Milpa, a Long-Standing Polyculture for Sustainable Agriculture
Abstract
1. Introduction
2. Methodology
3. What Is Milpa
3.1. Milpa, Defined as a Crop Site and Agro-Ecosystem
| Term a | Meaning a | Area | Reference |
|---|---|---|---|
| Milpa | Mil-pa (milli, sowing field; pa, in). “In the sowing field, inheritance”; or “in the maize field” | Mexico; Belize; Guatemala; El Salvador | [42,53,59,85] |
| Coamil | Coatl-mil (coatl, planting sitck; milli, sowing field). Maize field sowing with planting stick | Colima, Jalisco (Mexico) | [101] |
| Huamil | Huac-mil (huacqui, dry; milli, sowing field). Sowing field in drylands | Guanajuato (Mexico) | [143] |
| Calmil | Cal-mil (calli, house; milli, sowing field). Sowing field near to the house | State of México (Mexico) | [144] |
| Tonamil | Tona-mil (tonalli, warmth of the sun; milli, sowing field). Maize field sowing in winter season. | Tabasco, Oaxaca, Veracruz, Puebla (Mexico) | [114] |
| Xopamil | Xopa-mil (xopan, spring, raining; milli, sowing field). Maize field sowing in spring–summer or rainy season. | Veracruz, Puebla (Mexico) | [114] |
| Amilpa | A-mil-pa (atl, water; milli, milli, sowing field; pa, in). Maize sowing fields with irrigation | Valley of Mexico, Puebla, Tlaxcala, Guerrero, Michoacán, Jalisco (Mexico) | [114,145] |
| Cacaomile | Cacao-mile (cacahatl, cocoa; milli, sowing field). Field planted with cocoa trees | Tabasco, Chiapas, Oaxaca, Veracruz, Guerreo (Mexico) | [114,145] |
| Ahuacamile | Ahuaca-mile (ahuacatl, avocado; milli, sowing field). Field planted with avocado trees | State of México, Morelos, Puebla (Mexico) | [114,145] |
3.2. The Term Milpa Used to Describe a Type of Polyculture
3.3. Milpa as Cultivations System
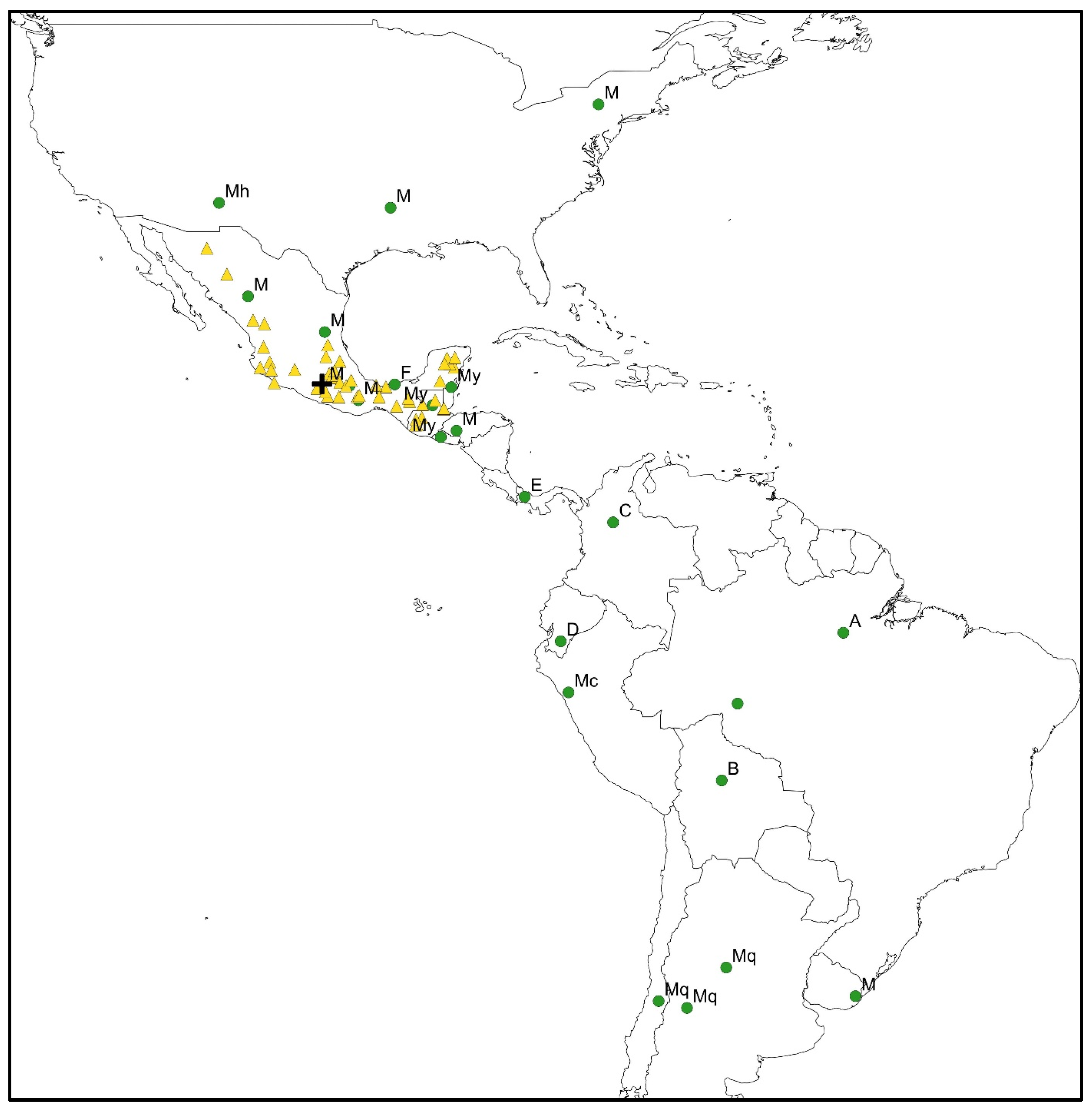
4. Origin and Configuration of the Milpa Polyculture
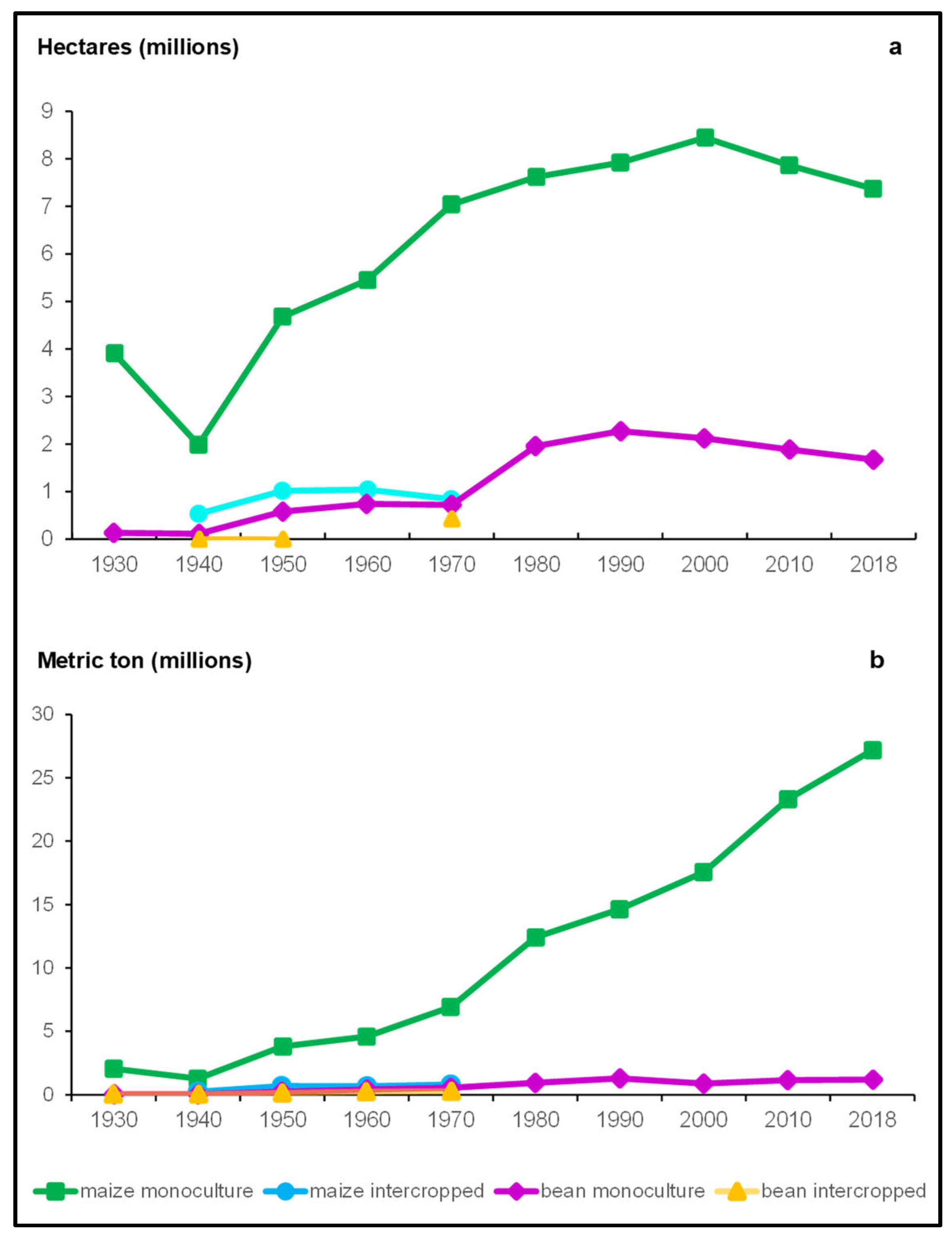
5. Milpa Polyculture at Present
6. Structural, Ecological and Agronomic Traits of Milpa Polyculture
6.1. Cultivated and Associated Diversity

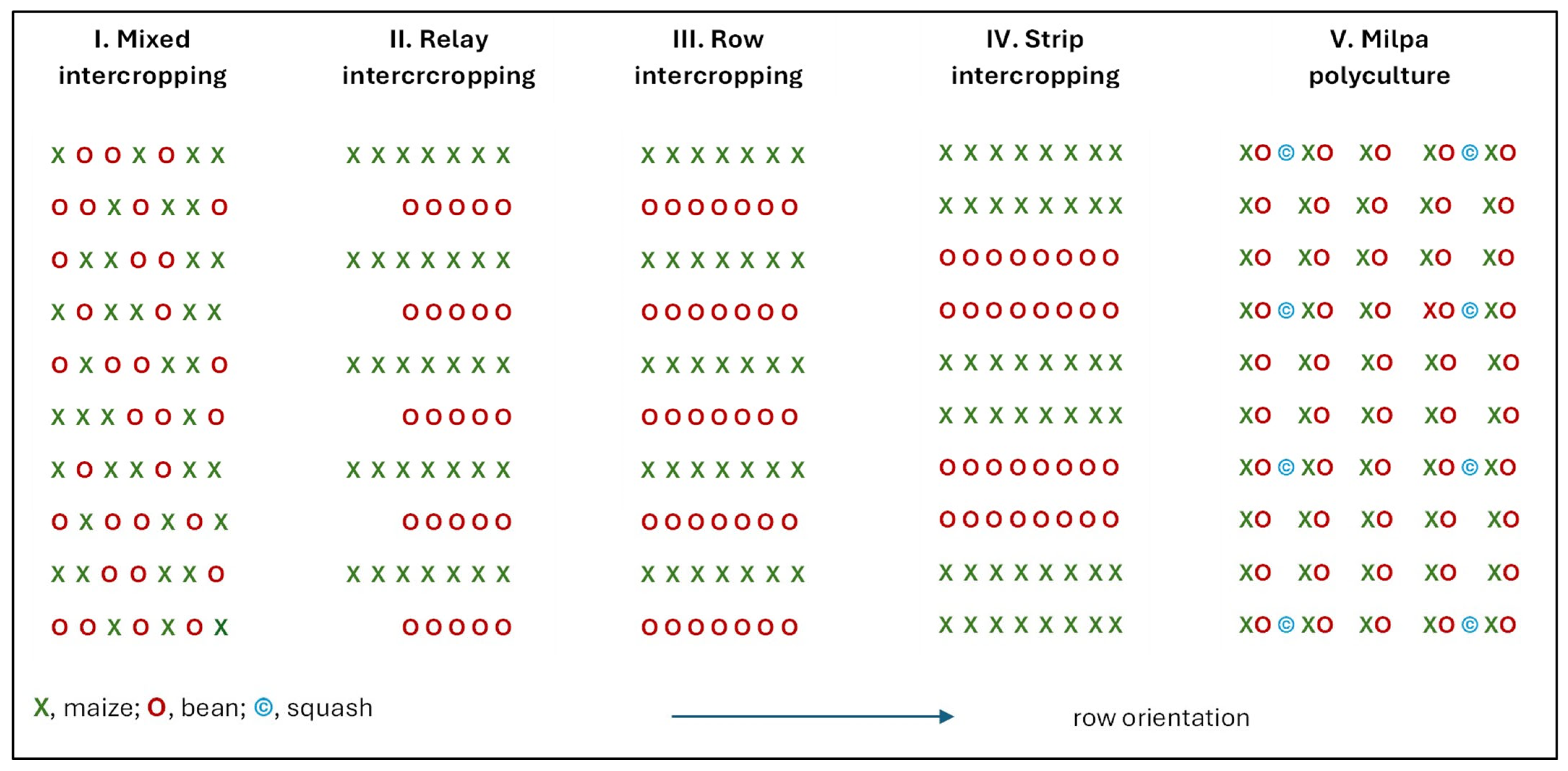
6.2. Intercrop Types
6.3. Complementarity
6.3.1. Niche Partitioning
6.3.2. Abiotic Facilitation
6.3.3. Biotic Feedback
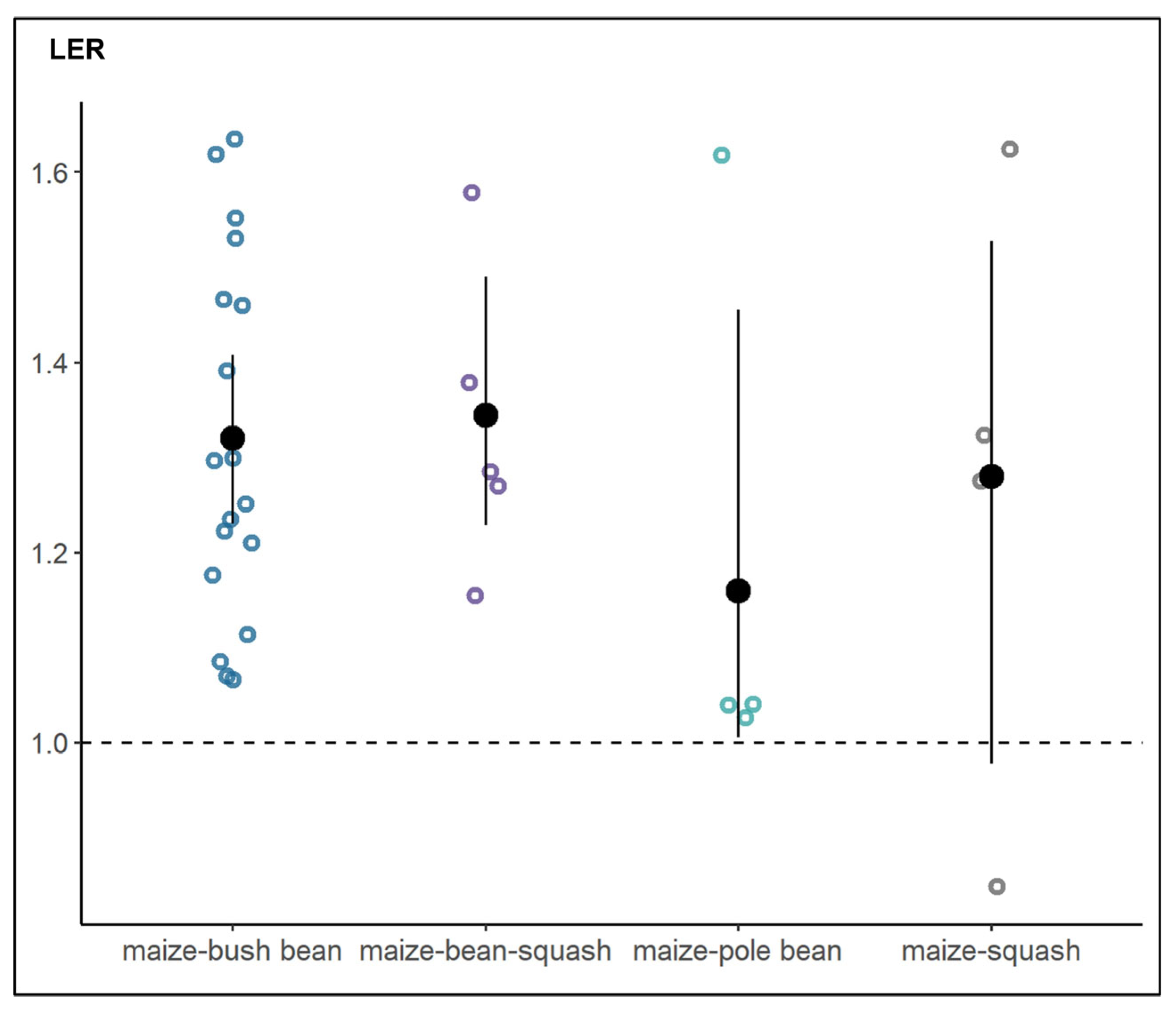
6.4. Productivity and Stability
7. Multifunctionality and Limitations of Milpa Polyculture
7.1. Milpa and Food
7.2. Milpa and the Economy
7.3. Milpa and Culture
7.4. Labor and Mechanization
8. Future Prospects
8.1. Microbiome
8.2. Breeding
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (Ed.) The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Tilman, D. Global Environmental Impacts of Agricultural Expansion: The Need for Sustainable and Efficient Practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Altieri, M.A. Convergence or Divide in the Movement for Sustainable and Just Agriculture. In Organic Fertilisation, Soil Quality and Human Health; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–9. [Google Scholar] [CrossRef]
- Cox, G.M.; Atkins, M.D. Agricultural Ecology. An Analysis of World Food Production Systems; W.H. Freeman and Company: San Francisco, CA, USA, 1979. [Google Scholar]
- Pingali, P.L. Green Revolution: Impacts, Limits, and the Path Ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef]
- Vitousek, P.M. Beyond Global Warming: Ecology and Global Change. Ecology 1994, 75, 1861–1876. [Google Scholar] [CrossRef]
- Renard, D.; Tilman, D. National Food Production Stabilized by Crop Diversity. Nature 2019, 571, 257–260. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of Increasing Plant Diversity in Sustainable Agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- MacLaren, C.; Mead, A.; van Balen, D.; Claessens, L.; Etana, A.; de Haan, J.; Haagsma, W.; Jäck, O.; Keller, T.; Labuschagne, J.; et al. Long-Term Evidence for Ecological Intensification as a Pathway to Sustainable Agriculture. Nat. Sustain. 2022, 5, 770–779. [Google Scholar] [CrossRef]
- Vandermeer, J.H. The Ecology of Intercropping; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar] [CrossRef]
- Willey, R.W. Intercropping-Its Importance and Research Need. Part 1. Competition and Yield Advantage. Field Crop Abstr. 1979, 32, 1–10. [Google Scholar]
- Casas, A.; Caballero, J.; Mapes, C.; Zárate, S. Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Bot. Sci. 1997, 61, 31–47. [Google Scholar] [CrossRef]
- Plucknett, D.L.; Smith, N.J.H. Historical Perspectives for Multiple Cropping. In Multiple Cropping Systems; Macmillan: New York, NY, USA, 1986; pp. 20–39. [Google Scholar]
- Denevan, W.M. 2 Prehistoric Agricultural Methods as Models for Sustainability. In Advances in Plant Pathology; Andrews, J.H., Tommerup, I.C., Eds.; Academic Press: Cambridge, MA, USA, 1995; Volume 11, pp. 21–43. [Google Scholar] [CrossRef]
- Francis, C.A. Multiple Cropping Systems; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Francis, C.A. Biological Efficiencies in Multiple-Cropping Systems. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1989; Volume 42, pp. 1–42. [Google Scholar] [CrossRef]
- Casas, A.; Otero-Arnaiz, A.; Pérez-Negrón, E.; Valiente-Banuet, A. In Situ Management and Domestication of Plants in Mesoamerica. Ann. Bot. 2007, 100, 1101–1115. [Google Scholar] [CrossRef]
- Moguel, P.; Toledo, V.M. Biodiversity Conservation in Traditional Coffee Systems of Mexico. Conserv. Biol. 1999, 13, 11–21. [Google Scholar] [CrossRef]
- Gómez-Pompa, A. On Maya Silviculture. Mex. Stud. Mex. 1987, 3, 1–17. [Google Scholar] [CrossRef]
- Ford, A.; Nigh, R. Origins of the Maya Forest Garden: Maya Resource Management. J. Ethnobiol. 2009, 29, 213–236. [Google Scholar] [CrossRef]
- Gliessman, S.R. Agroecology: The Ecology of Sustainable Food Systems; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Woldeamlak, A.; Grando, S.; Maatougui, M.; Ceccarelli, S. Hanfets, a Barley and Wheat Mixture in Eritrea: Yield, Stability and Farmer Preferences. Field Crops Res. 2008, 109, 50–56. [Google Scholar] [CrossRef]
- Ferrario, V. Learning from Agricultural Heritage? Lessons of Sustainability from Italian “Coltura Promiscua”. Sustainability 2021, 13, 8879. [Google Scholar] [CrossRef]
- Columela, L.J.M. Liber de Arboribvs; UNAM: Mexico City, Mexico, 2019. [Google Scholar]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; van der Werf, W. Temporal Niche Differentiation Increases the Land Equivalent Ratio of Annual Intercrops: A Meta-Analysis. Field Crops Res. 2015, 184, 133–144. [Google Scholar] [CrossRef]
- Paut, R.; Garreau, L.; Ollivier, G.; Sabatier, R.; Tchamitchian, M. A Global Dataset of Experimental Intercropping and Agroforestry Studies in Horticulture. Sci. Data 2024, 11, 5. [Google Scholar] [CrossRef]
- Tilman, D. Benefits of Intensive Agricultural Intercropping. Nat. Plants 2020, 6, 604–605. [Google Scholar] [CrossRef]
- Stomph, T.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; Gu, C.; Li, L.; Simon, J.; Jensen, E.S.; et al. Designing Intercrops for High Yield, Yield Stability and Efficient Use of Resources: Are There Principles? In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2020; Volume 160, pp. 1–50. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of Production in Intercropping Impact Yield Gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Martin-Guay, M.-O.; Paquette, A.; Dupras, J.; Rivest, D. The New Green Revolution: Sustainable Intensification of Agriculture by Intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving Intercropping: A Synthesis of Research in Agronomy, Plant Physiology and Ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Boudreau, M.A. Diseases in Intercropping Systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, P.T.; Gliessman, S.R.; Langenheim, J.H. Factors in the Suppression of Weeds by Squash Interplanted in Corn. Weed Biol. Manag. 2007, 7, 105–114. [Google Scholar] [CrossRef]
- Grof-Tisza, P.; Muller, M.H.; Gónzalez-Salas, R.; Bustos-Segura, C.; Benrey, B. The Mesoamerican Milpa Agroecosystem Fosters Greater Arthropod Diversity Compared to Monocultures. Agric. Ecosyst. Environ. 2024, 372, 109074. [Google Scholar] [CrossRef]
- Landaverde-González, P.; Quezada-Euán, J.J.G.; Theodorou, P.; Murray, T.E.; Husemann, M.; Ayala, R.; Moo-Valle, H.; Vandame, R.; Paxton, R.J. Sweat Bees on Hot Chillies: Provision of Pollination Services by Native Bees in Traditional Slash-and-Burn Agriculture in the Yucatán Peninsula of Tropical Mexico. J. Appl. Ecol. 2017, 54, 1814–1824. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Complementarity in Root Architecture for Nutrient Uptake in Ancient Maize/Bean and Maize/Bean/Squash Polycultures. Ann. Bot. 2012, 110, 521–534. [Google Scholar] [CrossRef]
- Li, C.; Lambers, H.; Jing, J.; Zhang, C.; Bezemer, T.M.; Klironomos, J.; Cong, W.-F.; Zhang, F. Belowground Cascading Biotic Interactions Trigger Crop Diversity Benefits. Trends Plant Sci. 2024, 29, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Wang, Z.-G.; Bao, X.-G.; Sun, J.-H.; Yang, S.-C.; Wang, P.; Wang, C.-B.; Wu, J.-P.; Liu, X.-R.; Tian, X.-L.; et al. Long-Term Increased Grain Yield and Soil Fertility from Intercropping. Nat. Sustain. 2021, 4, 943–950. [Google Scholar] [CrossRef]
- Lynam, J.K.; Sanders, J.H.; Mason, S.C. Economic and Risk in Multiple Cropping. In Multiple Cropping Systems; Macmillan: New York, NY, USA, 1986; pp. 250–266. [Google Scholar]
- Isakson, S.R. No Hay Ganancia En La Milpa: The Agrarian Question, Food Sovereignty, and the on-Farm Conservation of Agrobiodiversity in the Guatemalan Highlands. J. Peasant Stud. 2009, 36, 725–759. [Google Scholar] [CrossRef]
- Jodha, N.S. Intercropping in Traditional Farming Systems. J. Dev. Stud. 1980, 16, 427–442. [Google Scholar] [CrossRef]
- Norman, D.W. Rationalising Mixed Cropping Under Indigenous Conditions: The Example of Northern Nigeria. J. Dev. Stud. 1974, 11, 3–21. [Google Scholar] [CrossRef]
- Falkowski, T.B.; Chankin, A.; Diemont, S.A.W.; Pedian, R.W. More than Just Corn and Calories: A Comprehensive Assessment of the Yield and Nutritional Content of a Traditional Lacandon Maya Milpa. Food Secur. 2019, 11, 389–404. [Google Scholar] [CrossRef]
- Papendick, R.I.; Sánchez, P.A.; Triplett, G.B. Multiple Cropping; ASA, CSSA, SSSA: Madison, WI, USA, 1976. [Google Scholar]
- Litrico, I.; Violle, C. Diversity in Plant Breeding: A New Conceptual Framework. Trends Plant Sci. 2015, 20, 604–613. [Google Scholar] [CrossRef]
- Bourke, P.M.; Evers, J.B.; Bijma, P.; van Apeldoorn, D.F.; Smulders, M.J.M.; Kuyper, T.W.; Mommer, L.; Bonnema, G. Breeding Beyond Monoculture: Putting the “Intercrop” Into Crops. Front. Plant Sci. 2021, 12, 734167. [Google Scholar] [CrossRef]
- Ditzler, L.; Driessen, C. Automating Agroecology: How to Design a Farming Robot Without a Monocultural Mindset? J. Agric. Environ. Ethics 2022, 35, 2. [Google Scholar] [CrossRef] [PubMed]
- Erbach, D.C.; Lovely, W.G. Machinery Adaptation for Multiple Cropping. In Multiple Cropping; ASA-CSSA-SSSA: Madison, WI, USA, 1986. [Google Scholar]
- Sampoux, J.-P.; Giraud, H.; Litrico, I. Which Recurrent Selection Scheme to Improve Mixtures of Crop Species? Theoretical Expectations. G3 GenesGenomesGenetics 2020, 10, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J. Intercropping. In Agroecology; McGraw-Hill: New York, NY, USA, 1990; pp. 481–516. [Google Scholar]
- Aguilar, J.; Illsey, C.; Marielle, C. Los Sistemas Agrícolas de Maíz y Sus Procesos Técnicos. In Sin Maíz no Hay País; Conaculta: Mexico City, Mexico, 2003. [Google Scholar]
- Heindorf, C.; Reyes–Agüero, J.A.; van’t Hooft, A.; Fortanelli–Martínez, J. Inter- and Intraspecific Edible Plant Diversity of the Tének Milpa Fields in Mexico. Econ. Bot. 2019, 73, 489–504. [Google Scholar] [CrossRef]
- Linares, E.; Bye, R. La Milpa: Patrimonio Biológico y Cultural de México. In El Frijol, un Regalo de México al Mundo; Fundación Herdez: Mexico City, Mexico, 2012. [Google Scholar]
- Lopez-Ridaura, S.; Barba-Escoto, L.; Reyna-Ramirez, C.A.; Sum, C.; Palacios-Rojas, N.; Gerard, B. Maize Intercropping in the Milpa System. Diversity, Extent and Importance for Nutritional Security in the Western Highlands of Guatemala. Sci. Rep. 2021, 11, 3696. [Google Scholar] [CrossRef]
- Mt. Pleasant, J. Food Yields and Nutrient Analyses of the Three Sisters: A Haudenosaunee Cropping System. Ethnobiol. Lett. 2016, 7, 87–98. [Google Scholar] [CrossRef]
- Rodríguez-Robayo, K.J.; Méndez-López, M.E.; Molina-Villegas, A.; Juárez, L. What Do We Talk About When We Talk About Milpa? A Conceptual Approach to the Significance, Topics of Research and Impact of the Mayan Milpa System. J. Rural Stud. 2020, 77, 47–54. [Google Scholar] [CrossRef]
- Drexler, K. Government Extension, Agroecology, and Sustainable Food Systems in Belize Milpa Communities: A Socio-Ecological Systems Approach. J. Agric. Food Syst. Community Dev. 2020, 9, 85–97. [Google Scholar] [CrossRef]
- Teran, S.; Rasmussen, C.H. Genetic Diversity and Agricultural Strategy in 16th Century and Present-Day Yucatecan Milpa Agriculture. Biodivers. Conserv. 1995, 4, 363–381. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Corzo Marquez, A.R. Swidden Counts: A Petén, Guatemala, Milpa System. J. Anthropol. Res. 2015, 71, 69–93. [Google Scholar] [CrossRef]
- Daniels, A.E.; Painter, K.; Southworth, J. Milpa Imprint on the Tropical Dry Forest Landscape in Yucatan, Mexico: Remote Sensing & Field Measurement of Edge Vegetation. Agric. Ecosyst. Environ. 2008, 123, 293–304. [Google Scholar] [CrossRef]
- Cabrera, R.; García-López, H.; Aguirre-von-Wobeser, E.; Orozco-Avitia, J.A.; Gutiérrez-Saldaña, A.H. Amycolatopsis BX17: An Actinobacterial Strain Isolated from Soil of a Traditional Milpa Agroecosystem with Potential Biocontrol Against Fusarium graminearum. Biol. Control 2020, 147, 104285. [Google Scholar] [CrossRef]
- Aguirre-Noyola, J.L.; Rosenblueth, M.; Santiago-Martínez, M.G.; Martínez-Romero, E. Transcriptomic Responses of Rhizobium Phaseoli to Root Exudates Reflect Its Capacity to Colonize Maize and Common Bean in an Intercropping System. Front. Microbiol. 2021, 12, 740818. [Google Scholar] [CrossRef]
- Velasco-Murguía, A.; del Castillo, R.F.; Rös, M.; Rivera-García, R. Successional Pathways of Post-Milpa Fallows in Oaxaca, Mexico. For. Ecol. Manag. 2021, 500, 119644. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Sandoval-Castellanos, E.; Roessler, K.; Gaut, B.S.; Alcaraz, L.D.; Benítez, M.; Escalante, A.E. Seasonal Changes in a Maize-Based Polyculture of Central Mexico Reshape the Co-occurrence Networks of Soil Bacterial Communities. Front. Microbiol. 2017, 8, 02478. [Google Scholar] [CrossRef]
- Otero Prevost, D.E.; Delfín Gurri, F.; Mariaca Méndez, R.; Guízar Vázquez, F. La incorporación y el aumento de oferta de alimentos industrializados en las dietas de las unidades domésticas y su relación con el abandono del sistema de subsistencia propio en las comunidades rurales mayas de Yucatán, México. Cuad. Desarro. Rural 2017, 14, 1–16. [Google Scholar] [CrossRef]
- Drucker, P.; Heizer, R.F. A Study of the Milpa System of La Venta Island and Its Archaeological Implications. Southwest. J. Anthropol. 1960, 16, 36–45. [Google Scholar] [CrossRef]
- Moreno-Espíndola, I.P.; Ferrara-Guerrero, M.J.; Luna-Guido, M.L.; Ramírez-Villanueva, D.A.; De León-Lorenzana, A.S.; Gómez-Acata, S.; González-Terreros, E.; Ramírez-Barajas, B.; Navarro-Noya, Y.E.; Sánchez-Rodríguez, L.M.; et al. The Bacterial Community Structure and Microbial Activity in a Traditional Organic Milpa Farming System Under Different Soil Moisture Conditions. Front. Microbiol. 2018, 9, 02737. [Google Scholar] [CrossRef]
- Nigh, R.; Diemont, S.A. The Maya Milpa: Fire and the Legacy of Living Soil. Front. Ecol. Environ. 2013, 11, e45–e54. [Google Scholar] [CrossRef]
- Nadal, A.; Rañó, H.G. Environmental Impact of Changes in Production Strategies in Tropical Mexico. J. Sustain. Agric. 2011, 35, 180–207. [Google Scholar] [CrossRef]
- Birol, E.; Villalba, E.R. Estimating Mexican Farmers’ Valuation of Milpa Diversity and Genetically Modified Maize: A Choice Experiment Approach. Environmental Economy and Policy Research Working Papers. 2006. Available online: https://www.researchgate.net/publication/40724211_Estimating_the_value_of_milpa_diversity_and_genetically_modified_maize_to_farmers_in_Mexico_a_choice_experiment_approach (accessed on 2 August 2023).
- López Binnqüist, C.; Hidalgo Ledesma, R.; Panzo Panzo, F. “Keeping Our Milpa”: Maize Production and Management of Trees by Nahuas of the Sierra de Zongolica, Mexico. In Indigenous Knowledge: Enhancing Its Contribution to Natural Resources Management; CABI Books: Wallingford, UK, 2017; pp. 40–50. [Google Scholar] [CrossRef]
- Hostettler, U. Labor Regime and Social Justice. Consequences of Economic and Social Stratification Among Maya Peasants in Central Quintana Roo, Mexico. Anthropos 2002, 97, 107–116. [Google Scholar]
- García-Frapolli, E.; Ayala-Orozco, B.; Bonilla-Moheno, M.; Espadas-Manrique, C.; Ramos-Fernández, G. Biodiversity Conservation, Traditional Agriculture and Ecotourism: Land Cover/Land Use Change Projections for a Natural Protected Area in the Northeastern Yucatan Peninsula, Mexico. Landsc. Urban Plan. 2007, 83, 137–153. [Google Scholar] [CrossRef]
- Zizumbo-Villarreal, D.; Flores-Silva, A.; Colunga-García Marín, P. The Archaic Diet in Mesoamerica: Incentive for Milpa Development and Species Domestication. Econ. Bot. 2012, 66, 328–343. [Google Scholar] [CrossRef]
- Schmook, B.; van Vliet, N.; Radel, C.; Manzón-Che, M.d.J.; McCandless, S. Persistence of Swidden Cultivation in the Face of Globalization: A Case Study from Communities in Calakmul, Mexico. Hum. Ecol. 2013, 41, 93–107. [Google Scholar] [CrossRef]
- Bermeo, A.; Couturier, S.; Galeana Pizaña, M. Conservation of Traditional Smallholder Cultivation Systems in Indigenous Territories: Mapping Land Availability for Milpa Cultivation in the Huasteca Poblana, Mexico. Appl. Geogr. 2014, 53, 299–310. [Google Scholar] [CrossRef]
- van Dusen, M.E. Missing Markets, Migration and Crop Biodiversity in the Milpa System of Mexico: A Household-Farm Model. In Valuing Crop Biodiversity: On-Farm Genetic Resources and Economic Change; CABI Books: Wallingford, UK, 2005; pp. 63–77. [Google Scholar] [CrossRef]
- Quintana-Ascencio, P.F.; Gonzalez-Espinosa, M.; Ramirez-Marcial, N.; Dominguez-Vazquez, G.; Martinez-Ico, M. Soil Seed Banks and Regeneration of Tropical Rain Forest from Milpa Fields at the Selva Lacandona, Chiapas, Mexico. Biotropica 1996, 28, 192–209. [Google Scholar] [CrossRef]
- Ebel, R. Effects of Slash-and-Burn-Farming and a Fire-Free Management on a Cambisol in a Traditional Maya Farming System. Cienc. Sum 2018, 25, e15. [Google Scholar] [CrossRef]
- Ortiz-Ceballos, A.I.; Aguirre-Rivera, J.R.; Salgado-Garcia, S.; Ortiz-Ceballos, G. Maize–Velvet Bean Rotation in Summer and Winter Milpas: A Greener Technology. Agron. J. 2015, 107, 330–336. [Google Scholar] [CrossRef]
- Levasseur, V.; Olivier, A. The Farming System and Traditional Agroforestry Systems in the Maya Community of San Jose, Belize. Agrofor. Syst. 2000, 49, 275–288. [Google Scholar] [CrossRef]
- Pascual-Mendoza, S.; Manzanero-Medina, G.I.; Saynes-Vásquez, A.; Vásquez-Dávila, M.A. Agroforestry Systems of a Zapotec Community in the Northern Sierra of Oaxaca, Mexico. Bot. Sci. 2020, 98, 128–144. [Google Scholar] [CrossRef]
- Olson, M.B.; Morris, K.S.; Méndez, V.E. Cultivation of Maize Landraces by Small-Scale Shade Coffee Farmers in Western El Salvador. Agric. Syst. 2012, 111, 63–74. [Google Scholar] [CrossRef]
- Pérez-García, O.; del Castillo, R.F. The Decline of the Itinerant Milpa and the Maintenance of Traditional Agrobiodiversity: Crops and Weeds Coexistence in a Tropical Cloud Forest Area in Oaxaca, Mexico. Agric. Ecosyst. Environ. 2016, 228, 30–37. [Google Scholar] [CrossRef]
- Diemont, S.A.W.; Martin, J.F.; Levy-Tacher, S.I. Emergy Evaluation of Lacandon Maya Indigenous Swidden Agroforestry in Chiapas, Mexico. Agrofor. Syst. 2006, 66, 23–42. [Google Scholar] [CrossRef]
- Del Amo, R.S.; Ramos, P.J. Use and Management of Secondary Vegetation in a Humid-Tropical Area. Agrofor. Syst. 1993, 21, 27–42. [Google Scholar] [CrossRef]
- Hartter, J.; Lucas, C.; Gaughan, A.E.; Lizama Aranda, L. Detecting Tropical Dry Forest Succession in a Shifting Cultivation Mosaic of the Yucatán Peninsula, Mexico. Appl. Geogr. 2008, 28, 134–149. [Google Scholar] [CrossRef]
- Parsons, D.; Ramírez-Aviles, L.; Cherney, J.H.; Ketterings, Q.M.; Blake, R.W.; Nicholson, C.F. Managing Maize Production in Shifting Cultivation Milpa Systems in Yucatán, Through Weed Control and Manure Application. Agric. Ecosyst. Environ. 2009, 133, 123–134. [Google Scholar] [CrossRef][Green Version]
- Roncal-García, S.; Soto-Pinto, L.; Castellanos-Albores, J. Sistemas agroforestales y almacenamiento de carbono en comunidades indígenas de Chiapas, México. Interciencia 2008, 33, 200–206. [Google Scholar][Green Version]
- Moreno-Calles, A.I.; Casas, A.; García-Frapolli, E.; Torres-García, I. Traditional Agroforestry Systems of Multi-Crop “Milpa” and “Chichipera” Cactus Forest in the Arid Tehuacán Valley, Mexico: Their Management and Role in People’s Subsistence. Agrofor. Syst. 2012, 84, 207–226. [Google Scholar] [CrossRef]
- Reyna-Ramírez, C.A.; Rodríguez-Sánchez, L.M.; Vela-Correa, G.; Etchevers-Barra, J.; Fuentes-Ponce, M. Redesign of the Traditional Mesoamerican Agroecosystem Based on Participative Ecological Intensification: Evaluation of the Soil and Efficiency of the System. Agric. Syst. 2018, 165, 177–186. [Google Scholar] [CrossRef]
- Silva, C.; Vinuesa, P.; Eguiarte, L.E.; MartínEz-Romero, E.; Souza, V. Rhizobium etli and Rhizobium gallicum Nodulate Common Bean (Phaseolus vulgaris) in a Traditionally Managed Milpa Plot in Mexico: Population Genetics and Biogeographic Implications. Appl. Environ. Microbiol. 2003, 69, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S. The Intensification of Traditional Agriculture Among Yucatec Maya Farmers: Facing up to the Dilemma of Livelihood Sustainability. Hum. Ecol. 1993, 21, 87–102. [Google Scholar] [CrossRef]
- Molina-Anzures, M.F.; Chávez-Servia, J.L.; Gil-Muñoz, A.; López, P.A.; Hernández-Romero, E.; Ortiz-Torres, E. Productive efficiencies in corn, bean and squash (Cucurbita pepo L.) associations, intercropped with rows of fruit trees. Phyton 2016, 85, 36–50. [Google Scholar] [CrossRef]
- Pérez-García, O.; del Castillo, R.F. Shifts in Swidden Agriculture Alter the Diversity of Young Fallows: Is the Regeneration of Cloud Forest at Stake in Southern Mexico? Agric. Ecosyst. Environ. 2017, 248, 162–174. [Google Scholar] [CrossRef]
- García-Jácome, L.G.; García-Frapolli, E.; Bonilla-Moheno, M.; Rangel-Rivera, C.E.; Benítez, M.; Ramos-Fernández, G. Multiple Resource Use Strategies and Resilience of a Socio-Ecosystem in a Natural Protected Area in the Yucatan Peninsula, Mexico. Front. Sustain. Food Syst. 2020, 4, 522657. [Google Scholar] [CrossRef]
- Milan, A.; Ruano, S. Rainfall Variability, Food Insecurity and Migration in Cabricán, Guatemala. Clim. Dev. 2014, 6, 61–68. [Google Scholar] [CrossRef]
- Brush, S.B.; Tadesse, D.; van Dusen, E. Crop Diversity in Peasant and Industrialized Agriculture: Mexico and California. Soc. Nat. Resour. 2003, 16, 123–141. [Google Scholar] [CrossRef]
- Ávalos, H.C.; Chavero, E.L. La multifuncionalidad de agroecosistemas en la cuenca del río Cuitzmala, Jalisco, México. Agric. Soc. Desarro. 2019, 16, 513–537. [Google Scholar] [CrossRef][Green Version]
- de Loera, R.D.M.; Ferguson, B.G.; Rivera, H.R.P.; Charbonnier, F.S.J. Herbicidas en la milpa: Estrategias de aplicación y su impacto sobre el consumo de arvenses. Ecosistemas Recur. Agropecu. 2019, 6, 477–486. [Google Scholar] [CrossRef]
- Montes-Hernández, S.; Merrick, L.C.; Eguiarte, L.E. Maintenance of Squash (Cucurbita spp.) Landrace Diversity by Farmers’ Activities in Mexico. Genet. Resour. Crop Evol. 2005, 52, 697–707. [Google Scholar] [CrossRef]
- Trejo, D.; Barois, I.; Sangabriel-Conde, W. Disturbance and Land Use Effect on Functional Diversity of the Arbuscular Mycorrhizal Fungi. Agrofor. Syst. 2016, 90, 265–279. [Google Scholar] [CrossRef]
- Soto-Pinto, L.; Colmenares, S.E.; Kanter, M.B.; Cruz, A.L.; Lugo, E.E.; Hernández, B.H.; Jiménez-Soto, E. Contributions of Agroforestry Systems to Food Provisioning of Peasant Households: Conflicts and Synergies in Chiapas, Mexico. Front. Sustain. Food Syst. 2022, 5, 756611. [Google Scholar] [CrossRef]
- Zhang, C.; Postma, J.A.; York, L.M.; Lynch, J.P. Root Foraging Elicits Niche Complementarity-Dependent Yield Advantage in the Ancient ‘Three Sisters’ (Maize/Bean/Squash) Polyculture. Ann. Bot. 2014, 114, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Kapayou, D.G.; Herrighty, E.M.; Hill, C.G.; Camacho, V.C.; Nair, A.; Winham, D.M.; McDaniel, M.D. Reuniting the Three Sisters: Collaborative Science with Native Growers to Improve Soil and Community Health. Agric. Hum. Values 2023, 40, 65–82. [Google Scholar] [CrossRef]
- Amador, M.F.; Gliessman, S.R. An Ecological Approach to Reducing External Inputs Through the Use of Intercropping. In Agroecology: Researching the Ecological Basis for Sustainable Agriculture; Gliessman, S.R., Ed.; Springer: New York, NY, USA, 1990; pp. 146–159. [Google Scholar] [CrossRef]
- Ebel, R.; Cárdenas, J.G.P.; Miranda, F.S.; González, J.C. Manejo orgánico de la milpa: Rendimiento de maíz, frijol y calabaza en monocultivo y policultivo. Rev. Terra Latinoam. 2017, 35, 149–160. [Google Scholar] [CrossRef]
- Kirchhoff, P. Mesoamérica, Sus Límites Geográficos, Composición Étnica y Caracteres Culturales; ENAH: Mexico City, Mexico, 1960. [Google Scholar]
- Olmos, A. Arte de La Lengva Mexicana; Imprenta Nacional: Mexico City, Mexico, 1547. [Google Scholar]
- Siméon, R. Diccionario de La Lengua Náhuatl o Mexicana; Siglo XXI: Mexico City, Mexico, 1992. [Google Scholar]
- Molina, A. Arte de La Lengua Mexicana y Castellana; Casa de Pedro Ocharte: Mexico City, Mexico, 1571. [Google Scholar]
- Rojas-Rabiela, T. Las Siembras Del Ayer. La Agricultura Indígena Del Siglo XVI; SEP-CIESAS: Mexico City, Mexico, 1988. [Google Scholar]
- Brown, C.H. The Role of Nahuatl in the Formation of Mesoamerica as a Linguistic Area. Lang. Dyn. Change 2011, 1, 171–204. [Google Scholar] [CrossRef]
- Heindorf, C.; Reyes-Agüero, J.A.; Fortanelli-Martínez, J.; van ’t Hooft, A. More than Maize, Bananas, and Coffee: The Inter– and Intraspecific Diversity of Edible Plants of the Huastec Mayan Landscape Mosaics in Mexico1. Econ. Bot. 2021, 75, 158–174. [Google Scholar] [CrossRef]
- Stewart, C.; Stewart, R.D. Diccionario Amuzgo de San Pedro Amuzgos, Oaxaca; Instituto Lingüístico de Verano: Mexico City, Mexico, 2000. [Google Scholar]
- Anderson, J.L.; de Montague, D.G.; de Davies, W.P. Diccionario Chinanteco de Santiago Comaltepec, Ixtlán de Juárez, Oaxaca; Instituto Lingüístico de Verano: Mexico City, Mexico, 2021. [Google Scholar]
- Anderson, R.E.; Concepción, H. Diccionario Cuicateco; Instituto Lingüístico de Verano: Mexico City, Mexico, 1983. [Google Scholar]
- Hilton, K.S. Palabras y frases de las lenguas tarahumara y guarijio. An. Del Inst. Nac. De Antropol. E Hist. 1947, II, 307–313. [Google Scholar]
- Larsen, R.; Voigtlander, C. Vocabulario Huasteco; Instituto Lingüístico de Verano: Mexico City, Mexico, 1955. [Google Scholar]
- Kreger, G.A.S.; de Stairs, E.F.S.; Olivares, P.; Ponce, T.; Comonfort, L. Diccionario Huave de San Mateo Del Mar; Instituto Lingüístico de Verano: Mexico City, Mexico, 1981. [Google Scholar]
- Estrada, A.; Fardlow, L. Diccionario Práctico de La Lengua Kiliwa; INALI: Mexico City, Mexico, 2004. [Google Scholar]
- Carrera, C. Acercamiento Gramatical a La Lengua Mazateca de Mazatlán Villa de Flores, Oaxaca; INALI: Mexico City, Mexico, 2011. [Google Scholar]
- Méndez, R.M. La Milpa en el Sur de México. Ecofronteras 2011, 43, 22–26. [Google Scholar]
- Casas, A.; Viveros, J.L.; Caballero, J. Etnobotánica Mixteca: Sociedad, Cultura y Recursos Naturales En La Montaña de Guerrero; INI-CONACULTA: Mexico City, Mexico, 1994. [Google Scholar]
- Krumholz, J.A.; Dolson, M.K.; Hernández, M. Diccionario Popoloca de San Juan Atzingo, Puebla; Instituto Lingüístico de Verano: Mexico City, Mexico, 1995. [Google Scholar]
- Elson, B.F.; Gutierrez, G. Diccionario Popoluca de La Sierra Veracruz; Instituto Lingüístico de Verano: Mexico City, Mexico, 1999. [Google Scholar]
- Lathrop, M. Vocabulario Del Idioma Purépecha; Instituto Lingüístico de Verano: Mexico City, Mexico, 2007. [Google Scholar]
- Hilton, K.S. Diccionario Tarahumara de Samachique, Chihuahua, México; Instituto Lingüístico de Verano: Mexico City, Mexico, 1959. [Google Scholar]
- Bye, R.; Linares, E. Ethnobotany in the Sierra Tarahumara, Mexico: Mountains As Barriers, Conduits, and Generators of Plant-People Interactions and Relationships. In Ethnobotany of the Mountain Regions of Mexico; Springer: Cham, Switzerland, 2022; pp. 1–20. [Google Scholar] [CrossRef]
- Brambila, D. Diccionario Rarámuri—Castellano (Tarahumar); Obra Nacional de la Buena Prensa: Mexico City, Mexico, 1976. [Google Scholar]
- de la Cruz, E.; Gutierrez, S.; Jiménez, N.; García, C. Vocabulario Tepehua-Español-Tepehua; INALI: Mexico City, Mexico, 2013. [Google Scholar]
- Reid, A.A.; Bishop, R.G. Diccionario Totonaco de Xicotepec de Juárez, Puebla. Oaxaca; Instituto Lingüístico de Verano: Mexico City, Mexico, 1974. [Google Scholar]
- Aschmann, H.P. Vocabulario Totonaco de Papantla; Instituto Lingüístico de Verano: Mexico City, Mexico, 1973. [Google Scholar]
- Good, C. Diccionario Triqui de Chicahuaxtla. Oaxaca; Instituto Lingüístico de Verano: Mexico City, Mexico, 1979. [Google Scholar]
- Estrada, Z.; Buitimea, C.; Gurrola, A.E.; Castillo, M.E.; Carlón, A. Diccionario Yaqui-Español y Textos: Obra de Preservación Lingüística; Universidad de Sonora: Mexico City, Mexico, 2004. [Google Scholar]
- López, C.; Hidalgo, R.; Panzo, F. Vocabulario Breve Del Zapoteco de San Juan Guelavía; Instituto Lingüístico de Verano: Mexico City, Mexico, 2012. [Google Scholar]
- Pastor, S.; López, L. Consideraciones Sobre La Agricultura Prehispánica En El Sector Central de Las Sierras de Córdoba (Argentina). In Arqueología de La Agricultura, Casos de Estudios En La Región Andina Argentina; Ediciones Magna: Tucumán, Argentina, 2010; pp. 208–233. [Google Scholar]
- Poma de Ayala, F.G. Nueva Coronica y Buen Gobierno; Université de Paris: Paris, France, 1936. [Google Scholar]
- Sauer, C.O. Cultivated Plants of South and Central America. In Handbook of South American Indians; US Government Printing Office: New York, NY, USA, 1963; Volume 6, pp. 487–543. [Google Scholar]
- Conway, G.R. The Properties of Agroecosystems. Agric. Syst. 1987, 24, 95–117. [Google Scholar] [CrossRef]
- Palerm, J.V. La persinstencia y expansión de sistemas agrícolas tradicionales: El caso del huamil en el Bajío Mexicano. Monogr. Real Jardín Botánico Córdoba 1997, 5, 121–133. [Google Scholar]
- Evans, S.T. The Productivity of Maguey Terrace Agriculture in Central Mexico During the Aztec Period. Lat. Am. Antiq. 1990, 1, 117–132. [Google Scholar] [CrossRef]
- Sahagún, B. Historia General de Las Cosas de La Nueva España; Porrúa: Mexico City, Mexico, 1975. [Google Scholar]
- Debouck, D.G. Your Beans of the Last Harvest and the Possible Adoption of Bright Ideas. In Ethnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Lira, R., Casas, A., Blancas, J., Eds.; Springer: New York, NY, USA, 2016; pp. 367–387. [Google Scholar] [CrossRef]
- Sanjur, O.I.; Piperno, D.R.; Andres, T.C.; Wessel-Beaver, L. Phylogenetic Relationships Among Domesticated and Wild Species of Cucurbita (Cucurbitaceae) Inferred from a Mitochondrial Gene: Implications for Crop Plant Evolution and Areas of Origin. Proc. Natl. Acad. Sci. USA 2002, 99, 535–540. [Google Scholar] [CrossRef]
- Moreno-Calles, A.I.; Toledo, V.M.; Casas, A. Los sistemas agroforestales tradicionales de México: Una aproximación biocultural. Bot. Sci. 2013, 91, 375–398. [Google Scholar] [CrossRef]
- Tapia, S.M.; Gómez, E.G. Caracterización sociocultural de las milpas en dos ejidos del municipio de Tlaquiltenango, Morelos, México. Etnobiología 2015, 13, 94–109. [Google Scholar]
- Hernández-Xolocotzi, E.; Bello, E.; Levy, S. La Milpa En Yucatán, Un Sistema de Producción Agrícola Tradicional. Volume I; Colegio de Postgraduados: Mexico City, Mexico, 1995. [Google Scholar]
- Griffon, D.; Hernandez, M.-J.; Ramírez, D. Theoretical Clues for Agroecological Transitions: The Conuco Legacy and the Monoculture Trap. Front. Sustain. Food Syst. 2021, 5, 529271. [Google Scholar] [CrossRef]
- Rindos, D. The Origins of Agriculture: An Evolutionary Perspective; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Allaby, R.G.; Stevens, C.J.; Kistler, L.; Fuller, D.Q. Emerging Evidence of Plant Domestication as a Landscape-Level Process. Trends Ecol. Evol. 2022, 37, 268–279. [Google Scholar] [CrossRef]
- Hernández-Xolocotzi, E. Aspectos de La Domesticación de Plantas En México: Una Apreciación Personal. In Diversidad Biológica de México: Orígenes y Distribución; UNAM: Mexico City, Mexico, 1998; pp. 715–735. [Google Scholar]
- Casas, A.; Parra, F.; Blancas, J.; Rangel-Landa, S.; Vallejo, M.; Figueredo, C.J.; Moreno-Calles, A.I. Origen de La Domesticación y La Agricultura: Cómo y Por Qué. In Domesticación En El Continente Americano; UNAM-Universidad Agraria La Molina: Mexico City, Mexico, 2016; Volume 1, pp. 189–223. [Google Scholar]
- Wilkes, H.G. Hybridization of Maize and Teosinte, in Mexico and Guatemala and the Improvement of Maize. Econ. Bot. 1977, 31, 254–293. [Google Scholar] [CrossRef]
- Brücher, H. The Wild Ancestor of Phaseolus vulgaris in South America. In Genetic Resources of Phaseolus Beans: Their Maintenance, Domestication, Evolution and Utilization; Gepts, P., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 185–214. [Google Scholar] [CrossRef]
- Clement, C.R.; Casas, A.; Parra-Rondinel, F.A.; Levis, C.; Peroni, N.; Hanazaki, N.; Cortés-Zárraga, L.; Rangel-Landa, S.; Alves, R.P.; Ferreira, M.J.; et al. Disentangling Domestication from Food Production Systems in the Neotropics. Quaternary 2021, 4, 4. [Google Scholar] [CrossRef]
- Iriarte, J.; Elliott, S.; Maezumi, S.Y.; Alves, D.; Gonda, R.; Robinson, M.; de Souza, J.G.; Watling, J.; Handley, J. The Origins of Amazonian Landscapes: Plant Cultivation, Domestication and the Spread of Food Production in Tropical South America. Quat. Sci. Rev. 2020, 248, 106582. [Google Scholar] [CrossRef]
- Piperno, D.R. The Origins of Plant Cultivation and Domestication in the New World Tropics: Patterns, Process, and New Developments. Curr. Anthropol. 2011, 52, S453–S470. [Google Scholar] [CrossRef]
- Vavilov, N.I. Origin and Geography of Cultivated Plants; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Ardelean, C.F.; Becerra-Valdivia, L.; Pedersen, M.W.; Schwenninger, J.-L.; Oviatt, C.G.; Macías-Quintero, J.I.; Arroyo-Cabrales, J.; Sikora, M.; Ocampo-Díaz, Y.Z.E.; Rubio-Cisneros, I.I.; et al. Evidence of Human Occupation in Mexico Around the Last Glacial Maximum. Nature 2020, 584, 87–92. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Vigouroux, Y.; Goodman, M.M.; Sanchez, G.J.; Buckler, E.; Doebley, J. A Single Domestication for Maize Shown by Multilocus Microsatellite Genotyping. Proc. Natl. Acad. Sci. USA 2002, 99, 6080–6084. [Google Scholar] [CrossRef]
- Piperno, D.R.; Ranere, A.J.; Holst, I.; Iriarte, J.; Dickau, R. Starch Grain and Phytolith Evidence for Early Ninth Millennium B.P. Maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 5019–5024. [Google Scholar] [CrossRef]
- Smith, B.D. The Initial Domestication of Cucurbita Pepo in the Americas 10,000 Years Ago. Science 1997, 276, 932–934. [Google Scholar] [CrossRef]
- Piperno, D.R.; Dillehay, T.D. Starch Grains on Human Teeth Reveal Early Broad Crop Diet in Northern Peru. Proc. Natl. Acad. Sci. USA 2008, 105, 19622–19627. [Google Scholar] [CrossRef]
- Barrera-Redondo, J.; Sánchez-de la Vega, G.; Aguirre-Liguori, J.A.; Castellanos-Morales, G.; Gutiérrez-Guerrero, Y.T.; Aguirre-Dugua, X.; Aguirre-Planter, E.; Tenaillon, M.I.; Lira-Saade, R.; Eguiarte, L.E. The Domestication of Cucurbita argyrosperma as Revealed by the Genome of Its Wild Relative. Hortic. Res. 2021, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Pearsall, D.M. The Origins of Plant Cultivation in South America. In The Origins of Agriculture: An International Perspective; Smithsonian Institution: Washington, DC, USA, 1992; pp. 173–205. [Google Scholar]
- Kaplan, L.; Lynch, T.F. Phaseolus (Fabaceae) in Archaeology: AMS Radiocarbon Dates and their Significance for Pre-Columbian Agriculture. Econ. Bot. 1999, 53, 261–272. [Google Scholar] [CrossRef]
- Serrano-Serrano, M.L.; Hernández-Torres, J.; Castillo-Villamizar, G.; Debouck, D.G.; Sánchez, M.I.C. Gene Pools in Wild Lima Bean (Phaseolus lunatus L.) from the Americas: Evidences for an Andean Origin and Past Migrations. Mol. Phylogenet. Evol. 2010, 54, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Bitocchi, E.; Bellucci, E.; Giardini, A.; Rau, D.; Rodriguez, M.; Biagetti, E.; Santilocchi, R.; Zeuli, P.S.; Gioia, T.; Logozzo, G.; et al. Molecular Analysis of the Parallel Domestication of the Common Bean (Phaseolus vulgaris) in Mesoamerica and the Andes. New Phytol. 2013, 197, 300–313. [Google Scholar] [CrossRef]
- Kwak, M.; Kami, J.A.; Gepts, P. The Putative Mesoamerican Domestication Center of Phaseolus vulgaris Is Located in the Lerma–Santiago Basin of Mexico. Crop Sci. 2009, 49, 554–563. [Google Scholar] [CrossRef]
- Spataro, G.; Tiranti, B.; Arcaleni, P. Genetic Diversity and Structure of a Worldwide Collection of Phaseolus coccineus L. Theor. Appl. Genet. 2011, 122, 1281–1291. [Google Scholar] [CrossRef]
- Mina-Vargas, A.M.; McKeown, P.C.; Flanagan, N.S.; Debouck, D.G.; Kilian, A.; Hodkinson, T.R.; Spillane, C. Origin of Year-Long Bean (Phaseolus dumosus Macfady, Fabaceae) from Reticulated Hybridization Events Between Multiple Phaseolus Species. Ann. Bot. 2016, 118, 957–969. [Google Scholar] [CrossRef]
- Blair, M.W.; Pantoja, W.; Carmenza Muñoz, L. First Use of Microsatellite Markers in a Large Collection of Cultivated and Wild Accessions of Tepary Bean (Phaseolus acutifolius A. Gray). Theor. Appl. Genet. 2012, 125, 1137–1147. [Google Scholar] [CrossRef]
- Miranda-Colín, S. Origen de Phaseolus vulgaris L. (Frijol Común). Agrociencia 1967, 1, 99–109. [Google Scholar]
- Flannery, K.V. Guilá Naquitz, Archaic Foraging and Early Agriculture in Oaxaca, Mexico; Academic Press: New York, NY, USA, 1986. [Google Scholar]
- Yang, N.; Wang, Y.; Liu, X.; Jin, M.; Vallebueno-Estrada, M.; Calfee, E.; Chen, L.; Dilkes, B.P.; Gui, S.; Fan, X.; et al. Two Teosintes Made Modern Maize. Science 2023, 382, eadg8940. [Google Scholar] [CrossRef] [PubMed]
- Kistler, L.; Maezumi, S.Y.; de Souza, J.G.; Przelomska, N.A.S.; Costa, F.M.; Smith, O.; Loiselle, H.; Ramos-Madrigal, J.; Wales, N.; Ribeiro, E.R.; et al. Multiproxy Evidence Highlights a Complex Evolutionary Legacy of Maize in South America. Science 2018, 362, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Swarts, K.; Gutaker, R.M.; Benz, B.; Blake, M.; Bukowski, R.; Holland, J.; Kruse-Peeples, M.; Lepak, N.; Prim, L.; Romay, M.C.; et al. Genomic Estimation of Complex Traits Reveals Ancient Maize Adaptation to Temperate North America. Science 2017, 357, 512–515. [Google Scholar] [CrossRef]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a Model for Understanding Crop Evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef]
- Ford, R.I. Gardening and Farming Before A. D. 1000: Patterns of Prehistoric Cultivation North of Mexico. J. Ethnobiol. 1981, 1, 6–27. [Google Scholar]
- Jardel Peláez, E.J.; Graf Montero, S.H.; Santana, C.E.; Ávila Palafox, R. Biodiversité et viabilité de l’agriculture paysanne dans la Réserve de Biosphère Sierra de Manantlán, Mexique. Rev. D’ethnoécologie 2013, 3, 1426. [Google Scholar] [CrossRef]
- Salinas, A.D.; Bonet, A.; Gepts, P. The Wild Relative of Phaseolus vulgaris in Middle America. In Genetic Resources of Phaseolus Beans: Their Maintenance, Domestication, Evolution and Utilization; Gepts, P., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 163–184. [Google Scholar] [CrossRef]
- Azurdia, C. Maíz Silvestre de Guatemala: Distribución, Diversidad Genética y Conservación; Conap: Guatemala City, Guatemala, 2022. [Google Scholar]
- Lira, R. Recopilación y Análisis de Información de Las Especies de Los Géneros Cucurbita y Sechium En México; Informe final; Conabio: Mexico City, Mexico, 2009. [Google Scholar]
- Kato, A.; Mera, L.M.; Serratos, J.A.; Mapes, C.; Bye, R. Origen y Diversificación Del Maíz: Una Revisión Analítica; Conabio: Mexico City, Mexico, 2009. [Google Scholar]
- Piperno, D.R.; Moreno, J.E.; Iriarte, J.; Holst, I.; Lachniet, M.; Jones, J.G.; Ranere, A.J.; Castanzo, R. Late Pleistocene and Holocene Environmental History of the Iguala Valley, Central Balsas Watershed of Mexico. Proc. Natl. Acad. Sci. USA 2007, 104, 11874–11881. [Google Scholar] [CrossRef] [PubMed]
- Maezumi, S.Y.; Alves, D.; Robinson, M.; de Souza, J.G.; Levis, C.; Barnett, R.L.; de Oliveira, E.A.; Urrego, D.; Schaan, D.; Iriarte, J. The Legacy of 4500 Years of Polyculture Agroforestry in the Eastern Amazon. Nat. Plants 2018, 4, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, T.D.; Bonavia, D.; Goodbred, S.; Pino, M.; Vasquez, V.; Tham, T.R.; Conklin, W.; Splitstoser, J.; Piperno, D.; Iriarte, J.; et al. Chronology, Mound-Building and Environment at Huaca Prieta, Coastal Peru, from 13,700 to 4000 Years Ago. Antiquity 2012, 86, 48–70. [Google Scholar] [CrossRef]
- Planella, M.T.; Tagle, M.B. El Sitio Agroalfarero Temprano de La Granja: Un Aporte Desde La Perspectiva Arqueobotánica; Museo de Historia Natural: Santiago, Chile, 1998. [Google Scholar]
- Hart, J.P. Evolving the Three Sisters: The Changing Histories of Maize, Bean, and Squash in New York and the Greater Northeast. In Current Northeast Paleoethnobotany; State University Albany: New York, NY, USA, 2008; Volume II. [Google Scholar]
- Slotten, V.; Lentz, D.; Sheets, P. Landscape Management and Polyculture in the Ancient Gardens and Fields at Joya de Cerén, El Salvador. J. Anthropol. Archaeol. 2020, 59, 101191. [Google Scholar] [CrossRef]
- Iriarte, J.; Holst, I.; Marozzi, O.; Listopad, C.; Alonso, E.; Rinderknecht, A.; Montaña, J. Evidence for Cultivar Adoption and Emerging Complexity During the Mid-Holocene in the La Plata Basin. Nature 2004, 432, 614–617. [Google Scholar] [CrossRef]
- Gil, A.F. Zea mays on the South American Periphery: Chronology and Dietary Importance. Curr. Anthropol. 2003, 44, 295–300. [Google Scholar] [CrossRef]
- Watling, J.; Shock, M.P.; Mongeló, G.Z.; Almeida, F.O.; Kater, T.; De Oliveira, P.E.; Neves, E.G.; Hart, J.P. Direct Archaeological Evidence for Southwestern Amazonia as an Early Plant Domestication and Food Production Centre. PLoS ONE 2018, 13, e0199868. [Google Scholar] [CrossRef]
- Brugger, S.O.; Gobet, E.; van Leeuwen, J.F.N.; Ledru, M.-P.; Colombaroli, D.; van der Knaap, W.; Lombardo, U.; Escobar-Torrez, K.; Finsinger, W.; Rodrigues, L.; et al. Long-Term Man–Environment Interactions in the Bolivian Amazon: 8000 Years of Vegetation Dynamics. Quat. Sci. Rev. 2016, 132, 114–128. [Google Scholar] [CrossRef]
- Santos Vecino, G.; Monsalve Marín, C.A.; Correa Salas, L.V. Alteration of Tropical Forest Vegetation from the Pleistocene–Holocene Transition and Plant Cultivation from the End of Early Holocene Through Middle Holocene in Northwest Colombia. Quat. Int. 2015, 363, 28–42. [Google Scholar] [CrossRef]
- Pagán-Jiménez, J.R.; Guachamín-Tello, A.M.; Romero-Bastidas, M.E.; Constantine-Castro, A.R. Late Ninth Millennium B.P. Use of Zea mays L. at Cubilán Area, Highland Ecuador, Revealed by Ancient Starches. Quat. Int. 2016, 404, 137–155. [Google Scholar] [CrossRef]
- Stothert, K.E.; Piperno, D.R.; Andres, T.C. Terminal Pleistocene/Early Holocene Human Adaptation in Coastal Ecuador: The Las Vegas Evidence. Quat. Int. 2003, 109–110, 23–43. [Google Scholar] [CrossRef]
- Dickau, R.; Ranere, A.J.; Cooke, R.G. Starch Grain Evidence for the Preceramic Dispersals of Maize and Root Crops into Tropical Dry and Humid Forests of Panama. Proc. Natl. Acad. Sci. USA 2007, 104, 3651–3656. [Google Scholar] [CrossRef] [PubMed]
- Kistler, L.; Thakar, H.B.; VanDerwarker, A.M.; Domic, A.; Bergström, A.; George, R.J.; Harper, T.K.; Allaby, R.G.; Hirth, K.; Kennett, D.J. Archaeological Central American Maize Genomes Suggest Ancient Gene Flow from South America. Proc. Natl. Acad. Sci. USA 2020, 117, 33124–33129. [Google Scholar] [CrossRef]
- Cagnato, C. Gathering and Sowing Across the Central Maya Lowlands: A Review of Plant Use by Preceramic Peoples and the Early to Middle Preclassic Maya. Anc. Mesoam. 2021, 32, 486–501. [Google Scholar] [CrossRef]
- Pope, K.O.; Pohl, M.E.D.; Jones, J.G.; Lentz, D.L.; von Nagy, C.; Vega, F.J.; Quitmyer, I.R. Origin and Environmental Setting of Ancient Agriculture in the Lowlands of Mesoamerica. Science 2001, 292, 1370–1373. [Google Scholar] [CrossRef]
- Kennett, D.J.; Piperno, D.R.; Jones, J.G.; Neff, H.; Voorhies, B.; Walsh, M.K.; Culleton, B.J. Pre-Pottery Farmers on the Pacific Coast of Southern Mexico. J. Archaeol. Sci. 2010, 37, 3401–3411. [Google Scholar] [CrossRef]
- Smith, B.D. Reassessing Coxcatlan Cave and the Early History of Domesticated Plants in Mesoamerica. Proc. Natl. Acad. Sci. USA 2005, 102, 9438–9445. [Google Scholar] [CrossRef]
- Piperno, D.R.; Flannery, K.V. The Earliest Archaeological Maize (Zea mays L.) from Highland Mexico: New Accelerator Mass Spectrometry Dates and Their Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 2101–2103. [Google Scholar] [CrossRef]
- Brooks, R.H.; Kaplan, L.; Cutler, H.C.; Whitaker, T.W. Plant Material from a Cave on the Rio Zape, Durango, Mexico. Am. Antiq. 1962, 27, 356–369. [Google Scholar] [CrossRef]
- Smith, C.E. Prehistoric Plant Remains from Bat Cave. Bot. Mus. Leafl. Harv. Univ. 1950, 14, 157–180. [Google Scholar] [CrossRef]
- Perttula, T.K. Caddo Agriculture on the Western Frontier of the Eastern Woodlands. Plains Anthropol. 2008, 53, 79–105. [Google Scholar] [CrossRef]
- Hart, J.P.; Scarry, C.M. The Age of Common Beans (Phaseolus vulgaris) in the Northeastern United States. Am. Antiq. 1999, 64, 653–658. [Google Scholar] [CrossRef]
- Mariaca, R.; Pérez, J.; León, N.S.; López, A. La Milpa Tsotsil de Los Altos de Chiapas y Sus Recursos Fitogenéticos; El Colegio de la Frontera Sur: Mexico City, Mexico, 2007. [Google Scholar]
- Jiménez, C.E.A.; Gadámez, J.G.; Aguilar, F.B.M.; Hernández, F.G.; Solis, H.V. Eficiencia del policultivo maíz-frijol-calabaza bajo manejo orgánico en la Frailesca, Chiapas, México. Rev. Científica Agroecosistemas 2019, 7, 64–72. [Google Scholar]
- Ávila-González, H.; González-Elixondo, M.; Piedra-Leandro, N.L.; Castro-Castro, A.; Amador-Sierra, B.R.; Luna-Vargas, U.; Ramírez-Maciel, R. Agrobiodiversidad de Durango, Una Primera Aproximación; CONHACYT: Mexico City, Mexico, 2024. [Google Scholar]
- Gómez, N.O.; Hernández, C.; Cantú, M.A. Manejo y Uso Sustentable Del Agroecosistema En Guerrero. In Resultados En Conservación, Uso y Aprovechamiento Sustentable de Recursos Fitogenéticos Para La Alimentación y La Agricultura; SNICS: Mexico City, Mexico, 2015; pp. 113–118. [Google Scholar]
- Carrera-Valtierra, J.A. Manejo y Uso Sustentable Del Agroecosistema Milpa En Razas de Maíz Con Problemas de Pérdida de Diversidad En Michoacán. In Resultados En Conservación, Uso y Aprovechamiento Sustentable de Recursos Fitogenéticos Para La Alimentación y La Agricultura; SNICS: Mexico City, Mexico, 2015; pp. 80–83. [Google Scholar]
- Hernández-Márquez, I.; Rojas-Rojas, D. Conservación In Situ de Maíces Criollos Bajo El Sistema Milpa En El Estado de Morelos. In Resultados En Conservación, Uso y Aprovechamiento Sustentable de Recursos Fitogenéticos Para La Alimentación y La Agricultura; SNICS: Mexico City, Mexico, 2015; pp. 58–60. [Google Scholar]
- Hernández, G.J.; Gil, M.A.; López, P.A.; López, S.H.; González, R.G.; Valdivia, B.R. Manejo Integral Del Agroecosistema En Nayarit. In Resultados En Conservación, Uso y Aprovechamiento Sustentable de Recursos Fitogenéticos Para La Alimentación y La Agricultura; SNICS: Mexico City, Mexico, 2015; pp. 62–66. [Google Scholar]
- Guerrero, M.J.; Ortega, A.; Cota, O.; Cubedo, A. Manejo Integral Del Agroecosistema En Sonora. In Resultados En Conservación, Uso y Aprovechamiento Sustentable de Recursos Fitogenéticos Para La Alimentación y La Agricultura; SNICS: Mexico City, Mexico, 2015; pp. 96–99. [Google Scholar]
- Canul-Ku, J.; Ramírez-Vallejo, P.; Castillo-González, F.; Chávez-Servia, J.L. Diversidad morfológica de calabaza cultivada en el centro-oriente de Yucatán, México. Rev. Fitotec. Mex. 2005, 28, 339. [Google Scholar] [CrossRef]
- Pastor, S.; López, L.; Rivero, D. Access to Maize (Zea mays) & Its Manipulation in Hunter-Gatherer Contexts in Central Argentina (c 3000–2500 bp). Farming 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Landa, D. Relación de Las Cosas de Yucatán; CONACULTA: Mexico City, Mexico, 1994. [Google Scholar]
- Newson, L.A. The Demographic Collapse of Native Peoples of the Americas, 1492–1650. Proc. Br. Acad. 1993, 81, 247–288. [Google Scholar]
- Hernández, J.E.; León, J. Neglected Crops, 1492 from a Different Perspective; FAO: Rome, Italy, 1994. [Google Scholar]
- Orozco-Ramírez, Q.; Bocco, G.; Solís-Castillo, B. Cajete Maize in the Mixteca Alta Region of Oaxaca, Mexico: Adaptation, Transformation, and Permanence. Agroecol. Sustain. Food Syst. 2020, 44, 1162–1184. [Google Scholar] [CrossRef]
- Gaytán-Ávila, C.; Vibrans, H.; Navarro-Garza, H.; Jiménez-Velázquez, M. Manejo de huertos familiares periurbanos de San Miguel Tlaixpan, Texcoco, Estado de México. Bot. Sci. 2001, 69, 39–62. [Google Scholar] [CrossRef]
- Cortes, J.I.; Turrent, A.; Díaz, P.; Hernández, E.; Mendoza, R.; Aceves, E. Manual Para El Establecimiento y Manejo de Sistema Milpa Intercalada Con Árboles Frutales; Colegio de Postgraduados: Mexico City, Mexico, 2005. [Google Scholar]
- Baudoin, J.P.; Camarena, F.; Lobo, M. Improving Phaseolus Genotypes for Multiple Cropping Systems. Euphytica 1997, 96, 115–123. [Google Scholar] [CrossRef]
- Francis, C.A. Multiple Cropping Potentials of Beans and Maize. HortScience 1978, 13, 12–17. [Google Scholar] [CrossRef]
- Anonymous. Primer Censo Agrícola Nacional 1930. Resumen General; Departamento de la Estadística Nacional: Mexico City, Mexico, 1936. [Google Scholar]
- Anonymous. V Censos Agrícola-Ganadero y Ejidal. 1970. Resumen General; Dirección General de Estadística: Mexico City, Mexico, 1975. [Google Scholar]
- Anonymous. Segundo Censo Agrícola Ganadero de Los Estados Unidos Mexicanos 1940. Resumen General; Secretaría de Economía, Dirección General de Estadística: Mexico City, Mexico, 1951. [Google Scholar]
- Anonymous. Tercer Censo Agrícola, Ganadero y Ejidal 1950; Dirección General de Estadística: Mexico City, Mexico, 1956. [Google Scholar]
- Anonymous. IV Censos Agrícola-Ganadero y Ejidal. 1960; Dirección General de Estadística: Mexico City, Mexico, 1965. [Google Scholar]
- Anonymous. VI Censos Agrícola-Ganadero y Ejidal, 1981; Instituto Nacional de Estadística, Geografía e Informática: Mexico City, Mexico, 1990. [Google Scholar]
- Sistema de Información Agroalimentaria de Consulta (SIACON). Available online: https://www.gob.mx/agricultura/dgsiap/prensa/sistema-de-informacion-agroalimentaria-de-consulta-siacon?idiom=es (accessed on 20 March 2024).
- Sevilla, R.; Salhuana, W. General Description of the Plan and Execution of Latin American Maize Project (LAMP). In Final Report Latin American Maize Project; USDA: Fort Collins, CO, USA, 1997. [Google Scholar]
- Goodman, M.M.; Brown, W.L. Races of Corn. In Corn and Corn Improvement; ASA, CSSA, SSSA: Madison, WI, USA, 1988. [Google Scholar]
- Sanchez, G.J.J.; Goodman, M.M.; Stuber, C.W. Isozymatic and Morphological Diversity in the Races of Maize of Mexico. Econ. Bot. 2000, 54, 43–59. [Google Scholar] [CrossRef]
- Hernández-Xolocotzi, E. Commentary Upon: Plant Introduction and Germplasm of Phaseolus vulgaris and Other Food Legumes in Latin América. In Potentials of Field Beans and Other Food Legumes in Latin America; CIAT: Cali, Colombia, 1973; pp. 253–258. [Google Scholar]
- Barrera-Redondo, J.; Hernández-Rosales, H.S.; Cañedo-Torres, D.V.; Alegria, K.A.; Torres-Guevara, J.; Parra-Rondinel, F.A.; Torres-García, I.; Casas, A. Variedades locales y criterios de selección de especies domesticadas del género Cucurbita (Cucurbitaceae) en los Andes Centrales del Perú: Tomayquichua, Huánuco. Bot. Sci. 2020, 98, 101–116. [Google Scholar] [CrossRef]
- Woolley, J.; Lépiz, R.; de Aquino, T.; Voss, C.J. Bean Cropping Systems in the Tropics and Their Determinants. In Common Beans Research for Crop Improvement; CAB International-CIAT: Walingford, CT, USA, 1991; pp. 679–706. [Google Scholar]
- Nee, M. The Domestication of Cucurbita (Cucurbitaceae). Econ. Bot. 1990, 44, 56–68. [Google Scholar] [CrossRef]
- Staller, S.J. High Altitude Maize (Zea mays L.) Cultivation and Endemism in the Lake Titicaca Basin. J. Bot. Res. 2016, 1, 8–21. [Google Scholar] [CrossRef]
- Vigouroux, Y.; Glaubitz, J.C.; Matsuoka, Y.; Goodman, M.M.; Sanchez , G.J.; Doebley, J. Population Structure and Genetic Diversity of New World Maize Races Assessed by DNA Microsatellites. Am. J. Bot. 2008, 95, 1240–1253. [Google Scholar] [CrossRef]
- Delgado-Salinas, A.; Bibler, R.; Lavin, M. Phylogeny of the Genus Phaseolus (Leguminosae): A Recent Diversification in an Ancient Landscape. Syst. Bot. 2006, 31, 779–791. [Google Scholar] [CrossRef]
- CIAT (Centro Internacional de Agricultura Tropical). Subset Phaseolus acutifolius, P. coccineus, P. dumosus, P. lunatus, P. vulgaris & Traditional Cultivar/Landrace. 2025. Available online: https://www.genesys-pgr.org/a/v22KeX625W9y (accessed on 25 March 2025).
- González-Amaro, R.M.; Martínez-Bernal, A.; Basurto-Peña, F.; Vibrans, H. Crop and Non-Crop Productivity in a Traditional Maize Agroecosystem of the Highland of Mexico. J. Ethnobiol. Ethnomed. 2009, 5, 38. [Google Scholar] [CrossRef]
- Nations, J.D.; Nigh, R.B. The Evolutionary Potential of Lacandon Maya Sustained-Yield Tropical Forest Agriculture. J. Anthropol. Res. 1980, 36, 1–30. [Google Scholar] [CrossRef]
- Mota-Cruz, C. Plantas Comestibles En La Sierra Negra de Puebla, México. Master’s Dissertation, Colegio de Postgraduados, Montecillo, Mexico, 2008. [Google Scholar]
- Casas, A.; Farfán-Heredia, B.; Camou-Guerrero, A.; Torres-García, I.; Blancas Vázquez, J.J.; Rangel-Landa, S. Wild, Weedy and Domesticated Plants for Food Security and Sovereignty. In Ethnobotany of the Mountain Regions of Mexico; Springer: Cham, Switzerland, 2023; pp. 97–127. [Google Scholar] [CrossRef]
- Bye, R. Quelites—Ethnoecology of Edible Greens—Past, Present, and Future. J. Ethnobiol. 1981, 1, 109–123. [Google Scholar]
- Mapes, C.; Basurto, F. Biodiversity and Edible Plants of Mexico. In Ethnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Springer: New York, NY, USA, 2016; pp. 83–131. [Google Scholar] [CrossRef]
- Linares, E.; Bye, R. Las Especies Subutilizadas de La Milpa. Rev. Digit. Univ. 2015, 16, 5. [Google Scholar]
- Blancas, J.; Casas, A.; Rangel-Landa, S.; Moreno-Calles, A.; Torres, I.; Pérez-Negrón, E.; Solís, L.; Delgado-Lemus, A.; Parra, F.; Arellanes, Y.; et al. Plant Management in the Tehuacán-Cuicatlán Valley, Mexico. Econ. Bot. 2010, 64, 287–302. [Google Scholar] [CrossRef]
- Mota-Cruz, C. El Policultivo de Milpa, Una Experiencia Agroecológica En México. In Proceedings of the Cultivando Sistemas Alimentarios Locales de Base Agroecológica, Sevilla, Spain, 19–21 January 2023; p. 24. [Google Scholar]
- Thierfelder, C.; Mhlanga, B.; Nyagumbo, I.; Kalala, K.; Simutowe, E.; Chiduwa, M.; MacLaren, C.; Silva, J.V.; Ngoma, H. Two Crops Are Better than One for Nutritional and Economic Outcomes of Zambian Smallholder Farms, but Require More Labour. Agric. Ecosyst. Environ. 2024, 361, 108819. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and Ecosystem Stability in a Decade-Long Grassland Experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef]
- Barry, K.E.; Mommer, L.; van Ruijven, J.; Wirth, C.; Wright, A.J.; Bai, Y.; Connolly, J.; De Deyn, G.B.; de Kroon, H.; Isbell, F.; et al. The Future of Complementarity: Disentangling Causes from Consequences. Trends Ecol. Evol. 2019, 34, 167–180. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of Plant Diversity on Biomass Production Increase Through Time Because of Species Complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—Categorical Traits Dataset. Data from: TRY—A Global Data-Base of Plant Traits. 2012. Available online: https://www.try-db.org/TryWeb/Data.php#3 (accessed on 15 April 2024).
- Pérez-Hernández, R.G.; Cach-Pérez, M.J.; Aparicio-Fabre, R.; Wal, H.V.; Rodríguez-Robles, U. Physiological and Microclimatic Effects of Different Agricultural Management Practices with Maize. Bot. Sci. 2021, 99, 132–148. [Google Scholar] [CrossRef]
- Tsubo, M.; Walker, S.; Mukhala, E. Comparisons of Radiation Use Efficiency of Mono-/Inter-Cropping Systems with Different Row Orientations. Field Crops Res. 2001, 71, 17–29. [Google Scholar] [CrossRef]
- Anaya, A.L. Allelopathy as a Tool in the Management of Biotic Resources in Agroecosystems. Crit. Rev. Plant Sci. 1999, 18, 697–739. [Google Scholar] [CrossRef]
- Stern, W.R. Nitrogen Fixation and Transfer in Intercrop Systems. Field Crops Res. 1993, 34, 335–356. [Google Scholar] [CrossRef]
- Liao, H.; Zhou, Z.; Liu, Y.; Luo, Y.; Zhang, C.; Feng, Y.; Shu, Y.; Wang, J. ‘The Three Sisters’ (Maize/Bean/Squash) Polyculture Promotes the Direct and Indirect Defences of Maize Against Herbivores. Eur. J. Agron. 2024, 155, 127118. [Google Scholar] [CrossRef]
- Santos-Fita, D.; Piñera, E.J.N.; Baltazar, E.B.; Lugo, E.I.E.; Méndez, R.M.; Mendoza, P.A.M. La milpa comedero-trampa como una estrategia de cacería tradicional maya. Estud. Cult. Maya 2013, 42, 87–118. [Google Scholar] [CrossRef]
- Weih, M.; Karley, A.J.; Newton, A.C.; Kiær, L.P.; Scherber, C.; Rubiales, D.; Adam, E.; Ajal, J.; Brandmeier, J.; Pappagallo, S.; et al. Grain Yield Stability of Cereal-Legume Intercrops Is Greater Than Sole Crops in More Productive Conditions. Agriculture 2021, 11, 255. [Google Scholar] [CrossRef]
- Van der Werf, W.; Zhang, L.; Chunjie, L.I.; Ping, C.H.; Chen, F.E.; Zhan, X.U.; Zhang, C.; Chunfeng, G.U.; Bastiaans, L.; Makowski, D.; et al. Comparing Performance of Crop Species Mixtures and Pure Stands. Front. Agric. Sci. Eng. 2021, 8, 481–489. [Google Scholar] [CrossRef]
- Li, C.; Stomph, T.-J.; Makowski, D.; Li, H.; Zhang, C.; Zhang, F.; van der Werf, W. The Productive Performance of Intercropping. Proc. Natl. Acad. Sci. USA 2023, 120, e2201886120. [Google Scholar] [CrossRef] [PubMed]
- Htet, M.N.S.; Wang, H.; Yadav, V.; Sompouviseth, T.; Feng, B. Legume Integration Augments the Forage Productivity and Quality in Maize-Based System in the Loess Plateau Region. Sustainability 2022, 14, 6022. [Google Scholar] [CrossRef]
- Sangakkara, R.; Bandaranayake, S.; Attanayake, U.; Stamp, P. Impact of Associated Intercrops on Growth and Yield of Maize (Zea mays L.) in Major Seasons of South Asia. Maydica 2012, 57, 6–10. [Google Scholar]
- Kour, M.; Thakur, N.P.; Kumar, P.; Charak, A.S. Productivity and Profitability of Maize (Zea mays) as Influenced by Intercropping of Rajmash (Phaseolus vulgaris) and Nutrient Management Techniques Under Sub-Alpine Conditions of Jammu, India. Legume Res. 2016, 39, 970–975. [Google Scholar] [CrossRef]
- Charani, E.; Sharifi, P.; Aminpanah, H. The Competitive Ability of Maize (Zea mays L.)-Common Bean (Phaseolus vulgaris L.) Intercrops against Weeds. Rev. Fac. Agron. 2018, 35, 40–62. [Google Scholar]
- Zhanbota, A.; Noor, R.S.; Khan, A.I.; Wang, G.; Waqas, M.M.; Shah, A.N.; Ullah, S. A Two-Year Study on Yield and Yield Components of Maize-White Bean Intercropping Systems under Different Sowing Techniques. Agronomy 2022, 12, 240. [Google Scholar] [CrossRef]
- Bitew, Y.; Derebe, B.; Worku, A.; Chakelie, G. Response of Maize and Common Bean to Spatial and Temporal Differentiation in Maize-Common Bean Intercropping. PLoS ONE 2021, 16, e0257203. [Google Scholar] [CrossRef] [PubMed]
- Shumet, S.T.; Ayalew, T.; Roro, A.G.; Beshir, H.M. Intercropping and Rhizobium Inoculation Affected Microclimate and Performance of Common Bean (Phaseolus vulgaris L.) Varieties. Scientifica 2022, 2022, 3471912. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.H.; Swella, G.B.; Mushobozy, D.M.K. Effect of Plant Populations on the Incidence of Bean Stem Maggot (Ophiomyia spp.) in Common Bean Intercropped with Maize. Plant Prot. Sci. 2009, 45, 149–155. [Google Scholar] [CrossRef]
- Nassary, E.K.; Baijukya, F.; Ndakidemi, P.A. Assessing the Productivity of Common Bean in Intercrop with Maize Across Agro-Ecological Zones of Smallholder Farms in the Northern Highlands of Tanzania. Agriculture 2020, 10, 117. [Google Scholar] [CrossRef]
- Siame, J.; Willey, R.W.; Morse, S. The Response of Maize/Phaseolus Intercropping to Applied Nitrogen on Oxisols in Northern Zambia. Field Crops Res. 1998, 55, 73–81. [Google Scholar] [CrossRef]
- Nkhata, W.; Shimelis, H.; Chirwa, R. Productivity of Newly Released Common Bean (Phaseolus vulgaris L.) Varieties Under Sole Cropping and Intercropping with Maize (Zea mays L.). Front. Sustain. Food Syst. 2021, 5, 741177. [Google Scholar] [CrossRef]
- Mukhala, E.; De Jager, J.M.; Van Rensburg, L.D.; Walker, S. Dietary Nutrient Deficiency in Small-Scale Farming Communities in South Africa: Benefits of Intercropping Maize (Zea mays) and Beans (Phaseolus vulgaris). Nutr. Res. 1999, 19, 629–641. [Google Scholar] [CrossRef]
- Yilmaz, Ş.; Atak, M.; Erayman, M. Identification of Advantages of Maize-Legume Intercropping over Solitary Cropping Through Competition Indices in the East Mediterranean Region. Turk. J. Agric. For. 2008, 32, 111–119. [Google Scholar]
- Santalla, M.; Rodiño, A.P.; Casquero, P.A.; de Ron, A.M. Interactions of Bush Bean Intercropped with Field and Sweet Maize. Eur. J. Agron. 2001, 15, 185–196. [Google Scholar] [CrossRef]
- Francis, C.A.; Prager, M.; Tejada, G. Density Interactions in Tropical Intercropping. I. Maize (Zea mays L.) and Climbing Beans (Phaseolus vulgaris L.). Field Crops Res. 1982, 5, 163–176. [Google Scholar] [CrossRef]
- Francis, C.A.; Prager, M.; Tejada, G. Density Interactions in Tropical Intercropping. II. Maize (Zea mays L.) and Bush Beans (Phaseolus vulgaris L.). Field Crops Res. 1982, 5, 253–264. [Google Scholar] [CrossRef]
- Suárez, J.C.; Anzola, J.A.; Contreras, A.T.; Salas, D.L.; Vanegas, J.I.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Agronomic Performance Evaluation of Intercropping Two Common Bean Breeding Lines with a Maize Variety under Two Types of Fertilizer Applications in the Colombian Amazon Region. Agronomy 2022, 12, 307. [Google Scholar] [CrossRef]
- Albino-Garduño, R.; Turrent-Fernández, A.; Cortés-Flores, J.I.; Livera-Muñoz, M.; Mendoza-Castillo, M.C. Distribución de raíces y de radiación solar en el dosel de maíz y frijol intercalados. Agrociencia 2015, 49, 513–531. [Google Scholar]
- Covarrubias, M.A.M.; Ramos, B.G.A.; Meza, V.M.J.; Robles, K.P. Arreglos topológicos en el cultivo intercalado de maíz y frijol en el estado de Nayarit, México. Rev. Chapingo Ser. Agric. Trop. 2022, 2, 45–57. [Google Scholar] [CrossRef]
- Bildirici, N.; Aldemir, R.; Karsli, M.A.; Dogan, Y. Potential Benefits of Intercropping Corn with Runner Bean for Small-Sized Farming System. Asian-Australas. J. Anim. Sci. 2009, 22, 836–842. [Google Scholar] [CrossRef]
- Oljaca, S.; Cvetkovic, R.; Kovacevic, D.; Vasic, G.; Momirovic, N. Effect of Plant Arrangement Pattern and Irrigation on Efficiency of Maize (Zea mays) and Bean (Phaseolus vulgaris) Intercropping System. J. Agric. Sci. 2000, 135, 261–270. [Google Scholar] [CrossRef]
- Gebeyehu, S.; Simane, B.; Kirkby, R. Genotype × Cropping System Interaction in Climbing Beans (Phaseolus vulgaris L.) Grown as Sole Crop and in Association with Maize (Zea mays L.). Eur. J. Agron. 2006, 24, 396–403. [Google Scholar] [CrossRef]
- Dolijanović, Ž.; Momirović, N.; Oljača, S.; Simić, M.; Oljača, M.; Janošević, B. Productivity of Intercropping Maize (Zea mays L.) and Pumkins (Cucurbita maxima Duch.) Under Conventional vs. Conservation Farming System. Turk. J. Field Crops 2015, 20, 92–98. [Google Scholar]
- Mahmud, S.; Alam, M.; Rahman, M.; Amin, M.; Hassan, M. Productivity and Economics of Maize–Squash Intercropping at Different Planting Systems. J. Bangladesh Agric. Univ. 2018, 16, 23–26. [Google Scholar] [CrossRef]
- Chowdhury, J.A.; Kakon, S.S.; Begum, A.A.; Mian, M.A.K. Intercropping Squash with Maize Under Varying Planting System. Bangladesh Agron. J. 2018, 21, 19–24. [Google Scholar] [CrossRef]
- Mota Cruz, C. Dimensions de la diversité des maïs indigènes au Mexique. Rev. D’ethnoécologie 2021, 7453. [Google Scholar] [CrossRef]
- Bressani, R. Chemistry, Technology, and Nutritive Value of Maize Tortillas. Food Rev. Int. 1990, 6, 225–264. [Google Scholar] [CrossRef]
- Morales de León, J.C.; Bourges, H.; Camacho, M.E. Tablas de Composición de Alimentos y Productos Alimenticios; Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán: Mexico City, Mexico, 2016. [Google Scholar]
- Menchú, M.T.; Mendez, H. Tabla de Composición de Alimentos de Centroamérica, 2nd ed.; INCAP/OPS: Guatemala City, Guatemala, 2007. [Google Scholar]
- Méndez-Flores, O.; López, H.O.-D.; Castro-Quezada, I.; Olivo-Vidal, Z.; García-Miranda, R.; Rodríguez-Robles, U.; Irecta-Nájera, C.; López-Ramírez, G.; Sánchez-Chino, X. The Milpa as A Supplier of Bioactive Compounds: A Review. Food Rev. Int. 2023, 39, 1359–1376. [Google Scholar] [CrossRef]
- Mota-Cruz, C. Diversidad de Maíces Nativos y Aspectos Relacionados a Sus Usos Alimenticios. In Nuestra Cultura Alimentaria Del Maíz, Diversidad de Saberes y Prácticas; UNAM: Mexico City, Mexico, 2019; pp. 67–86. [Google Scholar]
- Echeverría, M.E.; Arroyo, L.E. Recetario Del Maíz; CONACULTA: Mexico City, Mexico, 2012. [Google Scholar]
- Keleman, A.; Hellin, J.; Flores, D. Diverse Varieties and Diverse Markets: Scale-Related Maize “Profitability Crossover” in the Central Mexican Highlands. Hum. Ecol. 2013, 41, 683–705. [Google Scholar] [CrossRef]
- King, A. Trade and Totomoxtle: Livelihood Strategies in the Totonacan Region of Veracruz, Mexico. Agric. Hum. Values 2007, 24, 29–40. [Google Scholar] [CrossRef]
- Hellin, J.; Keleman, A.; López, D.; Donnet, L.; Flores, D. La importancia de los nichos de mercado. Un estudio de caso del maíz azul y del maíz para pozole en México. Rev. Fitotec. Mex. 2013, 36, 315. [Google Scholar] [CrossRef]
- Bonfil, G. El Maíz, Fundamento de La Cultura Popular Mexicana; CONACULTA: Mexico City, Mexico, 1982. [Google Scholar]
- Méndez, R.M. La Milpa Maya Yucateca en el Siglo XVI: Evidencias Etnohistóricas y Conjeturas. Etnobiología 2015, 13, 1–25. [Google Scholar]
- Mota-Cruz, C. Ceremonias, Festividades y Ferias Del Maíz. In La Jornada del Campo, 127th ed.; La Jornada: Mexico City, México, 2018; p. 10. [Google Scholar]
- Esteva, G.; Marielle, C. Sin Maíz No Hay País; CONACULTA: Mexico City, Mexico, 2013. [Google Scholar]
- Álvarez-Buylla, E.; Carreón, A.; San Vicente, A. Haciendo Milpa, La Protección de Las Semillas y La Agricultura Campesina; UNAM-Semillas de Vida: Mexico City, Mexico, 2011. [Google Scholar]
- Boege, E. El Sistema Milpa y El Patrimonio Biocultural de Los Pueblos Indígenas y Comunidades Campesinas Equiparables de México. In Milpa, Pueblos de Maíz, Diversidad y Patrimonio Biocultural de México; Secretaría de Cultura: Mexico City, Mexico, 2021. [Google Scholar]
- Casagrande, M.; Alletto, L.; Naudin, C.; Lenoir, A.; Siah, A.; Celette, F. Enhancing Planned and Associated Biodiversity in French Farming Systems. Agron. Sustain. Dev. 2017, 37, 57. [Google Scholar] [CrossRef]
- Fonteyne, S.; Caamal, J.B.C.; Lopez-Ridaura, S.; Van Loon, J.; Balbuena, J.E.; Alcalá, L.O.; Hernández, F.M.; Odjo, S.; Verhulst, N. Review of Agronomic Research on the Milpa, the Traditional Polyculture System of Mesoamerica. Front. Agron. 2023, 5, 1115490. [Google Scholar] [CrossRef]
- Cuanalo-de la Cerda, H.E. Resultados de la investigación participativa en la Milpa Sin Quema. Rev. Terra Latinoam. 2006, 24, 401. [Google Scholar]
- Ortega-Paczka, R. Variedades y Razas Mexicanas de Maíz y Su Evaluación En Cruzamientos Con Líneas de Clima Templado Como Material de Partida Para Mejoramiento. Ph.D. Dissertation, N.I. Vavilov Plant Institute, St. Petersburg, Russia, 1985. [Google Scholar]
- Goodman, M.M.; Moreno, J.; Castillo, F.; Holley, R.N.; Carson, M.L. Using Tropical Maize Germplasm for Temperate Breeding. Maydica 2000, 45, 221–234. [Google Scholar]
- Tsai, S.M.; Da Silva, P.M.; Cabezas, W.L.; Bonetti, R. Variability in Nitrogen Fixation of Common Bean (Phaseolus vulgaris L.) Intercropped with Maize. Plant Soil 1993, 152, 93–101. [Google Scholar] [CrossRef]
- Martínez-Romero, E. Diversity of Rhizobium-Phaseolus vulgaris Symbiosis: Overview and Perspectives. Plant Soil 2003, 252, 11–23. [Google Scholar] [CrossRef]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.-L. Plant Biodiversity Promotes Sustainable Agriculture Directly and via Belowground Effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef]
- Veen, G.F.; Wubs, E.R.J.; Bardgett, R.D.; Barrios, E.; Bradford, M.A.; Carvalho, S.; De Deyn, G.B.; de Vries, F.T.; Giller, K.E.; Kleijn, D.; et al. Applying the Aboveground-Belowground Interaction Concept in Agriculture: Spatio-Temporal Scales Matter. Front. Ecol. Evol. 2019, 7, 300. [Google Scholar] [CrossRef]
- Ávila-Bello, C.H.; Hernández-Romero, Á.H.; Mendoza-Briseño, M.A.; Vázquez-Luna, D. Complex Systems, Agroecological Matrices, and Management of Forest Resources: An Example of an Application in Los Tuxtlas, Veracruz, Mexico. Sustainability 2018, 10, 3496. [Google Scholar] [CrossRef]
- Benitez, M.; Fornoni, J.; García-Barrios, L.; López, R. Frontiers in Ecology, Evolution and Complexity. In Frontiers in Ecology, Evolution and Complexity; Copit-arXive: Mexico City, Mexico, 2014; pp. 64–77. [Google Scholar]
- Mexican Government. Programa Sembrando Vida. Available online: https://www.gob.mx/bienestar/acciones-y-programas/programa-sembrando-vida (accessed on 20 August 2024).
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Hu, H.-W.; He, Z.-Y.; Cui, L.; Zhu, Y.-G.; He, J.-Z. Potential of Indigenous Crop Microbiomes for Sustainable Agriculture. Nat. Food 2021, 2, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sangabriel-Conde, W.; Maldonado-Mendoza, I.E.; Mancera-López, M.E.; Cordero-Ramírez, J.D.; Trejo-Aguilar, D.; Negrete-Yankelevich, S. Glomeromycota Associated with Mexican Native Maize Landraces in Los Tuxtlas, Mexico. Appl. Soil Ecol. 2015, 87, 63–71. [Google Scholar] [CrossRef]
- Higdon, S.M.; Pozzo, T.; Kong, N.; Huang, B.C.; Yang, M.L.; Jeannotte, R.; Brown, C.T.; Bennett, A.B.; Weimer, B.C.; Chen, J.-T. Genomic Characterization of a Diazotrophic Microbiota Associated with Maize Aerial Root Mucilage. PLoS ONE 2020, 15, e0239677. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen Fixation in a Landrace of Maize Is Supported by a Mucilage-Associated Diazotrophic Microbiota. PLOS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef]
- Bennett, A.B.; Pankievicz, V.C.S.; Ané, J.-M. A Model for Nitrogen Fixation in Cereal Crops. Trends Plant Sci. 2020, 25, 226–235. [Google Scholar] [CrossRef]
- Donald, C.M. The Breeding of Crop Ideotypes. Euphytica 1968, 17, 385–403. [Google Scholar] [CrossRef]
- Fréville, H.; Montazeaud, G.; Forst, E.; David, J.; Papa, R.; Tenaillon, M.I. Shift in Beneficial Interactions During Crop Evolution. Evol. Appl. 2022, 15, 905–918. [Google Scholar] [CrossRef]
- Biernaskie, J.M. Kin Selection Theory and the Design of Cooperative Crops. Evol. Appl. 2022, 15, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Griffing, B. Selection in Reference to Biological Groups. VI. Use of Extreme Forms of Nonrandom Groups to Increase Selection Efficiency. Genetics 1976, 82, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J. Selection for Improved Yield in Inter-Specific Mixtures or Intercrops. Theor. Appl. Genet. 1985, 69, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Haug, B.; Messmer, M.M.; Enjalbert, J.; Goldringer, I.; Forst, E.; Flutre, T.; Mary-Huard, T.; Hohmann, P. Advances in Breeding for Mixed Cropping—Incomplete Factorials and the Producer/Associate Concept. Front. Plant Sci. 2021, 11, 620400. [Google Scholar] [CrossRef] [PubMed]
- Firmat, C.; Litrico, I. Linking Quantitative Genetics with Community-Level Performance: Are There Operational Models for Plant Breeding? Front. Plant Sci. 2022, 13, 733996. [Google Scholar] [CrossRef]
- Bančič, J.; Werner, C.R.; Gaynor, R.C.; Gorjanc, G.; Odeny, D.A.; Ojulong, H.F.; Dawson, I.K.; Hoad, S.P.; Hickey, J.M. Modeling Illustrates That Genomic Selection Provides New Opportunities for Intercrop Breeding. Front. Plant Sci. 2021, 12, 605172. [Google Scholar] [CrossRef]
- Santamarina, C.; Mathieu, L.; Bitocchi, E.; Pieri, A.; Bellucci, E.; Di Vittori, V.; Susek, K.; Scossa, F.; Nanni, L.; Papa, R. Agroecological Genomics and Participatory Science: Optimizing Crop Mixtures for Agricultural Diversification. Trends Plant Sci. 2025. [Google Scholar] [CrossRef]



| Traits Evaluated | pLER Common Bean | pLER Maize | tLER (Intercrop) | Reference |
|---|---|---|---|---|
| Variety, spatial arrangement, inoculation | 7.5 | 0.68 | 3.09 | [278] |
| Climbing genotypes: maize varieties | 18 | 17.53 | 14.24 | [293] |
| Agro-ecological zone | 9.9 | 17.4 | 11.1 | [280] |
| Bean variety | 5.6 | 14.5 | 5.8 | [280] |
| CV | 10.25 | 12.52 | 8.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Cruz, C.; Casas, A.; Ortega-Paczka, R.; Perales, H.; Vega-Peña, E.; Bye, R. Milpa, a Long-Standing Polyculture for Sustainable Agriculture. Agriculture 2025, 15, 1737. https://doi.org/10.3390/agriculture15161737
Mota-Cruz C, Casas A, Ortega-Paczka R, Perales H, Vega-Peña E, Bye R. Milpa, a Long-Standing Polyculture for Sustainable Agriculture. Agriculture. 2025; 15(16):1737. https://doi.org/10.3390/agriculture15161737
Chicago/Turabian StyleMota-Cruz, Cecilio, Alejandro Casas, Rafael Ortega-Paczka, Hugo Perales, Ernesto Vega-Peña, and Robert Bye. 2025. "Milpa, a Long-Standing Polyculture for Sustainable Agriculture" Agriculture 15, no. 16: 1737. https://doi.org/10.3390/agriculture15161737
APA StyleMota-Cruz, C., Casas, A., Ortega-Paczka, R., Perales, H., Vega-Peña, E., & Bye, R. (2025). Milpa, a Long-Standing Polyculture for Sustainable Agriculture. Agriculture, 15(16), 1737. https://doi.org/10.3390/agriculture15161737







