Eco-Friendly Suppression of Grapevine Root Rot: Synergistic Action of Biochar and Trichoderma spp. Against Fusarium equiseti
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production and Characterization
2.2. Trichoderma aureoviride Inoculum Production
2.3. Fusarium equiseti Inoculum Production
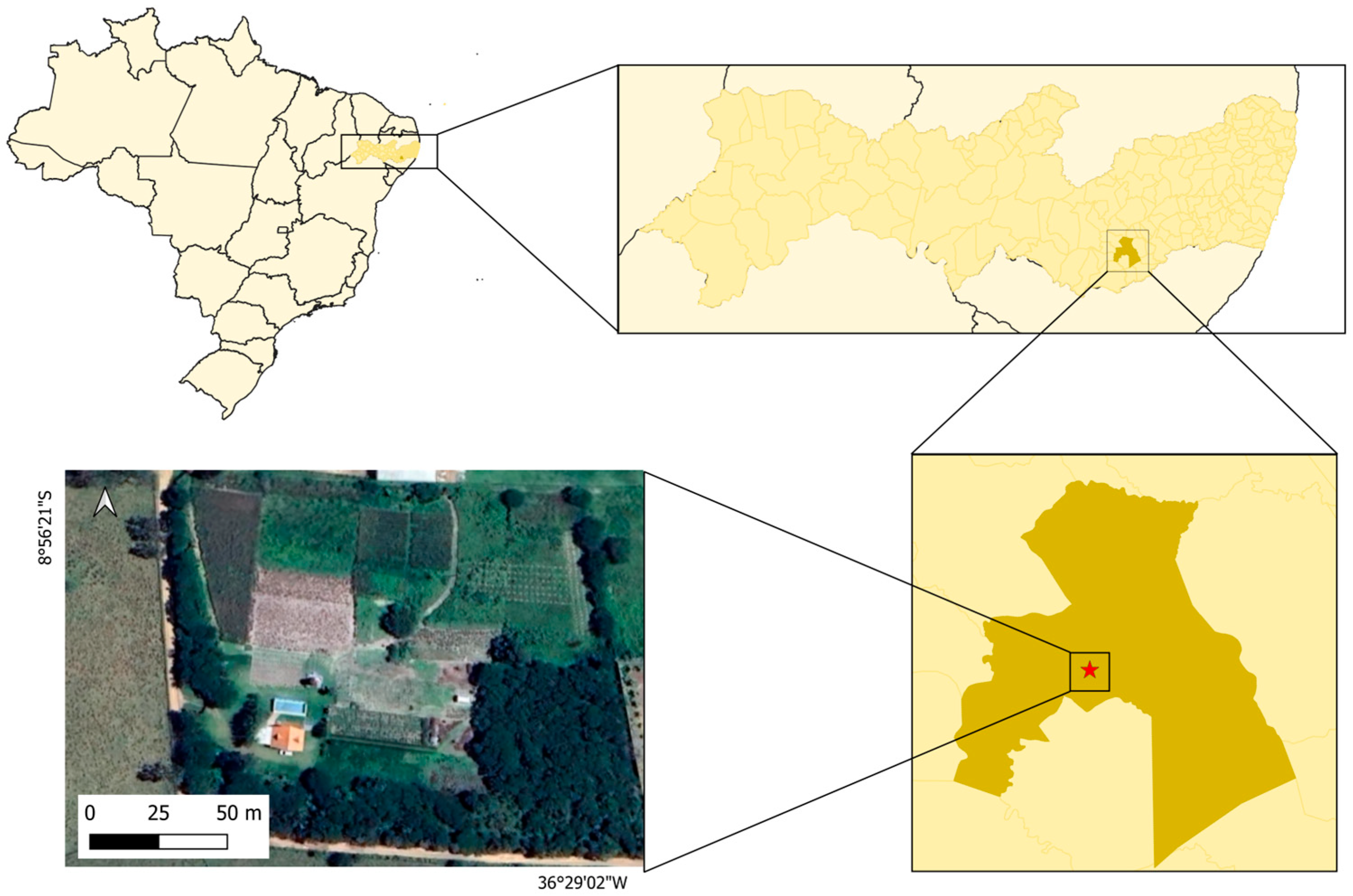
2.4. Microcosm Experiment
2.5. Statistical Analysis
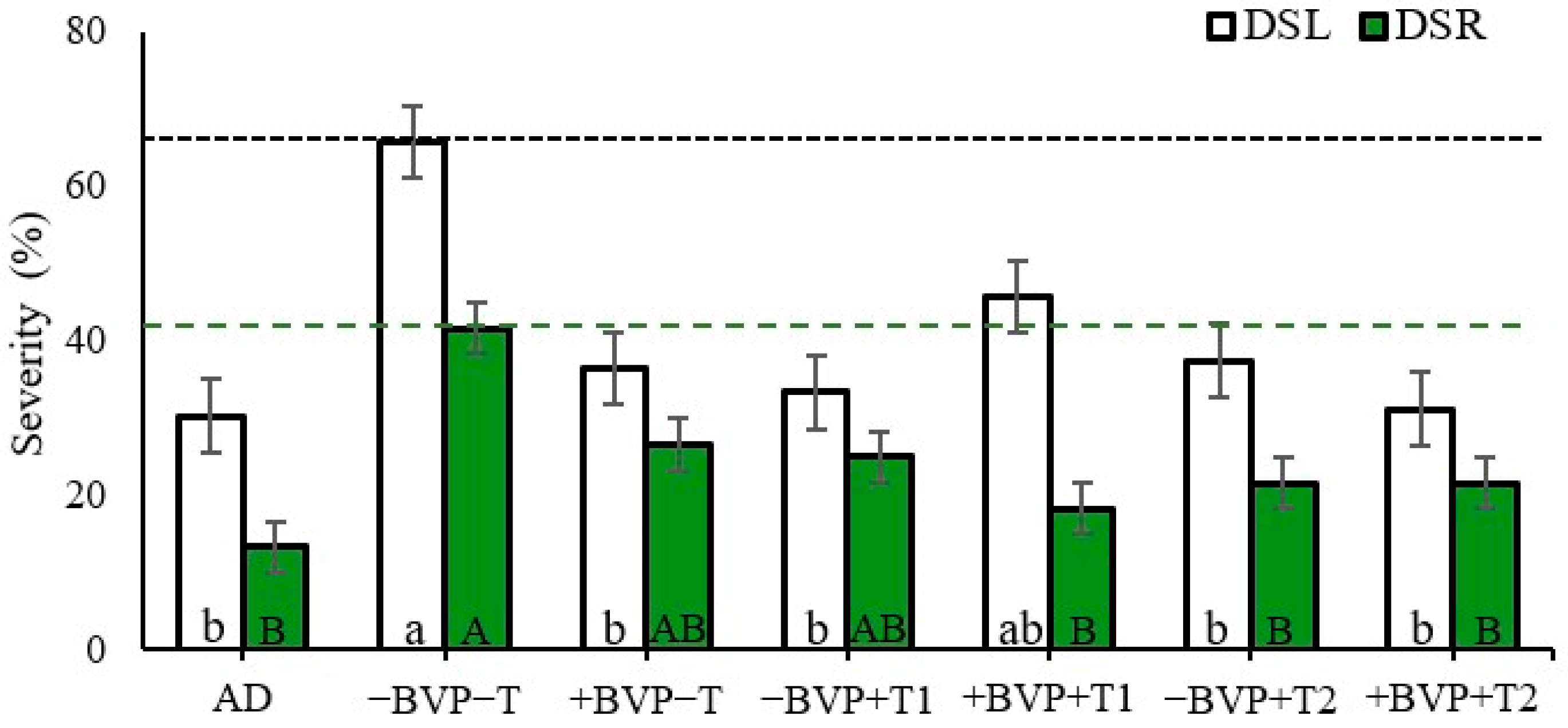
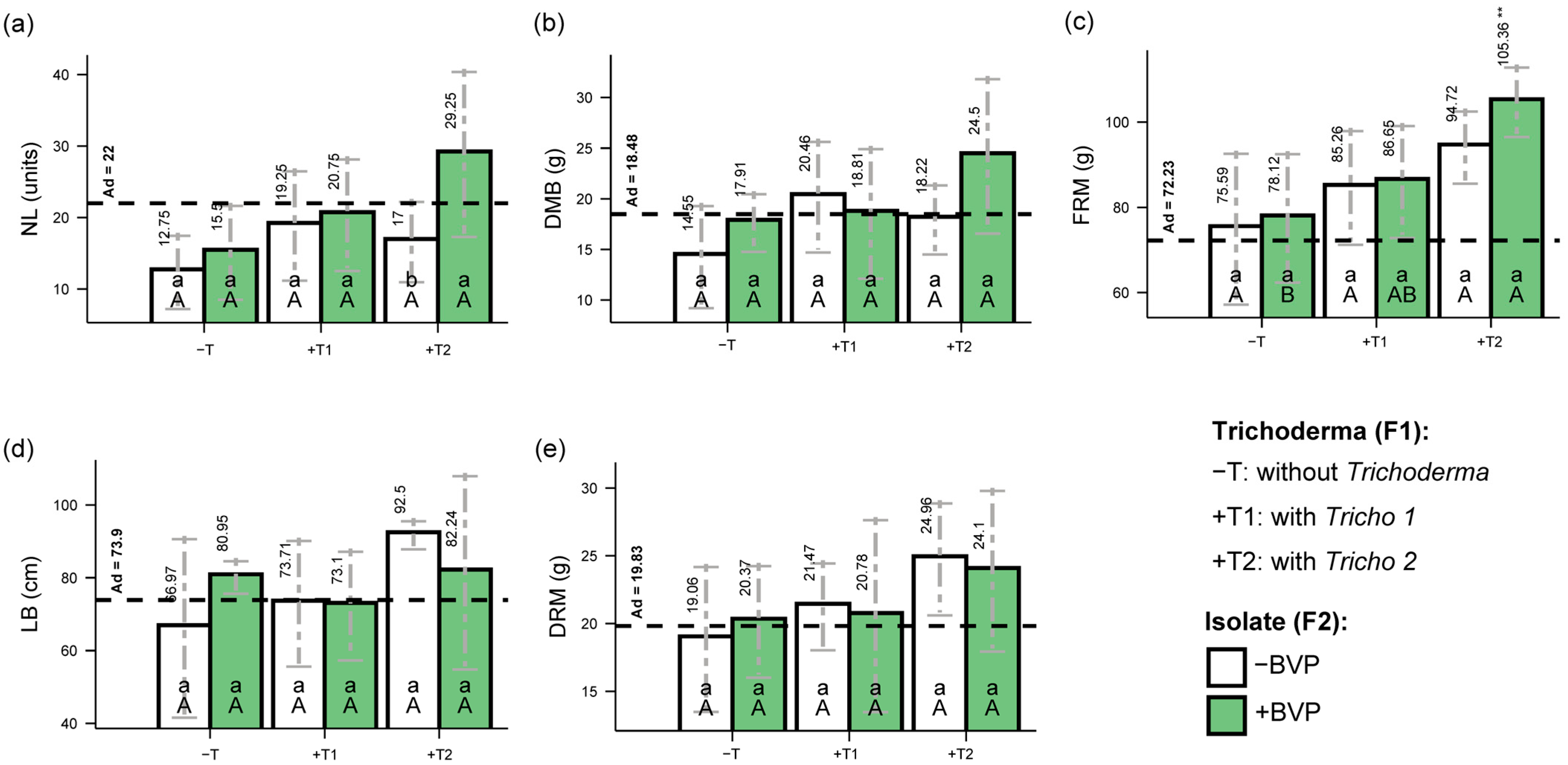
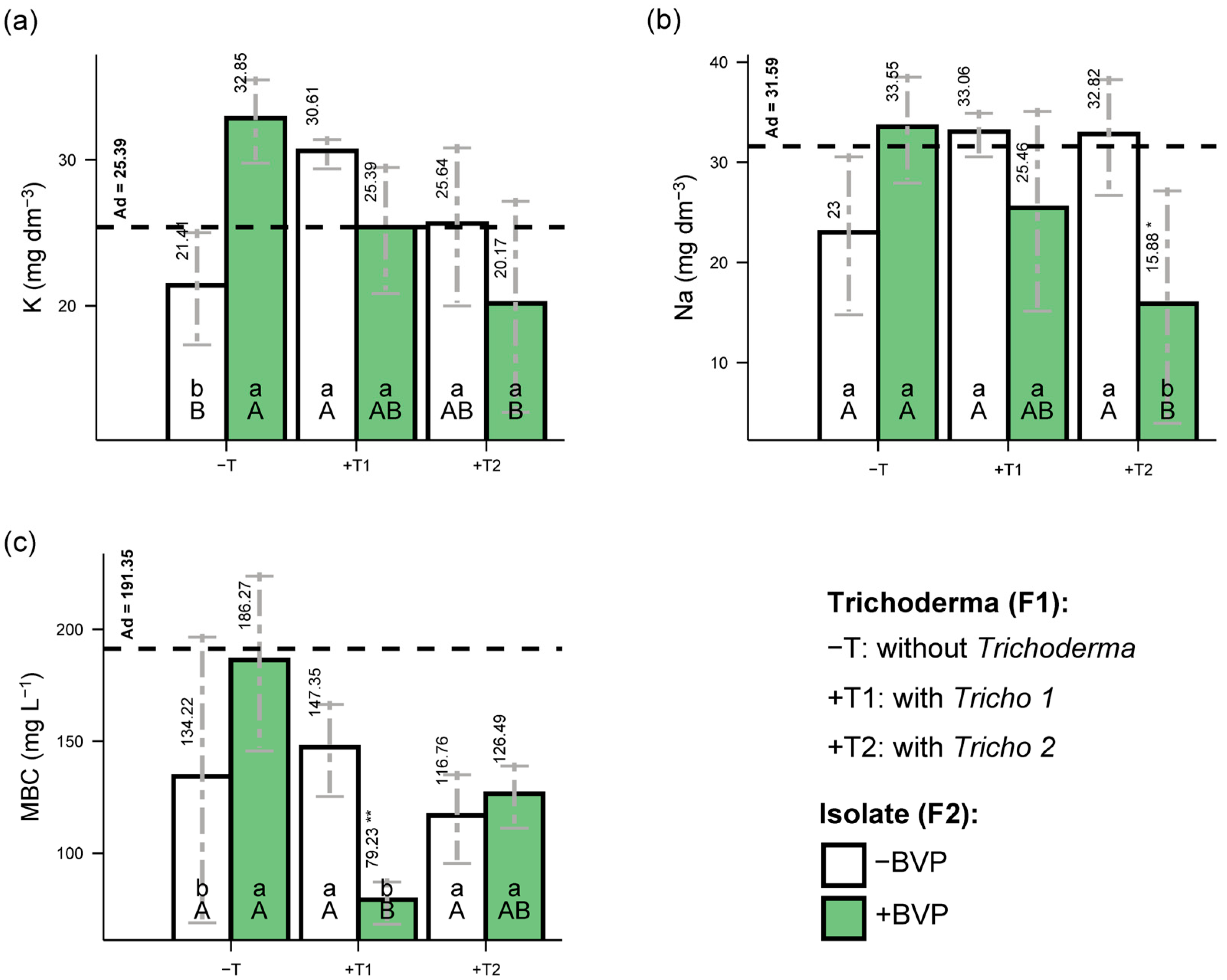
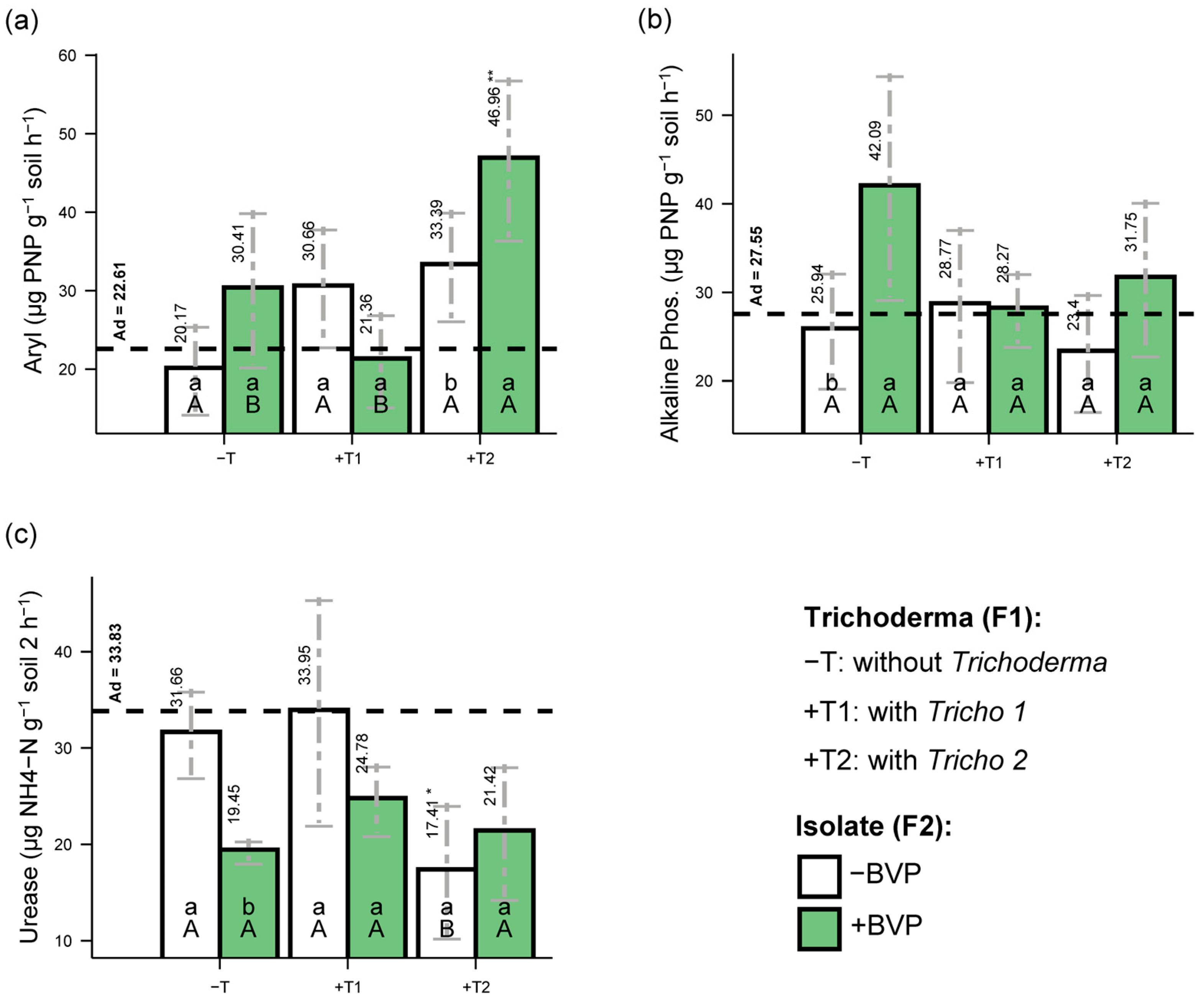
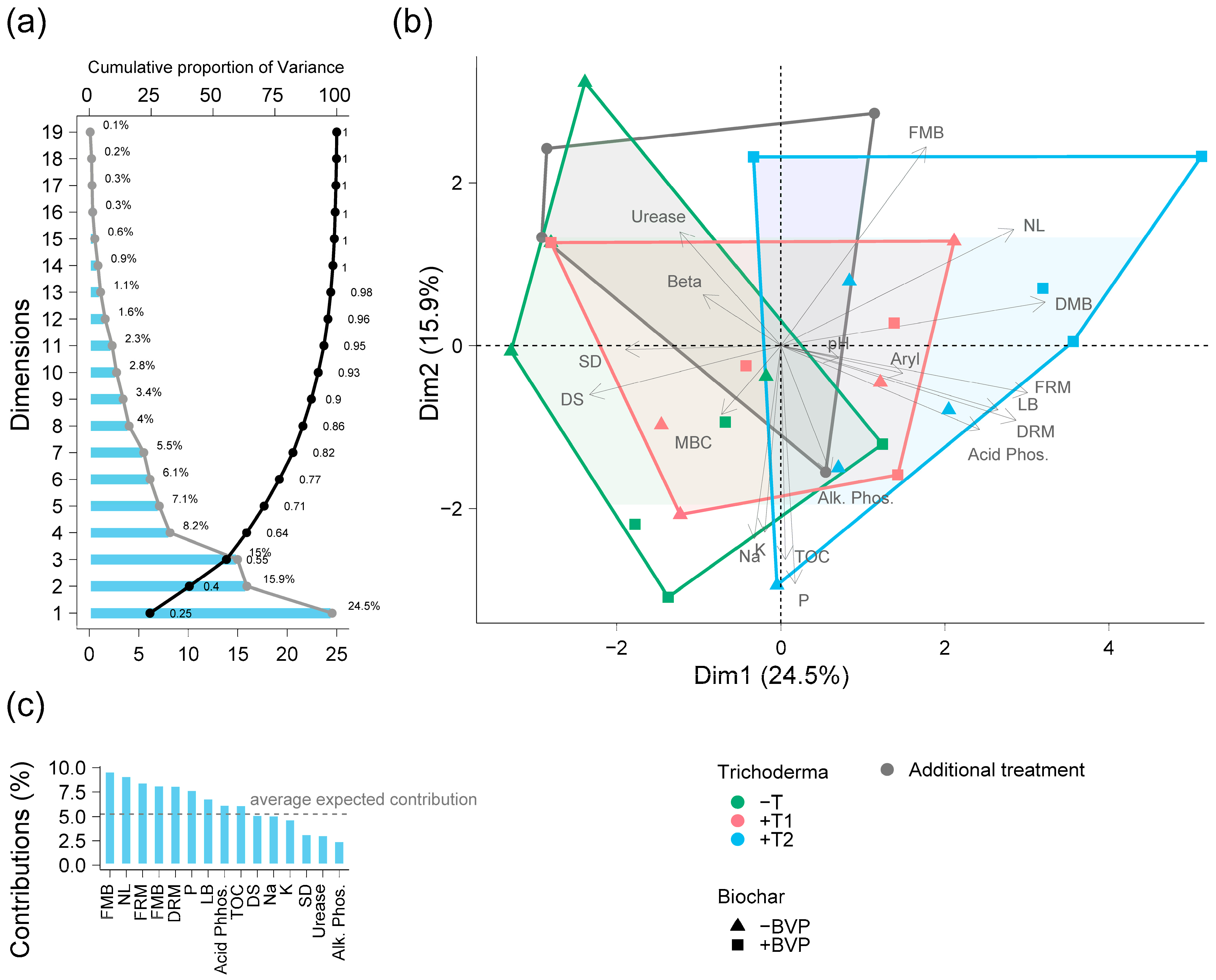
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| +BVP | with grapevine pruning biochar |
| −BVP | without biochar |
| T1 | Trichoderma aureoviride URM 6668 |
| T2 | Trichoderma aureoviride URM 3734 |
| NL | number of leaves |
| DMB | dry mass of branches |
| FRM | fresh root mass |
| LB | length of branches |
| DRM | dry root mass |
| MBC | microbial biomass carbon |
| Acid Phosp. | acid phosphatase |
| Alkaline Phos. | alkaline phosphatase |
| Beta | beta-glucosidase |
| Aryl | arylsulfatase |
| Ure | urease |
References
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, J.; Wang, Z.; Wu, P.; Wang, J.; Cai, X.; Zhao, D.; Yan, R. Graph-Structured Speculative Decoding. Graph-Structured Speculative Decoding. Trans. Assoc. Comput. Linguist. 2024, 11404–11415. [Google Scholar] [CrossRef]
- Li, Z.; Unzué-Belmonte, D.; Cornelis, J.T.; Linden, C.V.; Struyf, E.; Ronsse, F.; Delvaux, B. Effects of phytolithic rice-straw biochar, soil buffering capacity and pH on silicon bioavailability. Plant Soil. 2019, 438, 187–203. [Google Scholar] [CrossRef]
- Medeiros, E.V.; Moraes, M.C.H.S.; Costa, D.P.; Duda, G.P.; Silva, J.S.A.; Oliveira, J.B.; Lima, J.R.S.; Menezes, R.S.C.; Hammecker, C. Biochar and Trichoderma Aureoviride applied to the sandy soil: Effect on soil quality and watermelon growth. Not. Bot. Horti Agrobot. 2020, 48, 735–751. [Google Scholar] [CrossRef]
- Silva, L.G.; Andrade, C.A.; Bettiol, W. Biochar amendment increases soil microbial biomass and plant growth and suppresses Fusarium wilt in tomato. Trop. Plant Pathol. 2020, 45, 73–83. [Google Scholar] [CrossRef]
- De Medeiros, E.V.; Lima, N.T.; de Sousa Lima, J.R.; Pinto, K.M.S.; Costa, D.P.; Franco Junior, C.L.; Souza, R.M.S.; Hammecker, C. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: A critical review. Phytoparasitica 2021, 49, 713–726. [Google Scholar] [CrossRef]
- De Medeiros, E.V.; Pereira, A.P.A.; Silva, A.C.; Albuquerque, M.F.; Rocha, E.; Farias, L.; Ferraz, R. Biochar and Trichoderma as an eco-friendly and low-cost alternative to improve soil chemical and biological properties. Waste Biomass Valorization 2023, 14, 3507–3520. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Correia, K.C.; Barbosa, M.A.G.; Câmara, M.P.S.; Gramaje, D.; Michereff, S.J. Characterization of Phaeoacremonium isolates associated with Petri disease of table grape in Northeastern Brazil, with description of Phaeoacremonium nordesticola sp. nov. Eur. J. Plant Pathol. 2017, 149, 695–709. [Google Scholar] [CrossRef]
- Ali, A.; Elrys, A.; Liu, L.; Xia, Q.; Wang, B.; Li, Y.; Dan, X.; Iqbal, M.; Zhao, J.; Huang, X.; et al. Deciphering the Synergies of Reductive Soil Disinfestation Combined with Biochar and Antagonistic Microbial Inoculation in Cucumber Fusarium Wilt Suppression Through Rhizosphere Microbiota Structure. Microb. Ecol. 2022, 85, 980–997. [Google Scholar] [CrossRef]
- Da Silva, J.S.A.; de Medeiros, E.V.; Costa, D.P.; de Souza, C.A.F.; de Oliveira, J.B.; da França, R.F.; Souza-Motta, C.M.; Lima, J.R.d.S.; Hammecker, C. Biochar and Trichoderma aureoviride URM 5158 as alternatives for the management of cassava root rot. Appl. Soil. Ecol. 2022, 172, 104353. [Google Scholar] [CrossRef]
- ASAD, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Anothai, J.; Chairin, T. Development of integrated factor modeling: Inhibiting Ganoderma lignocellulosic enzymes while promoting Trichoderma sporulation for enhanced plant disease control. Physiol. Mol. Plant Pathol. 2024, 133, 102382. [Google Scholar] [CrossRef]
- Khan, R.; Najeeb, S.; Chen, J.; Wang, R.; Zhang, J.; Hou, J.; Liu, T. Insights into the molecular mechanism of Trichoderma stimulating plant growth and immunity against phytopathogens. Physiol. Plant. 2023, 175, e14132023. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.; Schmoll, M.; Esquivel-Ayala, B.; González-Esquivel, C.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef]
- Devi, K.S.; Devi, P.S.; Sinha, B.; Singh, L.N.K.; Maibam, N.; Chanu, T.W.; Devi, H.C. Effects of bio priming of rice seeds with native Trichoderma spp. isolated from rice rhizospheric soil. J. Pharmacogn. Phytochem. 2019, 8, 1968–1971. [Google Scholar]
- Liu, B.; Ji, S.; Zhang, H.; Wang, Y.; Liu, Z. Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol. Res. 2020, 235, 126445. [Google Scholar] [CrossRef]
- Lees, A.K.; Hawkins, N.J.; Baker, S.C.; Wilkinson, R.E. New chemical fungicides in relation to risk for resistance development. Trop. Plant Pathol. 2019, 44, 271–280. [Google Scholar] [CrossRef]
- Kellar, C.R.; Hassell, K.L.; Long, S.M.; Myers, J.H.; Golding, L.; Rose, G.; Pettigrove, V. Ecological evidence links adverse biological effects to pesticide and metal contamination in an urban Australian watershed. J. Appl. Ecol. 2014, 51, 426–439. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Marfo, T.D. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Muller, A.; Sumser, H.; Horren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Samen, F.; Nasrallah, M.; Alfaqih, M.; Alananbeh, K. Prevalence and pathogenicity of fungi associated with grapevine trunk diseases in Jordan. Phytopathol. Mediterr. 2023, 62, 255–268. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clement, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef]
- Billones-Baaijens, R.; Úrbez-Torres, J.R.; Ridgway, H.J. Virulence affected by assay parameters during grapevine pathogenicity studies with Botryosphaeriaceae nursery isolates. Plant Pathol. 2013, 62, 1214–1225. [Google Scholar] [CrossRef]
- Hrycan, J.; Theilmann, J.; Mahovlic, A.; Boulé, J.; Úrbez-Torres, J.R. Health status of ready-to-plant grapevine nursery material in Canada regarding young vine decline fungi. Plant Dis. 2023, 107, 3708–3717. [Google Scholar] [CrossRef]
- González, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42. [Google Scholar] [CrossRef]
- Fischer, M.; Peighami-Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar] [CrossRef]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar] [CrossRef]
- Vilvert, E.; Costa, M.D.; Cangahuala-Inocente, G.C.; Lovato, P.E. Root Proteomic Analysis of Grapevine Rootstocks Inoculated with Rhizophagus irregularis and Fusarium oxysporum f. sp. herbemontis. Rev. Bras. Ciênc. Solo 2017, 41, e0160134. [Google Scholar] [CrossRef]
- Silva-Valderrama, I.; Toapanta, D.; Miccono, M.D.L.A.; Lolas, M.; Díaz, G.A.; Cantu, D.; Castro, A. Biocontrol potential of grapevine endophytic and rhizospheric fungi against trunk pathogens. Front. Microbiol. 2021, 11, 614620. [Google Scholar] [CrossRef]
- Akgül, D.S.; Güngör Savas, N.; Yildiz, M.; Bülbül, I.; Özarslandan, M. Current status of grapevine trunk disease pathogens on asymptomatic nursery-produced grapevines in Türkiye. Phytopathol. Mediterr. 2023, 60, 151–163. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil. Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Lima, J.R.S.; Silva, W.M.; Medeiros, E.V.; Duda, G.P.; Correa, M.M.; Filho Martins, A.P.; Clermont-Dauphin, C.; Antonino, A.C.D.; Hammecker, C. Effect of biochar on physicochemical properties of a sandy soil and maize growth in a greenhouse experiment. Geoderma 2018, 319, 14–23. [Google Scholar] [CrossRef]
- Nair, R.R.; Schaate, A.; Klepzig, L.F.; Turcios, A.E.; Lecinski, J.; Shamsuyeva, M.; Endres, H.-J.; Papenbrock, J.; Behrens, P.; Weichgrebe, D. Physico-chemical characterization of walnut shell biochar from uncontrolled pyrolysis in a garden oven and surface modification by ex-situ chemical magnetization. Clean. Technol. Environ. Policy 2023, 25, 2727–2746. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; Melo, I.C.N.D.; Melo, L.C.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; EMBRAPA: Brasília, Brazil, 2017; 15p. [Google Scholar]
- da Silva, J.A.T.; de Medeiros, E.V.; Tenório, D.D.A.; Moreira, K.A.; Nascimento, T.C.E.d.S.; Souza-Motta, C. Trichoderma aureoviride URM 5158 and Trichoderma hamatum URM 6656 are biocontrol agents that act against cassava root rot through different mechanisms. J. Phytopathol. 2016, 164, 1003–1011. [Google Scholar] [CrossRef]
- Steffen, G.P.; Maldaner, J. BOLETIM TÉCNICO: Pesquisa e desenvolvimento. In Metodologia Para Multiplicação de Trichoderma sp. Em Substratos Orgânicos; SEAPDR/DDPA: Porto Alegre, Brazil, 2019; 22p. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Tokeshi, H.; Galli, F. Variabilidade de Fusarium oxysporum f. sp. lycopersici (Wr) Sny & Hans em São Paulo. An. Esc. Super. Agric. Luiz Queiroz 1966, 23, 195–209. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil. Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Mendonça, E.D.S.; Matos, E.D.S. Soil Organic Matter: Methods of Analysis. 2005. Available online: https://www.ciodaterra.com.br/materia-organica-do-solo-metodos-de-analises?srsltid=AfmBOopx79zLFMoNfIK9asAjqq3VppRf1-zeBjAgFGwhB2Q7dwu0jiSS (accessed on 3 April 2025). (In Portuguese).
- Bartlett, R.J.; Ross, D.S. Colorimetric Determination of Oxidizable Carbon in Acid Soil Solutions. Soil. Sci. Soc. Am. J. 1988, 52, 1191–1192. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil. Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil. Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Factors Affecting Soil Arylsulfatase Activity. Soil. Sci. Soc. Am. J. 1970, 34, 427–429. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fert. Soils 1988, 6, 68. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 24 January 2025).
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston. 2023. Available online: http://www.rstudio.com/ (accessed on 24 January 2025).
- Kassambara, A.; Mundt, F.; Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 24 January 2025).
- Contreras-Cornejo, H.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- Arinbasarova, A.; Botin, A.; Medentsev, A.; Makrushin, K.; Vetcher, A.; Stanishevskiy, Y. Synthesis of Extracellular L-lysine-α-oxidase along with Degrading Enzymes by Trichoderma cf. aureoviride Rifai VKM F-4268D: Role in Biocontrol and Systemic Plant Resistance. J. Fungi 2024, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Mazrou, Y.; Makhlouf, A.; Hassan, M.; Baazeem, A.; Hamad, A.; Farid, M. Influence of chitinase production on the antagonistic activity of Trichoderma against plant-pathogenic fungi. J. Environ. Biol. 2020, 41, 1501–1510. [Google Scholar] [CrossRef]
- Loc, N.; Huy, N.; Quang, H.; Lan, T.; Ha, T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus. Trichoderma asperellum PQ34. Mycology 2019, 11, 38–48. [Google Scholar] [CrossRef]
- Sharma, A.; Salwan, R.; Sharma, V. Extracellular proteins of Trichoderma and their role in plant health. S. Afr. J. Bot. 2022, 147, 359–369. [Google Scholar] [CrossRef]
- Dong-Mei, S.; Qian, Y. Antagonism of Trichoderma aureoviride against Fusarium spp. which causes soybean root rot. Chin. J. Oil Crop Sci. 2005, 27, 58–61. [Google Scholar]
- Guo, L.; Yu, H.; Niu, W.; Kharbach, M. Biochar Promotes Nitrogen Transformation and Tomato Yield by Regulating Nitrogen-Related Microorganisms in Tomato Cultivation Soil. Agronomy 2021, 11, 381. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, T.; Hou, Y.; Bol, R.; Zhang, X.; Zhang, M.; Yu, N.; Meng, J.; Zou, H.; Wang, J. Review on the effects of biochar amendment on soil microorganisms and enzyme activity. J. Soils Sediments, 2024; in press. [Google Scholar] [CrossRef]
- Rahmanian, M.; Khadem, A. The effects of biochar on soil extra- and intracellular enzyme activity. Biomass Convers. Biorefin. 2023; in press. [Google Scholar] [CrossRef]
- Zeng, L.; Zimmerman, A.; Huang, R. Adsorption of extracellular enzymes by biochar: Impacts of enzyme and biochar properties. Geoderma 2024, 441, 117082. [Google Scholar] [CrossRef]
- Wu, H.; Foster, X.; Kazemian, H.; Diby, I.; Kaliaguine, S.; Vaneeckhaute, C. Nutrient recovery from urine: Urea adsorption onto biochar integrated with Na-chabazite as urease inhibitor. Resour. Conserv. Recycl. 2025, 198, 107955. [Google Scholar] [CrossRef]
- Bello, A.; Liu, W.; Chang, N.; Erinle, K.; Deng, L.; Egbeagu, U.; Babalola, B.; Yue, H.; Sun, Y.; Wei, Z.; et al. Deciphering biochar compost co-application impact on microbial communities mediating carbon and nitrogen transformation across different stages of corn development. Environ. Res. 2022, 219, 115123. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, K.; Cui, M.; Chen, Z.; Dai, Y.; Qian, X.; Chen, Z. Meta-Analysis Study on the Role of Biochar on Soil Nitrogen Cycling. J. Soil Sci. Plant Nutr. 2024; in press. [Google Scholar] [CrossRef]
- Jam, E.; Khomari, S.; Ebadi, A.; Goli-Kalanpa, E.; Ghavidel, A. Influences of peanut hull-derived biochar, Trichoderma harzianum and supplemental phosphorus on hairy vetch growth in Pb- and Zn-contaminated soil. Environ. Geochem. Health 2023, 45, 9411–9432. [Google Scholar] [CrossRef]
| Attributes | Grapevine Pruning Biochar (BVP) * |
|---|---|
| pH | 9.64 |
| CEC | 17.3 |
| C (g kg−1) | 381.7 |
| N 1 (g kg−1) | 14.7 |
| C/N | 26 |
| P (g kg−1) | 8.9 |
| K (g kg−1) | 14.5 |
| Ca (g kg−1) | 12.8 |
| Mg (g kg−1) | 4.2 |
| Na (g kg−1) | 4.8 |
| Sulfur (g kg−1) | 3209.4 |
| Fe (mg kg−1) | 3631.9 |
| Cu (mg kg−1) | 36.8 |
| Mn (mg kg−1) | 362.9 |
| Zn (mg kg−1) | 3205.5 |
| B 2 (g kg−1) | 23.8 |
| EC (dS m−1) | 1.01 |
| pH | EC | OM | P | S-SO42− | Ca2+ | Mg2+ | K+ | Na+ | Al3+ |
| (H2O) | (dS m−1) | (g kg−1) | (mg kg−1) | (cmolc kg−1) | |||||
| 5.37 | 0.42 | 5.5 | 15.5 | 1.8 | 1.04 | 0.6 | 0.14 | 0.02 | 0.08 |
| H+Al | SB | CEC | V | Fe2+ | Mn2+ | Cu2+ | Zn2+ | B | |
| (cmolc kg−1) | (%) | (cmolc kg−1) | |||||||
| 1.16 | 1.8 | 2.96 | 60.69 | 50.1 | 15.9 | 0.34 | 1.6 | 0.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Mota, S.E.O.; Barros, J.A.d.; Pinto, K.M.S.; Santos, J.E.C.C.; Vieira, A.d.P.; Lima, E.M.d.; Costa, D.P.d.; Duda, G.P.; Lima, J.R.d.S.; da Silva, M.M.; et al. Eco-Friendly Suppression of Grapevine Root Rot: Synergistic Action of Biochar and Trichoderma spp. Against Fusarium equiseti. Agriculture 2025, 15, 1774. https://doi.org/10.3390/agriculture15161774
da Mota SEO, Barros JAd, Pinto KMS, Santos JECC, Vieira AdP, Lima EMd, Costa DPd, Duda GP, Lima JRdS, da Silva MM, et al. Eco-Friendly Suppression of Grapevine Root Rot: Synergistic Action of Biochar and Trichoderma spp. Against Fusarium equiseti. Agriculture. 2025; 15(16):1774. https://doi.org/10.3390/agriculture15161774
Chicago/Turabian Styleda Mota, Sabrina Esposito Oliveira, Jamilly Alves de Barros, Kedma Maria Silva Pinto, José Eduardo Cordeiro Cezar Santos, Alberto dos Passos Vieira, Elisiane Martins de Lima, Diogo Paes da Costa, Gustavo Pereira Duda, José Romualdo de Sousa Lima, Mairon Moura da Silva, and et al. 2025. "Eco-Friendly Suppression of Grapevine Root Rot: Synergistic Action of Biochar and Trichoderma spp. Against Fusarium equiseti" Agriculture 15, no. 16: 1774. https://doi.org/10.3390/agriculture15161774
APA Styleda Mota, S. E. O., Barros, J. A. d., Pinto, K. M. S., Santos, J. E. C. C., Vieira, A. d. P., Lima, E. M. d., Costa, D. P. d., Duda, G. P., Lima, J. R. d. S., da Silva, M. M., Souza, C. A. F. d., Oliveira, R. J. V. d., Hammecker, C., & Medeiros, E. V. d. (2025). Eco-Friendly Suppression of Grapevine Root Rot: Synergistic Action of Biochar and Trichoderma spp. Against Fusarium equiseti. Agriculture, 15(16), 1774. https://doi.org/10.3390/agriculture15161774







