fluorescence excitation bands, such as F440/F690 and F440/F735, have been used as indicators of environmental stress, while the F690/F735 ratio has been shown to correlate with chlorophyll content [47,48]. Essential ChlF parameters for evaluating photosynthetic performance are summarized in Table 1. Among them, the maximum quantum yield of photosystem II (Fv/Fm) is a key physiological indicator for assessing the functional integrity of PSII reaction centers, particularly under various abiotic and biotic stress conditions such as drought, salinity, heavy metal toxicity, temperature extremes, and pathogen or pest infestation [49,50,51].

Table 1.
The parameters of ChlF for the assessment of photosynthesis.
Currently, two principal methodologies are employed for the detection of OJIP transients: the Plant Efficiency Analyzer (PEA) and the Pulse Amplitude Modulation (PAM) fluorometer. The PEA system utilizes non-modulated saturating light pulses to induce chlorophyll fluorescence emission in plant samples, with high temporal resolution (microsecond to second scale) to precisely capture the dynamic characteristics of the OJIP curve, as shown in Figure 3a. In contrast, the PAM technology incorporates modulated light sources to effectively discriminate photochemically active fluorescence signals from non-photochemical background interference, significantly improving both data reliability and measurement precision as shown in Figure 3b [44]. Specifically, PAM measurements begin by detecting the minimum chlorophyll fluorescence (F0) using an extremely weak measuring light that does not affect the photosynthetic process. Subsequently, actinic light and a series of very short saturation pulses are applied to induce variable fluorescence emission. These very short saturation pulses are sufficiently brief and weak so as not to alter the state of the photosynthetic reaction centers, allowing for accurate measurement of true chlorophyll fluorescence. Moreover, the actinic light temporarily exposes the plant to intense illumination to achieve a saturated fluorescence level [52,53]. The characteristic fluorescence signals obtained through PAE and PAM measurements are illustrated in Figure 3.
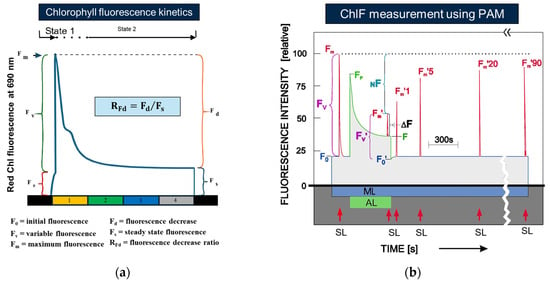
Figure 3.
(a) Dynamic characteristics of the OJIP curve captured by a Plant Efficiency Analyzer (PEA). (b) Scheme of the ChlF induction kinetics as measured in dark-adapted leaves with a Pulse Amplitude Modulation (PAM) fluorometer [51].
2.2. Instrumentation for Active ChlF Detection
The work principle of an active ChlF sensor is shown schematically in Figure 4. Due to the inherently weak nature of chlorophyll fluorescence signals, the effective extraction of fluorescence signals is a critical issue in active ChlF sensors. Therefore, it is necessary to systematically optimize each module according to the working principle of active chlorophyll fluorescence detection. The core components of an active chlorophyll fluorescence sensor typically include an excitation light source, a light detection unit, and a signal processing unit with built-in algorithms. Each component requires careful engineering optimization to enhance the signal-to-noise ratio and ensure accurate measurement of fluorescence parameters [54].
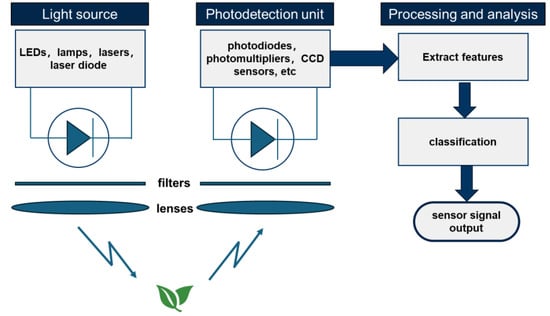
Figure 4.
The work principle of an active ChlF sensor.
2.2.1. Light Source for ChlF Excitation
The selection of the light source in a chlorophyll fluorescence detection system has a direct impact on the system’s performance. In recent years, light-emitting diodes (LEDs) with high efficiency and low power consumption and lasers with high directivity and energy density have become frequently used light sources [55]. Lasers have high directivity and energy density, which is advantageous in regional excitation applications because the light beam can be focused on a small area [56]. Conversely, the collimation of the laser allows a reduction in the number of optical components and is often used in portable devices. However, the high cost and comparatively low efficiency of lasers make them less cost-effective than LEDs for certain large area applications [57]. While LEDs offer the optimum light intensity/cost ratio, the cosine emission law governing the light emitted by LEDs results in uneven light distribution, which in turn affects the intensity of the ChlF excited in different areas. In SSWM, a rectangular or linear light source is typically required for illumination. It is therefore necessary to shape the LED light source to ensure the desired illuminating shape and uniformity of the light source. In order to shape the light emitted, the uniformity of irradiance can be enhanced in an LED array by optimizing the number of LEDs, the array spacing, and the array height [58]. Meanwhile, advancements in freeform lens design technology have made it possible to miniaturize and enhance the portability of rectangular light sources with uniform illumination [59,60,61].
Given that chlorophyll predominantly absorbs blue (430–470 nm) and red light (640–680 nm), these two wavebands are frequently employed to excite ChlF. The red light has a greater penetrating ability than the blue light and can penetrate deeper into the leaves. However, its longer wavelength causes stronger scattering, resulting in a lower excitation efficiency compared to the blue light [47,62,63]. Therefore, some active ChlF sensors do not rely solely on a single-wavelength light source for excitation; instead, they often employ multi-color fluorescence imaging to enhance spectral resolution and data richness. For instance, one of the most widely used fluorescence instruments in many laboratories is the Pulse Amplitude Modulation (PAM) fluorometer. This device typically employs three types of visible light sources for fluorescence excitation: weak pulse-modulated measuring light, continuous non-saturating actinic light, and saturating pulse light. For instance, the PAM 101 fluorometer (Heinz Walz GmbH, Effeltrich, Germany) is equipped with a red LED light source (PAM-102L), a far-red LED (PAM-102FR), and a saturating pulse lamp (KL-1500), which covers a measurement range of approximately 705–740 nm [64].
2.2.2. Photodetection Unit of ChlF Sensor
Once the excitation source of chlorophyll has been determined, the subsequent step is to select the appropriate ChlF detector. Since fluorescence emission occurs isotopically and irradiance follows the inverse square law [65]—where the detected radiant flux decreases proportionally to the inverse square of the distance—the separation between the sample area and receiving optics must be minimized to maintain detectable signal levels. Subsequently, appropriate methodologies must be employed to discern the plant’s response to the excitation light. The current methods for detecting the excited ChlF include the frequency domain, analyzed by the reflectance spectrum, and the time domain, analyzed by ChlF intensity. Reflectance spectroscopy measures the proportion of light reflected at specific wavelengths and is widely used to assess vegetation by analyzing its reflectance spectrum. Typical spectral sensing systems consist of a light source, detector, optical guide, wavelength selector, and signal processing unit. Spectrometers, as key detectors, capture visible and near-infrared reflectance from plant surfaces [66]. Several commercial spectrometers offer varying capabilities. For example, the LI-COR LI-180™ captures blue and far-red wavelengths; Spectral Evolution’s SR-6500™ and SR-6500A™ provide ultra-high resolution (1.5–3.8 nm) across 350–2500 nm; NanoLambda’s XL-500™, smartphone-compatible via Bluetooth, covers 390–1000 nm at 10–40 nm resolution; and Apogee’s SS-110™ and SS-120™ offer 3 nm resolution and wide field-of-view (25–180°) across 340–820 nm and 635–1100 nm ranges.
ChlF intensity detection employs a filter and an optical sensing element to detect the light intensity of ChlF, with the aim of extracting the characteristics of the OJIP curve. Among the ChlF intensity detection sensors, photodiodes, photomultiplier tubes (PMTs), and CCD sensors are the most used optical sensing devices. Photodiodes are a widely utilized instrument for detecting fluorescence, offering a rapid response, low cost, and straightforward optical characteristics [67]. Normally, photodiode detection requires high-gain transimpedance amplifiers (TIAs) to ensure sufficient signal strength. For example, the Walz Junior-PAM system uses a gain of up to 3.3 × 105, while custom designs by Bates et al. (2019) and Leeuw et al. (2013) reach gains of 2.2 × 106 and 5 × 106, respectively [68,69]. However, very high gain is limited by the operational amplifier’s internal gain and increases the risk of oscillation. To improve stability, a damping capacitor is typically added in parallel with the feedback resistor. While this reduces oscillations, it also slows the amplifier’s response due to the Resistive Capacitor’s time constant, which can delay the signal rise from microseconds to tens of milliseconds. Therefore, the gain and damping must be carefully balanced based on the photodiode and light conditions [70]. PMTs represent a specialized class of vacuum tube devices that utilize the phenomenon of secondary electron emission through a cascade multiplication process, enabling exceptional sensitivity in the detection and measurement of extremely weak optical signals, particularly in low-radiation environments. Such instruments are well suited to high-precision fluorescence measurements in low-light environments [70,71,72].
Compared to the photodiodes and PMTs, the CCD imaging sensors provide the possibility for chlorophyll fluorescence detection systems to capture two-dimensional spatial chlorophyll fluorescence responses in plant leaves [73]. These systems generate pixel-resolution fluorescence parameter maps encoded with pseudo-color scales ranging from black (0.000) to red, yellow, green, blue, and pink (1.000), representing the spatial heterogeneity of fluorescence characteristics [48,73]. The CCD imaging module features single-photon-level sensitivity, a wide dynamic range (>16-bit digitization), and rapid response dynamics (millisecond-scale exposure control), ensuring precise spatiotemporal resolution of fluorescence signals. By integrating image processing algorithms, quantitative analysis of fluorescence characteristics in different leaf regions can be performed, revealing spatial dynamics of photosynthetic parameters such as PSII photochemical efficiency (Fv/Fm) and non-photochemical quenching (NPQ). This capability provides critical decision-making support for site-specific weed management (SSWM) in precision agriculture.
The performance of active ChlF-based weed detection systems scales variably across field sizes and weed density gradients. In large, uniformly infested fields, spray volume tends to increase proportionally with area, whereas in sparse or patchy infestations, intermittent canopy signal detection results in weaker correlations between weed presence and spray output. This scaling behavior is strongly influenced by engineering parameters. A narrower field of view (FOV) or higher detection threshold can reduce false positives in low-density areas but may increase the risk of missing partially occluded weeds in dense canopies. Conversely, a wider FOV and lower threshold improve detection continuity in high-density stands but may trigger unnecessary activations in heterogeneous backgrounds. Operational parameters, particularly vehicle travel speed, also play a key role: higher speeds reduce the number of samples collected per unit area, disproportionately affecting detection accuracy under low-density conditions. From the perspective of photodetection unit design, these scaling characteristics imply the need to optimize the balance among optical collection geometry, threshold adjustment range, and signal integration time. In sparse weed scenarios, the system should feature high sensitivity and a low-noise amplification chain to capture weak fluorescence signals; in contrast, in dense infestations, it requires faster response times and higher instantaneous sampling rates to avoid signal saturation or triggering delays. Furthermore, integrating an adjustable field-of-view optical system with adaptive threshold algorithms can enable the photodetection unit to maintain an optimal signal-to-noise ratio and detection stability across varying field sizes and weed distributions, thereby supporting scalable, cross-scenario precision spraying.
2.3. Processing and Analysis of Active ChlF Data
The inherent complexity of species-specific fluorescence induction curves limits the utility of conventional parameters such as Fv/Fm, which are widely adopted in plant stress studies, for accurate plant species discrimination. To overcome this limitation, the integration of multiple parameters is required, necessitating the use of advanced feature processing algorithms. The discrimination of crops/weeds in precision agriculture primarily employs two methodological frameworks. The first approach involves classical machine learning techniques that rely on handcrafted features and supervised learning algorithms such as SVM and Random Forest. The second approach utilizes deep learning architectures, particularly convolutional neural networks (CNNs) with automated feature extraction capabilities [74].
Conventional machine learning can be divided into two principal categories, unsupervised learning and supervised learning methods. In unsupervised learning, the objective is to classify objects based on their similarities or attributes, as observed in unlabeled fluorescence signals [75]. Given that SSWM only considers two variables, weeds and crops, due to the stochastic nature of the population categories in which weeds occur in the field, it is not possible to ascertain the number of weed species. This renders some clustering algorithms that do not necessitate a known number of clusters more appropriate, including hierarchical clustering, binarization clustering, the Dirichlet process Gaussian mixture model (DPGMM), and affinity propagation [76]. The hierarchical clustering method is based on a distance measure between clusters. It involves the iterative merging or splitting of clusters in order to form a cluster relationship tree, with the aim of constructing a more adaptive clustering system [77]. Iterative clustering represents a variant of the agglomerative hierarchical clustering method. It involves the iterative merging of clusters based on a relative distance metric until a pre-defined stopping criterion is reached. DPGMM is an infinite Gaussian mixture model that does not require the number of Gaussian components to be known in advance. Rather, it is capable of automatically determining the appropriate number of clusters based on the data [76]. The affinity propagation algorithm generates prototypes and completes the clustering process through an iterative procedure whereby the likelihood of each point being a prototype is updated, as is the likelihood of each point being selected by a specific prototype [76,78].
The supervised learning process necessitates the utilization of known fluorescence signals for the extraction of a set of representative features, which are subsequently employed in the classification of plant species through the application of a statistical classifier. Given the considerable number of features involved in the extraction of ChlF information for the classification of plants, it is essential to implement a suitable feature selection process in order to reduce the presence of redundant information, thereby enhancing the robustness and efficiency of the classifier. After the pre-processing and feature selection of ChlF spectra, an appropriate statistical classifier is employed to categorize the specific ChlF transient according to a pre-specified classification scheme. Commonly used classifiers include the linear discriminant classifier (LDC), quadratic discriminant classifier (QDC), Fisher linear discriminant classifier (FLDC), k-nearest neighbor classifier (k-NNC), neighbor classifier (NNC), artificial neural network classifier (NEURC), support vector classifier (SVC), and nearest mean classifier (NMC) [79].
3. Weed Detection Based on ChlF
3.1. Rapid Plant Detection
In the aforementioned first scenario of SSWM, the primary task of the system is to detect the green vegetation targets (i.e., weeds) and distinguish them from non-target background elements such as bare soil, crop residues, and stones. Given that the classification task is relatively straightforward, the focus of the detection system lies in how to ensure adequate signal strength and response speed within the limited acquisition time window when using low-cost optoelectronic sensors under variable ambient light and rapid outdoor motion conditions. The use of existing spectrometers necessitates the acquisition of a dark spectrum and a reference spectrum prior to the acquisition of spectral data. The high cost of the hardware and the considerable volume of data produced impact the processing speed, which in turn restricts the application of these instruments in SSWM. In response to the aforementioned technical limitations, Huete et al. [80] and Hanks and Beck [81] proposed the use of weeds and soil reflectance as a means of detection. This was based on the observation that weed reflectance is significantly lower than that of soil in the red (R) spectrum, while soil reflectance is almost equal in the R and near-infrared (NIR) spectra. Two downward-facing photodiodes with filters were employed for the detection of R and NIR light, respectively. The density of ground weeds is detected and estimated by calculating the ratio of reflectance in the red and NIR spectra. This technique of distinguishing between weeds and soil based on (R + NIR)/NIR reflectance ratios has been successfully commercialized. Weed Control Australia (WCA), headquartered in Western Australia, was the first to develop a precision spraying system based on the (R + NIR)/NIR reflectance ratio method, aiming to distinguish between weeds and bare ground through spectral differences. Subsequently, Patchen Technology in California developed the first-generation WeedSeeker system in the 1990s. Building on this foundation, Rainbow Agricultural Services partnered with Oklahoma State University to establish a joint venture company, NTech Industries, which now manages the technology and has since advanced it to a second-generation product. The use of reflectance spectroscopy has proven effective in distinguishing weeds from bare soil, thereby significantly reducing herbicide application. However, accurate detection of small weeds requires initial system calibration on weed-free surfaces that possess reflectance properties similar to the target area. Without periodic recalibration, substantial variations in surface characteristics during operation can adversely affect system performance. Currently, the Weed-It system (Rometron B.V., Haaksbergen, The Netherlands) utilizes high-frequency modulated LED light sources to excite chlorophyll fluorescence signals in plants (within the 650–700 nm wavelength range). These signals are received through a combination of optical filters and photodiodes and are subsequently processed via a lock-in amplifier that synchronizes with the modulation frequency of the excitation light to extract the desired fluorescence signal. Commercially available active ChlF detection sensors are shown in Figure 5.
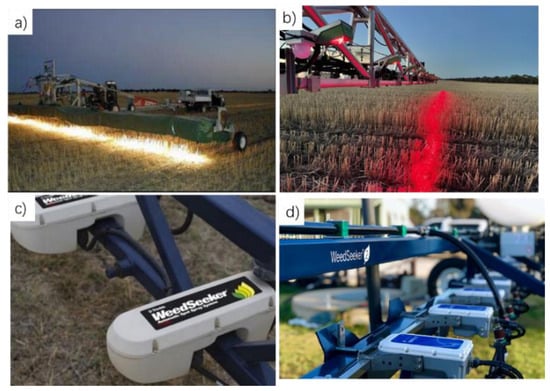
Figure 5.
Commercially available active ChlF detection sensors. (a) Weed Control Australia (WCA); (b) the Weed-It system (Sensor Technology Ltd.); (c) the first-generation WeedSeeker system; (d) the second-generation WeedSeeker system.
These commercially active weed-detection sensors differ notably in resolution, sensitivity, and operational performance. The WeedSeeker (Gen 1) delivers a spray resolution of 12–15 in (0.18–0.23 m2) with a minimum detectable target of ~0.28 cm2, operating effectively at speeds up to 16 km h−1, though requiring manual calibration under variable field conditions. The WeedSeeker 2 improves on this by providing a spray resolution of ~50 cm width (0.50 m2) and detecting weeds as small as 1 cm2 with millisecond-level response time, sustaining detection at speeds up to 20 km h−1 and operating at up to 40 km h−1 with automatic calibration. The WEED-IT Quadro further enhances performance, offering a spray resolution of 100 cm width (1.10 m2) with a 1 cm2 detection limit and ≤10 ms response time, enabling accurate operation at speeds up to 25 km h−1 and tracking up to 50 km h−1, with high robustness to fluctuating illumination (Table 2).

Table 2.
Comparative specifications of commercial active ChlF weed-detection sensors.
In addition to the commercially available active ChlF sensors for soil and vegetation detection, several researchers have developed similar types of sensors. For instance, Deng et al. [82] designed a green plant sensor using the reflectance ratio at 850 nm and 650 nm as a vegetation detection index, with a threshold value set at 5.54 [82], and Wang et al. [62] optimized the selection of spectral bands and identified blue light as having the highest excitation efficiency for vegetation and developed a low-cost green plant sensor using a blue light source [62].
3.2. Crop/Weed Classification Based on Active ChlF
During the cultivation period, the use of active ChlF sensors for weed detection necessitates distinguishing between crops and weeds both within and between crop rows, thereby imposing increased complexity on the classification system. As mentioned above, the ChlF properties of plants can be divided into frequency-domain properties represented by spectra and time-domain properties represented by ChlF transient curves. For active fluorescence spectroscopy to detect vegetation, although Longchamps et al. [83] and Panneton et al. [84] successfully classified monocotyledons and dicotyledons through ultraviolet-induced fluorescence spectroscopy within the 400–800 nm spectral range, the methodology demonstrated limitations in distinguishing certain species with similar spectral characteristics, particularly evident in the case of maize (Zea mays) and foxtail (Setaria spp.). The spectral reflectance characteristics of plants exhibit dynamic variations in response to developmental changes and environmental modifications. During early growth stages, crops and weeds demonstrate comparable spectral reflectance patterns, as evidenced by multiple studies [28,83,85]. Furthermore, environmental stressors, including but not limited to salinity gradients and water availability, have been shown to significantly alter plant spectral reflectance properties [86]. Therefore, relying solely on simple band ratios or single fluorescence signals is insufficient to meet the accuracy requirements for vegetation classification. Plant optical reflectance is strongly influenced by observation geometry, exhibiting pronounced angular dependence. In polarimetric bidirectional reflectance studies, variations in azimuth and zenith angles alter the balance between diffuse, specular, and secondary reflections within the canopy, leading to distinct spectral–polarimetric signatures for different plant architectures [87]. Yang et al. [88] proposed that the intensity and shape of steady-state laser-induced fluorescence (LIF) spectra of plant species depend on the fluorescence emission angle. By constructing pseudocolor images using fluorescence wavelength and emission angle as axes, the spatial distribution characteristics of the fluorescence spectra can be leveraged for vegetation identification. This method was effectively applied to distinguish a range of species, including dicots such as Camphora officinarum and Cinnamomum kotoense, and monocots such as Scindapsus aureus, Dracaena sanderiana, paddy rice (Oryza sativa), and bamboo.
Vegetation classification based on ChlF transients has demonstrated considerable potential due to the species-specific nature of fluorescence signals. Tyystjärvi et al. [89] utilized a pulse-amplitude modulation fluorometer (PAM 101, Heinz Walz GmbH, Effeltrich, Germany) to measure ChlF transients in seven plant species, identifying distinct fluorescence patterns that they termed “fluorescence fingerprinting”. Building upon this concept, Codrea et al. [90] proposed an improved classification framework by incorporating a genetic algorithm-based feature selection method with a feedforward neural network trained via backpropagation. Their system effectively classified nine plant species—including five angiosperms (birch, maize, tobacco, stinging nettle, and rye), one gymnosperm (pine), and three cryptogams (common haircap moss, rusty peat moss, and a lichen species)—achieving classification accuracies exceeding 90%. However, conventional ChlF transient measurements typically require a dark adaptation period ranging from 10 min to 2 h to establish a stable fluorescence baseline [73], which severely limits the efficiency and scalability of real-time vegetation monitoring in field conditions, particularly in SSWM applications. To address this limitation, Keränen et al. [91,92] trained a multilayer perceptron (MLP) neural network to classify the fluorescence profiles obtrained using a PEA fluorometer. Their model achieved an average classification accuracy of 86% across six plant species—quackgrass, radish, beetroot, field pennycress, rye, and wheat—using the full 1-second OJIP transient. Remarkably, even when the acquisition window was shortened to just 0.1 s, the model still maintained a classification accuracy of 76% -. Further advancing this approach, Tyystjärvi et al. [93] demonstrated that a 1-second fluorescence curve, obtained after only 1.2 s of shading under natural light conditions, could be effectively used for crop/weed classification. The study focused on two crop species (maize and barley) and six common weed species (annual bluegrass, lamb’s quarters, scentless mayweed, Canada thistle, corn speedwell, and common chickweed). The classification accuracy reached as high as 95%, and the influence of varying ambient light intensities on model performance was surprisingly minimal. The direct contact between chlorophyll fluorescence (ChlF) probes and plant leaves can improve classification performance while reducing the need for dark adaptation, but their limited detection range at the leaf level restricts practical applications in the field. To overcome this limitation, Mishra et al. [94] utilized a non-contact imaging approach with the FluorCam system (Photon Systems Instruments Ltd., Brno, Czech Republic) under ambient laboratory conditions to capture ChlF images of three Lamiaceae species—Ocimum basilicum, Origanum vulgare, and Origanum majorana. By evaluating eight classifiers and four feature selection methods, they found that combining a sequential forward floating selection (SFFS) with a quadratic discriminant classifier (QDC) achieved optimal species discrimination, reaching over 97% accuracy in mixed-species potted samples [94]. Building on this imaging-based strategy, Mattila et al. [95] employed the Handy FluorCam FC 1000-H to measure ChlF images of field-collected wild dandelion (Taraxacum officinale) and greenhouse-grown oat (Avena sativa, cv. Aslak). Using a greedy algorithm for feature extraction and integrating ChlF with leaf texture features, they further improved classification accuracy to 98%.
Given the established relationship between ChlF and plant phenomics and genomics [96,97], ChlF has been widely utilized in controlled-environment breeding programs for various purposes, including screening of plant mutants [98,99,100], selecting cold- and waterlogging-tolerant crop varieties [101,102,103], and identifying mutant populations in different algal species [92]. The application of ChlF in screening plant variants has fully demonstrated its sensitivity to genetic and physiological variations. Given that commercial crop cultivars typically exhibit high genetic uniformity and display greater phenotypic diversity compared to wild weeds, the use of ChlF for crop/weed classification represents an effective approach. However, it is essential to consider that ChlF transients in vegetation are also influenced by environmental conditions [104], which may affect the reliability of ChlF-based binary classification between crops and weeds. To ensure the robustness of chlorophyll fluorescence-based classification under variable field conditions, it is necessary to develop and analyze extensive datasets that capture a wide range of environmental factors.
To further enhance the performance of fluorescence fingerprinting, additional optical strategies may be considered. These include increasing kinetic detail by measuring responses under varying light intensities, optimizing the use of the O–I–J–P phases of the Kautsky curve [67], and employing high-order polynomial fitting of specific curve segments instead of simple linear regressions to extract more nuanced features. Moreover, the use of higher-sensitivity fluorescence detection systems, such as advanced PAM or PEA fluorometers, may significantly improve data resolution and the overall reliability of the detection system.
4. Evaluation of the Effectiveness of SSWM Based on ChlF
The evolution of herbicide resistance has been documented in various weed management scenarios since the first reports of herbicide-resistant weeds in 1957 [105,106,107]. With the increasing prevalence of herbicide-resistant weed populations, a corresponding decline in herbicide efficacy has been observed. Consequently, managing herbicide resistance has become a critical component of sustainable soil and water management practices. Early detection of resistant weeds is critical because herbicide-resistant weeds can rapidly proliferate and establish persistent seedbanks if not addressed promptly. Once resistance becomes widespread, control measures often require higher chemical doses, the use of multiple herbicide modes of action, or more intensive mechanical interventions, all of which increase production costs and environmental risks. Furthermore, delayed identification reduces the window of opportunity for implementing integrated weed management strategies, thereby undermining the long-term sustainability of cropping systems. However, traditional approaches in weed science research have primarily relied on four methodological approaches for the diagnosis of herbicide resistance: (1) controlled environment bioassays conducted under greenhouse conditions, (2) biochemical characterization assays, (3) molecular genetic analyses, and (4) analytical chemistry-based assays [108]. However, these conventional methods are typically laboratory- or greenhouse-based and are time-consuming, making it difficult to manage weed resistance at an early stage [109,110]. To address this challenge, Barbagallo [111] and Riethmuller-Haage et al. [112] proposed using ChlF to quantify the impact of herbicides on weeds and gained insights into their mode of action. In many weed species, the Fv/Fm ratio, which represents the maximum quantum yield of photosystem II, often exhibits a marked alteration following herbicide treatment. Susceptible species typically display a rapid decline in the Fv/Fm value, whereas resistant species tend to maintain a relatively stable ratio [111,112]. In a study by Wang et al. [113,114], the WEED-PAM sensor was used to monitor resistance in blackgrass (Alopecurus myosuroides). Following the application of acetolactate synthase (ALS) inhibitors, the Fv/Fm value of the susceptible variety significantly decreased within 24 h, declining from 0.7 to a range of 0.6–0.65. In contrast, the resistant variety exhibited minimal variation in its Fv/Fm value. After treatment with PSII inhibitors, the Fv/Fm value of the sensitive variety decreased to 0.25 within three to four days, while the resistant variety remained around 0.6. Mechanistically, herbicides that target photosynthetic pathways disrupt the electron transport chain within PSII, leading to energy imbalance and photoinhibition. These physiological impairments are rapidly reflected in altered fluorescence emission patterns—particularly in Fv/Fm and the kinetics of OJIP transients—well before visible injury symptoms develop. Early detection of resistant weeds is critical because herbicide-resistant weeds can rapidly proliferate and establish persistent seedbanks if not addressed promptly. Once resistance becomes widespread, control measures often require higher chemical doses, the use of multiple herbicide modes of action, or more intensive mechanical interventions, all of which increase production costs and environmental risks. Furthermore, delayed identification reduces the window of opportunity for implementing integrated weed management strategies, thereby undermining the long-term sustainability of cropping systems.
Active ChlF sensors enable precise assessment of herbicide efficacy, providing a scientific basis for optimizing application rates. Real-time monitoring of photosynthetic performance allows for timely feedback and adaptive adjustment of herbicide strategies, ensuring effective weed control while minimizing environmental impact. The Fv/Fm ratio is commonly used to detect herbicide resistance but is also sensitive to environmental stresses such as drought or salinity [115]. Thus, declines in Fv/Fm cannot be attributed solely to herbicide effects. However, herbicide-induced reductions are generally more rapid and severe than those from abiotic stressors [113], making Fv/Fm a useful indicator. Given that herbicide resistance detection primarily involves assessing whether photosynthetic efficiency is severely inhibited, it may be feasible to replace the requirement for extended dark adaptation with features extracted from the full ChlF response curve. This suggests a promising possibility: integrating weed/crop classification algorithms with herbicide resistance detection algorithms into a unified framework. By doing so, platforms such as PEA or PAM fluorometers equipped with active ChlF sensors could be transformed into core perception modules for SSWM. Such systems could continue monitoring ChlF responses of residual plants either during the return pass after herbicide application or within 3–5 days post-treatment. By analyzing these responses, weeds potentially harboring herbicide resistance genes could be identified, enabling the implementation of targeted follow-up measures. This integrated approach would contribute to a more comprehensive and adaptive weed management strategy, facilitating effective control of both susceptible and resistant weed populations.
5. Conclusions
At present, active chlorophyll fluorescence (ChlF) sensors have demonstrated maturity in the rapid detection of weeds during the seedling stage. Commercial systems such as WeedSeeker, which is based on the differential reflectance of chlorophyll excited by specific wavelengths (typically in the blue or red spectrum), and Weed-it, which detects fluorescence responses, have shown reliable performance in detecting green vegetation and have been widely applied in site-specific spraying within precision agriculture. However, significant challenges remain in applying active ChlF sensors under complex field conditions throughout the growing season for distinguishing weeds from crops and detecting herbicide-resistant biotypes.
In field conditions, the growth history of vegetation can cause variability in ChlF transient characteristics, potentially reducing the reliability of binary crop–weed classification based on ChlF measurements. In recent years, deep learning has rapidly advanced as a powerful tool for modeling complex spatial patterns and nonlinear physiological responses in ChlF image analysis. Convolutional neural networks (CNNs) can automatically extract key features from ChlF images to distinguish stressed from healthy plants [116]. Fully convolutional network architectures, such as U-Net, enable fine-scale segmentation of stress-affected regions at the leaf level [117]. Generative adversarial networks (GANs) can augment datasets, expand training samples, and improve classification robustness [118]. Furthermore, integrating deep learning with temporal modeling has shown significant advantages: Sun et al. characterized the photosynthetic dynamics of drought-stressed mutants through time-series ChlF imaging [119], while Dong et al. [120] combined ChlF features with bidirectional long short-term memory (BiLSTM) networks to achieve high-accuracy classification of cold-stressed tomato seedlings. These approaches not only improve model performance but also enhance interpretability, allowing AI outputs to make more physiologically meaningful inferences about plant status. However, there is still a severe lack of publicly available, standardized ChlF image datasets—particularly those acquired under natural field illumination and annotated with ground-truth labels such as crop/weed species, stress types, and resistance status. There is a pressing need to establish large-scale annotated datasets under field conditions, encompassing well-labeled samples across diverse environments, plant types, and stress levels. In the future, as dataset size expands, integrating physical models and fluorescence kinetics into deep learning frameworks holds great potential for further improving physiological interpretability and cross-environment generalization.
Compared with traditional RGB or multispectral imaging systems, active ChlF imaging involves greater technical complexity and cost. Fluorescence imaging typically requires a controlled excitation light source (e.g., LEDs or lasers), specialized optical filters to isolate weak fluorescence signals, and highly sensitive detectors such as CCD or scientific CMOS cameras. These hardware requirements—combined with the need for precise synchronization, calibration, and noise suppression—make current commercial chlorophyll fluorescence imaging devices prohibitively expensive. While there have been research efforts toward developing low-cost ChlF sensors, these remain at the experimental stage and are generally limited to small-scale sample detection. Therefore, comprehensive studies are needed to determine the spatial and temporal resolution requirements for accurate crop–weed discrimination and early herbicide resistance detection, in order to guide the design of low-cost, scalable ChlF imaging systems suitable for high-throughput field image acquisition.
Integrating active ChlF sensors into autonomous or vehicle-mounted platforms [121] requires the use of cameras or push-broom scanners to collect modulated fluorescence signals. Fluorescence measurements also require short periods of dark adaptation or low ambient light to achieve a stable optical system. In principle, this can be achieved by shading the field with the tractor or installing light-blocking panels on the tractor to provide brief dark adaptation, combined with a continuously focused downward light beam for plant identification. This necessitates the development of a tractor-compatible, scalable hardware platform capable of real-time fluorescence imaging, coupled with fast excitation protocols or machine learning–based compensation strategies to minimize dark adaptation requirements.
Overall, active ChlF sensors, as a promising sensing tool, provide critical support for perception-based SSWM due to their high sensitivity to plant physiological status. Their potential extends beyond early-stage weed detection to include intra- and inter-row weed identification during the growing season and post-herbicide application assessment, enabling the establishment of a full-process weed monitoring system. Furthermore, ChlF-based vegetation sensing technologies hold broad application prospects beyond weed detection, such as in fertilization management and irrigation regulation, facilitating precision variable-rate fertilization and spraying in smart agriculture. Despite current limitations in hardware resolution, imaging speed, and cost, the unique advantages of active ChlF sensors in sensing plant physiological phenotypes and their deep integration potential in digital agriculture present a promising outlook for future research and industrial deployment.
Author Contributions
Conceptualization, J.H., Z.Z. (Zhenjiang Zhou), Z.Z. (Zhao Zhang), A.W. and T.W.; methodology, J.H., Y.X., X.B. and L.Z.; validation, L.Z., A.W. and T.W.; formal analysis, J.H. and A.W.; investigation, Y.X., X.B. and Z.Z. (Zhenjiang Zhou); resources, A.W.; writing—original draft preparation, J.H.; writing—review and editing, A.W. and T.W.; supervision, A.W. and T.W.; project administration, A.W. and L.Z.; funding acquisition, A.W. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 320011417), the China Postdoctoral Science Foundation (No. 2025T180690), the Zhenjiang Science & Technology Program (No. NY2024020), the Modern Agricultural Machinery Equipment and Technology Promotion Project of Jiangsu Province (No. NJ2024-26), open funding from the Jiangsu Province and Education Ministry Co-sponsored Synergistic Innovation Center of Modern Agricultural Equipment (No. XTCX2010), and the Zhejiang Key Laboratory of Intelligent Sensing and Robotics for Agriculture (No. 2025QSZD2504).
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that the naming of commercial products in this manuscript is solely for the purpose of providing specific information and does not imply endorsement or recommendation by the authors or their affiliated institutions.
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results; UN DESA: New York, NY, USA, 2022; Available online: https://population.un.org/wpp/ (accessed on 18 August 2025).
- Ronay, I.; Nisim Lati, R.; Kizel, F. Spectral Mixture Analysis for Weed Traits Identification under Varying Resolutions and Growth Stages. Comput. Electron. Agric. 2024, 220, 108859. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Parven, A.; Meftaul, I.M.; Venkateswarlu, K.; Megharaj, M. Herbicides in Modern Sustainable Agriculture: Environmental Fate, Ecological Implications, and Human Health Concerns. Int. J. Environ. Sci. Technol. 2025, 22, 1181–1202. [Google Scholar] [CrossRef]
- Nørremark, M.; Griepentrog, H.W.; Nielsen, J.; Søgaard, H.T. The Development and Assessment of the Accuracy of an Autonomous GPS-Based System for Intra-Row Mechanical Weed Control in Row Crops. Biosyst. Eng. 2008, 101, 396–410. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, X.; Fu, H.; Tan, H.; Zhai, C.; Chen, L. Design and Experimental Evaluation of a Smart Intra-Row Weed Control System for Open-Field Cabbage. Agronomy 2025, 15, 112. [Google Scholar] [CrossRef]
- Jia, W.; Tai, K.; Wang, X.; Dong, X.; Ou, M. Design and Simulation of Intra-Row Obstacle Avoidance Shovel-Type Weeding Machine in Orchard. Agriculture 2024, 14, 1124. [Google Scholar] [CrossRef]
- Kuang, Y.; Yu, H.; Qi, F.; Zhou, X.; Li, X.; Zhou, H. Developing Herbicide-Resistant Crops through Genome Editing Technologies: A Review. Crop Prot. 2024, 183, 106745. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate Toxicity for Animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Sharma, K.K.; Tripathy, V.; Gopal, M.; Walia, S. Good Agricultural Practices and Monitoring of Herbicide Residues in India. In Herbicide Residue Research in India. Environmental Chemistry for a Sustainable World; Springer: Singapore, 2019; pp. 443–465. [Google Scholar]
- Zhang, H.-Y.; Goncalves, P.; Copeland, E.; Qi, S.-S.; Dai, Z.-C.; Li, G.-L.; Wang, C.-Y.; Du, D.-L.; Thomas, T. Invasion by the Weed Conyza Canadensis Alters Soil Nutrient Supply and Shifts Microbiota Structure. Soil Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Determination of Pesticides and Their Degradation Products in Soil: Critical Review and Comparison of Methods. TrAC Trends Anal. Chem. 2004, 23, 772–789. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Singh, J. Contaminants in Agriculture and Environment: Health Risks and Remediation. In Contaminants in Agriculture and Environment: Health Risks and Remediation; Agro Environ Media-Agriculture and Environmental Science Academy: Haridwar, India, 2019; pp. 1–301. [Google Scholar]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 18 August 2025).
- Ministry of Agriculture, Forestry and Fisheries (MAFF). The Revised Pesticide Control Act; Government of Japan: Tokyo, Japan, 2018. Available online: https://www.maff.go.jp/e/ (accessed on 18 August 2025).
- U.S. Environmental Protection Agency (EPA). Summary of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA); US EPA: Washington, DC, USA, 2022. Available online: https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act (accessed on 18 August 2025).
- Gutjahr, C.; Sökefeld, M.; Gerhards, R. Evaluation of Two Patch Spraying Systems in Winter Wheat and Maize. Weed Res. 2012, 52, 510–519. [Google Scholar] [CrossRef]
- Gerhards, R.; Oebel, H. Practical Experiences with a System for Site-specific Weed Control in Arable Crops Using Real-time Image Analysis and GPS-controlled Patch Spraying. Weed Res. 2006, 46, 185–193. [Google Scholar] [CrossRef]
- Biller, R.H. Reduced Input of Herbicides by Use of Optoelectronic Sensors. J. Agric. Eng. Res. 1998, 71, 357–362. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, D.; Zhou, W.; Currie, K. Laser and Optical Radiation Weed Control: A Critical Review. Precis. Agric. 2024, 25, 2033–2057. [Google Scholar] [CrossRef]
- Brodie, G. The Use of Physics in Weed Control. In Non-Chemical Weed Control; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–59. [Google Scholar]
- Slaughter, D.C.; Giles, D.K.; Downey, D. Autonomous Robotic Weed Control Systems: A Review. Comput. Electron. Agric. 2008, 61, 63–78. [Google Scholar] [CrossRef]
- Memon, M.S.; Chen, S.; Shen, B.; Liang, R.; Tang, Z.; Wang, S.; Zhou, W.; Memon, N. Automatic Visual Recognition, Detection and Classification of Weeds in Cotton Fields Based on Machine Vision. Crop Prot. 2025, 187, 106966. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Paap, A.; Ahderom, S.; Apopei, B.; Alameh, K. Plant Discrimination by Support Vector Machine Classifier Based on Spectral Reflectance. Comput. Electron. Agric. 2018, 148, 250–258. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, W.; Wei, X. A Review on Weed Detection Using Ground-Based Machine Vision and Image Processing Techniques. Comput. Electron. Agric. 2019, 158, 226–240. [Google Scholar] [CrossRef]
- AlSuwaidi, A.; Veys, C.; Hussey, M.; Grieve, B.; Yin, H. Hyperspectral Selection Based Algorithm for Plant Classification. In Proceedings of the 2016 IEEE International Conference on Imaging Systems and Techniques (IST), Chania, Greece, 4–6 October 2016; pp. 395–400. [Google Scholar]
- López-Granados, F. Weed Detection for Site-specific Weed Management: Mapping and Real-time Approaches. Weed Res. 2011, 51, 1–11. [Google Scholar] [CrossRef]
- Peteinatos, G.G.; Weis, M.; Andújar, D.; Rueda Ayala, V.; Gerhards, R. Potential Use of Ground-Based Sensor Technologies for Weed Detection. Pest Manag. Sci. 2014, 70, 190–199. [Google Scholar] [CrossRef]
- Tao, T.; Wei, X. STBNA-YOLOv5: An Improved YOLOv5 Network for Weed Detection in Rapeseed Field. Agriculture 2024, 15, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, G.; Du, X.; Shaheen, N.; Mao, H. Recognition of Weeds at Asparagus Fields Using Multi-Feature Fusion and Backpropagation Neural Network. Int. J. Agric. Biol. Eng. 2021, 14, 190–198. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Chen, S.; Memon, M.S.; Shen, B.; Guo, J.; Du, Z.; Tang, Z.; Guo, X.; Memon, H. Identification of Weeds in Cotton Fields at Various Growth Stages Using Color Feature Techniques. Ital. J. Agron. 2024, 19, 100021. [Google Scholar] [CrossRef]
- Falkowski, P.; Kolber, Z. Variations in Chlorophyll Fluorescence Yields in Phytoplankton in the World Oceans. Funct. Plant Biol. 1995, 22, 341. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Thorp, K.R.; Tian, L.F. Performance Study of Variable-Rate Herbicide Applications Based on Remote Sensing Imagery. Biosyst. Eng. 2004, 88, 35–47. [Google Scholar] [CrossRef]
- Liu, J.; Abbas, I.; Noor, R.S. Development of Deep Learning-Based Variable Rate Agrochemical Spraying System for Targeted Weeds Control in Strawberry Crop. Agronomy 2021, 11, 1480. [Google Scholar] [CrossRef]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.-E.; et al. Global and Time-Resolved Monitoring of Crop Photosynthesis with Chlorophyll Fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, E1327–E1333. [Google Scholar] [CrossRef]
- Frankenberg, C.; O’Dell, C.; Guanter, L.; McDuffie, J. Remote Sensing of Near-Infrared Chlorophyll Fluorescence from Space in Scattering Atmospheres: Implications for Its Retrieval and Interferences with Atmospheric CO2 Retrievals. Atmos. Meas. Tech. 2012, 5, 2081–2094. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Zhang, Y.; Wolf, S.; Zhou, S.; Joiner, J.; Guanter, L.; Verma, M.; Sun, Y.; Yang, X.; et al. On the Relationship between Sub-Daily Instantaneous and Daily Total Gross Primary Production: Implications for Interpreting Satellite-Based SIF Retrievals. Remote Sens. Env. 2018, 205, 276–289. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking Chlorophyll a Fluorescence to Photosynthesis for Remote Sensing Applications: Mechanisms and Challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote Sensing of Solar-Induced Chlorophyll Fluorescence (SIF) in Vegetation: 50 years of Progress. Remote Sens. Env. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Konanz, S.; Kocsányi, L.; Buschmann, C. Advanced Multi-Color Fluorescence Imaging System for Detection of Biotic and Abiotic Stresses in Leaves. Agriculture 2014, 4, 79–95. [Google Scholar] [CrossRef]
- Nedbal, L.; Soukupová, J.; Kaftan, D.; Whitmarsh, J.; Trtílek, M. Kinetic Imaging of Chlorophyll Fluorescence Using Modulated Light. Photosynth. Res. 2000, 66, 3–12. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar]
- Baker, N.R. Applications of Chlorophyll Fluorescence Can Improve Crop Production Strategies: An Examination of Future Possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Lazár, D. Chlorophyll a Fluorescence Induction1Dedicated to Docent Jan Nauš on the Occasion of His 50th Birthday.1. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1999, 1412, 1–28. [Google Scholar] [CrossRef]
- Buschmann, C.; Lichtenthaler, H.K. Principles and Characteristics of Multi-Colour Fluorescence Imaging of Plants. J. Plant Physiol. 1998, 152, 297–314. [Google Scholar] [CrossRef]
- Chaerle, L.; Van Der Straeten, D. Imaging Techniques and the Early Detection of Plant Stress. Trends Plant Sci. 2000, 5, 495–501. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; Van Der Straeten, D. Monitoring and Screening Plant Populations with Combined Thermal and Chlorophyll Fluorescence Imaging. J. Exp. Bot. 2007, 58, 773–784. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to Correctly Determine the Different Chlorophyll Fluorescence Parameters and the Chlorophyll Fluorescence Decrease RFd of Leaves with the PAM Fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Havaux, M. Stress Tolerance of Photosystem II in Vivo. Plant Physiol. 1992, 100, 424–432. [Google Scholar] [CrossRef]
- Pfündel, E.E. Simultaneously Measuring Pulse-Amplitude-Modulated (PAM) Chlorophyll Fluorescence of Leaves at Wavelengths Shorter and Longer than 700 Nm. Photosynth. Res. 2021, 147, 345–358. [Google Scholar] [CrossRef]
- Vlaovic, J.; Balen, J.; Grgic, K.; Zagar, D.; Galic, V.; Simic, D. An Overview of Chlorophyll Fluorescence Measurement Process, Meters and Methods. In Proceedings of the 2020 International Conference on Smart Systems and Technologies (SST), Osijek, Croatia, 14 October 2020; pp. 245–250. [Google Scholar]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Romero-Troncoso, R.d.J.; Guevara-Gonzalez, R.G.; Millan-Almaraz, J.R. Instrumentation in Developing Chlorophyll Fluorescence Biosensing: A Review. Sensors 2012, 12, 11853–11869. [Google Scholar] [CrossRef]
- Che, X.; Fang, W.; Dong, X.; Yang, Y.; Guan, X.; Tian, Z. Research on a Small-Volume Portable Non-Contact Chlorophyll-b Measurement Instrument Based on Laser Spectroscopy. In Proceedings of the Conference on Spectral Technology and Applications (CSTA 2024), Dalian, China, 4 December 2024; Wang, Z., Ding, H., Eds.; SPIE: Bellingham, WA, USA, 2024; p. 135. [Google Scholar]
- Li, Z.; Ji, J.; Xu, M. Designation of Rapid Detection System for Chlorophyll Fluorescence Parameters Based on LED Irradiation. In Proceedings of the 2012 International Conference on Graphic and Image Processing, Singapore, 6–7 October 2012; Zhu, Z., Ed.; Proceedings of SPIE; SPIE—International Society for Optics and Photonics: Bellingham, WA, USA, 2013; Volume 8768, p. 87682I. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Jing, M.; Chen, M.-L.; Yang, F.; Ding, M.; Zhang, Q. Simulation Study of Detection System on LED Stimulated Plant Chlorophyll Fluorescence. In Proceedings of the Seventh Symposium on Novel Photoelectronic Detection Technology and Applications, Nanjing, China, 12 March 2021; Chu, J., Yu, Q., Jiang, H., Su, J., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 40. [Google Scholar]
- Moiseev, M.A.; Doskolovich, L.L.; Kazanskiy, N.L. Design of High-Efficient Freeform LED Lens for Illumination of Elongated Rectangular Regions. Opt. Express 2011, 19, A225. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, Z.; Ma, D. Efficient and Compact Freeform Optics Design for Customized LED Lighting. Opt. Laser Technol. 2023, 167, 109775. [Google Scholar] [CrossRef]
- Wu, R.; Zheng, Z.; Li, H.; Liu, X. Optimization Design of Irradiance Array for LED Uniform Rectangular Illumination. Appl. Opt. 2012, 51, 2257. [Google Scholar] [CrossRef]
- Wang, A.; Li, W.; Men, X.; Gao, B.; Xu, Y.; Wei, X. Vegetation Detection Based on Spectral Information and Development of a Low-cost Vegetation Sensor for Selective Spraying. Pest Manag. Sci. 2022, 78, 2467–2476. [Google Scholar] [CrossRef]
- Agati, G. Response of the in Vivo Chlorophyll Fluorescence Spectrum to Environmental Factors and Laser Excitation Wavelength. Pure Appl. Opt. J. Eur. Opt. Soc. Part A 1998, 7, 797–807. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Langsdorf, G.; Lenk, S.; Buschmann, C. Chlorophyll Fluorescence Imaging of Photosynthetic Activity with the Flash-Lamp Fluorescence Imaging System. Photosynthetica 2005, 43, 355–369. [Google Scholar] [CrossRef]
- Ryer, A. Light Measurement Handbook; International Light: Newburyport, MA, USA, 1997. [Google Scholar]
- Vrindts, E.; De Baerdemaeker, J.; Ramon, H. Weed Detection Using Canopy Reflection. Precis. Agric. 2002, 3, 63–80. [Google Scholar] [CrossRef]
- Strasser, R.J.; Govindjee. The Fo and the O-J-I-P Fluorescence Rise in Higher Plants and Algae. In Regulation of Chloroplast Biogenesis; Springer: Boston, MA, USA, 1992; pp. 423–426. [Google Scholar]
- Bates, H.; Zavafer, A.; Szabó, M.; Ralph, P.J. A Guide to Open-JIP, a Low-Cost Open-Source Chlorophyll Fluorometer. Photosynth. Res. 2019, 142, 361–368. [Google Scholar] [CrossRef]
- Leeuw, T.; Boss, E.; Wright, D. In Situ Measurements of Phytoplankton Fluorescence Using Low Cost Electronics. Sensors 2013, 13, 7872–7883. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Dong, K.; Mattos, E.; van Iersel, M.W. A Very Low-Cost Pulse-Amplitude Modulated Chlorophyll Fluorometer. Comput. Electron. Agric. 2022, 203, 107438. [Google Scholar] [CrossRef]
- Bürling, K.; Hunsche, M.; Noga, G. Use of Blue–Green and Chlorophyll Fluorescence Measurements for Differentiation between Nitrogen Deficiency and Pathogen Infection in Winter Wheat. J. Plant Physiol. 2011, 168, 1641–1648. [Google Scholar] [CrossRef]
- Hunsche, M.; Bürling, K.; Noga, G. Spectral and Time-Resolved Fluorescence Signature of Four Weed Species as Affected by Selected Herbicides. Pestic. Biochem. Physiol. 2011, 101, 39–47. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of Chlorophyll Fluorescence Imaging Technique in Horticultural Research: A Review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Deep Learning in Agriculture: A Survey. Comput. Electron. Agric. 2018, 147, 70–90. [Google Scholar] [CrossRef]
- Behmann, J.; Mahlein, A.-K.; Rumpf, T.; Römer, C.; Plümer, L. A Review of Advanced Machine Learning Methods for the Detection of Biotic Stress in Precision Crop Protection. Precis. Agric. 2015, 16, 239–260. [Google Scholar] [CrossRef]
- Hall, D.; Dayoub, F.; Kulk, J.; McCool, C. Towards Unsupervised Weed Scouting for Agricultural Robotics. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 13–17 May 2017; pp. 5223–5230. [Google Scholar]
- Johnson, S.C. Hierarchical Clustering Schemes. Psychometrika 1967, 32, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.J.; Dueck, D. Clustering by Passing Messages Between Data Points. Science 2007, 315, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine Learning for High-Throughput Stress Phenotyping in Plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.R.; Jackson, R.D.; Post, D.F. Spectral Response of a Plant Canopy with Different Soil Backgrounds. Remote Sens. Env. 1985, 17, 37–53. [Google Scholar] [CrossRef]
- Hanks, J.E.; Beck, J.L. Sensor-Controlled Hooded Sprayer for Row Crops. Weed Technol. 1998, 12, 308–314. [Google Scholar] [CrossRef]
- Deng, W.; Zhao, C.-J.; He, X.-K.; Chen, L.-P.; Zhang, L.-D.; Wu, G.-W.; Mueller, J.; Zhai, C.-Y. Study on Spectral Detection of Green Plant Target. Spectrosc. Spectr. Anal. 2010, 30, 2179–2183. [Google Scholar] [CrossRef]
- Longchamps, L.; Panneton, B.; Samson, G.; Leroux, G.D.; Thériault, R. Discrimination of Corn, Grasses and Dicot Weeds by Their UV-Induced Fluorescence Spectral Signature. Precis. Agric. 2010, 11, 181–197. [Google Scholar] [CrossRef]
- Panneton, B.; Guillaume, S.; Samson, G.; Roger, J.-M. Discrimination of Corn from Monocotyledonous Weeds with Ultraviolet (UV) Induced Fluorescence. Appl. Spectrosc. 2011, 65, 10–19. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of Photosynthetic Radiation-use Efficiency with Spectral Reflectance. N. Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The Reflectance at the 950–970 Nm Region as an Indicator of Plant Water Status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- He, Q.; Zhan, J.; Liu, X.; Dong, C.; Tian, D.; Fu, Q. Multispectral Polarimetric Bidirectional Reflectance Research of Plant Canopy. Opt. Lasers Eng. 2025, 184, 108688. [Google Scholar] [CrossRef]
- Yang, J.; Gong, W.; Shi, S.; Du, L.; Sun, J.; Zhu, B.; Ma, Y.; Song, S. Vegetation Identification Based on Characteristics of Fluorescence Spectral Spatial Distribution. RSC Adv. 2015, 5, 56932–56935. [Google Scholar] [CrossRef]
- Tyystjärvi, E.; Keränen, M.; Koski, A.; Nevalainen, O.; Aro, E.-M. Chlorophyll Fluorescence Can Be Used to Identify Plant Species Automatically. In Photosynthesis: Mechanisms and Effects; Springer: Dordrecht, The Netherlands, 1998; pp. 3857–3860. [Google Scholar]
- Codrea, C.M.; Aittokallio, T.; Keränen, M.; Tyystjärvi, E.; Nevalainen, O.S. Feature Learning with a Genetic Algorithm for Fluorescence Fingerprinting of Plant Species. Pattern Recognit. Lett. 2003, 24, 2663–2673. [Google Scholar] [CrossRef]
- Keränen, M.; Aro, E.-M.; Tyystjärvi, E.; Nevalainen, O. Automatic Plant Identification with Chlorophyll Fluorescence Fingerprinting. Precis. Agric. 2003, 4, 53–67. [Google Scholar] [CrossRef]
- Keränen, M.; Aro, E.-M.; Nevalainen, O.; Tyystjärvi, E. Toxic and Non-Toxic Nodularia Strains Can Be Distinguished from Each Other and from Eukaryotic Algae with Chlorophyll Fluorescence Fingerprinting. Harmful Algae 2009, 8, 817–822. [Google Scholar] [CrossRef]
- Tyystjärvi, E.; Nørremark, M.; Mattila, H.; Keränen, M.; Hakala-Yatkin, M.; Ottosen, C.-O.; Rosenqvist, E. Automatic Identification of Crop and Weed Species with Chlorophyll Fluorescence Induction Curves. Precis. Agric. 2011, 12, 546–563. [Google Scholar] [CrossRef]
- Mishra, A.; Matouš, K.; Mishra, K.B.; Nedbal, L. Towards Discrimination of Plant Species by Machine Vision: Advanced Statistical Analysis of Chlorophyll Fluorescence Transients. J. Fluoresc. 2009, 19, 905–913. [Google Scholar] [CrossRef]
- Mattila, H.; Valli, P.; Pahikkala, T.; Teuhola, J.; Nevalainen, O.S.; Tyystjärvi, E. Comparison of Chlorophyll Fluorescence Curves and Texture Analysis for Automatic Plant Identification. Precis. Agric. 2013, 14, 621–636. [Google Scholar] [CrossRef]
- Herritt, M.; Dhanapal, A.P.; Purcell, L.C.; Fritschi, F.B. Identification of Genomic Loci Associated with 21chlorophyll Fluorescence Phenotypes by Genome-Wide Association Analysis in Soybean. BMC Plant Biol. 2018, 18, 312. [Google Scholar] [CrossRef]
- Rapacz, M.; Wójcik-Jagła, M.; Fiust, A.; Kalaji, H.M.; Kościelniak, J. Genome-Wide Associations of Chlorophyll Fluorescence OJIP Transient Parameters Connected With Soil Drought Response in Barley. Front. Plant Sci. 2019, 10, 78. [Google Scholar] [CrossRef]
- Codrea, M.C.; Hakala-Yatkin, M.; Kårlund-Marttila, A.; Nedbal, L.; Aittokallio, T.; Nevalainen, O.S.; Tyystjärvi, E. Mahalanobis Distance Screening of Arabidopsis Mutants with Chlorophyll Fluorescence. Photosynth. Res. 2010, 105, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Sonoike, K. Screening of Mutants Using Chlorophyll Fluorescence. J. Plant Res. 2021, 134, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Shtaya, M.J.Y. Screening for Genetic Variation in the Circadian Clock in Barley Landraces from Palestine. J. Plant Growth Regul. 2024, 44, 3236–3242. [Google Scholar] [CrossRef]
- Basavaraj, P.; Jangid, K.K.; Babar, R.; Rane, J.; Boraiah, K.; Harisha, C.; Halli, H.; Pradhan, A.; Tripathi, K.; Sammi Reddy, K.; et al. Non-Invasive Measurements to Identify Mungbean Genotypes for Waterlogging Tolerance. PeerJ 2024, 12, e16872. [Google Scholar] [CrossRef]
- Mishra, A.; Heyer, A.G.; Mishra, K.B. Chlorophyll Fluorescence Emission Can Screen Cold Tolerance of Cold Acclimated Arabidopsis Thaliana Accessions. Plant Methods 2014, 10, 38. [Google Scholar] [CrossRef]
- Qin, X.; Xing, D.; Wu, Y.; Wang, W.; Li, M.; Solangi, K. Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants. Agronomy 2022, 12, 2758. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, M.; Mukai, K.; Shimasaki, Y.; Oshima, Y. Effects of Elevated Irradiance, Temperature, and Rapid Shifts of Salinity on the Chlorophyll a Fluorescence (OJIP) Transient of Chattonella Marina Var. Antiqua. J. Fac. Agric. Kyushu Univ. 2019, 64, 293–300. [Google Scholar] [CrossRef]
- Clay, S.A. Near-term Challenges for Global Agriculture: Herbicide-resistant Weeds. Agron. J. 2021, 113, 4463–4472. [Google Scholar] [CrossRef]
- Prather, T.S.; DiTomaso, J.M.; Holt, J.S. History, Mechanisms, and Strategies for Prevention and Management of Herbi-cide Resistant Weeds. Proc. Calif. Weed Sci. Soc. 2000, 52, 155–160. [Google Scholar]
- Gaines, T.A.; Busi, R.; Küpper, A. Can New Herbicide Discovery Allow Weed Management to Outpace Resistance Evolution? Pest Manag. Sci. 2021, 77, 3036–3041. [Google Scholar] [CrossRef]
- Roland, B.; Andrea, F.; Lothar, L.; Martin, H.; Bernd, L.; Juan Pedro, R.-S.A.; Harry, S. Weed Resistance Diagnostic Technologies to Detect Herbicide Resistance in Cereal-Growing Areas. A Review. In Proceedings of the Tagungsband/25. Deutsche Arbeitsbesprechung über Fragen der Unkrautbiologie und -bekämpfung, Braunschweig, Germany, 13–15 March 2012; Julius Kühn-Institut: Braunschweig, Germany, 2012; pp. 75–80. [Google Scholar]
- Burgos, N.R.; Tranel, P.J.; Streibig, J.C.; Davis, V.M.; Shaner, D.; Norsworthy, J.K.; Ritz, C. Review: Confirmation of Resistance to Herbicides and Evaluation of Resistance Levels. Weed Sci. 2013, 61, 4–20. [Google Scholar] [CrossRef]
- Streibig, J.C. Herbicide Bioassay. Weed Res. 1988, 28, 479–484. [Google Scholar] [CrossRef]
- Barbagallo, M. Role of Magnesium in Insulin Action, Diabetes and Cardio-Metabolic Syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Riethmuller-Haage, I.; Bastiaans, L.; Kropff, M.J.; Harbinson, J.; Kempenaar, C. Can Photosynthesis-Related Parameters Be Used to Establish the Activity of Acetolactate Synthase–Inhibiting Herbicides on Weeds? Weed Sci. 2006, 54, 974–982. [Google Scholar] [CrossRef]
- Wang, P.; Peteinatos, G.G.; Gerhards, R. In Field Identification of Herbicide Resistant Apera Spica-Venti Using Chlorophyll Fluorescence. Adv. Anim. Biosci. 2017, 8, 283–287. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Gerhards, R. Chlorophyll Fluorescence Response to Herbicide Stress in Alopecurus Myosuroides. In Proceedings of the 27th German Conference on Weed Biology and Weed Control, Braunschweig, Germany, 23–25 February 2016. [Google Scholar]
- Rosenqvist, E.; van Kooten, O. Chlorophyll Fluorescence: A General Description and Nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Springer: Boston, MA, USA, 2003; pp. 31–77. [Google Scholar]
- Deng, Y.; Xin, N.; Zhao, L.; Shi, H.; Deng, L.; Han, Z.; Wu, G. Precision Detection of Salt Stress in Soybean Seedlings Based on Deep Learning and Chlorophyll Fluorescence Imaging. Plants 2024, 13, 2089. [Google Scholar] [CrossRef]
- Sapoukhina, N.; Boureau, T.; Rousseau, D. Plant Disease Symptom Segmentation in Chlorophyll Fluorescence Imaging with a Synthetic Dataset. Front. Plant Sci. 2022, 13, 969205. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Xiao, Q.; Bai, X.; Wu, B.; Wu, N.; Zhao, Y.; Wang, J.; Feng, L. End-to-End Fusion of Hyperspectral and Chlorophyll Fluorescence Imaging to Identify Rice Stresses. Plant Phenomics 2022, 2022, 9851096. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, Y.; Xu, H.; He, Y.; Cen, H. Time-Series Chlorophyll Fluorescence Imaging Reveals Dynamic Photosynthetic Fingerprints of Sos Mutants to Drought Stress. Sensors 2019, 19, 2649. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, J.; Ji, W.; Wei, W.; Men, Y. Classification of Tomato Seedling Chilling Injury Based on Chlorophyll Fluorescence Imaging and DBO-BiLSTM. Front. Plant Sci. 2024, 15, 1409200. [Google Scholar] [CrossRef]
- Gong, L.; Gao, B.; Sun, Y.; Zhang, W.; Lin, G.; Zhang, Z.; Li, Y.; Liu, C. PreciseSLAM: Robust, Real-Time, LiDAR–Inertial–Ultrasonic Tightly-Coupled SLAM With Ultraprecise Positioning for Plant Factories. IEEE Trans. Ind. Inform. 2024, 20, 8818–8827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).