Pollination Deficit: A Key Limitation of Fruit Set in Northward-Expanded Camellia Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
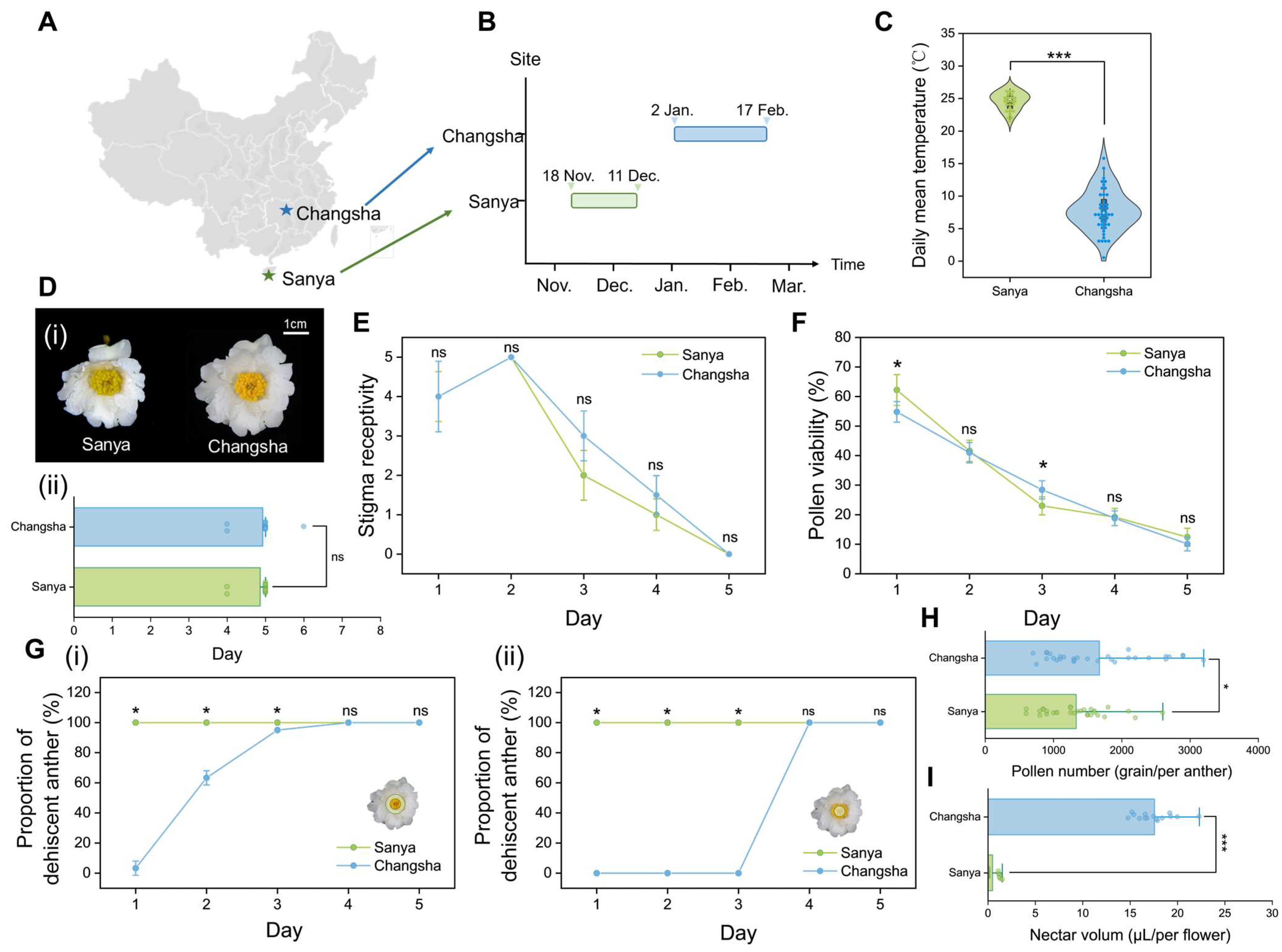
2.2. Cultivation Site Environment
2.3. Anthesis and Temperature During Anthesis
2.4. Flower Longevity Recording
2.5. Observation of Anther Dehiscence
2.6. Pollen Viability and Stigma Receptivity Assays
2.7. Pollen Number and Nectar Volume Quantification
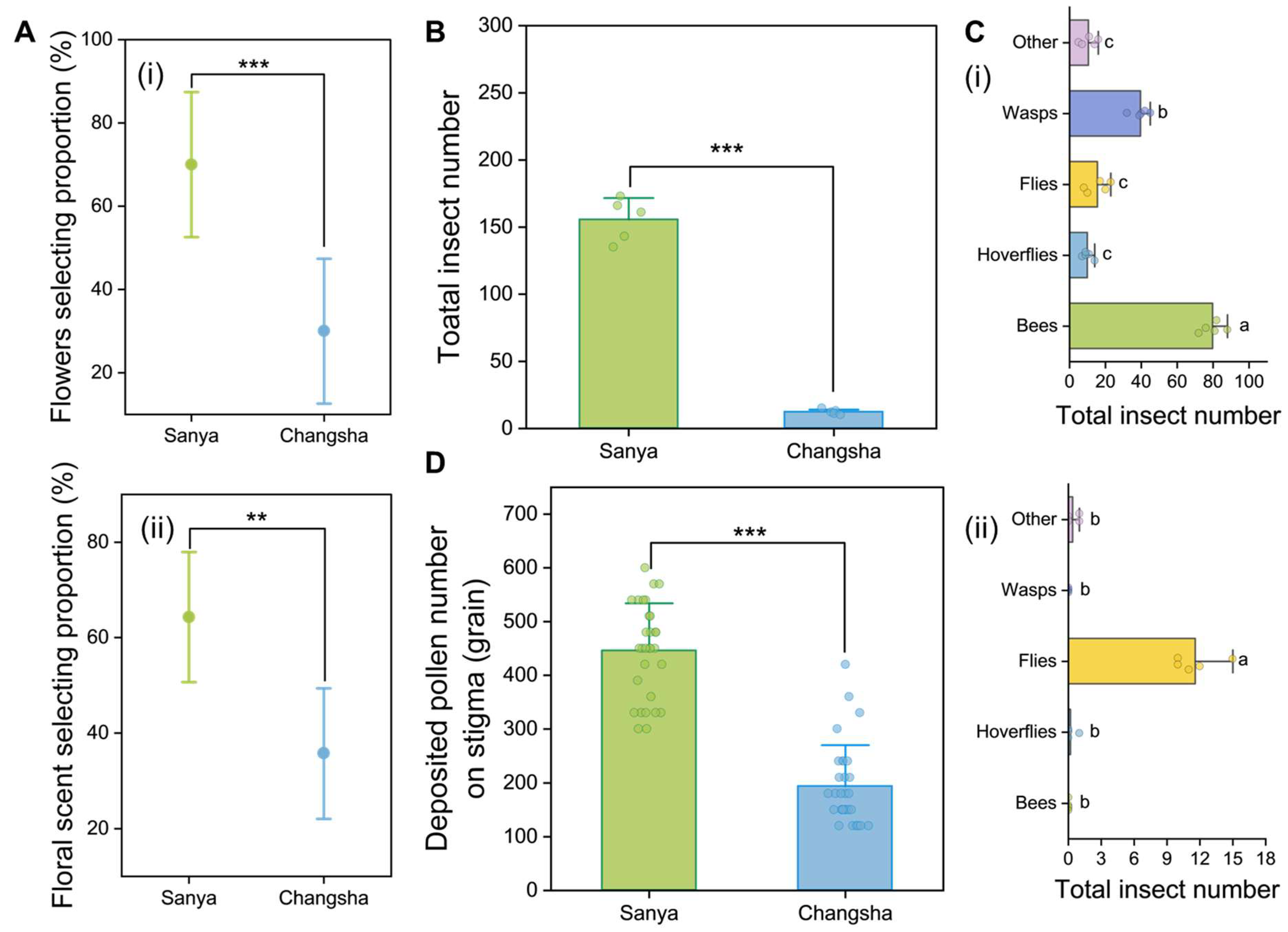
2.8. Observation of Flower-Visiting Preferences of A. cerana
2.9. Observation of Flower-Visiting Insects
2.10. Quantification of Stigma Pollen Deposition
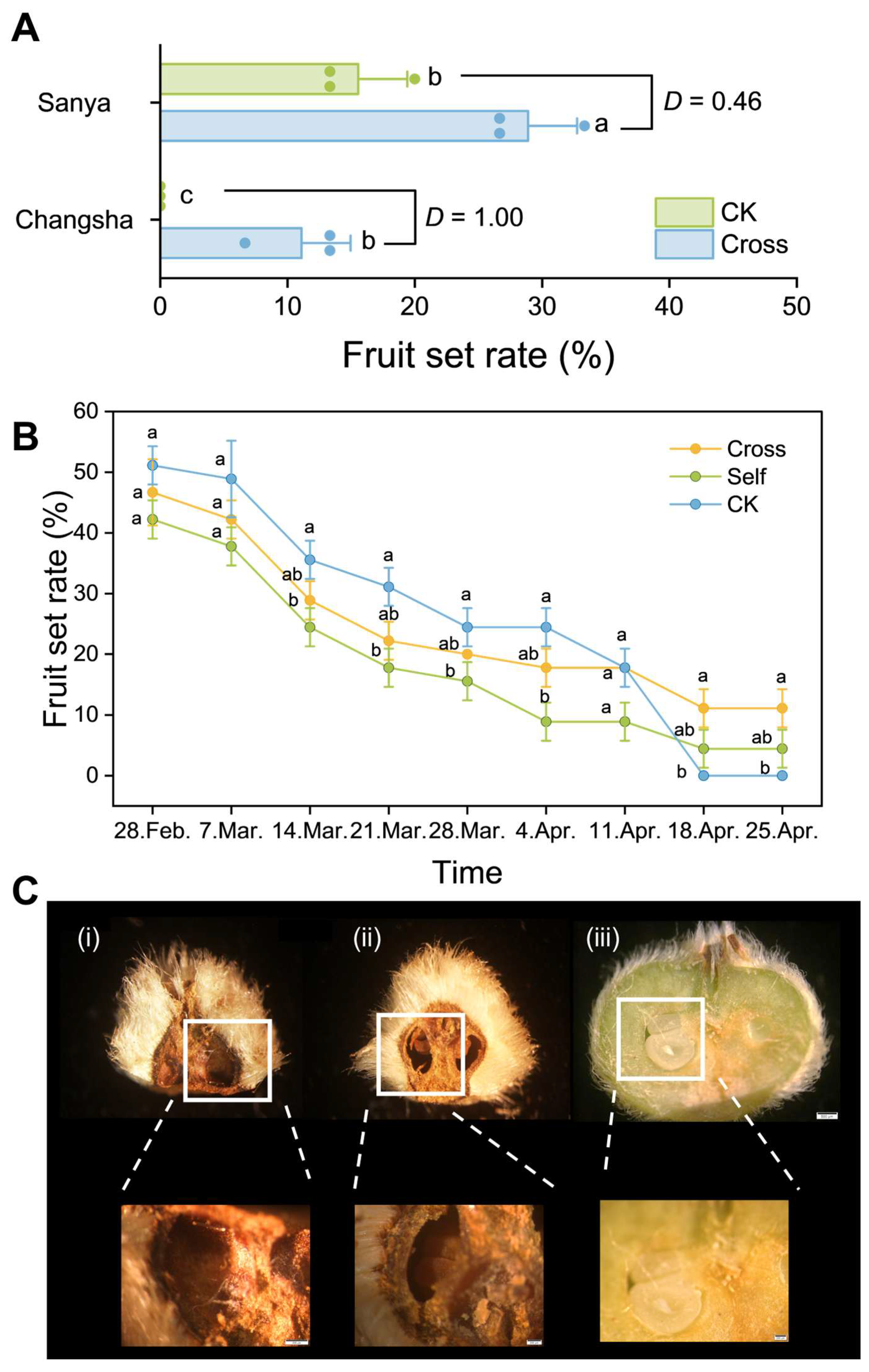
2.11. Pollination Treatments and Fruit Development Monitoring
2.12. Statistical Analysis
3. Results
3.1. Environmental Conditions of Different Sites
3.2. Impact of Northward Expansion on the C. hainanica Flower
3.2.1. Anthesis and Flower Longevity
3.2.2. Stigma Receptivity and Pollen Viability
3.2.3. Anther Dehiscence
3.2.4. Nectar Volume and Pollen Number
3.3. Impact of Northward Expansion on Pollination of C. hainanica
3.3.1. Bee Attractiveness of C. hainanica Flower
3.3.2. Flower-Visiting Insects
3.3.3. Pollen Deposition on Stigmas
3.4. Impact of Northward Expansion on C. hainanica Fruiting
3.4.1. Fruit Set Rate
3.4.2. Fruit Development of Changsha C. hainanica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, J.; Xiao, X.; Zhang, G.; Menarguez, M.A.; Choi, C.Y.; Qin, Y.; Luo, P.; Zhang, Y.; Moore, B. Northward expansion of paddy rice in northeastern Asia during 2000–2014. Geophys. Res. Lett. 2016, 43, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, G.; Wu, J.; Pu, Y.; Liu, L.; Ma, L.; Fan, T.; Wang, W.; Zhang, Y.; Lei, J.; et al. Feasibility analysis of expanding winter rapeseed northwards in China. Agric. For. Meteorol. 2025, 361, 110297. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z. Improving Northeast China’s soybean and maize planting structure through subsidy optimization considering climate change and comparative economic benefit. Land Use Policy 2024, 146, 107319. [Google Scholar] [CrossRef]
- Guga, S.; Bole, Y.; Riao, D.; Bilige, S.; Wei, S.; Li, K.; Zhang, J.; Tong, Z.; Liu, X. The challenge of chilling injury amid shifting maize planting boundaries: A case study of Northeast China. Agric. Syst. 2025, 222, 104166. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; de Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Zu, K.; Chen, F.; Li, Y.; Shrestha, N.; Fang, X.; Ahmad, S.; Nabi, G.; Wang, Z. Climate change impacts flowering phenology in Gongga Mountains, Southwest China. Plant Divers. 2024, 46, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Etterson, J.R.; Mazer, S.J. How climate change affects plants’ sex lives. Science 2016, 353, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Dorken, M.E.; Eckert, C.G. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J. Ecol. 2001, 89, 339–350. [Google Scholar] [CrossRef]
- Nathan, M. Plant-Insect Interactions in a Shifting Coastal Ecosystem: Avicennia germinans and Its Associated Arthropods. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2020. [Google Scholar]
- Wu, W.; Gao, X.; Hu, J.; Yu, Y.; Shu, Q. Physiological responses of northern Camellia oleifera to the habitats in different altitudes. J. Anhui Agric. Univ. 2019, 46, 655–659. [Google Scholar] [CrossRef]
- He, Q.; Zhou, G. Climate-associated distribution of summer maize in China from 1961 to 2010. Agric. Ecosyst. Environ. 2016, 232, 326–335. [Google Scholar] [CrossRef]
- Liang, H.; Qi, H.; Wang, C.; Wang, Y.; Liu, M.; Chen, J.; Sun, X.; Xia, T.; Feng, S.; Chen, C.; et al. Analysis of the complete mitogenomes of three high economic value tea plants (Tea-oil Camellia) provide insights into evolution and phylogeny relationship. Front. Plant Sci. 2025, 16, 1549185. [Google Scholar] [CrossRef]
- Liang, H.; Qi, H.; Chen, J.; Wang, Y.; Liu, M.; Sun, X.; Wang, C.; Xia, T.; Feng, X.; Feng, S.; et al. Assembly and analysis of the first complete mitochondrial genome sequencing of main Tea-oil Camellia cultivars Camellia drupifera (Theaceae): Revealed a multi-branch mitochondrial conformation for Camellia. BMC Plant Biol. 2025, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hu, J.; Duan, W.; Shen, Y.; Cao, Z.; Shu, Q. Study on the chemical components of shoots and leaves of Camellia oleifera from different elevations. Pak. J. Bot. 2015, 47, 1111–1114. [Google Scholar]
- Gu, W.; Xiao, X.; Ran, Z.; Yan, C.; Jiang, D.; Zhou, L.; An, M.; Li, Z. Distribution prediction and adaptability analysis of section camellia plants (Camellia Genus) in China based on the MaxEnt model. Ecol. Evol. 2025, 15, e71365. [Google Scholar] [CrossRef]
- Wu, D.; Wang, P.; Huo, Z.; Yuan, X.; Jiang, H.; Yang, J.; Tang, J.; Ma, Y. Changes in climate suitability for oil-tea (Camellia oleifera Abel) production in China under historical and future climate conditions. Agric. For. Meteorol. 2022, 316, 108843. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, Q.; Li, Z.; Zhong, Q.; Zhou, S.; Chen, F.; Zhang, M.; Li, Y.; Li, P.; Xie, C.; et al. Transcriptome and metabolome analysis reveals the mechanism of key nutrient formation in Hainan oil-camellia (Camellia hainanica) growth cycle. Ind. Crops Prod. 2025, 230, 121122. [Google Scholar] [CrossRef]
- Polce, C.; Garratt, M.P.; Termansen, M.; Ramirez-Villegas, J.; Challinor, A.J.; Lappage, M.G.; Boatman, N.D.; Crowe, A.; Endalew, A.M.; Potts, S.G.; et al. Climate-driven spatial mismatches between British orchards and their pollinators: Increased risks of pollination deficits. Glob. Change Biol. 2014, 20, 2815–2828. [Google Scholar] [CrossRef]
- Bezerra, A.D.M.; Pacheco Filho, A.J.S.; Bomfim, I.G.A.; Smagghe, G.; Freitas, B.M. Agricultural area losses and pollinator mismatch due to climate changes endanger passion fruit production in the Neotropics. Agric. Syst. 2019, 169, 49–57. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, K.; Zhong, W.-P.; Chen, P.; Fan, X.-M.; Yuan, D.-Y. Optimization of in vitro pollen germination and pollen viability tests for Castanea mollissima and Castanea henryi. Sci. Hortic. 2020, 271, 109481. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Wang, R.; Chen, L.; Peng, S.; Ma, L.; Luo, J.; Tang, W. Study on pollen viability of Camellia oleifera improved varieties from different species. J. Cent. South Univ. For. Technol. 2016, 36, 1–5. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Sastri, D.C. Stigma-surface esterase activity and stigma receptivity in some taxa characterized by wet stigmas. Ann. Bot. 1981, 47, 53–64. [Google Scholar] [CrossRef]
- Li, Q.; Sun, M.; Liu, Y.; Liu, B.; van der Werf, W.; Bianchi, F.J.J.A.; Lu, Y. Synthetic Nasonov gland pheromone enhances abundance and visitation of honeybee, Apis mellifera, in Korla fragrant pear, Pyrus sinkiangensis. Agric. For. Entomol. 2023, 25, 365–374. [Google Scholar] [CrossRef]
- Zhong, Y.-H.; Zhao, D.-X.; Wang, S.-J.; Han, W.-S.; Zhao, S.; Liu, J.-F.; Gao, J.-L. An observation on pollinating insect and their flower-visiting behavior on Camellia oleifera Abel. Apic. China 2020, 71, 63–66. [Google Scholar]
- Yuan, B.; Hu, G.-X.; Zhang, X.-X.; Yuan, J.-K.; Fan, X.-M.; Yuan, D.-Y. What are the best pollinator candidates for Camellia oleifera: Do not forget hoverflies and flies. Insects 2022, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Layek, U.; Baghira, N.K.; Das, A.; Kundu, A.; Karmakar, P. Dependency of Crops on Pollinators and Pollination Deficits: An Approach to Measurement Considering the Influence of Various Reproductive Traits. Agriculture 2023, 13, 1563. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Gao, J.; Wang, L. Latitudinal and climate effects on key plant traits in Chinese forest ecosystems. Glob. Ecol. Conserv. 2019, 17, e00527. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Gao, F.; Chen, M.; Jiang, Y.; Zhao, H.; Ma, W. Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles. Insects 2022, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, J.; He, Z.; Miao, C.; Wu, J.; Ma, D.; Zhao, P. Pollinator peaking earlier than flowering is more detrimental to plant fecundity. Sci. Total Environ. 2024, 917, 170458. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, J.; Feng, L.; Liu, S.; Li, J.; Qiao, W.; Song, Y.; Zhang, Z.; Cheng, Y.; Zhang, L.; et al. A combination of long-day suppressor genes contributes to the northward expansion of rice. Front. Plant Sci. 2020, 11, 864. [Google Scholar] [CrossRef]
- Lee, K.J. The Effects of Warming on Floral Traits. Master’s Thesis, Australian National University, Canberra, Australia, 2023. [Google Scholar]
- He, J.-C.; Li, J.-A.; Ren, S.-S.; Wang, Y.; Yan, J.-D. Effects of photoperiod on flowering and physiological characteristics of Camellia oleifera. Non-Wood For. Res. 2023, 41, 273–281. [Google Scholar] [CrossRef]
- Maeda, T.; Hiraiwa, M.K.; Shimomura, Y.; Oe, T. Weather conditions affect pollinator activity, fruit set rate, and yield in Japanese apricot. Sci. Hortic. 2023, 307, 111522. [Google Scholar] [CrossRef]
- Robertshaw, A. Effects of Temperature and Pollinator Availability on Plant Reproductive Success in the Indiana Spring Ephemeral Community. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2015. [Google Scholar]
- Williams, C.E. Mating system and pollination biology of the spring-flowering shrub, Dirca palustris. Plant Species Biol. 2004, 19, 101–106. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Wang, A.; He, M. Floral characters and breeding system of Hemerocallis lilio-asphodelus. J. Hunan Agric. Univ. Nat. Sci. 2016, 42, 58–63. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Gituru, R.W.; Yang, C.-F.; Guo, Y.-H. Exposure to water increased pollen longevity of pondweed (Potamogeton spp.) indicates different mechanisms ensuring pollination success of angiosperms in aquatic habitat. Evol. Ecol. 2010, 24, 939–953. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, C. The true identity of the “Second Pollen Morphology” of Camellia oleifera-stomium cells. Horticulturae 2022, 8, 347. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Huang, L.; Zhao, J.; Li, S.; Lu, J.; Li, X.; Yang, T. Identification of the effects of low temperature on grain-setting rate of different types of late-season rice (Oryza sativa) during heading. Field Crops Res. 2024, 318, 109584. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.A.; Wang, N.; Gu, Y.; Zhang, F.; Tan, X. The effects of low temperature stress on the flowering, fruiting and physiological characteristics of two Camellia oleifera cultivars. Plant Physiol. J. 2020, 56, 681–692. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, B.; Yuan, D.; Hu, F.; Su, X.; Fan, X.; Luo, Y. Flower visiting behavior and pollination effect of Bombus terrestris (Hymenoptera: Apidae) in Camellia oleifera plantation. Acta Entomol. Sin. 2025, 68, 311–320. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Manzoor, S.H.; Li, T.; Igathinathane, C.; Li, W.; Zhang, M.; Mhamed, M.; Javidan, S.M.; Abdelhamid, M. A comprehensive review of autonomous flower pollination techniques: Progress, challenges, and future directions. Comput. Electron. Agric. 2025, 237, 110577. [Google Scholar] [CrossRef]
- Xiong, W.; Reynolds, M.; Xu, Y. Climate change challenges plant breeding. Curr. Opin. Plant Biol. 2022, 70, 102308. [Google Scholar] [CrossRef] [PubMed]
| Site | Sanya | Changsha |
|---|---|---|
| Coordinates | 18.25° N, 109.50° E | 28.20° N, 112.97° E |
| Soil type | Red soil | Red soil |
| Annual temperature in 2024 | 26.7 °C | 18.8 °C |
| Annual precipitation in 2024 | 2080.1 mm | 1736.1 mm |
| Climate type | Tropical monsoon | Humid subtropical monsoon |
| Climate | High temperature and humidity, no frost | Hot summer and old winter, synchronized rain–heat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Deng, Z.-H.; Zhang, N.-N.; Huang, Z.-C.; Su, X.-L.; Lu, Y.-Y.; Zong, Z.-Y.; Yuan, D.-Y.; Fan, X.-M.; Hu, F.-L. Pollination Deficit: A Key Limitation of Fruit Set in Northward-Expanded Camellia Orchards. Agriculture 2025, 15, 1717. https://doi.org/10.3390/agriculture15161717
Yuan B, Deng Z-H, Zhang N-N, Huang Z-C, Su X-L, Lu Y-Y, Zong Z-Y, Yuan D-Y, Fan X-M, Hu F-L. Pollination Deficit: A Key Limitation of Fruit Set in Northward-Expanded Camellia Orchards. Agriculture. 2025; 15(16):1717. https://doi.org/10.3390/agriculture15161717
Chicago/Turabian StyleYuan, Bin, Zhi-Hui Deng, Ning-Ning Zhang, Zhi-Chu Huang, Xiao-Ling Su, Yuan-Yuan Lu, Ze-Yue Zong, De-Yi Yuan, Xiao-Ming Fan, and Fu-Liang Hu. 2025. "Pollination Deficit: A Key Limitation of Fruit Set in Northward-Expanded Camellia Orchards" Agriculture 15, no. 16: 1717. https://doi.org/10.3390/agriculture15161717
APA StyleYuan, B., Deng, Z.-H., Zhang, N.-N., Huang, Z.-C., Su, X.-L., Lu, Y.-Y., Zong, Z.-Y., Yuan, D.-Y., Fan, X.-M., & Hu, F.-L. (2025). Pollination Deficit: A Key Limitation of Fruit Set in Northward-Expanded Camellia Orchards. Agriculture, 15(16), 1717. https://doi.org/10.3390/agriculture15161717







