Molecular Insights into Powdery Mildew Pathogenesis and Resistance in Cucurbitaceous Crops
Abstract
1. Introduction
2. Etiology and Biology of Powdery Mildew Pathogens
2.1. Major Causal Agents in Cucurbit and Pathogen Distribution
2.2. Symptoms
2.3. Biology, Lifecycle, and Environmental Conditions for Infection
2.3.1. Overwintering
2.3.2. Infection
2.3.3. Appressoria and Haustoria Formation
2.3.4. Sporulation
3. Molecular Mechanisms of Pathogenesis
3.1. Pathogen Effectors and Host Manipulation
3.2. Host–Pathogen Molecular Interactions
4. Plant Defense Responses Against Powdery Mildew
4.1. Recognition of Powdery Mildew Pathogens
4.1.1. PRRs (Pattern Recognition Receptors) and R Proteins
4.1.2. Downstream Signaling Pathways (SA, JA, and ROS Bursts)
4.1.3. Crosstalk and Integration of SA, JA, and ROS Pathways
4.1.4. Pathogen Strategies to Maintain Host Cell Viability and Later Stages of Pathogenesis
4.2. Natural and Engineered Resistance Mechanisms
5. Breeding and Biotechnological Strategies for Resistance
5.1. Conventional Breeding Approaches
5.2. Genomics-Assisted Breeding and Gene Editing
5.3. Transgenic and RNAi-Based Strategies
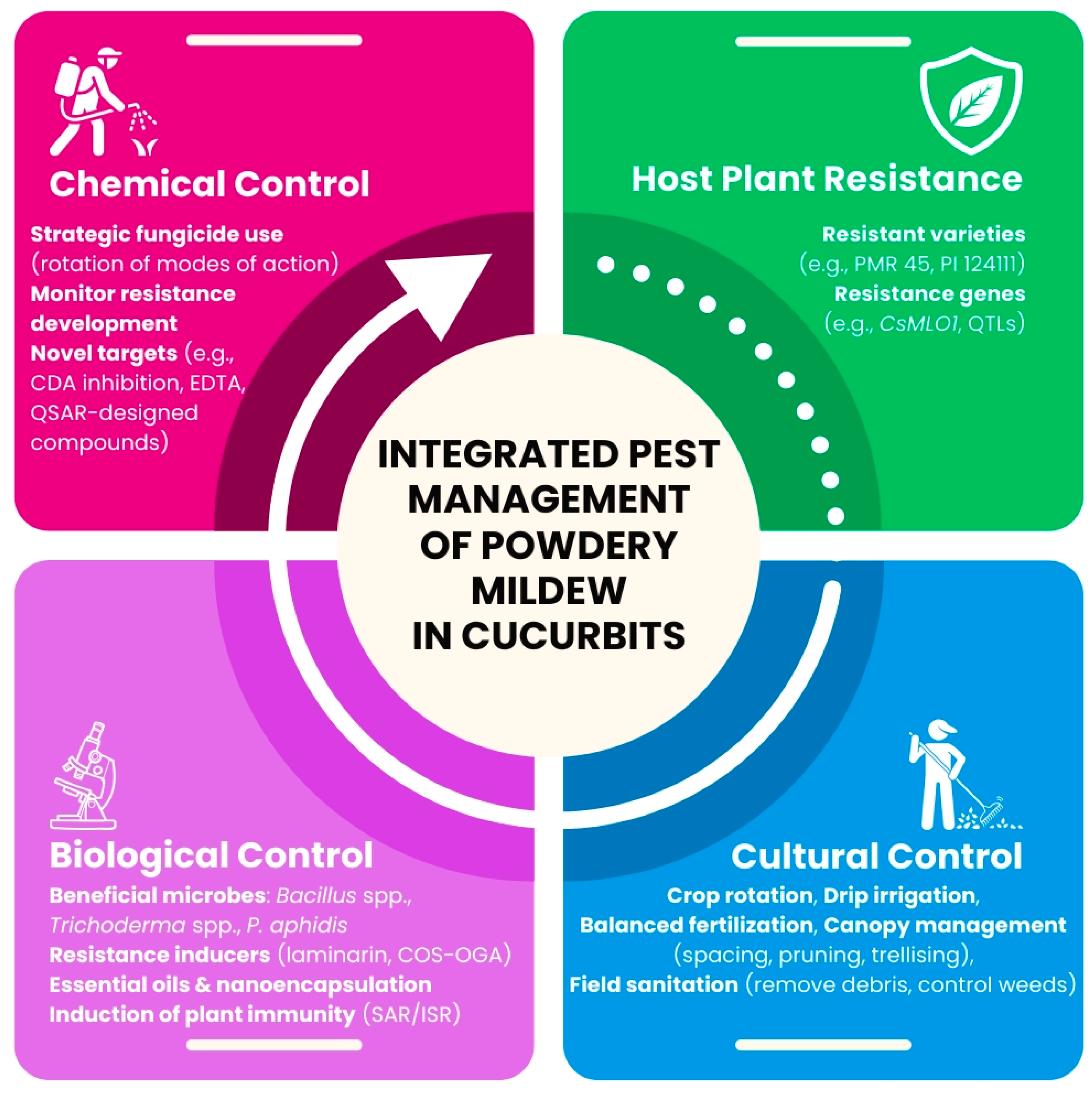
6. Integrated Management Strategies
6.1. Host Plant Resistance
6.2. Cultural Control
6.3. Biological Control
6.4. Chemical Control
6.5. Forecasting Model of Powdery Mildew Disease in Cucurbits
- Machine Learning (e.g., CNN-LSTM): These models can fuse quantitative disease information with environmental data to predict disease incidence [170].
- Hyperspectral and Terahertz Technology: These methods use spectral data from leaves to detect and identify powdery mildew, even in its early stages [171].
7. Climate Change Impacts on Powderly Mildew
8. Future Perspectives
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Wei, M.; Yan, Y.; Yu, C.; Cheng, S.; Sun, Y.; Zhu, X.; Wei, L.; Wang, H.; Miao, L. Research advances in genetic mechanisms of major cucumber diseases resistance. Front. Plant Sci. 2022, 13, 862486. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Pandey, S.; Yandell, B.S.; Pathak, M.; Weng, Y. QTL mapping of powdery mildew resistance in WI 2757 cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2013, 126, 2149–2161. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, Y.; Yang, J.; Liao, D.; Liu, P.; Wen, C.; Yun, T. Identification of powdery mildew resistance-related genes in butternut squash (Cucurbita moschata). Int. J. Mol. Sci. 2024, 25, 10896. [Google Scholar] [CrossRef]
- Xu, X.; Yu, T.; Xu, R.; Shi, Y.; Lin, X.; Xu, Q.; Qi, X.; Weng, Y.; Chen, X. Fine mapping of a dominantly inherited powdery mildew resistance major-effect QTL, Pm1.1, in cucumber identifies a 41.1 kb region containing two tandemly arrayed cysteine-rich receptor-like protein kinase genes. Theor. Appl. Genet. 2016, 129, 507–516. [Google Scholar] [CrossRef]
- Liu, P.N.; Miao, H.; Lu, H.W.; Cui, J.Y.; Tian, G.L.; Wehner, T.C.; Gu, X.F.; Zhang, S.P. Molecular mapping and candidate gene analysis for resistance to powdery mildew in Cucumis sativus stem. Genet. Mol. Res. 2017, 16, gmr16039680. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xu, X.; Shi, Y.; Qi, X.; Chen, X. Elucidation of the molecular responses of a cucumber segment substitution line carrying Pm5.1 and its recurrent parent triggered by powdery mildew by comparative transcriptome profiling. BMC Genom. 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Grumet, R.; McCreight, J.D.; McGregor, C.; Weng, Y.; Mazourek, M.; Reitsma, K.; Labate, J.; Davis, A.; Fei, Z. Genetic resources and vulnerabilities of major cucurbit crops. Genes 2021, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, J.; Xu, B.; Zhou, J. Differential responses of Cucurbita pepo to Podosphaera xanthii reveal the mechanism of powdery mildew disease resistance in pumpkin. Front. Plant Sci. 2021, 12, 633221. [Google Scholar] [CrossRef]
- Xu, X.; Du, Y.; Li, S.; Tan, M.; Sohail, H.; Liu, X.; Qi, X.; Yang, X.; Chen, X. A genome-wide association study reveals molecular mechanism underlying powdery mildew resistance in cucumber. Genome Biol. 2024, 25, 252. [Google Scholar] [CrossRef]
- Jiang, L.; Xiao, X. Research progress on powdery mildew in cucurbitaceae plants, a systematic review. Mol. Pathog. 2024, 15, 155–169. [Google Scholar] [CrossRef]
- Kousik, C.S.; Donahoo, R.S.; Webster, C.G.; Turechek, W.W.; Adkins, S.T.; Roberts, P.D. Outbreak of cucurbit powdery mildew on watermelon fruit caused by Podosphaera xanthii in southwest Florida. Plant Dis. 2011, 95, 1586–1587. [Google Scholar] [CrossRef] [PubMed]
- Maia, G.S.; Gevens, A.J. Characterizing cucurbit powdery mildew in north central Florida. Proc. Fla. State Hortic. Soc. 2009, 122, 247–249. [Google Scholar]
- Nuñez-Palenius, H.G.; Hopkins, D.; Cantliffe, D.J. Powdery mildew of cucurbits in Florida. Univ. Fla. IFAS Ext. 2006, 20, HS1067/HS321. [Google Scholar] [CrossRef]
- Seal, D.; Zhang, S.; Ozores-Hampton, M.; Dittmar, P.; Li, Y.; Klassen, W.; Wang, Q.; Olczyk, T. Summer squash production in Miami-Dade County, Florida. Univ. Fla. IFAS Ext. 2022, 4, HS-861 TR012. [Google Scholar] [CrossRef]
- Reports on Plant Disease. RPD No. 925—Powdery Mildew of Cucurbits. University of Illinois Extension 1999. Available online: https://ipm.illinois.edu/diseases/series900/rpd925/?utm_source=chatgpt.com (accessed on 5 August 2025).
- Wahul, S.M.; Jagtap, G.P.; Rewale, K.A.; Bhosale, R.P. In vitro evaluation of various fungicides against Erysiphe cichoracearum in polyhouse. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1611–1617. [Google Scholar] [CrossRef]
- Wahul, S.M.; Jagtap, G.P.; Rewale, K.A.; Bhosale, R.P. Survey on powdery mildew of cucumber in Aurangabad and Jalna districts, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1618–1624. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yan, Y.; Wang, W.; Gebretsadik, K.; Qi, X.; Xu, Q.; Chen, X. Comparative proteomic analysis of cucumber powdery mildew resistance between a single-segment substitution line and its recurrent parent. Hortic. Res. 2019, 6, 115. [Google Scholar] [CrossRef]
- Li, H.; Khan, I.U.; Anarjan, M.B.; Hussain, M.; Lee, S. The mutant STAY-GREEN (Cssgr) in cucumber interacts with the CSEP30 protein to elicit a defense response against Podosphaera xanthii. Mol. Breed. 2024, 44, 67. [Google Scholar] [CrossRef]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of the Erysiphales (Powdery Mildews); CBS Biodiversity Series 11; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012; pp. 1–324. 1–40, 60, 165–166, 322–324. [Google Scholar]
- Lebeda, A.; Křístková, E.; Sedláková, B.; McCreight, J.D.; Coffey, M.D. Cucurbit powdery mildews: Methodology for objective determination and denomination of races. Eur. J. Plant Pathol. 2016, 144, 399–410. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: www.indexfungorum.org (accessed on 28 June 2025).
- McCreight, J.D.; Coffey, M.D.; Sedlaková, B.; Lebeda, A. Cucurbit powdery mildew of melon incited by Podosphaera xanthii: Global and western U.S. perspectives. In Cucurbitaceae 2012, Proceedings of the Xth Eucarpia Meeting on Genetics and Breeding of Cucurbitaceae; Sari, N., Solmaz, I., Arars, V., Eds.; Cukurova University: Adana, Turkey, 2012; pp. 181–189. [Google Scholar]
- Rabelo, H.d.O.; Santos, L.d.S.; Diniz, G.M.M.; Marin, M.V.; Braz, L.T.; McCreight, J.D. Identificação de raças de oídio das cucurbitáceas e reação de genótipos de meloeiro. Pesqui. Agropecu. Trop. 2017, 47, 440–447. [Google Scholar] [CrossRef]
- El-Kazzaz, M.K. Sphaerotheca fuliginea (Schlecht. ex Fr.) Poll., the causal agent of powdery mildew on many cucurbits in Egypt. Egypt. J. Phytopathol. 1981, 13, 65–66. [Google Scholar]
- El-Ammari, S.S.; Khan, M.W. Incidence and intensity of powdery mildew of cucurbits in Libya. In Proceedings of the 8th Congress of the Mediterranean Phytopathological Union; Assigbetse, K., Henni, J., Boisson, C., Eds.; Institut Agronomique et Veterinaire Hassan II: Rabat, Morocco, 1990; pp. 389–391. [Google Scholar]
- Endo, T.; El Guilli, M.; Farih, A.; Tantaoui, A. Identification of powdery mildew fungus on Moroccan cucurbitaceous plants. Al Awamia 2012, 125–126, 119–141. [Google Scholar]
- Rudich, J.; Karchi, Z.; Eshed, N. Evidence for two races of the pathogen causing powdery mildew of Muskmelon in Israel. Isr. J. Agric. Res. 1969, 19, 41–46. [Google Scholar]
- Trecate, L.; Sedláková, B.; Mieslerová, B.; Manstretta, V.; Rossi, V.; Lebeda, A. Effect of temperature on infection and development of powdery mildew on cucumber. Plant Pathol. 2019, 68, 1165–1178. [Google Scholar] [CrossRef]
- Bardin, M.; Carlier, J.; Nicot, P.C. Genetic differentiation in the French population of Erysiphe cichoracearum, a causal agent of powdery mildew of cucurbits. Plant Pathol. 1999, 48, 531–540. [Google Scholar] [CrossRef]
- Vakalounakis, D.J.; Klironomou, E. Race and mating type identification of powdery mildew on cucurbits in Greece. Plant Pathol. 1995, 44, 1033–1038. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; Vicente, A.D.; Torés, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Křístková, E.; Lebeda, A.; Sedláková, B. Species spectra, distribution and host range of cucurbit powdery mildews in the Czech Republic, and in some other European and Middle Eastern countries. Phytoparasitica 2009, 37, 337–350. [Google Scholar] [CrossRef]
- Miazzi, M.; Laguardia, C.; Faretra, F. Variation in Podosphaera xanthii on cucurbits in southern Italy. J. Phytopathol. 2011, 159, 538–545. [Google Scholar] [CrossRef]
- Tomason, Y.; Gibson, P.T. Fungal characteristics and varietal reactions of powdery mildew species on cucurbits in the steppes of Ukraine. Agron. Res. 2006, 4, 549–562. [Google Scholar]
- Nagy, G.S.; Kiss, L. A check-list of powdery mildew fungi of Hungary. Acta Phytopathol. Entomol. Hung. 2006, 41, 79–91. [Google Scholar] [CrossRef]
- Velkov, N.; Masheva, S. Species and races composition of powdery mildew on cucurbits in Bulgaria. Rep. Cucurbit Genet. Coop. 2002, 25, 7–10. [Google Scholar]
- Braun, U.; Shin, H.D.; Takamatsu, S.; Meeboon, J.; Kiss, L.; Lebeda, A.; Kitner, M.; Götz, M. Phylogeny and taxonomy of Golovinomyces orontii revisited. Mycol. Prog. 2019, 18, 335–357. [Google Scholar] [CrossRef]
- Lebeda, A.; Křístková, E.; Sedláková, B.; Coffey, M.D.; McCreight, J.D. Gaps and perspectives of pathotype and race determination in Golovinomyces cichoracearum and Podosphaera xanthii. Mycoscience 2011, 52, 159–164. [Google Scholar] [CrossRef]
- Rur, M.; Rämert, B.; Hökeberg, M.; Vetukuri, R.R.; Grenville-Briggs, L.; Liljeroth, E. Screening of alternative products for integrated pest management of cucurbit powdery mildew in Sweden. Eur. J. Plant Pathol. 2018, 150, 127–138. [Google Scholar] [CrossRef]
- Wu, T.Y.; Kirschner, R. A brief global review on the species of cucurbit powdery mildew fungi and new records in Taiwan. Mycol. Iran. 2017, 4, 85–91. [Google Scholar] [CrossRef]
- Lebeda, A. The genera and species spectrum of cucumber powdery mildew in Czechoslovakia. Phytopath. Z. 1983, 108, 71–79. [Google Scholar] [CrossRef]
- Lebeda, A.; Sedlakova, B.; Krıstkova, E.; Vysoudil, M. Long-lasting changes in the species spectrum of cucurbit powdery mildew in the Czech Republic—Influence of air temperature changes or random effect? Plant Prot. Sci. 2009, 45, S41–S47. [Google Scholar] [CrossRef]
- Lebeda, A.; Sedlakova, B.; Krıstkova, E.; Widrlechner, M.P.; Kosman, E. Understanding pathogen population structure and virulence variation for efficient resistance breeding to control cucurbit powdery mildews. Plant Pathol. 2021, 70, 1364–1377. [Google Scholar] [CrossRef]
- Lebeda, A.; Křístková, E.; Mieslerová, B.; Dhillon, N.P.S.; James, D.; McCreight, J.D. Status, gaps and perspectives of powdery mildew resistance research and breeding in cucurbits. Crit. Rev. Plant Sci. 2024, 43, 211–290. [Google Scholar] [CrossRef]
- Li, Y.H. Powdery Mildew of Cucurbits. Connecticut Agricultural Experiment Station 2012. Available online: https://homegarden.cahnr.uconn.edu/factsheets/powdery-mildew-of-cucurbits/ (accessed on 13 August 2025).
- Zitter, T.A.; Hopkins, D.L.; Thomas, C.E. Compendium of Cucurbit Diseases; Amer Phytopathological Society Press: Saint Paul, MN, USA, 1996. [Google Scholar]
- Bojórquez-Ramos, C.; León-Félix, J.; Allende-Molar, R.; Muy-Rangel, M.D.; Carrillo-Fasio, J.A.; Valdez-Torres, J.B.; López-Soto, F.S.M.; García-Estrada, R.S. Characterization of powdery mildew in cucumber plants under greenhouse conditions in the Culiacan Valley, Sinaloa, Mexico. Afr. J. Agric. Res. 2012, 7, 3237–3248. [Google Scholar] [CrossRef]
- Schuh, M.; Grabowski, M. University of Minnesota Extension. Powdery Mildew of Cucurbits 2025. Available online: https://extension.umn.edu/disease-management/powdery-mildew-cucurbits (accessed on 20 June 2025).
- Sitterly, W.R. Powdery mildews of cucurbits. In The Powdery Mildews; Spencer, D.M., Ed.; Academic Press: London, UK, 1978; pp. 359–379. [Google Scholar]
- McGrath, M.T. Powdery Mildew of Cucurbits. Cornell Cooperative Extension 1997. Available online: https://hdl.handle.net/1813/43293 (accessed on 20 June 2025).
- Pirondi, A.; Pérez-García, A.; Portillo, I.; Battistini, G.; Turan, C.; Brunelli, A.; Collina, M. Occurrence of chasmothecia and mating type distribution of Podosphaera xanthii, a causal agent of cucurbit powdery mildew in northern Italy. Plant Pathol. J. 2015, 97, 307–313. [Google Scholar] [CrossRef]
- University of Illinois Extension. Powdery Mildew of Cucurbit 2012. Available online: http://extension.cropsciences.illinois.edu/fruitveg/pdfs/925_powdery_mildew.pdf (accessed on 20 June 2025).
- Reuveni, R.; Rotem, J. Effect of humidity on epidemiological patterns of the powdery mildew (Sphaerotheca fuliginea) on squash. Phytoparasitica 1974, 2, 25–33. [Google Scholar] [CrossRef]
- Tsatsia, H.; Jackson, G. Pacific Pests, Pathogens & Weeds. 2021. Available online: https://apps.lucidcentral.org/ppp_v9/text/web_full/entities/cucumber_powdery_mildew_063.htm (accessed on 20 June 2025).
- Pérez-Garcia, A.; Olalla, L.; Rivera, E.; Del Pino, D.; Cánovas, I.; De Vicente, A.; Torés, J.A. Development of Sphaerotheca fusca on susceptible, resistant, and temperature-sensitive resistant melon cultivars. Mycol. Res. 2001, 105, 1216–1222. [Google Scholar] [CrossRef]
- Pirondi, A. Epidemiology and Population Genetics of Podosphaera fusca and Golovinomyces orontii, Causal Agent of Cucurbit Powdery Mildew. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2013. [Google Scholar]
- Wang, Z.; Du, Y.; Li, S.; Xu, X.; Chen, X. A complete genome sequence of Podosphaera xanthii isolate YZU573, the causal agent of powdery mildew isolated from cucumber in China. Pathogens 2023, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Vela-Corcía, D.; Bautista, R.; De Vicente, A.; Spanu, P.D.; Pérez-García, A. De novo analysis of the epiphytic transcriptome of the cucurbit powdery mildew fungus Podosphaera xanthii and identification of candidate secreted effector proteins. PLoS ONE 2016, 11, e0163379. [Google Scholar] [CrossRef]
- Leiva-Mora, M.; Capdesuñer, Y.; Villalobos-Olivera, A.; Moya-Jiménez, R.; Saa, L.R.; Martínez-Montero, M.E. Uncovering the Mechanisms: The role of biotrophic fungi in activating or suppressing plant defense responses. J. Fungi 2024, 10, 635. [Google Scholar] [CrossRef]
- Martel, A.; Ruiz-Bedoya, T.; Breit-McNally, C.; Laflamme, B.; Desveaux, D.; Guttman, D.S. The ETS-ETI cycle: Evolutionary processes and metapopulation dynamics driving the diversification of pathogen effectors and host immune factors. Curr. Opin. Plant Biol. 2021, 62, 102011. [Google Scholar] [CrossRef]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Chang, J.; Yang, W. Infection strategies and pathogenicity of biotrophic plant fungal pathogens. Front. Microbiol. 2022, 13, 799396. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cruz, J.; Romero, D.; de la Torre, F.N.; Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. The Functional characterization of Podosphaera xanthii candidate effector genes reveals novel target functions for fungal pathogenicity. Mol. Plant Microbe Interact. 2018, 31, 914–931. [Google Scholar] [CrossRef]
- Polonio, Á.; Fernández-Ortuño, D.; de Vicente, A.; Pérez-García, A. A haustorial-expressed lytic polysaccharide monooxygenase from the cucurbit powdery mildew pathogen Podosphaera xanthii contributes to the suppression of chitin-triggered immunity. Mol. Plant Pathol. 2021, 22, 580–601. [Google Scholar] [CrossRef]
- Bakhat, N.; Jiménez-Sánchez, A.; Ruiz-Jiménez, L.; Padilla-Roji, I.; Velasco, L.; Pérez-García, A.; Fernández-Ortuño, D. Fungal effector genes involved in the suppression of chitin signaling as novel targets for the control of powdery mildew disease via a nontransgenic RNA interference approach. Pest Manag. Sci. 2025, 81, 3452–3463. [Google Scholar] [CrossRef]
- De Jonge, R.; Van Esse, H.P.; Kombrink, A.; Shinya, T.; Desaki, Y.; Bours, R.; Van Der Krol, S.; Shibuya, N.; Joosten, M.H.A.J.; Thomma, B.P.H.J. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science 2010, 329, 953–955. [Google Scholar] [CrossRef]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.; et al. Effector-mediated suppression of chitin-triggered immunity by magnaporthe oryzae is necessary for rice blast disease. Plant Cell 2012, 24, 322–335. [Google Scholar] [CrossRef]
- Tanaka, K.; Nguyen, C.T.; Liang, Y.; Cao, Y.; Stacey, G. Role of LysM receptors in chitin-triggered plant innate immunity. Plant Signal. Behav. 2013, 8, e22598. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.M.; Polonio, Á.; Zanni, R.; Romero, D.; Gálvez, J.; Fernández-Ortuño, D.; Pérez-García, A. Chitin deacetylase, a novel target for the design of agricultural fungicides. J. Fungi 2021, 7, 1009. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cruz, J.; Romero, D.; Hierrezuelo, J.; Thon, M.; de Vicente, A.; Pérez-García, A. Effectors with chitinase activity (EWCAs), a family of conserved, secreted fungal chitinases that suppress chitin-triggered immunity. Plant Cell 2021, 33, 1319–1340. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cruz, J.M.; Polonio, Á.; Ruiz-Jiménez, L.; Vielba-Fernández, A.; Hierrezuelo, J.; Romero, D.; de Vicente, A.; Fernández-Ortuño, D.; Pérez-García, A. Suppression of chitin-triggered immunity by a new fungal chitin-binding effector resulting from alternative splicing of a chitin deacetylase gene. J. Fungi 2022, 8, 1022. [Google Scholar] [CrossRef]
- Bakhat, N.; Vielba-Fernández, A.; Padilla-Roji, I.; Martínez-Cruz, J.; Polonio, Á.; Fernández-Ortuño, D.; Pérez-García, A. Suppression of chitin-triggered immunity by plant fungal pathogens: A case study of the cucurbit powdery mildew fungus Podosphaera xanthii. J. Fungi 2023, 9, 771. [Google Scholar] [CrossRef]
- Dickman, M.B.; Fluhr, R. Centrality of host cell death in plant-microbe interactions. Annu. Rev. Phytopathol. 2013, 51, 543–570. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Riaz, S.; Morales-Cruz, A.; Amrine, K.C.H.; McGuire, B.; Gubler, W.D.; Walker, M.A.; Cantu, D. Adaptive genomic structural variation in the grape powdery mildew pathogen, Erysiphe necator. BMC Genom. 2014, 15, 1081. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ai, G.; Xia, C.; Pan, W.; Yin, Z.; Dou, D. Different requirement of immunity pathway components by oomycete effectors-induced cell death. Phytopathol. Res. 2022, 4, 4. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef]
- Pan, J.; Tan, J.; Wang, Y.; Zheng, X.; Owens, K.; Li, D.; Li, Y.; Weng, Y. STAYGREEN (CsSGR) is a candidate for the anthracnose (Colletotrichum orbiculare) resistance locus cla in Gy14 cucumber. Theor. Appl. Genet. 2018, 131, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, J.; Wu, Z.; VandenLangenberg, K.; Wehner, T.C.; Wen, C.; Zheng, X.; Owens, K.; Thornton, A.; Bang, H.H.; et al. STAYGREEN, STAY HEALTHY: A loss-of-susceptibility mutation in the STAYGREEN gene provides durable, broad-spectrum disease resistances for over 50 years of US cucumber production. New Phytol. 2019, 221, 415–430. [Google Scholar] [CrossRef]
- Van Schie, C.C.N.; Takken, F.L.W. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, I.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 1992, 63, 141–152. [Google Scholar] [CrossRef]
- Devoto, A.; Piffanelli, P.; Nilsson, I.M.; Wallin, E.; Panstruga, R.; Von Heijne, G.; Schulze-Lefert, P. Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J. Biol. Chem. 1999, 274, 34993–35004. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Santillán Martínez, M.; Hermans, F.W.K.; Vriezen, W.H.; Visser, R.G.F.; Bai, Y.; Schouten, H.J. A transposable element insertion in the susceptibility gene CsaMLO8 results in hypocotyl resistance to powdery mildew in cucumber. BMC Plant Biol. 2015, 15, 243. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Zhang, Q.; Zhang, X.; Ma, Z.; Hong, Y.; Zhang, L.; Chen, S. The reverse mutation of CsMLO8 results in susceptibility to powdery mildew via inhibiting cell wall apposition formation and cell death in cucumber (Cucumis sativus L.). Sci. Hortic. 2023, 313, 11894. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, C.; Xi, Y.; Shao, Q.; Li, L.; Luan, S. A receptor-channel trio conducts Ca2+ signalling for pollen tube reception. Nature 2022, 607, 534–539. [Google Scholar] [CrossRef]
- Ma, M.; Yang, L.; Hu, Z.; Mo, C.; Geng, S.; Zhao, X.; He, Q.; Xiao, L.; Lu, L.; Wang, D.; et al. Multiplex gene editing reveals cucumber MILDEW RESISTANCE LOCUS O family roles in powdery mildew resistance. Plant Physiol. 2024, 195, 1069–1088. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, X.; Sun, W.; Zhang, G.; Wang, Z.; Mi, Q.; Ding, Z.; Zhu, Z.; Gao, P. Molecular mechanism of the CmDSK2b-CmMLO5 module in regulating powdery mildew resistance in melon. Sci. Hortic. 2025, 349, 114239. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Bijsterbosch, G.; Visser, R.G.F.; Schouten, H.J.; Bai, Y. Functional characterization of cucumber (Cucumis sativus L.) Clade V MLO genes. BMC Plant Biol. 2017, 17, 80. [Google Scholar] [CrossRef]
- Dong, S.; Liu, X.; Han, J.; Miao, H.; Beckles, D.M.; Bai, Y.; Liu, X.; Guan, J.; Yang, R.; Gu, X.; et al. CsMLO8/11 are required for full susceptibility of cucumber stem to powdery mildew and interact with CsCRK2 and CsRbohD. Hortic. Res. 2024, 11, uhad295. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Kimura, S.; Hunter, K.; Vaahtera, L.; Tran, H.C.; Citterico, M.; Vaattovaara, A.; Rokka, A.; Stolze, S.C.; Harzen, A.; Meißner, L.; et al. CRK2 and C-terminal phosphorylation of NADPH oxidase RBOHD regulate reactive oxygen species production in arabidopsis. Plant Cell 2020, 32, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, G.; Iovieno, P.; Ricciardi, L.; Lotti, C.; Filippone, E.; Pavan, S.; Ercolano, M.R. Evolutionary conservation of MLO gene promoter signatures. BMC Plant Biol. 2019, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, N.; Amanullah, S.; Gao, P. Genome-wide identification, evolution, and expression analysis of MLO gene family in melon (Cucumis melo L.). Front Plant Sci. 2023, 14, 1144317. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, W.; Du, Y.; Wang, Z.; Liu, X.; Tan, M.; Lin, X.; Xu, J.; Cai, C.; Qi, X.; et al. A single-nucleotide substitution in the leucine-rich-repeat-only gene CsLRR1 confers powdery mildew resistance in cucumber. Plant Commun. 2024, 5, 100774. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, L.; Yu, Y.; Ma, Z.; Yin, Y.; Zhou, S.; Yu, Y.; Cui, N.; Meng, X.; Fan, H. Cucumis sativus CsbZIP90 suppresses Podosphaera xanthii resistance by modulating reactive oxygen species. Plant Sci. 2024, 339, 111945. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jin, Z.; Chen, K.; Gao, W.; Wang, M.; Wang, Y.; Chen, P.; Yue, H.; Li, Y. Mapping, cloning, and functional characterization of CsPBGD in leaf necrosis and its potential role in disease resistance in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2025, 138, 136. [Google Scholar] [CrossRef]
- Meng, X.; Yu, Y.; Zhao, J.; Cui, N.; Song, T.; Yang, Y.; Fan, H. The two translationally controlled tumor protein genes, CsTCTP1 and CsTCTP2, are negative modulators in the Cucumis sativus defense response to Sphaerotheca fuliginea. Front Plant Sci. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, S.; Qu, M.; Yang, Y.; Chen, Q.; Meng, X.; Fan, H. Cucumber (Cucumis sativus L.) translationally controlled tumor protein interacts with CsRab11A and promotes activation of target of rapamycin in response to Podosphaera xanthii. Plant J. 2024, 119, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yin, Y.; Jiang, Y.; Zhao, J.; Ma, L.; Li, Z.; Chen, Q.; Meng, X.; Fan, H. Unraveling the role of TARGET OF RAPAMYCIN in the immune response of Cucumis sativus to Podosphaera xanthii. Physiol. Plant. 2025, 177, e70350. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Wang, L.; Tong, P.; Xing, Q.; Qi, H. An ethylene response factor negatively regulates red light induced resistance of melon to powdery mildew by inhibiting ethylene biosynthesis. Int. J. Biol. Macromol. 2025, 307, 141867. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Rout, P.; Ullah, I.; Nag, S.R.; Reddy, V.V.; Kumar, G.; Kumar, R.; He, S.; Wu, H. Genome-wide identification and molecular characterization of CRK gene family in cucumber (Cucumis sativus L.) under cold stress and Sclerotium rolfsii infection. BMC Genom. 2023, 24, 219. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kang, H.-W. β-Aminobutyric acid and powdery mildew infection enhanced the activation of defense-related genes and salicylic acid in cucumber (Cucumis sativus L.). Genes 2023, 14, 2087. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Q.; Bao, X.; Gong, X.; Zhao, L.; Chen, J.; Liu, L.; Li, R. Transcriptomic Profiling Reveals Regulatory Pathways of Tomato in Resistance to Verticillium Wilt Triggered by VdR3e. Plants 2025, 14, 1243. [Google Scholar] [CrossRef]
- Tek, M.I.; Calis, O. Mechanisms of resistance to powdery mildew in cucumber. Phytopathol. Mediterr. 2022, 61, 119–127. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Ghanadian, S.M. Celery flavonoid-rich extract significantly reduces cucumber powdery mildew severity and enhances plant defense responses. Sci. Rep. 2025, 15, 10589. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Wang, J. Increasing the activities of protective enzymes is an important strategy to improve resistance in cucumber to powdery mildew disease and melon aphid under different infection/infestation patterns. Front. Plant Sci. 2022, 13, 950538. [Google Scholar] [CrossRef]
- Meng, S.; Chao, S.; Xiong, M.; Cheng, L.; Sun, Y.; Wang, L.; Chen, Y.; Jane, S.J.; Luo, C.; Chen, J. CaSun1, a SUN family protein, governs the pathogenicity of Colletotrichum camelliae by recruiting CaAtg8 to promote mitophagy. Hortic. Res. 2025, 12, uhaf121. [Google Scholar] [CrossRef]
- Janicka, M.; Reda, M.; Mroczko, E.; Wdowikowska, A.; Kabała, K. Jasmonic acid effect on Cucumis sativus L. growth is related to inhibition of plasma membrane proton pump and the uptake and assimilation of nitrates. Cells 2023, 12, 2263. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Jęcz, T.; Ciereszko, I.; Kononowicz, A.K. Jasmonic acid as a mediator in plant response to necrotrophic fungi. Cells 2023, 12, 1027. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Jiang, W.-J.; Yu, H.-J. The expression profiling of the lipoxygenase (LOX) family genes during fruit development, abiotic stress and hormonal treatments in cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2012, 13, 2481–2500. [Google Scholar] [CrossRef] [PubMed]
- Khan, V.; Jha, A.; Princi; Seth, T.; Iqbal, N.; Umar, S. Exploring the role of jasmonic acid in boosting the production of secondary metabolites in medicinal plants: Pathway for future research. Ind. Crops Prod. 2024, 220, 119227. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef]
- Singh, Y.; Nair, A.M.; Verma, P.K. Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant Commun. 2021, 2, 100142. [Google Scholar] [CrossRef]
- Meng, X.; Yu, Y.; Song, T.; Yu, Y.; Cui, N.; Ma, Z.; Chen, L.; Fan, H. Transcriptome sequence analysis of the defense responses of resistant and susceptible cucumber strains to Podosphaera xanthii. Front. Plant Sci. 2022, 13, 872218. [Google Scholar] [CrossRef]
- Liu, X.; Gu, X.; Lu, H.; Liu, P.; Miao, H.; Bai, Y.; Zhang, S. Identification of novel loci and candidate genes for resistance to powdery mildew in a resequenced cucumber germplasm. Genes 2021, 12, 584. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Y.; He, H.; Guo, C.; Zhu, W.; Pan, J.; Li, D.; Lian, H.; Pan, J.; Cai, R. Loss-of-function mutations in CsMLO1 confer durable powdery mildew resistance in cucumber (Cucumis sativus L.). Front. Plant Sci. 2015, 6, 1155. [Google Scholar] [CrossRef]
- Shnaider, Y.; Elad, Y.; Rav-David, D.; Pashkovsky, E.; Leibman, D.; Kravchik, M.; Shtarkman-Cohen, M.; Gal-On, A.; Spiegelman, Z. Development of powdery mildew resistance in cucumber using CRISPR/Cas9-mediated mutagenesis of CsaMLO8. Phytopathology 2023, 113, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, W.L.; LaPlant, K.E.; Bell, D.C.; Jahn, M.M.; Mazourek, M. Cultivar-based introgression mapping reveals wild species-derived Pm-0, the major powdery mildew resistance locus in squash. PLoS ONE 2016, 11, e0167715. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.S.; Ramireddy, S.; Reddy, U.K. Widening genetic diversity using embryo rescue in cucurbit crops: A review. Plants 2024, 13, 1320. [Google Scholar] [CrossRef] [PubMed]
- Alavilli, H.; Lee, J.-J.; You, C.-R.; Poli, Y.; Kim, H.-J.; Jain, A.; Song, K. GWAS reveals a novel candidate gene CmoAP2/ERF in pumpkin (Cucurbita moschata) involved in resistance to powdery mildew. Int. J. Mol. Sci. 2022, 23, 6524. [Google Scholar] [CrossRef]
- Shimomura, K.; Sugiyama, M.; Kawazu, Y.; Yoshioka, Y. Identification of quantitative trait loci for powdery mildew resistance in highly resistant cucumber (Cucumis sativus L.) using ddRAD-seq analysis. Breed Sci. 2021, 71, 326–333. [Google Scholar] [CrossRef]
- Sakata, Y.; Kubo, N.; Morishita, M.; Kitadani, E.; Sugiyama, M.; Hirai, M. QTL analysis of powdery mildew resistance in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2006, 112, 243–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, B.; Tan, X.; Zhu, C.; Wu, T.; Feng, S.; Yang, Q.; Shen, S.; Yu, T.; et al. Polyploidy events shaped the expansion of transcription factors in Cucurbitaceae and exploitation of genes for tendril development. Hortic. Plant J. 2022, 8, 562–574. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Zheng, Y.; Guo, J.; Yuan, S.; Fu, A.; Bai, C.; Zhao, X.; Zheng, S.; Wen, C.; et al. Cucurbitaceae genome evolution, gene function, and molecular breeding. Horticulturae 2022, 9, uhab057. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Xie, X.; Gu, X.; Zhao, J.; Ping, X.; Li, Y.; Yang, Y.; Mao, Z.; Xie, B. High-quality chromosome-level genomes of Cucumis metuliferus and Cucumis melo provide insight into Cucumis genome evolution. Plant J. 2021, 107, 136–148. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. RNA interference for improving disease resistance in plants and its relevance in this clustered regularly interspaced short palindromic repeats-dominated era in terms of dsRNA-based biopesticides. Front. Plant Sci. 2022, 13, 885128. [Google Scholar] [CrossRef]
- Padilla-Roji, I.; Ruiz-Jiménez, L.; Bakhat, N.; Vielba-Fernández, A.; Pérez-García, A.; Fernández-Ortuño, D. RNAi technology: A new path for the research and management of obligate biotrophic phytopathogenic fungi. Int. J. Mol. Sci. 2023, 24, 9082. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Jang, Y.J.; Park, J.Y.; Ryu, J.; Lee, G.P. Virus-induced gene silencing for in planta validation of gene function in cucurbits. Plant Physiol. 2022, 190, 2366–2379. [Google Scholar] [CrossRef]
- Dos Santos, C.; Franco, O.L. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Morishita, M.; Sugiyama, K.; Saito, T.; Sakata, Y. Powdery mildew resistance in cucumber. Jpn. Agric. Res. Q. 2003, 37, 7–14. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Kraus, C.; Markakis, E.; Alves, A.; Armengol, J.; Eichmeier, A.; Compant, S.; Gramaje, D. Fungal trunk diseases of fruit trees in Europe: Pathogens, spread and future directions. Phytopathol. Mediterr. 2022, 61, 563–599. [Google Scholar] [CrossRef]
- Tan, J.; Zhong, L.; Fan, S.; Cheng, S.; Gao., Y.; Zhang, P.; Miao, L.; Wang, H. Methods for seedling identification of cucumber resistance to powdery mildew and its effect on the growth of cucumber seedlings. Veg. Res. 2024, 4, e030. [Google Scholar] [CrossRef]
- McGrath, M.T. Fungicide resistance in cucurbit powdery mildew: Experiences and challenges. Plant Dis. 2001, 85, 236–245. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Shtienberg, D.; Elad, Y.; Bornstein, M.; Ziv, G.; Grava, A.; Cohen, S. Polyethylene mulch modifies greenhouse microclimate and reduces infection of Phytophthora infestans in tomato and Pseudoperonospora cubensis in cucumber. Phytopathology 2010, 100, 97–104. [Google Scholar] [CrossRef]
- Jewett, T.J.; Jarvis, W.R. Management of the greenhouse microclimate in relation to disease control: A review. Agronomie 2001, 21, 351–366. [Google Scholar] [CrossRef]
- Pfeufer, E.; Gauthier, N.; Bradley, C.A. Powdery Mildew. Available online: https://plantpathology.ca.uky.edu/sites/plantpathology.ca.uky.edu/files/PPFS-GEN-02.pdf (accessed on 22 June 2025).
- Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; Moreno, J. Effect of horticultural waste composting on infected plant residues with pathogenic bacteria and fungi: Integrated and localized sanitation. Waste Manag. 2007, 27, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P. What Is Drip Irrigation and Why Is It Beneficial? Available online: https://agri-route.com/blogs/news/what-is-drip-irrigation-and-why-is-it-beneficial (accessed on 22 June 2025).
- Dik, A.J.; Verhaar, M.A.; Bélanger, R.R. Comparison of three biological control agents against cucumber powdery mildew (Sphaerotheca fuliginea) in semi-commercial-scale glasshouse trials. Eur. J. Plant Pathol. 1998, 104, 413–423. [Google Scholar] [CrossRef]
- Kiss, L. A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Manag. Sci. 2003, 59, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, E.A.D.; Abd-Elsyed, M.H.F.; Ebrahiem, A.M.Y. Biological control of cucumber powdery mildew (Podosphaera xanthii) (Castagne) under greenhouse conditions. Egypt. J. Biol. Pest Control. 2020, 30, 65. [Google Scholar] [CrossRef]
- Romero, D.; De Vicente, A.; Zeriouh, H.; Cazorla, F.M.; Fernández-Ortuño, D.; Torés, J.A.; Pérez-García, A. Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon. Plant Pathol. 2007, 56, 976–986. [Google Scholar] [CrossRef]
- Pugliese, M.; Monchiero, M.; Gullino, M.L.; Garibaldi, A. Application of laminarin and calcium oxide for the control of grape powdery mildew on Vitis vinifera cv. Moscato. J. Plant Dis. Prot. 2018, 125, 477–482. [Google Scholar] [CrossRef]
- van Aubel, G.; Buonatesta, R.; Van Cutsem, P. COS-OGA: A novel oligosaccharidic elicitor that protects grapes and cucumbers against powdery mildew. Crop Prot. 2014, 65, 129–137. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Y.; Li, J.; Xu, M.; Wei, Q.; Wang, Y. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front. Microbiol. 2015, 6, 883. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Zeriouh, H.; Romero, D.; Cubero, J.; de Vicente, A.; Pérez-García, A. The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate- and salicylic acid-dependent defence responses. Microb. Biotechnol. 2013, 6, 264–274. [Google Scholar] [CrossRef]
- Trupo, M.; Magarelli, R.A.; Martino, M.; Larocca, V.; Giorgianni, A.; Ambrico, A. Crude lipopeptides from culture of Bacillus subtilis strain ET-1 against Podosphaera xanthii on Cucumis melo. J. Nat. Pestic. Res. 2023, 4, 100032. [Google Scholar] [CrossRef]
- Gafni, A.; Calderon, C.E.; Harris, R.; Buxdorf, K.; Dafa-Berger, A.; Zeilinger-Reichert, E.; Levy, M. Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front. Plant Sci. 2015, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance cerevisane (cell walls of Saccharomyces cerevisiae strain LAS117). EFSA J. 2014, 12, 3583. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Hashem, M.; Alshehri, A.M.; Alamri, S.; Eid, E.M.; Ziedan, E.S.H.E.; Alrumman, S.A. Effective Management of cucumber powdery mildew with essential oils. Agriculture 2021, 11, 1177. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Zarei, A. Chitosan nanoparticle encapsulation of celery seed essential oil: Antifungal activity and defense mechanisms against cucumber powdery mildew. Carbohydr. Polym. Tech. 2024, 8, 100531. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Ghanadian, M. Characterization, biochemical defense mechanisms, and antifungal activities of chitosan nanoparticle-encapsulated spinach seed essential oil. J. Agric. Food Res. 2025, 22, 102016. [Google Scholar] [CrossRef]
- Paduch-Cichal, E.; Szyndel, M.S.; Schollenberger, M.; Wakuliński, W. Fitopatologia Szczegółowa. Choroby Roślin Ogrodniczych; SGGW: Warszawa, Poland, 2010. [Google Scholar]
- Gołębniak, B. Choroby powodowane przez grzyby z typu Ascomycota (workowce). In Fitopatologia, Choroby Roślin Uprawnych; Kryczyński, S., Webera, Z., Eds.; PWRiL: Poznań, Poland, 2011; Volume 2, pp. 362–363. [Google Scholar]
- McGrath, M.T. Conventional Fungicide Recommendations for Cucurbit Powdery Mildew. Cornell University, LIHREC. 2023. Available online: https://www.vegetables.cornell.edu/pest-management/disease-factsheets/cucurbit-powdery-mildew/ (accessed on 2 July 2025).
- EPPO. List of Databases on Registered Plant Protection Products in the EPPO Region. 2025. Available online: https://www.eppo.int/ACTIVITIES/plant_protection_products/registered_products (accessed on 6 August 2025).
- Wyszukiwarka Środków Ochrony Roślin—Zastosowanie—Ministerstwo Rolnictwa i Rozwoju Wsi—Portal Gov.pl. Available online: https://www.gov.pl/web/rolnictwo/wyszukiwarka-srodkow-ochrony-roslin---zastosowanie (accessed on 17 June 2025).
- Kim, M.J.; Kim, Y.K.; Park, S.H.; Park, J.H.; Hong, S.J.; Shim, C.K. Control of cucumber powdery mildew using resistant cultivars and organic agricultural materials. J. Microbiol. Biotechnol. 2025, 35, e2409030. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Kang, X.; Wang, J.; Wu, Y.; Yao, Z.; Zhou, Y.; Li, Y.; Fu, Y.; Huang, Y.; et al. Adding sulfur to soil improved cucumber plants’ resistance to powdery mildew. Agronomy 2024, 14, 1799. [Google Scholar] [CrossRef]
- Hendricks, K.E.M.; Roberts, P.D. Evaluation of the sensitivity of Podosphaera xanthii to several fungicides for management of powdery mildew on squash in Florida. Crop Prot. 2023, 172, 106328. [Google Scholar] [CrossRef]
- Babadoost, M.; Sulley, S.; Xiang, Y. Sensitivities of cucurbit powdery mildew fungus (Podosphaera xanthii) to fungicides. Plant Health Prog. 2020, 21, 272–277. [Google Scholar] [CrossRef]
- Zanni, R.; Martínez-Cruz, J.; Gálvez-Llompart, M.; Fernández-Ortuño, D.; Romero, D.; García-Domènech, R.; Pérez-García, A.; Gálvez, J. Rational design of chitin deacetylase inhibitors for sustainable agricultural use based on molecular topology. J. Agric. Food Chem. 2022, 70, 13118–13131. [Google Scholar] [CrossRef]
- Sapak, Z.; Salam, M.U.; Minchinton, E.J.; MacManus, G.P.V.; Joyce, D.C.; Galea, V.J. POMICS: A simulation disease model for timing fungicide applications in management of powdery mildew of cucurbits. Phytopathology 2017, 107, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Jeong, H.; Jung, J.Y.; Park, J.; Park, J.; Park, H.; Yoon, J.; Jung, S.H.; Sim, C.B.; Kim, K.H.; et al. Predicting the onset date of cucumber powdery mildew based on growing degree days and leaf wetness duration in greenhouse environment. Plant Pathol J. 2025, 41, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Chen, T.; Zhang, X.; Taha, M.F.; Yang, N.; Mao, H.; Shi, Q. Cucumber downy mildew disease prediction using a CNN-LSTM approach. Agriculture 2024, 14, 1155. [Google Scholar] [CrossRef]
- Xu, J.T.; Zhang, Z.; Guo, Y.H.; Feng, L.Y.; Ning, X.F. Detection of cucumber powdery mildew based on spectral and image information. J. Biosyst. Eng. 2023, 48, 115–122. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P. Range-expanding pests and pathogens in a warming world. Annu. Rev. Phytopathol. 2015, 53, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Change 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Roy, B.A.; Güsewell, S.; Harte, J. Response of plant pathogens and herbivores to a warming experiment. Ecology 2004, 85, 2570–2581. [Google Scholar] [CrossRef]
- UMass Extension Vegetable Program. Available online: https://www.umass.edu/agriculture-food-environment/vegetable/fact-sheets/cucurbits-powdery-mildew (accessed on 17 June 2025).
- Cucumber (Cucumis Sativus)-Powdery Mildew. Available online: https://pnwhandbooks.org/plantdisease/host-disease/cucumber-cucumis-sativus-powdery-mildew (accessed on 17 June 2025).



| PAMP | Effector | Chemical Nature of the Effector | Function of the Effector in Host Plant | Host Putative Target of Fungal Effector | References |
|---|---|---|---|---|---|
| NA | 53 CSEPs, 87 putative effector candidates | Containing the N-terminal conserved motif Y/F/WxC, with the WxC motif more abundant | P. xanthii pathogenesis; elevated expression at the beginning of the infection process at 24 hpi, when the primary appressoria are mostly formed. | NA | [58,59] |
| NA | PEC019 (PxPLBE1) | Phospholipid-binding protein | P. xanthii pathogenesis; elevated expression during the early stage of pathogenesis at 24 hpi. Modulation of plant cell membrane organization. | Targeting host–cell plasma membrane | [63] |
| NA | PEC032 (PxMLE1) | α-Mannosidase | P. xanthii pathogenesis; elevated expression during the early stage of pathogenesis at 24 hpi. Host–cell glycosylation | Interaction with α-mannose | |
| Cellulose | PEC054 (PxCLBE1) | Cellulose-binding protein | P. xanthii pathogenesis; elevated expression during the early stage of pathogenesis at 24 hpi. Sequesters cellulose fragments (cellopentaose), preventing cellulose recognition by the plant. | Suppression of cellulose-triggered immunity | |
| Chitin | PxLPMO (PHEC27213) | Lytic polysaccharide monooxygenases containing a putative chitin-binding domain 3 located from amino acids 115 to 128 | Binds and catalyzes colloidal chitin and chitooligosaccharides; suppression of chitin-triggers immunity during haustorium development. | May indirectly target putative melon homologs of plant chitin receptors | [64, 65, 66, 67,68] |
| Chitin | PxCDA | Chitin deacetylases | Converts chitin into chitosan by hydrolyzing the N-acetamido group in N-acetylglucosamine units. Chitosan has reduced affinity for plant chitin receptors; suppression of chitin signaling by avoiding recognition. | May indirectly target putative melon homologs of plant chitin receptors | [65,67,68,69] |
| Chitin | PxEWCAs | Chitinase | Degrades chitin fragments. The degraded chitin oligomers exhibit reduced affinity for plant chitin receptors, preventing the activation of chitin-triggered immunity. | May indirectly target putative melon homologs of plant chitin receptors | [65,67,68,70] |
| Chitin | PxCHBE (PxCDA3) | A truncated version of chitin deacetylase resulting from an alternative splicing of the PxCDA gene, which lacked most of the chitin deacetylase activity domain but retained the carbohydrate-binding module. | Bands to the chitin oligomers, preventing activation of the chitin signaling, localizing in plant papillae where chitin is densely accumulated at pathogen penetration sites. | May indirectly target putative melon homologs of plant chitin receptors (e.g., CEBiP, CERK1) | [65,67,68,71] |
| NA | CSEP30, CSEP47, CSEP48 | Secreted fungal protein | Induces cell death in cucumber. | Interact with CsSGR in susceptible cucumber genotype S6 and the mutant Cssgr (Q108R) in resistant cucumber genotype Gy14 | [19] |
| CSEP30∆SP | Mature form of the secreted fungal protein | Induces dry necrotic lesions on the abaxial surfaces of leaves and defense response. | Interacts with Cssgr in resistant cucumber genotype Gy14 |
| Gene Type | Gene/Locus | Species | Function/Mechanism | Reference |
|---|---|---|---|---|
| Susceptibility gene | CsaMLO8 | Cucumis sativus (cucumber) | MLO-like gene; loss of function confers PM resistance | [119] |
| Susceptibility gene | CsMLO1, CsMLO11 | Cucumis sativus | Other MLO family members interacting with CsaMLO8 | [85] |
| Resistance gene | Csa5G623470 (MLO-like) | Cucumis sativus | Candidate gene within pm-s locus; associated with PM resistance | [5] |
| Resistance gene | CsCPK11 | Cucumis sativus | Calcium-dependent protein kinase; positive regulator of resistance | [85] |
| Resistance gene | CRK (Pm1.1) | Cucumis sativus | Cysteine-rich receptor-like kinase; dominantly inherited PM resistance gene | [4] |
| Regulatory genes | STN7, WRKY22, D6PKL1 | Cucumis sativus | Regulators of ROS production and hypersensitive response (HR) | [104] |
| Major QTLs | Pm5.1, Pm5.2 | Cucumis sativus | Major loci linked to phosphate transporter gene CsGy5G015960; confer durable resistance | [9] |
| Organ-specific QTLs | Chr. 1, 2, 5, 6 | Cucumis sativus | Organ-specific expression patterns of PM resistance | [1] |
| Crop | Variety/Line Name | Resistance Level | Breeding Method | Reference/Source |
|---|---|---|---|---|
| Cucumber | PI 197088-5 | Highly resistant (temperature-independent resistance) | Backcross breeding for resistance genes | [133,134] |
| Cucumber | Natsufushinari | Resistant (at high temperature) | Pure line selection/backcross breeding | [133] |
| Cucumber | Jinza 1 hao, 808, SC-8 | Resistant | Selection | [133] |
| Cucumber | R1461 | Highly resistant | Seedling disease resistance screening | [135] |
| Cucumber | BK2 | Resistant | Seedling disease resistance screening | [135] |
| Cucumber | 9930, H136 | Susceptible | Selection | [135] |
| Cucurbita | YD26 (C. moschata) | Highly resistant | Hybridization and selection | [135] |
| Cucurbita | SF02 (C. moschata) | Highly susceptible | Selection | [3] |
| Cucurbita | Varieties with Pm-0 locus | Resistant | Wide hybridization and introgression | [3] |
| Zucchini | Varieties carrying CpPM10.1 | Resistant | Fine mapping, backcross breeding | [3] |
| Pumpkin | Commercial cultivars with Pm-0 introgression | Resistant | Wide hybridization and backcross breeding | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawełkowicz, M.; Głuchowska, A.; Mirzwa-Mróz, E.; Zieniuk, B.; Yin, Z.; Zamorski, C.; Przybysz, A. Molecular Insights into Powdery Mildew Pathogenesis and Resistance in Cucurbitaceous Crops. Agriculture 2025, 15, 1743. https://doi.org/10.3390/agriculture15161743
Pawełkowicz M, Głuchowska A, Mirzwa-Mróz E, Zieniuk B, Yin Z, Zamorski C, Przybysz A. Molecular Insights into Powdery Mildew Pathogenesis and Resistance in Cucurbitaceous Crops. Agriculture. 2025; 15(16):1743. https://doi.org/10.3390/agriculture15161743
Chicago/Turabian StylePawełkowicz, Magdalena, Agata Głuchowska, Ewa Mirzwa-Mróz, Bartłomiej Zieniuk, Zhimin Yin, Czesław Zamorski, and Arkadiusz Przybysz. 2025. "Molecular Insights into Powdery Mildew Pathogenesis and Resistance in Cucurbitaceous Crops" Agriculture 15, no. 16: 1743. https://doi.org/10.3390/agriculture15161743
APA StylePawełkowicz, M., Głuchowska, A., Mirzwa-Mróz, E., Zieniuk, B., Yin, Z., Zamorski, C., & Przybysz, A. (2025). Molecular Insights into Powdery Mildew Pathogenesis and Resistance in Cucurbitaceous Crops. Agriculture, 15(16), 1743. https://doi.org/10.3390/agriculture15161743







