Integration of Hyperspectral Imaging and Chemometrics for Internal Quality Evaluation of Packaged and Non-Packaged Fresh Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
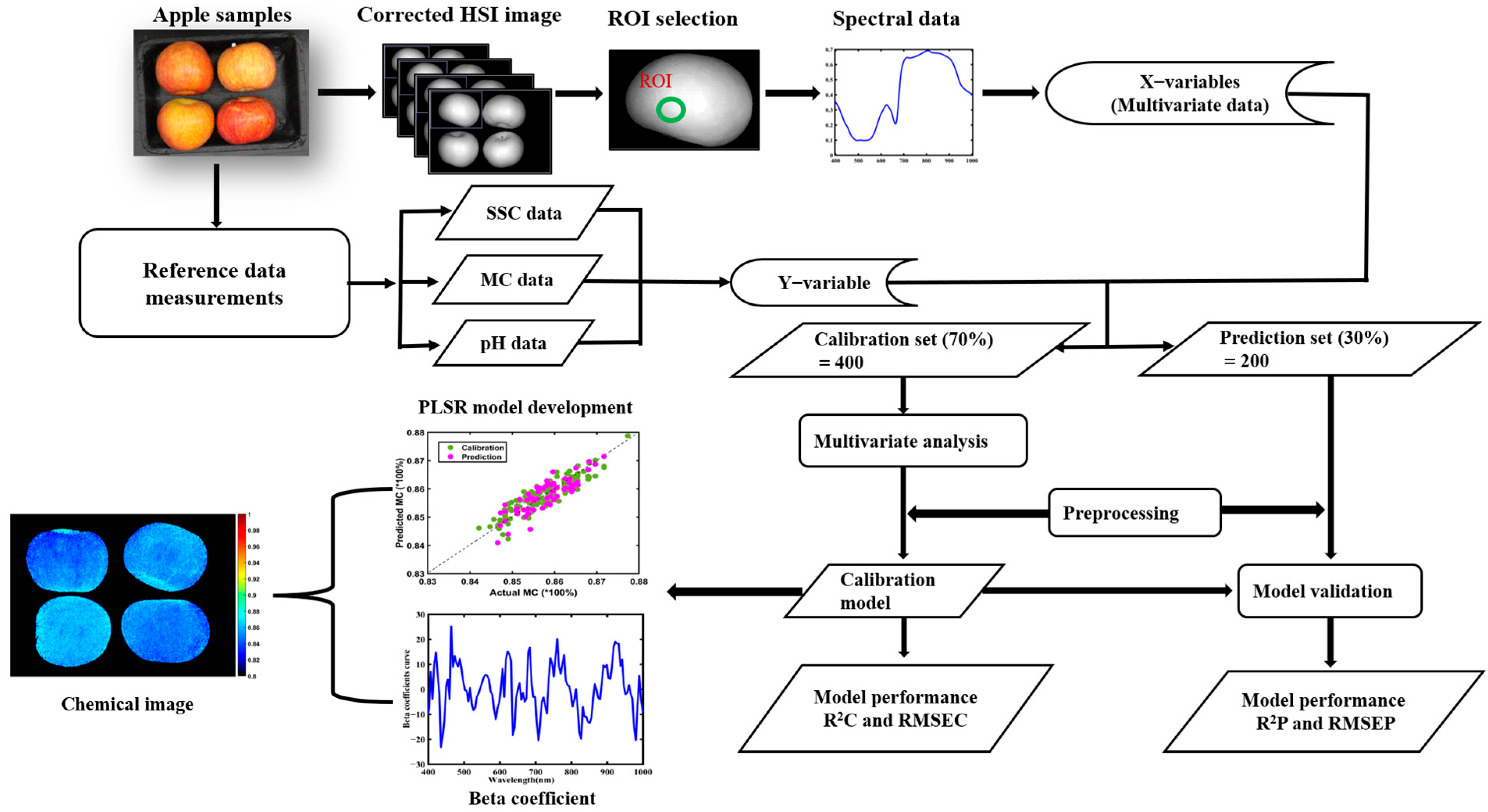
2.2. Hyperspectral Imaging System
2.2.1. Experimental Configuration of the HSI System
2.2.2. HSI Image Acquisition and Data Extraction
2.3. Reference Value Measurements
2.4. Data Processing and Chemometric Analysis
2.5. Chemical Image Development
3. Results
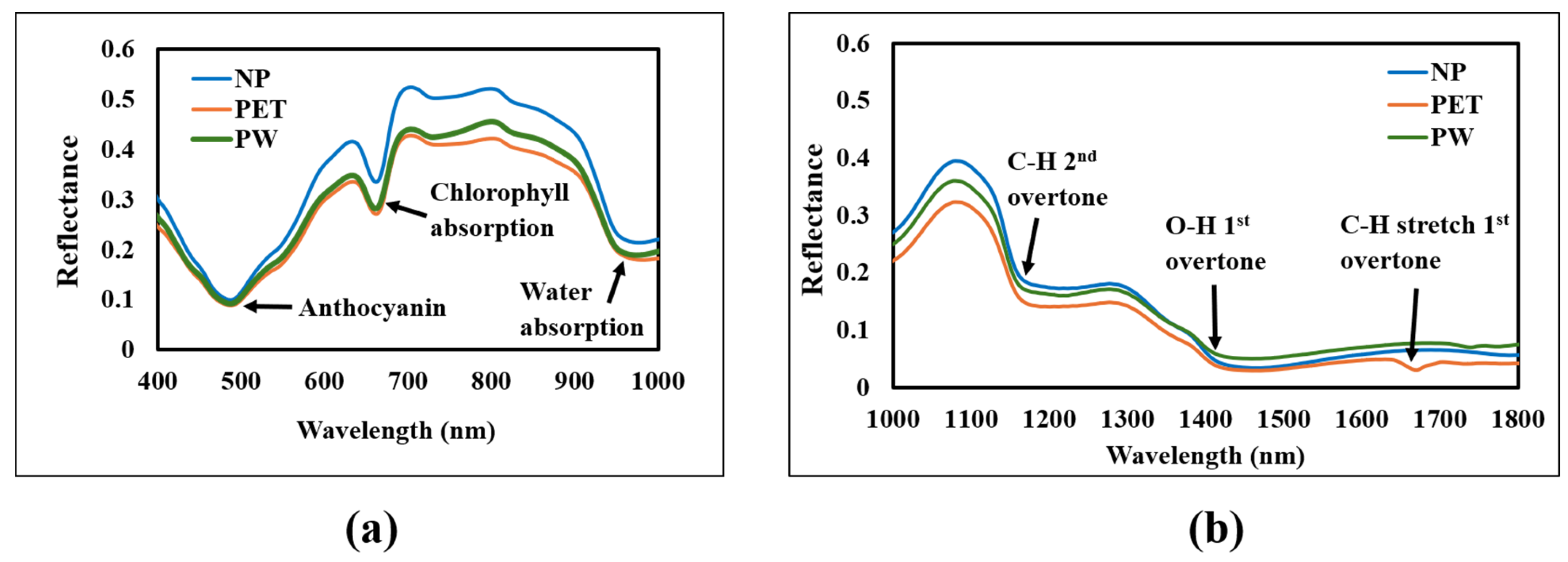
3.1. Package Spectral Characteristics
3.2. Reference Value Results
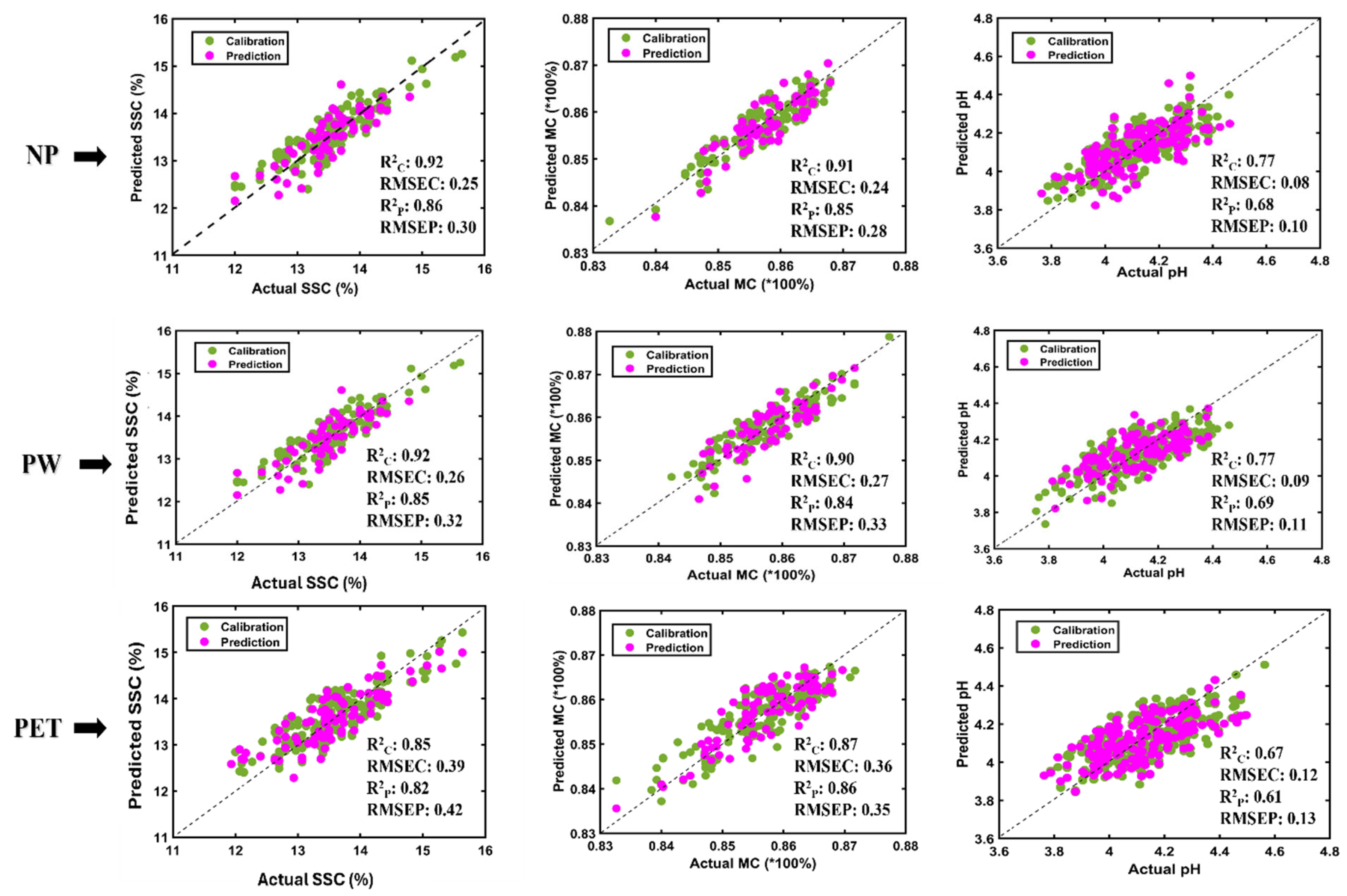
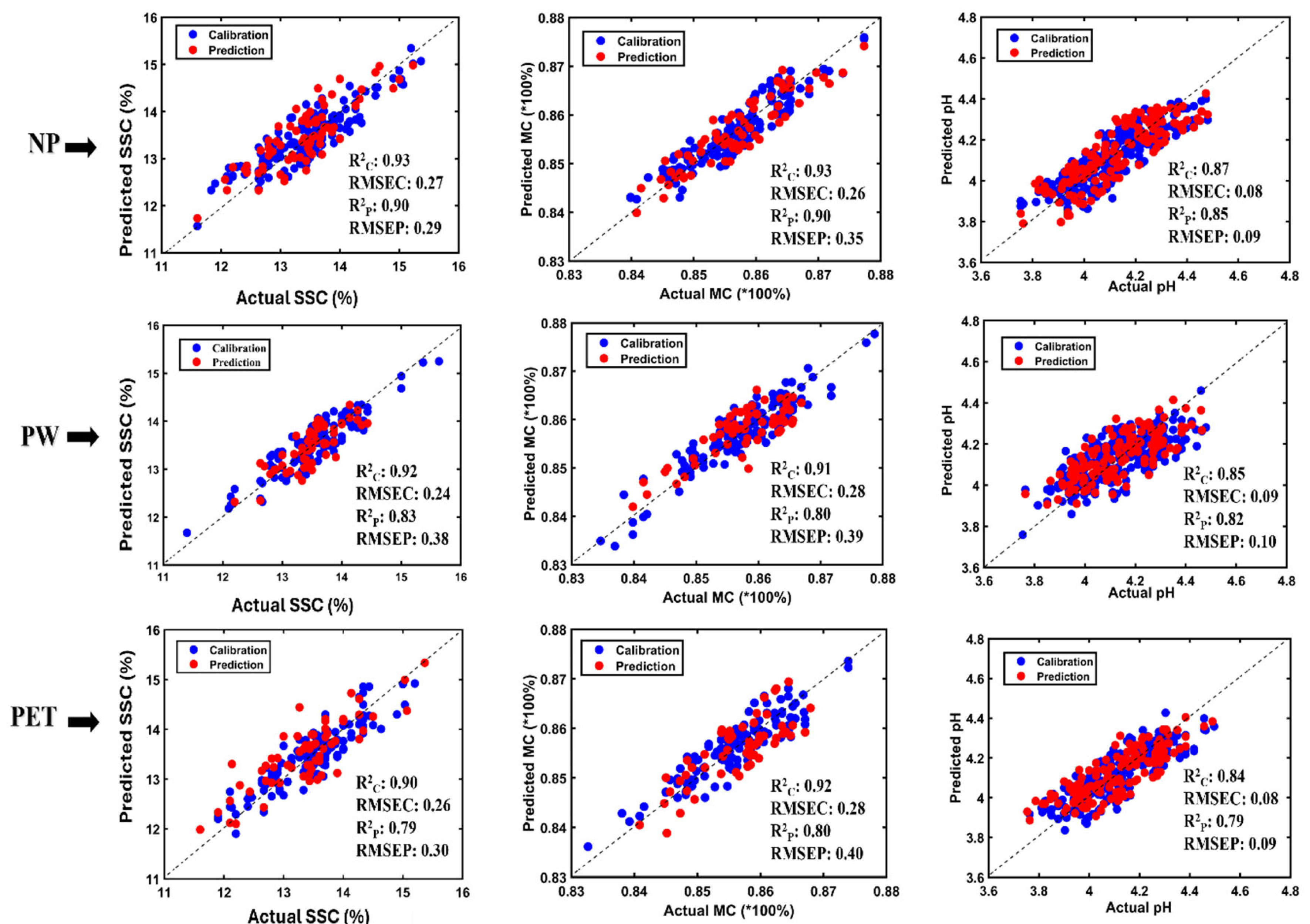
3.3. Partial Least Square Regression (PLSR) Model
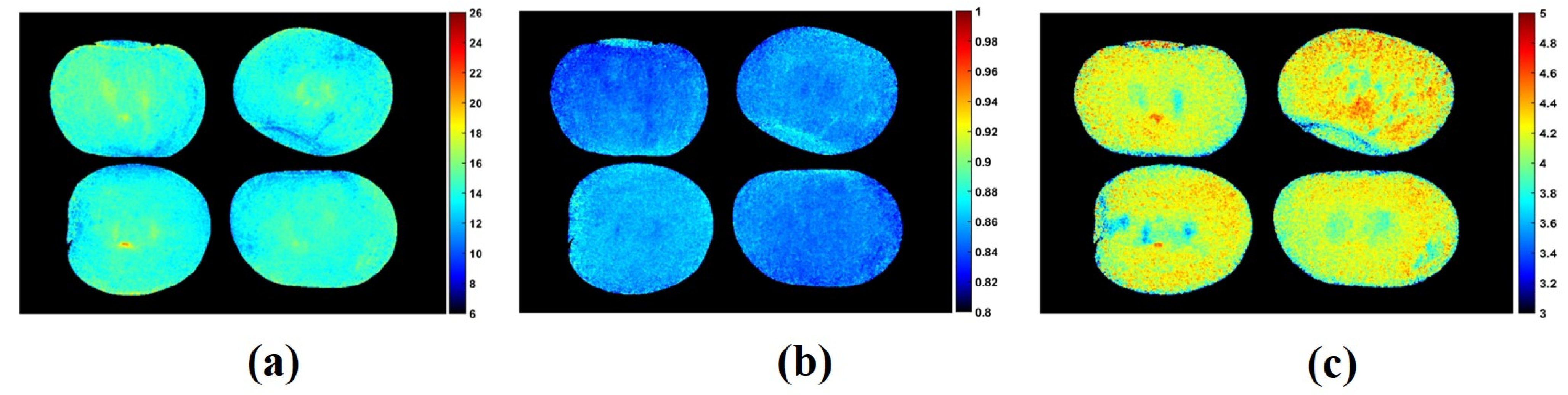
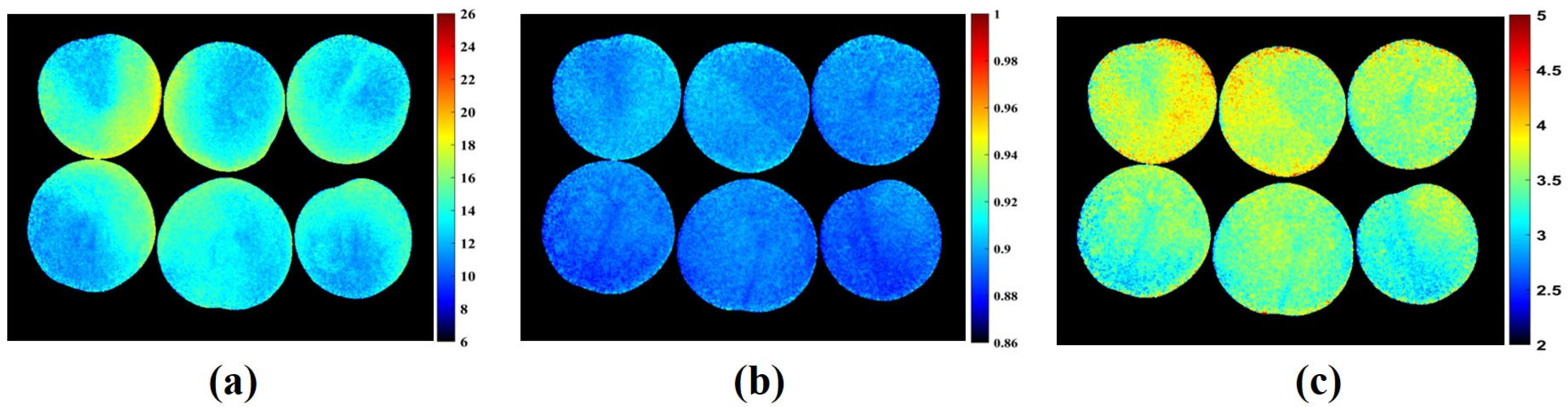
3.4. Chemical Visualization and Mapping of Apple and Plum Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Jamal Deen, M. Fruit Quality Monitoring with Smart Packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and Challenges in the Development of Bio-Based Barrier Coating Materials for Paper/Cardboard Food Packaging; a Review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.D.; Hamam, Y.; Sadiku, E.R.; Ndambuki, J.M.; Kupolati, W.K.; Jamiru, T.; Eze, A.A.; Snyman, J. Need for Sustainable Packaging: An Overview. Polymers 2022, 14, 4430. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Montalvo, L. Repurposing Waste Plastics into Cleaner Asphalt Pavement Materials: A Critical Literature Review. J. Clean. Prod. 2021, 280, 124355. [Google Scholar] [CrossRef]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A Comprehensive Review of Natural Fibers and Their Composites: An Eco-Friendly Alternative to Conventional Materials. Results Eng. 2023, 19, 101271. [Google Scholar] [CrossRef]
- Sarkar, S.; Aparna, K. Research Trends in Home Science and Extension; AkiNik Publications: New Delhi, India, 2020. [Google Scholar] [CrossRef]
- He, X.; Pu, Y.; Chen, L.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. A Comprehensive Review of Intelligent Packaging for Fruits and Vegetables: Target Responders, Classification, Applications, and Future Challenges. Compr. Rev. Food Sci. Food Saf. 2023, 22, 842–881. [Google Scholar] [CrossRef]
- Dirpan, A.; Djalal, M.; Kamaruddin, I. Application of an Intelligent Sensor and Active Packaging System Based on the Bacterial Cellulose of Acetobacter Xylinum to Meat Products. Sensors 2022, 22, 544. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yangb, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-Destructive Monitoring of Apple Ripeness Using an Aldehyde Sensitive.Pdf. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Keshri, N.; Truppel, I.; Herppich, W.B.; Geyer, M.; Weltzien, C.; Mahajan, P.V. In-Situ Measurement of Fresh Produce Respiration Using a Modular Sensor-Based System. Sensors 2020, 20, 3589. [Google Scholar] [CrossRef]
- Adiani, V.; Gupta, S.; Variyar, P.S. A Simple Time Temperature Indicator for Real Time Microbial Assessment in Minimally Processed Fruits. J. Food Eng. 2021, 311, 110731. [Google Scholar] [CrossRef]
- Shao, P.; Liu, L.; Yu, J.; Zheng, L.; Sun, P. Novel Aldehyde Sensitive Bio-Based Colorimetric Film for Kiwi Fruit Freshness Monitoring. LWT 2022, 159, 113177. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food Packaging’s Materials: A Food Safety Perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Wang, B.; Jianfei, S.; Lianming, X.; Junjie, L.; Zhenhe, W.; Pei, L.; Yemin, G.; Sun, X. The Applications of Hyperspectral Imaging Technology for Agricultural Products Quality Analysis: A Review. Food Rev. Int. 2023, 39, 1043–1062. [Google Scholar] [CrossRef]
- Aline, U.; Bhattacharya, T.; Faqeerzada, M.A.; Kim, M.S.; Baek, I.; Cho, B.-K. Advancement of Non-Destructive Spectral Measurements for the Quality of Major Tropical Fruits and Vegetables: A Review. Front. Plant Sci. 2023, 14, 1240361. [Google Scholar] [CrossRef]
- Park, S.J.; Yoon, H.I.; Lee, H.; Kim, M.; Yang, J.; Jung, D.; Ahn, J.Y.; Park, S.H. Evaluating the Accuracy of Machine Learning Classification Models for Similar Herbal Medicine Using Hyperspectral Imaging. J. Biosyst. Eng. 2024, 49, 156–166. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, Z.; Cheng, X.; Cai, X.; Cao, M.; Guo, W. Prediction of Soluble Solids Content Using Near-Infrared Spectra and Optical Properties of Intact Apple and Pulp Applying PLSR and CNN. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123402. [Google Scholar] [CrossRef] [PubMed]
- Semyalo, D.; Kim, Y.; Omia, E.; Arief, M.A.A.; Kim, H.; Sim, E.-Y.; Kim, M.S.; Baek, I.; Cho, B.-K. Nondestructive Identification of Internal Potato Defects Using Visible and Short-Wavelength Near-Infrared Spectral Analysis. Agriculture 2024, 14, 2014. [Google Scholar] [CrossRef]
- Kaparapu, J.; Pragada, P.M.; Geddada, M.N.R. Fruits and Vegetables and Its Nutritional Benefits BT. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 241–260. ISBN 978-3-030-42319-3. [Google Scholar]
- Tian, Y.; Sun, J.; Zhou, X.; Yao, K.; Tang, N. Detection of Soluble Solid Content in Apples Based on Hyperspectral Technology Combined with Deep Learning Algorithm. J. Food Process. Preserv. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Kavuncuoğlu, E.; Çetin, N.; Yildirim, B.; Nadimi, M.; Paliwal, J. Exploration of Machine Learning Algorithms for PH and Moisture Estimation in Apples Using VIS-NIR Imaging. Appl. Sci. 2023, 13, 8391. [Google Scholar] [CrossRef]
- Li, B.; Cobo-Medina, M.; Lecourt, J.; Harrison, N.B.; Harrison, R.J.; Cross, J.V. Application of Hyperspectral Imaging for Nondestructive Measurement of Plum Quality Attributes. Postharvest Biol. Technol. 2018, 141, 8–15. [Google Scholar] [CrossRef]
- Siche, R.; Vejarano, R.; Aredo, V.; Velasquez, L.; Saldaña, E.; Quevedo, R. Evaluation of Food Quality and Safety with Hyperspectral Imaging (HSI). Food Eng. Rev. 2016, 8, 306–322. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D.W. Hyperspectral Imaging Technique for Evaluating Food Quality and Safety during Various Processes: A Review of Recent Applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Lan, W.; Jaillais, B.; Renard, C.M.G.C.; Leca, A.; Chen, S.; Le Bourvellec, C.; Bureau, S. A Method Using near Infrared Hyperspectral Imaging to Highlight the Internal Quality of Apple Fruit Slices. Postharvest Biol. Technol. 2021, 175, 111497. [Google Scholar] [CrossRef]
- Metlenkin, D.A.; Platov, Y.T.; Platova, R.A.; Zhirkova, E.V.; Teneva, O.T. Non-Destructive Identification of Defects and Classification of Hass Avocado Fruits with the Use of a Hyperspectral Image. Agron. Res. 2022, 20, 326–340. [Google Scholar] [CrossRef]
- Mishra, P.; Chauhan, A.; Pettersson, T. Seeing through Plastics: A Novel Combination of NIR Hyperspectral Imaging and Spectral Orthogonalization for Detecting Fresh Fruit inside Plastic Packaging to Support Automated Barcode Less Checkouts in Supermarkets. Food Control 2023, 150, 109762. [Google Scholar] [CrossRef]
- Kharbach, M.; Alaoui Mansouri, M.; Taabouz, M.; Yu, H. Current Application of Advancing Spectroscopy Techniques in Food Analysis: Data Handling with Chemometric Approaches. Foods 2023, 12, 2753. [Google Scholar] [CrossRef]
- Burnett, A.C.; Anderson, J.; Davidson, K.J.; Ely, K.S.; Lamour, J.; Li, Q.; Morrison, B.D.; Yang, D.; Rogers, A.; Serbin, S.P. A Best-Practice Guide to Predicting Plant Traits from Leaf-Level Hyperspectral Data Using Partial Least Squares Regression. J. Exp. Bot. 2021, 72, 6175–6189. [Google Scholar] [CrossRef]
- Yu, W.; Lee, S.-J.; Cho, H. Partial Least Squares Regression Trees for Multivariate Response Data with Multicollinear Predictors. IEEE Access 2024, 12, 36636–36644. [Google Scholar] [CrossRef]
- Shawky, E.; Selim, D.A. NIR Spectroscopy-Multivariate Analysis for Discrimination and Bioactive Compounds Prediction of Different Citrus Species Peels. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 1–7. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, H.; Feng, Q.; Xu, L.; Lin, Q.; Cai, K. Rapid Detection of Pomelo Fruit Quality Using Near-Infrared Hyperspectral Imaging Combined With Chemometric Methods. Front. Bioeng. Biotechnol. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, P.; Niu, T.; He, D.; Wang, M.; Yang, H.; Zhao, X. Soluble Solid Content and Firmness Index Assessment and Maturity. Food Chem. 2022, 370, 131013. [Google Scholar] [CrossRef]
- Hapuarachchi, N.S.; Trueman, S.J.; Kämper, W.; Farrar, M.B.; Wallace, H.M.; Nichols, J.; Bai, S.H. Hyperspectral Imaging of Adaxial and Abaxial Leaf Surfaces for Rapid Assessment of Foliar Nutrient Concentrations in Hass Avocado. Remote Sens. 2023, 15, 3100. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J. Advanced Chemometrics toward Robust Spectral Analysis for Fruit Quality Evaluation. Trends Food Sci. Technol. 2024, 150, 104612. [Google Scholar] [CrossRef]
- Tunny, S.S.; Kurniawan, H.; Amanah, H.Z.; Baek, I.; Kim, M.S.; Chan, D.; Faqeerzada, M.A.; Wakholi, C.; Cho, B.K. Hyperspectral Imaging Techniques for Detection of Foreign Materials from Fresh-Cut Vegetables. Postharvest Biol. Technol. 2023, 201, 112373. [Google Scholar] [CrossRef]
- De Oliveira, T.S.; Costa, A.M.M.; Cabral Correa, L.M.; Freitas-Silva, O.; Tonon, R.V. Physical and Biological Properties of Alginate-Based Cinnamon Essential Oil. Int. J. Biol. Macromol. 2024, 275, 133627. [Google Scholar] [CrossRef] [PubMed]
- Semyalo, D.; Kwon, O.; Wakholi, C.; Min, H.J.; Cho, B.K. Nondestructive Online Measurement of Pineapple Maturity and Soluble Solids Content Using Visible and Near-Infrared Spectral Analysis. Postharvest Biol. Technol. 2024, 209, 112706. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Z.; Wang, M.; Zhao, L.; Yu, Z.; Xia, C.; Huang, X. Leaching Kinetic Study of Sulfuric Acid Roasted Mixed-Type Rare Earth Concentrate for Reducing the Solid-Waste Production and Chemical Consumption. J. Clean. Prod. 2020, 260, 120989. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effects of Nonthermal Plasma Treatment on Decontamination and Sprouting of Radish (Raphanus Sativus L.) Seeds. Food Bioprocess Technol. 2017, 10, 1093–1102. [Google Scholar] [CrossRef]
- Rahman, A.; Kandpal, L.M.; Lohumi, S.; Kim, M.S.; Lee, H.; Mo, C.; Cho, B.K. Nondestructive Estimation of Moisture Content, PH and Soluble Solid Contents in Intact Tomatoes Using Hyperspectral Imaging. Appl. Sci. 2017, 7, 109. [Google Scholar] [CrossRef]
- Morais, C.L.M.; Paraskevaidi, M.; Cui, L.; Fullwood, N.J.; Isabelle, M.; Lima, K.M.G.; Martin-Hirsch, P.L.; Sreedhar, H.; Trevisan, J.; Walsh, M.J. Standardization of Complex Biologically Derived Spectrochemical Datasets. Nat. Protoc. 2019, 14, 1546–1577. [Google Scholar] [CrossRef]
- Dorrepaal, R.; Malegori, C.; Gowen, A. Tutorial: Time Series Hyperspectral Image Analysis. J. Near Infrared Spectrosc. 2016, 24, 89–107. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Amanah, H.Z.; Wakholi, C.; Perez, M.; Faqeerzada, M.A.; Tunny, S.S.; Masithoh, R.E.; Choung, M.G.; Kim, K.H.; Lee, W.H.; Cho, B.K. Near-Infrared Hyperspectral Imaging (Nir-Hsi) for Nondestructive Prediction of Anthocyanins Content in Black Rice Seeds. Appl. Sci. 2021, 11, 4841. [Google Scholar] [CrossRef]
- Aulia, R.; Kim, Y.; Amanah, H.; Andi, A.; Kim, H.; Kim, H.; Lee, W.-H.; Kim, K.-H.; Baek, J.-H.; Cho, B.-K. Non-Destructive Prediction of Protein Contents of Soybean Seeds Using Near-Infrared Hyperspectral Imaging. Infrared Phys. Technol. 2022, 127, 104365. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Zuo, C.; Jiang, M.; Song, J.; Ding, F.; Tu, K.; Lan, W.; Pan, L. Exploring the Variability and Heterogeneity of Apple Firmness Using Visible and Near-Infrared Hyperspectral Imaging. LWT 2024, 192, 115704. [Google Scholar] [CrossRef]
- Çetin, N.; Karaman, K.; Kavuncuoğlu, E.; Yıldırım, B.; Jahanbakhshi, A. Using Hyperspectral Imaging Technology and Machine Learning Algorithms for Assessing Internal Quality Parameters of Apple Fruits. Chemom. Intell. Lab. Syst. 2022, 230, 104650. [Google Scholar] [CrossRef]
- Castillo-Girones, S.; Van Belleghem, R.; Wouters, N.; Munera, S.; Blasco, J.; Saeys, W. Detection of Subsurface Bruises in Plums Using Spectral Imaging and Deep Learning with Wavelength Selection. Postharvest Biol. Technol. 2024, 207, 112615. [Google Scholar] [CrossRef]
- Horbowicz, M.; Grzesiuk, A.; DĘBski, H.; Kosson, R. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human. Veg. Crop. Res. Bull. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Knaeps, E.; Sterckx, S.; Garaba, S.; Meire, D. Spectral Reflectance of Marine Macroplastics in the VNIR and SWIR Measured in a Controlled Environment. Sci. Rep. 2021, 11, 5436. [Google Scholar] [CrossRef]
- Chen, X.; Kroell, N.; Althaus, M.; Pretz, T.; Pomberger, R.; Greiff, K. Enabling Mechanical Recycling of Plastic Bottles with Shrink Sleeves through Near-Infrared Spectroscopy and Machine Learning Algorithms. Resour. Conserv. Recycl. 2023, 188, 106719. [Google Scholar] [CrossRef]
- Alassali, A.; Fiore, S.; Kuchta, K. Assessment of Plastic Waste Materials Degradation through near Infrared Spectroscopy. Waste Manag. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Tiecher, T.; Moura-Bueno, J.M.; Caner, L.; Minella, J.P.G.; Evrard, O.; Ramon, R.; Naibo, G.; Barros, C.A.P.; Silva, Y.J.A.B.; Amorim, F.F. Improving the Quantification of Sediment Source Contributions Using Different Mathematical Models and Spectral Preprocessing Techniques for Individual or Combined Spectra of Ultraviolet–Visible, near-and Middle-Infrared Spectroscopy. Geoderma 2021, 384, 114815. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Donnell, C.P.; Esquerre, C.; Downey, G. Influence of Polymer Packaging Films on Hyperspectral Imaging Data in the Visible–near-Infrared (450–950 Nm) Wavelength Range. Appl. Spectrosc. 2010, 64, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Koinig, G.; Friedrich, K.; Rutrecht, B.; Oreski, G.; Barretta, C.; Vollprecht, D. Influence of Reflective Materials, Emitter Intensity and Foil Thickness on the Variability of near-Infrared Spectra of 2D Plastic Packaging Materials. Waste Manag. 2022, 144, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pan, L.; Lu, L. Prediction of TVB-N Content in Beef with Packaging Films Using Visible-near Infrared Hyperspectral Imaging. Food Control 2023, 147, 109562. [Google Scholar] [CrossRef]
- Pourdarbani, R.; Sabzi, S.; Arribas, J.I. Nondestructive Estimation of Three Apple Fruit Properties at Various Ripening Levels with Optimal Vis-NIR Spectral Wavelength Regression Data. Heliyon 2021, 7, e07942. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D.; Khoshtaghaza, M.H. Rapid Measurement of Apple Quality Parameters Using Wavelet De-Noising Transform with Vis/NIR Analysis. Sci. Hortic. 2019, 252, 7–13. [Google Scholar] [CrossRef]
- Mehrubeoglu, M.; Van Sickle, A.; Turner, J. Detection and Identification of Plastics Using SWIR Hyperspectral Imaging. In Imaging Spectrometry XXIV: Applications, Sensors, and Processing; SPIE: Bellingham, WA, USA, 2020; Volume 11504, pp. 85–95. [Google Scholar]
- Xin, Z.; Ju, S.; Zhang, D.; Zhou, X.-G.; Guo, S.; Pan, Z.; Wang, L.; Cheng, T. Construction of Spectral Detection Models to Evaluate Soluble Solids Content and Acidity in Dangshan Pear Using Two Different Sensors. Infrared Phys. Technol. 2023, 131, 104632. [Google Scholar] [CrossRef]
- Nguyen Van, L.; Lee, G. Optimizing Stacked Ensemble Machine Learning Models for Accurate Wildfire Severity Mapping. Remote Sens. 2025, 17, 854. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Kim, Y.-N.; Kim, H.; Akter, T.; Kim, H.; Park, M.-S.; Kim, M.S.; Baek, I.; Cho, B.-K. Hyperspectral Imaging System for Pre-and Post-Harvest Defect Detection in Paprika Fruit. Postharvest Biol. Technol. 2024, 218, 113151. [Google Scholar] [CrossRef]
- Signoroni, A.; Conte, M.; Plutino, A.; Rizzi, A. Spatial–Spectral Evidence of Glare Influence on Hyperspectral Acquisitions. Sensors 2020, 20, 4374. [Google Scholar] [CrossRef]




| System Components | HSI Vis-NIR | HSI SWIR |
|---|---|---|
| Light source | 100 Watt-Quartz tungsten halogen (QTH) line light. | 100 Watt-Quartz tungsten halogen (QTH) line light (SWIR via quartz fiber bundles). |
| Imaging spectrograph | (Vis-NIR, Headwall Photonics, Fitchburg, MA, USA) with 400–1000 nm wavelength range, and 4.7 nm spectral resolution. | (SWIR, Headwall Photonics, Fitchburg, MA, USA) with 1000–2500 nm wavelength range, and 5.9 nm spectral resolution. |
| Image sensors | Electron multiplying charge-coupled device (EMCCD) with pixels: 1004 × 1002 (Spatial × Spectral channels). | A mercury cadmium telluride (MCT; HgCdTe) with Pixels: 320 × 256 (Spatial × Spectral channels). |
| Objective lens | Focal length: 23 mm, f/1.4 | Focal length: 25 mm, f/1.4 |
| Fruit | Parameters | Sample Numbers | Mean ± SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Apple | SSC (%) | 200 | 13.49 ± 1.20 | 11.03 | 15.80 |
| MC (%) | 200 | 86 ± 2 | 80 | 91 | |
| Ph | 200 | 4.14 ± 0.20 | 3.74 | 4.75 | |
| Plum | SSC (%) | 200 | 10.52 ± 1.64 | 7 | 14 |
| MC (%) | 200 | 90 ± 2 | 87 | 93 | |
| pH | 200 | 3.48 ± 0.16 | 3.55 | 3.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aline, U.; Semyalo, D.; Pahlawan, M.F.R.; Akter, T.; Faqeerzada, M.A.; Kim, S.-Y.; Oh, D.; Cho, B.-K. Integration of Hyperspectral Imaging and Chemometrics for Internal Quality Evaluation of Packaged and Non-Packaged Fresh Fruits. Agriculture 2025, 15, 1718. https://doi.org/10.3390/agriculture15161718
Aline U, Semyalo D, Pahlawan MFR, Akter T, Faqeerzada MA, Kim S-Y, Oh D, Cho B-K. Integration of Hyperspectral Imaging and Chemometrics for Internal Quality Evaluation of Packaged and Non-Packaged Fresh Fruits. Agriculture. 2025; 15(16):1718. https://doi.org/10.3390/agriculture15161718
Chicago/Turabian StyleAline, Umuhoza, Dennis Semyalo, Muhammad Fahri Reza Pahlawan, Tanjima Akter, Mohammad Akbar Faqeerzada, Seo-Young Kim, Dayoung Oh, and Byoung-Kwan Cho. 2025. "Integration of Hyperspectral Imaging and Chemometrics for Internal Quality Evaluation of Packaged and Non-Packaged Fresh Fruits" Agriculture 15, no. 16: 1718. https://doi.org/10.3390/agriculture15161718
APA StyleAline, U., Semyalo, D., Pahlawan, M. F. R., Akter, T., Faqeerzada, M. A., Kim, S.-Y., Oh, D., & Cho, B.-K. (2025). Integration of Hyperspectral Imaging and Chemometrics for Internal Quality Evaluation of Packaged and Non-Packaged Fresh Fruits. Agriculture, 15(16), 1718. https://doi.org/10.3390/agriculture15161718









