Spatial Analysis of Soil Acidity and Available Phosphorus in Coffee-Growing Areas of Pichanaqui: Implications for Liming and Site-Specific Fertilization
Abstract
1. Introduction
2. Materials and Methods
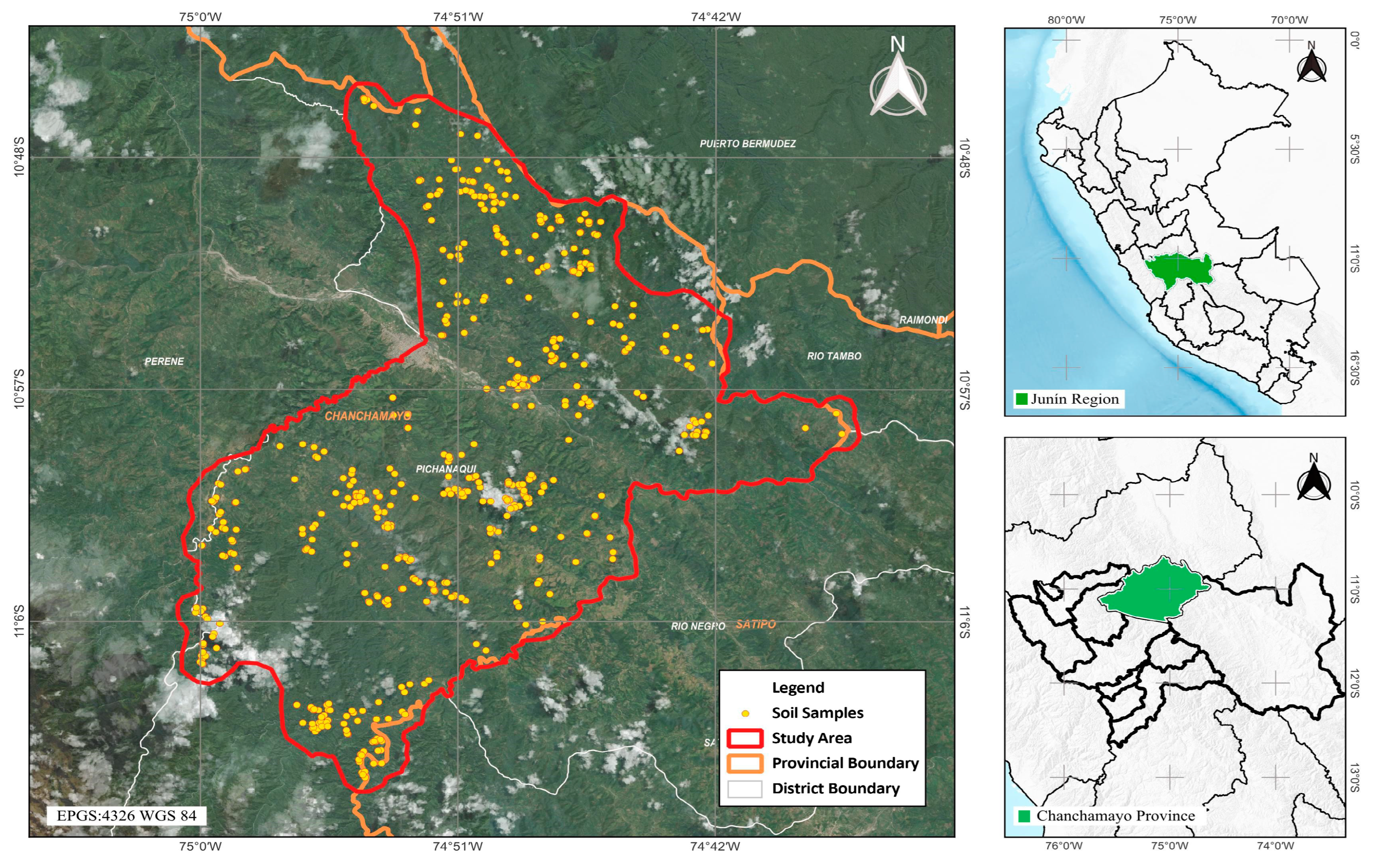
2.1. Study Area
2.2. Soil Sampling
2.3. Analysis of Soils
2.4. Extraction and Processing of Geospatial Variables
2.5. Crop Description
2.6. Estimation of Liming Requirement of Soils
2.7. Estimation of Phosphorus Fertilization Requirements
2.8. Multivariate Statistical Analysis
2.9. Non-Parametric Comparative Statistical Analysis
2.10. Geostatistical Interpolation
- (a)
- Semivariogram Equation
- (b)
- Ordinary Kriging Interpolation
2.11. Model Assessment
3. Results
3.1. Analysis Interpretation in the Pichanaqui District
3.2. Spearman Correlation Analysis of Soil Physical-Chemical Variables and Their Relationship with Acidity and Available Phosphorus
3.3. Principal Component Analysis of Edaphoclimatic Variables of the Coffee Agroecosystem in Pichanaqui
3.4. Estimation of Liming Requirements for Coffee Plantation
3.5. Evaluation of the of Phosphorous Fertilization Requirement in Coffee Crops
3.6. Spatial Variation of Soil Acidity and P Deficiency in the District of Pichanaqui
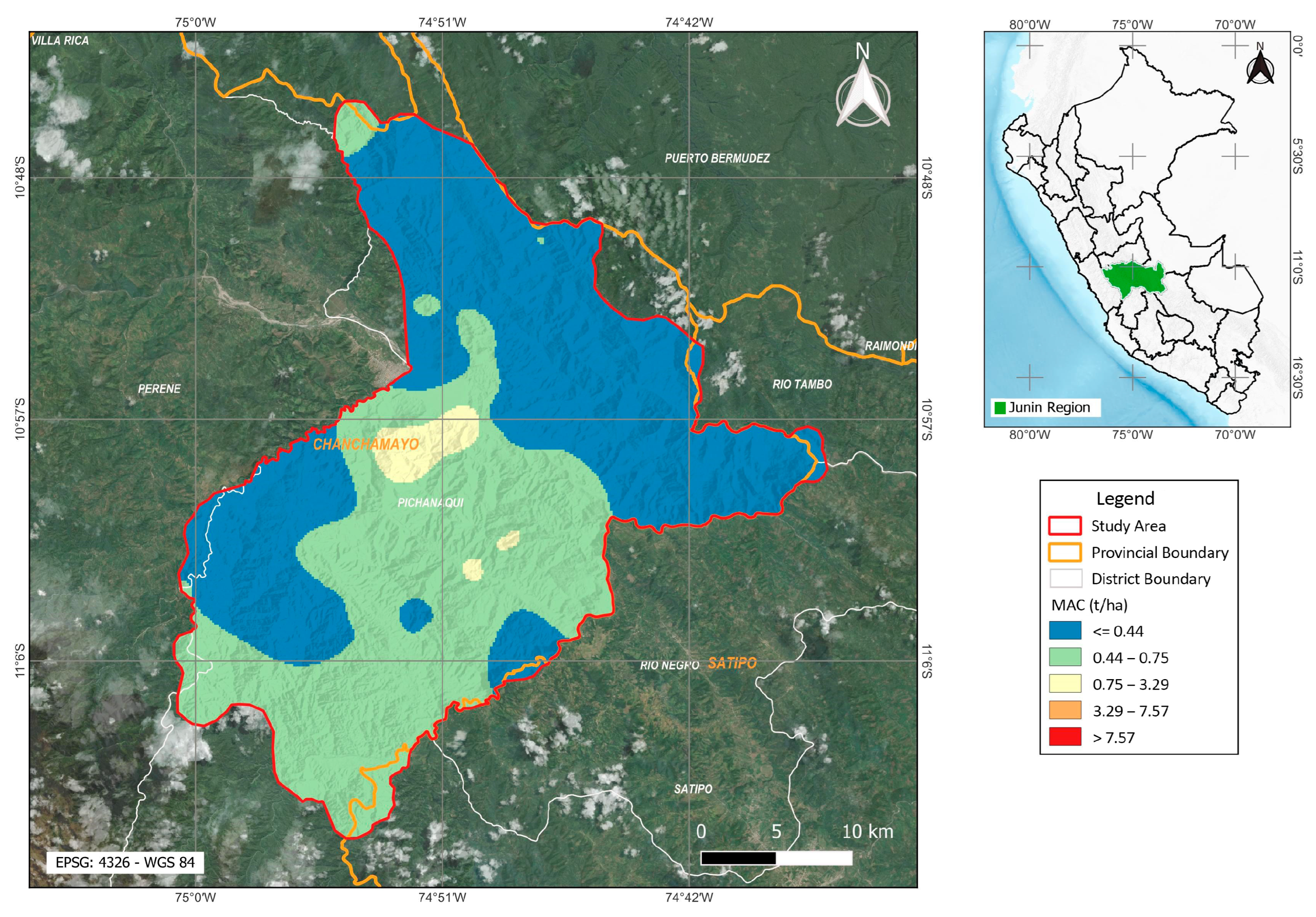
3.7. Spatial Variation of Liming and Phosphorus Fertilization Requirements in the Coffee Crop in Pichanaqui
4. Discussion
4.1. Critical Acidity Indicators and Their Impact on Coffee Agronomic Management in Pichanaqui Soils
4.2. Pedogenetic and Edaphoclimatic Factors That Limit P Availability in Coffee Plantations in Pichanaqui
4.3. Spatial Analysis of Liming Requirement in Pichanaqui Coffee Plantations
4.4. Spatial Analysis of Phosphorus Fertilization Requirements in Pichanaquis’ Coffee Plantations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Pancsira, J. International Coffee Trade: A Literature Review. J. Agric. Inform. 2022, 13. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Development Report 2022-23: Beyond Coffee—Towards a Circular Coffee Economy; International Coffee Organization: London, UK, 2023. [Google Scholar]
- National Institute of Statistics and Informatics. Caracteristicas Sociodemograficas Del Productor Agropecuario En El Peru, IV Censo Nacional Agropecuario 2012; INEI: Lima, Peru, 2012; Available online: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1057/libro.pdf (accessed on 10 April 2025).
- Integrated Agricultural Statistics System. El Agro En Cifras 2024 [Boletín Estadístico Mensual]; Ministerio de Desarrollo Agrario y Riego, Dirección General de Estadística, Seguimiento y Evaluación de Políticas (DGESEP): Lima, Peru, 2024. [Google Scholar]
- Torrez, V.; Benavides-Frias, C.; Jacobi, J.; Speranza, C.I. Ecological Quality as a Coffee Quality Enhancer. A Review. Agron. Sustain. Dev. 2023, 43, 19. [Google Scholar] [CrossRef] [PubMed]
- Latin American Model Forest Network Pichanaki. 2015. Available online: https://imfn.net/regional-networks/pichanaki-model-forest/ (accessed on 10 May 2025).
- Kochian, L.V. Cellular Mechanisms of Aluminum Toxicity and Resistance in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- FAO Suelos Ácidos|Portal de Suelos de La. Available online: https://www.fao.org/soils-portal/soil-management/manejo-de-suelos-problematicos/suelos-acidos/es/ (accessed on 29 May 2025).
- Sadeghian, S. La acidez del suelo, una limitante común para la producción de café. Av. Téc. Cenicafé 2016, 1–12. Available online: https://www.cenicafe.org/es/publications/avt0466.pdf (accessed on 11 June 2025).
- Zhang, Y.; Zhou, J.; Ren, H.; Chen, H. Characterization of Forest Soil Acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve. Sustainability 2024, 16, 7051. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Fast Root Growth Responses, Root Exudates, and Internal Detoxification as Clues to the Mechanisms of Aluminium Toxicity and Resistance: A Review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Blancaflor, E.B.; Jones, D.L.; Gilroy, S. Alterations in the Cytoskeleton Accompany Aluminum-Induced Growth Inhibition and Morphological Changes in Primary Roots of Maize1. Plant Physiol. 1998, 118, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, S.; Amenós, M.; Poschenrieder, C.; Barceló, J. Root Cell Patterning: A Primary Target for Aluminium Toxicity in Maize. J. Exp. Bot. 2005, 56, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Syndrome of Aluminum Toxicity and Diversity of Aluminum Resistance in Higher Plants. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 264, pp. 225–252. ISBN 978-0-12-374263-6. [Google Scholar]
- Sit, I.; Young, M.A.; Kubicki, J.D.; Grassian, V.H. Distinguishing Different Surface Interactions for Nucleotides Adsorbed onto Hematite and Goethite Particle Surfaces through ATR-FTIR Spectroscopy and DFT Calculations. Phys. Chem. Chem. Phys. 2023, 25, 20557–20566. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Fujiwara, T.; Šamaj, J.; Baluška, F.; Yang, Z.; Osawa, H.; Maeda, T.; Mori, T.; Volkmann, D.; Matsumoto, H. Aluminum-Induced 1→3-β-d-Glucan Inhibits Cell-to-Cell Trafficking of Molecules through Plasmodesmata. A New Mechanism of Aluminum Toxicity in Plants. Plant Physiol. 2000, 124, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Eticha, D.; Staß, A.; Horst, W.J. Localization of Aluminium in the Maize Root Apex: Can Morin Detect Cell Wall-Bound Aluminium? J. Exp. Bot. 2005, 56, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- López Báez, W.; Castro Mendoza, I.; Salinas Cruz, E.; Reynoso Santos, R.; López Martínez, J. Propiedades de Los Suelos Cafetaleros En La Reserva de La Biósfera El Triunfo, Chiapas, México. Rev. Mex. Cienc. Agríc. 2016, 7, 607–618. [Google Scholar]
- Demidchik, V. Reactive Oxygen Species and Oxidative Stress in Plants. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2012; pp. 24–58. ISBN 978-1-84593-996-0. [Google Scholar]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Duputel, M.; Devau, N.; Brossard, M.; Jaillard, B.; Jones, D.L.; Hinsinger, P.; Gérard, F. Citrate Adsorption Can Decrease Soluble Phosphate Concentration in Soils: Results of Theoretical Modeling. Appl. Geochem. 2013, 35, 120–131. [Google Scholar] [CrossRef]
- Penn, C.; Camberato, J. A Critical Review on Soil Chemical Processes That Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- McDowell, R.W.; Sharpley, A.N. Phosphorus Solubility and Release Kinetics as a Function of Soil Test P Concentration. Geoderma 2003, 112, 143–154. [Google Scholar] [CrossRef]
- Olibone, D.; Rosolem, C.A. Phosphate Fertilization and Phosphorus Forms in an Oxisol under No-Till. Sci. Agric. 2010, 67, 465–471. [Google Scholar] [CrossRef]
- Rheinheimer, D.D.S.; Anghinoni, I. Distribuição Do Fósforo Inorgânico Em Sistemas de Manejo de Solo. Pesqui. Agropecuária Bras. 2001, 36, 151–160. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Functional Relationships of Soil Acidification, Liming, and Greenhouse Gas Flux. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 139, pp. 1–71. ISBN 978-0-12-804773-6. [Google Scholar]
- Slattery, W.; Coventry, D. Response of Wheat, Triticale, Barley, and Canola to Lime on Four Soil Types in North-Eastern Victoria. Aust. J. Exp. Agric. 1993, 33, 609. [Google Scholar] [CrossRef]
- Thomas, G.W.; Hargrove, W.L. The Chemistry of Soil Acidity. In Agronomy Monographs; Adams, F., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1984; pp. 3–56. ISBN 978-0-89118-207-8. [Google Scholar]
- Fageria, N.K.; Nascente, A.S. Management of Soil Acidity of South American Soils for Sustainable Crop Production. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 128, pp. 221–275. ISBN 978-0-12-802139-2. [Google Scholar]
- Haynes, R.J.; Ludecke, T.E. Effect of Lime and Phosphorus Applications on Concentrations of Available Nutrients and on P, Al and Mn Uptake by Two Pasture Legumes in an Acid Soil. Plant Soil 1981, 62, 117–128. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Chang, S.X.; Zhang, Q. Liming Effects on Soil pH and Crop Yield Depend on Lime Material Type, Application Method and Rate, and Crop Species: A Global Meta-Analysis. J. Soils Sediments 2019, 19, 1393–1406. [Google Scholar] [CrossRef]
- Slattery, J.F.; Coventry, D.R.; Slattery, W.J. Rhizobial Ecology as Affected by the Soil Environment. Aust. J. Exp. Agric. 2001, 41, 289. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Zhan, Y.; Zheng, X.; Zhou, M.; Yan, G.; Wang, L.; Werner, C.; Butterbach-Bahl, K. Potential Benefits of Liming to Acid Soils on Climate Change Mitigation and Food Security. Glob. Chang. Biol. 2021, 27, 2807–2821. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, M.A.; Khan Tarin, M.W.; Jingxia, G.; Yanhui, C.; Guo, W. Soil Liming Effects on CH4, N2O Emission and Cd, Pb Accumulation in Upland and Paddy Rice. Environ. Pollut. 2019, 248, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Royer-Tardif, S.; Whalen, J.; Rivest, D. Can Alkaline Residuals from the Pulp and Paper Industry Neutralize Acidity in Forest Soils without Increasing Greenhouse Gas Emissions? Sci. Total Environ. 2019, 663, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Hénault, C.; Bourennane, H.; Ayzac, A.; Ratié, C.; Saby, N.P.A.; Cohan, J.-P.; Eglin, T.; Gall, C.L. Management of Soil pH Promotes Nitrous Oxide Reduction and Thus Mitigates Soil Emissions of This Greenhouse Gas. Sci. Rep. 2019, 9, 20182. [Google Scholar] [CrossRef] [PubMed]
- Fenn, L.B.; Kissel, D.E. Ammonia Volatilization from Surface Applications of Ammonium Compounds on Calcareous Soils: IV. Effect of Calcium Carbonate Content. Soil Sci. Soc. Am. J. 1975, 39, 631–633. [Google Scholar] [CrossRef]
- Lalande, R.; Gagnon, B.; Royer, I. Impact of Natural or Industrial Liming Materials on Soil Properties and Microbial Activity. Can. J. Soil Sci. 2009, 89, 209–222. [Google Scholar] [CrossRef]
- Barber, S.A. Liming Materials and Practices. In Agronomy Monographs; Adams, F., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 171–209. ISBN 978-0-89118-207-8. [Google Scholar]
- Buni, A. Effects of Liming Acidic Soils on Improving Soil Properties and Yield of Haricot Bean. J. Environ. Anal. Toxicol. 2014, 5. [Google Scholar] [CrossRef]
- Chen, S.; Lin, B.; Li, Y.; Zhou, S. Spatial and Temporal Changes of Soil Properties and Soil Fertility Evaluation in a Large Grain-Production Area of Subtropical Plain, China. Geoderma 2020, 357, 113937. [Google Scholar] [CrossRef]
- Henríquez, C.; Killorn, R.; Bertsch, F.; Sancho, F. La Geostadística En El Estudio de La Variación Espacial de La Fertilidad Del Suelo Mediante El Uso Del Interpolador Kriging. Agron. Costarric. 2005, 29, 73–81. [Google Scholar] [CrossRef]
- Hengl, T. A Practical Guide to Geostatistical Mapping; Office for Official Publications of the European Communities: Luxembourg, 2009; ISBN 978-90-90-24981-0. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- ISO 11464 Soil Quality—Pretreatment of Samples for Physico-Chemical Analysis. Available online: https://www.iso.org/standard/37718.html (accessed on 2 April 2025).
- NOM-021-RECNAT-2000 NOM-021-RECNAT-2000; Specifications of Fertility, Salinity, and Soil Classification. Study, Sampling and Analysis 2002. Natural Resources and Environment Secretary. Mexican Official Standard: Ciudad de Mexico, Mexico, 2000.
- United States. Environmental Protection Agency. Office of Solid Waste and Emergency Response. Method 9045D: Soil and Waste pH. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods; Soil Waste PH; US Environmental Protection Agency, Office of Solid Waste and Emergency Respons: Washington, DC, USA, 2004; pp. 1–5. [Google Scholar]
- ISO 11265 Soil Quality—Determination of Specific Electrical Conductivity. Available online: https://www.iso.org/es/contents/data/standard/01/92/19243.html (accessed on 2 April 2025).
- ISO 11261 Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. Available online: https://www.iso.org/standard/19239.html (accessed on 2 April 2025).
- Bazán Tapia, R. Manual de Procedimientos de los Análisis de Suelos y Agua con Fines de Riego; Instituto Nacional de Innovación Agraria: La Molina District, Peru, 2017. [Google Scholar]
- Hengl, T.; Mendes De Jesus, J.; Heuvelink, G.B.M.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global Gridded Soil Information Based on Machine Learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [PubMed]
- ISRIC World Soil SoilGrids250m 2.0. Available online: https://soilgrids.org/ (accessed on 28 May 2025).
- Aybar-Camacho, C.; Lavado-Casimiro, W.; Sabino, E.; Ramírez, S.; Huerta, J.; Felipe-Obando, O. Atlas de Zonas de Vida Del Perú-: Guía Explicativa; Servicio Nacional de Meteorología e Hidrología del Perú: Lima, Peru, 2017. [Google Scholar]
- IUSS Working Group. WRB World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; Roma, Italy, 2014. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/bcdecec7-f45f-4dc5-beb1-97022d29fab4/content (accessed on 19 July 2025).
- Yost, R.; Uehara, G.; Wade, M.; Sudjadi, M.; Widjaja-adhi, I.P.G.; Li, Z.-C. Expert Systems in Agriculture: Determining Lime Recommendations for Soils of the Humid Tropics; Hawaii, USA, 1988. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/RES-089.pdf (accessed on 17 May 2025).
- Cate, R.B.; Nelson, L.A. A Rapid Method for Correlation of Soil Test Analyses with Plant Response Data; International Soil Testing series; N.C. State University Agricultural Experiment Station: Raleigh, NC, USA, 1965. [Google Scholar]
- Osmond, D.L.; Smyth, T.J.; Yost, R.S.; Hoag, D.L.; Reid, W.S.; Branch, W.; Wang, H. Nutrient Management Support System (NuMaSS), Version 2.2; CRSPs: Delhi, India, 2007. [Google Scholar]
- Van Raij, B.; Cantarella, H.; Cangiani, F.A. Recomendações de Adubação e Calagem Para o Estado de São Paulo. Camp. Inst. Agronômico 1997, 2, 285. [Google Scholar]
- Alvarez, V.V.H.; Ribeiro, A.C. Recomendações Para o uso de Corretivos e Fertilizantes em Minas Gerais: 5. Aproximação; SBCS: Viçosa, Brazil, 1999. [Google Scholar]
- Defelipo, B.; Braga, J.; Spies, C. Comparação Entre Métodos de Determinação Da Necessidade de Calcário de Solos de Minas Gerais. Viçosa 1972, 13, 111–136. [Google Scholar]
- Teixeira, W.G.; Reis, J.; Freitas, J.; Alvarez, V. Determinação Da Necessidade de Calagem Para o Cafeeiro Considerando a Acidez Potencial. In Proceedings of the 20th Congreso Latinoamericano 16th Congreso Peruano de la Ciencia del Suelo, Cuzco, Peru, 9–15 November 2014; pp. 9–15. [Google Scholar]
- Quispe, K.; Mejía, S.; Carbajal, C.; Alejandro, L.; Verástegui, P.; Solórzano, R. Spatial Variability of Soil Acidity and Lime Requirements for Potato Cultivation in the Huánuco Highlands. Agriculture 2024, 14, 2286. [Google Scholar] [CrossRef]
- Khalajabadi, S.S. Manejo integrado de nutrientes para una caficultura sostenible. Suelos Ecuat. 2014, 44, 74–89. [Google Scholar]
- McFarland, M.L.; Haby, V.A.; Redmon, L.A.; Bade, D.H. The Texas A&M University System. 2024. Available online: https://forages.tamu.edu/wp-content/uploads/sites/26/legacy-files/PDF/scs2001-05.pdf (accessed on 19 July 2025).
- Redmon, L.A.; McFarland, M.L.; Haby, V.A.; Bade, D.H. The Texas A&M University System. 2024. Available online: https://soiltesting.tamu.edu/soiltesting/wp-content/uploads/sites/13/2023/05/SCS-2001-06.pdf (accessed on 19 July 2025).
- Malavolta, E. Nutrição Mineral e Adubação Do Cafeeiro; Associacao Brasileira para Pesquisa da Potassa e do Fosfato (Piracicaba) y itora Agronômica Ceres: Sao Paulo, Brazil, 1990; ISBN 85-318-0005-6. [Google Scholar]
- Sadeghian, S. Nutrición de Cafetales; Cenicafé: Chinchiná, Colombia, 2013. [Google Scholar]
- Tunçay, T. Comparison Quality of Interpolation Methods to Estimate Spatial Distribution of Soil Moisture Content. Commun. Soil Sci. Plant Anal. 2021, 52, 353–374. [Google Scholar] [CrossRef]
- Bock, M.; Böhner, J.; Köthe, R.; Conrad, O.; Ringeler, A. Methods for Creating Functional Soil Databases and Applying Digital Soil Mapping with SAGA GIS. RC Sci. Tech. Rep. Off. Off. Publ. Eur. Communities Luxemb. 2007, 1, 31–37. [Google Scholar]
- Martínez, C.R. Modelado Geoestadístico de Fertilidad de Un Terreno Agrícola Mediante Mapas de Kriging Que Interpolan Los Datos de Análisis Químico de Suelos. Prod. Agropecu. Desarro. Sosten. 2020, 9, 63–84. [Google Scholar] [CrossRef]
- Villatoro, M.; Henríquez, C.; Sancho, F. Comparación de los interpoladores IDW y Kriging en la variación espacial de pH, Ca, CICE y P del suelo. Agron. Costarric. 2008. [Google Scholar] [CrossRef]
- Bromberg, F.; Pérez, D.S. Interpolación Espacial Mediante Aprendizaje de Máquinas en Viñedos de la Provincia de Mendoza, Argentina. In Proceedings of the XIII Argentine Symposium on Artificial Intelligence (ASAI 2012), La Plata, Argentina, 27–28 August 2012. [Google Scholar]
- Beltrán Rodríguez, A.; Cebrián Guajardo, A.C.; Castillo-Mateo, J. Estadística Espacial para datos punto Referenciados; Universidad de Zaragoza: Zaragoza, Spain, 2023. [Google Scholar]
- Fernández Villafañez, S. Métodos de Regresión y Clasificación Basados en Árboles; Universidad de Valladolid: Valladolid, Spain, 2022. [Google Scholar]
- Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; U.S. Department of Agriculture Handbook 18; Government Printing Office: Washington, DC, USA, 2017. [Google Scholar]
- Sadeghian, S.; Díaz Marín, C. Corrección de la acidez del suelo: Alteraciones químicas del suelo. Rev. Cenicafé 2020, 7–20. [Google Scholar] [CrossRef]
- Takala, B. Soil Acidity and Its Management Options in Western Ethiopia. J. Environ. Earth Sci. 2019, 9, 2224–3216. [Google Scholar]
- Valencia Aristizabal, G.; Carrillo Pachon, I.; Estrada Hoyos, L. Fertilización Cafetal Según Análisis Suelos. In 50 años de Cenicafé 1938 1988: Conferencias Conmemorativas; 1989; pp. 97–103. Available online: https://biblioteca.cenicafe.org/bitstream/10778/713/1/1%2050%20a%C3%B1os%20de%20Cenicaf%C3%A9%201938%201988.pdf (accessed on 19 July 2025).
- Sadeghian, S. Fertilidad del suelo y nutrición del café en Colombia: Guía práctica. Bol. Téc. Cenicafé 2008, 32, 1–44. [Google Scholar] [CrossRef]
- Cenicafé. Composición elemental de frutos de café y extracción de nutrients. 2006. Available online: https://www.cenicafe.org/es/publications/arc057(04)251-261.pdf (accessed on 2 April 2025).
- Foy, C.D. Physiological Effects of Hydrogen, Aluminum, and Manganese Toxicities in Acid Soil. In Agronomy Monographs; Adams, F., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1984; pp. 57–97. ISBN 978-0-89118-207-8. [Google Scholar]
- Rosas Arellano, J.; Escamilla Prado, E.; Ruiz Rosado, O. Relación de los nutrimentos del suelo con las características físicas y sensoriales del café orgánico. Terra Latinoam. 2008, 26, 375–384. [Google Scholar]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Eekhout, T.; Larsen, P.; De Veylder, L. Modification of DNA Checkpoints to Confer Aluminum Tolerance. Trends Plant Sci. 2017, 22, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Wintgens, J.N. Coffee—Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders and Researchers; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-3-527-33253-3. [Google Scholar]
- Kollie, W.S.; Byalebeka, J.; Basamba, T.A. Effects of Liming on Acid Ferrasols for Sustainable Crop Production in Uganda—A Review. Int. J. Plant Soil Sci. 2023, 35, 111–124. [Google Scholar] [CrossRef]
- Sabino Rojas, E. Servicio Nacional de Meteorología e Hidrología del Perú; SENAMHI: Lima, Peru, 2019. [Google Scholar]
- Weil, R.; Brady, N. The Nature and Properties of Soils; Pearson: London, UK, 2017; Volume 1104. [Google Scholar]
- Von Uexküll, H.R.; Mutert, E. Global Extent, Development and Economic Impact of Acid Soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Auler, A.C.; Caires, E.F.; Pires, L.F.; Galetto, S.L.; Romaniw, J.; Charnobay, A.C. Lime Effects in a No-Tillage System on Inceptisols in Southern Brazil. Geoderma Reg. 2019, 16, e00206. [Google Scholar] [CrossRef]
- Aviles, D.; Berglund, K.; Wesström, I.; Joel, A. Effect of Liming Products on Soil Detachment Resistance, Measured with a Cohesive Strength Meter. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 48–55. [Google Scholar] [CrossRef]
- Huisa Altamirano, D. La Calidad del Suelo en Campos de Agricultura Intensiva de Café (Coffea arabica) VAR. CATIMOR en el Anexo Alto Pitocuna del Distrito de Río Negro. Satipo. 2018; Universidad Continental: Huancayo, Peru, 2020. [Google Scholar]
- Liao, W.; Xu, R.; Stone, A.T. Adsorption of Phosphorus Oxyanions at the FeOOH(Goethite)/Water Interface: The Importance of Basicity. Environ. Sci. Technol. 2021, 55, 14389–14396. [Google Scholar] [CrossRef] [PubMed]
- Tanada, S.; Kabayama, M.; Kawasaki, N.; Sakiyama, T.; Nakamura, T.; Araki, M.; Tamura, T. Removal of Phosphate by Aluminum Oxide Hydroxide. J. Colloid Interface Sci. 2003, 257, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ganta, P.B.; Morshedizad, M.; Kühn, O.; Leinweber, P.; Ahmed, A.A. The Binding of Phosphorus Species at Goethite: A Joint Experimental and Theoretical Study. Minerals 2021, 11, 323. [Google Scholar] [CrossRef]
- Wang, X.; Phillips, B.L.; Boily, J.-F.; Hu, Y.; Hu, Z.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide. Soil Syst. 2019, 3, 20. [Google Scholar] [CrossRef]
- Van Emmerik, T.J.; Sandström, D.E.; Antzutkin, O.N.; Angove, M.J.; Johnson, B.B. 31 P Solid-State Nuclear Magnetic Resonance Study of the Sorption of Phosphate onto Gibbsite and Kaolinite. Langmuir 2007, 23, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.W.; Syers, J.K. The Fate of Phosphorus during Pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Nishigaki, T.; Tsujimoto, Y.; Rakotoson, T.; Rabenarivo, M.; Andriamananjara, A.; Asai, H.; Andrianary, H.B.; Rakotonindrina, H.; Razafimbelo, T. Soil Phosphorus Retention Can Predict Responses of Phosphorus Uptake and Yield of Rice Plants to P Fertilizer Application in Flooded Weathered Soils in the Central Highlands of Madagascar. Geoderma 2021, 402, 115326. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bayer, C.; Torrent, J.; Barrón, V. Mineralogy and Phosphorus Adsorption in Soils of South and Central-West Brazil under Conventional and No-Tillage Systems. Acta Sci. Agron. 2014, 36, 379. [Google Scholar] [CrossRef]
- Grand, S.; Lavkulich, L.M. Potential Influence of Poorly Crystalline Minerals on Soil Chemistry in P Odzols of Southwestern C Anada. Eur. J. Soil Sci. 2013, 64, 651–660. [Google Scholar] [CrossRef]
- Díaz Poveda, V.C.; Sadeghian, S. Phosphorus Fixation and Its Relationship with Soils Chemical Properties of the Coffee Zone of Huila, Colombia. Coffee Sci. 2023, 18, e182122. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.D.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 6th ed.; Pearson Education India: Noida, India, 2016; Volume 8, ISBN 978-93-325-7034-4. [Google Scholar]
- Jenny, H. Factors of Soil Formation: A System of Quantitative Pedology; Courier Corporation: Chelmsford, MA, USA, 1994; ISBN 978-0-486-68128-3. [Google Scholar]
- Van Lierop, W. Soil pH and Lime Requirement Determination. In SSSA Book Series; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 73–126. ISBN 978-0-89118-862-9. [Google Scholar]
- Alvarado, A.; Molina, E.; Cabalceta, G. Acidez y encalado de suelos. In Tecnología de Suelos: Estudio de Casos; Prensas de la Universidad de Zaragoza: Zaragoza, Spain, 2010; pp. 69–99. ISBN 978-84-92774-97-5. [Google Scholar]
- Teixeira, W.G.; Víctor Hugo Alvarez, V.; Neves, J.C.L.; Paulucio, R.B. Evaluation of Traditional Methods for Estimating Lime Requirement in Brazilian Soils. Rev. Bras. Ciênc. Solo 2020, 44, e0200078. [Google Scholar] [CrossRef]
- Shoemaker, H.E.; McLean, E.O.; Pratt, P.F. Buffer Methods for Determining Lime Requirement of Soils With Appreciable Amounts of Extractable Aluminum. Soil Sci. Soc. Am. J. 1961, 25, 274–277. [Google Scholar] [CrossRef]
- Adams, F.; Evans, C.E. A Rapid Method for Measuring Lime Requirement of Red-Yellow Podzolic Soils. Soil Sci. Soc. Am. J. 1962, 26, 355–357. [Google Scholar] [CrossRef]
- Mehlich, A. New Buffer pH Method for Rapid Estimation of Exchangeable Acidity and Lime Requirement of Soils. Commun. Soil Sci. Plant Anal. 1976, 7, 637–652. [Google Scholar] [CrossRef]
- Nolla, A.; Anghinoni, I. Métodos Utilizados Para a Correção Da Acidez Do Solo No Brasil. Fac. Agron. Universidade Fed. Rio Gd. Sul 2004, 6, 97–111. [Google Scholar]
- Teixeira, W.G.; Víctor Hugo Alvarez, V.; Neves, J.C.L. New Methods for Estimating Lime Requirement to Attain Desirable pH Values in Brazilian Soils. Rev. Bras. Ciênc. Solo 2020, 44, e0200008. [Google Scholar] [CrossRef]
- Reis, T.H.P.; Guimarães, P.T.G.; Furtini Neto, A.E.; Guerra, A.F.; Curi, N. Soil Phosphorus Dynamics and Availability and Irrigated Coffee Yield. Rev. Bras. Ciênc. Solo 2011, 35, 503–515. [Google Scholar] [CrossRef]
- Siman, F.C.; Andrade, F.V.; Stauffer, E.; Mendonça, E.D.S. Reservoirs of P and Fertilizer with Technology on the Path to Sustainability in Coffee Production. Front. Sustain. Food Syst. 2024, 8, 1403744. [Google Scholar] [CrossRef]
- Villamagua, M.A.; Castillo, M.G.G.; Sarango, R.d.C.R.; Vásquez, E.; Manosalvas, C.A.V.; Erraez, R.M.M. Efecto del encalado sobre la acidez del suelo, la disponibilidad de nutrientes y el crecimiento del cafeto (Coffea arabica L.) en Pueblo Nuevo, cantón Loja, Ecuador. Bosques Latid. Cero 2021, 11, 166–180. [Google Scholar]
- Teshale, E.; Kufa, T.; Regassa, A. Effects of Lime on Phosphorus Availability and Nutrient Uptake of Hybrid Coffee (Coffea arabica L.) Seedlings Under Acidic Nursery Soil. Agric. For. Fish. 2021, 10, 21. [Google Scholar] [CrossRef]
- Benevenute, P.A.N.; Pereira, F.A.C.; Barbosa, S.M.; Da Silva, R.F.; Domingues, M.I.S.; Marques Filho, A.C.; De Oliveira, G.C.; Silva, B.M. Deep Soil Tillage in the Coffee Planting Furrow Has Long-Lasting Benefits for Improving Soil Physical Quality and Enhancing Plant Vigor in Dense Soils. Soil Tillage Res. 2025, 248, 106448. [Google Scholar] [CrossRef]
- Abruña, F.; Vicente-Chandler, J.; Becerra, L.A.; Bosque Lugo, R. Effects of Liming and Fertilization on Yields and Foliar Composition of High-Yielding Sun-Grown Coffee in Puerto Rico. J. Agric. Univ. P. R. 1965, 49, 413–428. [Google Scholar] [CrossRef]
- Parecido, R.J.; Soratto, R.P.; Perdoná, M.J.; Gitari, H.I.; Dognani, V.; Santos, A.R.; Silveira, L. Liming Method and Rate Effects on Soil Acidity and Arabica Coffee Nutrition, Growth, and Yield. J. Soil Sci. Plant Nutr. 2021, 21, 2613–2625. [Google Scholar] [CrossRef]
- Teshale, E. Lime and Phosphorus Rates Response on Dry Matter Production and Partitioning of Hybrid of Coffee. Am. J. Heterocycl. Chem. 2021, 7, 1. [Google Scholar] [CrossRef]
- Silva, E.R.O.D.; Silva, T.L.D.; Wei, M.C.F.; Souza, R.A.D.; Molin, J.P. Spatial and Temporal Variability Management for All Farmers: A Cell-Size Approach to Enhance Coffee Yields and Optimize Inputs. Plants 2025, 14, 169. [Google Scholar] [CrossRef] [PubMed]









| Method | Formula | Detail | Reference |
|---|---|---|---|
| Combined Method | Estimate just a liming dose if EAP > PAS | [56] | |
| Mx+ Method | Without restriction | [57] | |
| NuMaSS Method | Estimate a liming dose when EAP > PAS; in addition, when ECEC > 4.5, F value is 2.5, otherwise it is 1.3 | [58] | |
| Method of Bases Saturation | It is applied when EBP < 60% | [59] | |
| Method of Minas Gerais 5 A | Buffer power is estimated according to clay percentage | [60] | |
| It is applied when the EAP is higher than 25% and Ca+2 + Mg+2 is higher than 3.5 cmol Kg | |||
| It is applied when the PAS is higher than 25% and Ca+2 + Mg+2 is less than 3.5 cmol Kg−1 | |||
| pHOM Method | It is applied just when the pH is less than 6 | [61] | |
| Method of potential acidity | Without restriction | [62] | |
| Method of potential acidity modified | It is applied when EAP is less than 25% | [63] |
| Variable | Units | Mean | SD | Var | CV | Skewness | Kurtosis | Min | Max | P25 | Median | P75 | Shapiro |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | % | 58.94 | 15.95 | 254.36 | 27.06 | −0.02 | −0.69 | 8.00 | 91.50 | 47.48 | 58.15 | 70.55 | 0.00 |
| Silt | % | 24.69 | 12.09 | 146.05 | 48.95 | 0.37 | 0.34 | 1.50 | 84.20 | 15.70 | 24.60 | 32.60 | 0.00 |
| Clay | % | 16.37 | 8.24 | 67.84 | 50.31 | 1.24 | 1.32 | 1.80 | 48.50 | 10.60 | 13.80 | 20.80 | 0.00 |
| EC | dS m−1 | 1.02 | 2.46 | 6.07 | 241.53 | 9.20 | 91.11 | 0.01 | 29.45 | 0.40 | 0.63 | 0.89 | 0.00 |
| pH | unit | 4.54 | 0.90 | 0.82 | 19.93 | 1.08 | 0.45 | 3.40 | 7.30 | 3.80 | 4.20 | 5.10 | 0.00 |
| OM | % | 3.62 | 1.93 | 3.72 | 53.33 | 1.04 | 0.96 | 0.40 | 11.10 | 2.20 | 3.20 | 4.63 | 0.00 |
| N | % | 0.18 | 0.10 | 0.01 | 52.72 | 1.04 | 0.98 | 0.02 | 0.56 | 0.11 | 0.16 | 0.23 | 0.00 |
| Pav | mg kg−1 | 4.74 | 3.80 | 14.46 | 80.16 | 6.27 | 74.71 | 0.07 | 57.01 | 2.55 | 3.70 | 6.53 | 0.00 |
| Kav | mg kg−1 | 76.72 | 46.91 | 2200.27 | 61.14 | 1.62 | 4.20 | 18.90 | 373.80 | 42.30 | 63.75 | 104.70 | 0.00 |
| Ca+2 | cmol kg−1 | 3.55 | 4.25 | 18.06 | 119.87 | 2.35 | 7.31 | 0.03 | 31.97 | 0.78 | 1.78 | 4.96 | 0.00 |
| Mg+2 | cmol kg−1 | 1.01 | 1.10 | 1.20 | 108.57 | 2.37 | 8.44 | 0.02 | 8.72 | 0.26 | 0.64 | 1.37 | 0.00 |
| K+ | cmol kg−1 | 0.16 | 0.12 | 0.01 | 77.51 | 1.56 | 3.95 | 0.01 | 0.92 | 0.07 | 0.13 | 0.23 | 0.00 |
| Na+ | cmol kg−1 | 0.01 | 0.02 | 0.00 | 343.71 | 4.77 | 26.14 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 |
| Basicity | cmol kg−1 | 4.72 | 5.12 | 26.23 | 108.56 | 2.23 | 6.75 | 0.25 | 36.97 | 1.24 | 2.66 | 6.90 | 0.00 |
| EBP | % | 74.25 | 21.99 | 483.34 | 29.61 | −0.40 | −1.14 | 15.06 | 100.00 | 55.33 | 79.03 | 94.96 | 0.00 |
| Al+3 | cmol kg−1 | 0.53 | 0.43 | 0.18 | 80.73 | 1.61 | 9.47 | 0.00 | 4.23 | 0.21 | 0.46 | 0.87 | 0.00 |
| H+ | cmol kg−1 | 0.21 | 0.52 | 0.27 | 248.69 | 21.21 | 478.46 | 0.00 | 12.00 | 0.10 | 0.18 | 0.25 | 0.00 |
| Acidity | cmol kg−1 | 0.74 | 0.71 | 0.50 | 95.84 | 7.99 | 123.05 | 0.00 | 12.18 | 0.34 | 0.71 | 1.10 | 0.00 |
| EAP | % | 25.75 | 21.99 | 483.34 | 85.37 | 0.40 | −1.14 | 0.00 | 84.94 | 5.04 | 20.98 | 44.68 | 0.00 |
| ECEC | cmol kg−1 | 5.46 | 4.88 | 23.84 | 89.49 | 2.32 | 7.43 | 0.81 | 36.97 | 2.30 | 3.47 | 7.38 | 0.00 |
| CEC | cmol kg−1 | 16.83 | 1.56 | 2.42 | 9.24 | 0.46 | −0.17 | 13.00 | 22.40 | 15.68 | 16.70 | 17.80 | 0.00 |
| CEC-ECEC | cmol kg−1 | 11.38 | 5.38 | 28.91 | 47.26 | −1.95 | 5.90 | −21.57 | 20.39 | 9.24 | 12.87 | 14.94 | 0.00 |
| ECP | % | 52.41 | 21.54 | 464.08 | 41.10 | −0.03 | −1.12 | 0.62 | 93.04 | 33.43 | 52.33 | 71.60 | 0.00 |
| EMP | % | 17.49 | 10.74 | 115.33 | 61.41 | 1.70 | 4.93 | 1.21 | 83.33 | 10.31 | 15.00 | 22.00 | 0.00 |
| EPP | % | 4.23 | 4.01 | 16.10 | 94.95 | 2.40 | 8.28 | 0.17 | 28.84 | 1.56 | 3.09 | 5.47 | 0.00 |
| ESP | % | 0.12 | 0.52 | 0.27 | 419.77 | 6.12 | 42.31 | 0.00 | 4.76 | 0.00 | 0.00 | 0.00 | 0.00 |
| CMK | unit | 44.22 | 61.69 | 3805.60 | 139.51 | 3.46 | 16.71 | 1.20 | 545.50 | 9.57 | 22.06 | 49.12 | 0.00 |
| CM | unit | 4.32 | 3.83 | 14.65 | 88.63 | 3.19 | 14.77 | 0.01 | 32.14 | 2.14 | 3.35 | 5.09 | 0.00 |
| MK | unit | 10.36 | 15.05 | 226.50 | 145.27 | 3.50 | 17.32 | 0.22 | 141.50 | 2.25 | 4.71 | 11.67 | 0.00 |
| BD | g cm−3 | 1.11 | 0.06 | 0.00 | 5.20 | −0.04 | −0.49 | 0.95 | 1.28 | 1.07 | 1.11 | 1.16 | 0.00 |
| Method | Name | Mean | SD | Var | CV | Skewness | Kurtosis | Min | P25 | Median | P75 | Max | Shapiro |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined | MC | 0.18 | 0.28 | 0.08 | 158.95 | 1.56 | 1.72 | 0.00 | 0.00 | 0.00 | 0.33 | 1.4 | 0.00 |
| Cate and Nelson | MX | 0.79 | 0.60 | 0.36 | 75.92 | 0.59 | 0.07 | 0.00 | 0.32 | 0.69 | 1.31 | 3.23 | 0.00 |
| NuMaSS | NM | 0.29 | 0.46 | 0.21 | 158.09 | 1.53 | 1.61 | 0.00 | 0.00 | 0.00 | 0.55 | 2.34 | 0.00 |
| Bases Saturation | MSB | 0.71 | 1.38 | 1.90 | 194.15 | 2.20 | 4.58 | 0.00 | 0.00 | 0.00 | 0.78 | 7.55 | 0.00 |
| Minas Gerais 5A | MG5A | 1.04 | 1.36 | 1.86 | 130.89 | 0.66 | −1.37 | 0.00 | 0.00 | 0.00 | 2.58 | 4.25 | 0.00 |
| Integration of pH and organic material | MPM | 0.91 | 0.76 | 0.57 | 83.03 | 1.05 | 0.74 | 0.00 | 0.33 | 0.76 | 1.32 | 3.69 | 0.00 |
| Potential acidity | MAC | 0.47 | 0.40 | 0.14 | 78.75 | 1.20 | 5.54 | 0.00 | 0.17 | 0.44 | 0.75 | 3.29 | 0.00 |
| Modified potential acidity | MACM | 1.06 | 0.83 | 0.69 | 78.30 | 0.86 | 3.92 | 0.00 | 0.41 | 1.04 | 1.76 | 7.16 | 0.00 |
| Soil Property | Model | Nugget | Sill | Range | PSV | Cross-Validation | ||
|---|---|---|---|---|---|---|---|---|
| C0 | C0 + C | m | (C/C0 + C) | 1 R2 | 2 RMSE | 3 MAE | ||
| Clay (%) | Exponential | 0.13 | 0.28 | 31,826.72 | 0.54 | 0.36 | 0.41 | 0.33 |
| Silt (%) | Exponential | 0.11 | 0.46 | 31,826.72 | 0.77 | 0.49 | 0.50 | 0.40 |
| Sand (%) | Exponential | 0.08 | 0.15 | 31,826.72 | 0.47 | 0.35 | 0.09 | 0.07 |
| pH | Spherical | 0.32 | 0.64 | 31,826.72 | 0.50 | 0.85 | 0.10 | 0.08 |
| OM (%) | Exponential | 2.32 | 4.56 | 31,826.72 | 0.49 | 0.78 | 0.40 | 0.32 |
| EC (dS m−1) | Exponential | 0.22 | 0.49 | 31,826.72 | 0.55 | 0.49 | 0.15 | 0.12 |
| P Bray (mg kg−1) | Linear | 5.84 | 6.94 | 31,826.72 | 0.16 | 0.63 | 0.52 | 0.41 |
| K (mg kg−1) | Gaussian | 0.73 | 0.81 | 31,826.72 | 0.10 | 0.62 | 0.27 | 0.22 |
| N (%) | Gaussian | 0.01 | 0.01 | 31,826.72 | 0.38 | 0.78 | 0.07 | 0.06 |
| H+ (mEq 100 g−1) | Spherical | 0.01 | 0.02 | 31,826.72 | 0.33 | 0.50 | 0.21 | 0.17 |
| Al+3 (mEq 100 g−1) | Spherical | 0.09 | 0.15 | 31,826.72 | 0.41 | 0.81 | 0.01 | 0.00 |
| CECe (mEq 100 g−1) | Exponential | 0.41 | 0.94 | 31,826.72 | 0.56 | 0.27 | 0.68 | 0.54 |
| Ca+2 (mEq 100 g−1) | Gaussian | 0.37 | 0.63 | 31,826.72 | 0.41 | 0.77 | 0.23 | 0.18 |
| Mg+2 (mEq 100 g−1) | Linear | 0.53 | 0.67 | 31,826.72 | 0.22 | 0.63 | 0.27 | 0.21 |
| K+ (mEq 100 g−1) | Spherical | 0.00 | 0.01 | 31,826.72 | 0.73 | 0.82 | 0.03 | 0.03 |
| Na+ (mEq 100 g−1) | Spherical | 0.02 | 0.03 | 31,826.72 | 0.39 | 0.54 | 0.16 | 0.13 |
| BD | Exponential | 0.02 | 0.18 | 31,826.72 | 0.87 | 0.71 | 0.04 | 0.03 |
| Acidity | Exponential | 0.67 | 2.12 | 31,826.72 | 0.68 | 0.35 | 0.09 | 0.07 |
| Basicity | Exponential | 0.13 | 0.22 | 31,826.72 | 0.41 | 0.37 | 0.38 | 0.31 |
| EAP (%) | Exponential | 0.66 | 2.07 | 31,826.72 | 0.68 | 0.50 | 0.98 | 0.79 |
| ECP (%) | Exponential | 0.18 | 0.27 | 31,826.72 | 0.35 | 0.26 | 0.44 | 0.36 |
| EMP (%) | Exponential | 44.76 | 69.23 | 31,826.72 | 0.35 | 0.62 | 1.67 | 1.33 |
| EPP (%) | Exponential | 6.74 | 9.21 | 31,826.72 | 0.27 | 0.83 | 1.66 | 1.33 |
| ESP (%) | Exponential | 0.03 | 0.07 | 31,826.72 | 0.60 | 0.22 | 0.23 | 0.18 |
| Lime and DAP Requirement | Model | Nugget | Sill | Range | PSV | Cross-Validation | ||
|---|---|---|---|---|---|---|---|---|
| C0 | C0 + C | m | (C/C0 + C) | 1 R2 | 2 RMSE | 3 MAE | ||
| MC (t ha−1) | Exponential | 0.02 | 0.07 | 31,826.72 | 0.74 | 0.26 | 0.25 | 0.19 |
| MX (t ha−1) | Exponential | 0.06 | 0.12 | 31,826.72 | 0.53 | 0.35 | 0.27 | 0.22 |
| NM (t ha−1) | Exponential | 0.09 | 0.16 | 31,826.72 | 0.45 | 0.32 | 0.38 | 0.30 |
| MSB (t ha−1) | Exponential | 0.22 | 0.31 | 31,826.72 | 0.27 | 0.26 | 0.48 | 0.38 |
| MG (t ha−1) | Exponential | 0.89 | 1.73 | 31,826.72 | 0.49 | 0.53 | 0.93 | 0.83 |
| MPM (t ha−1) | Exponential | 0.04 | 0.95 | 31,826.72 | 0.96 | 0.79 | 0.35 | 0.41 |
| MAC (t ha−1) | Gaussian | 0.05 | 0.17 | 31,826.72 | 0.73 | 0.59 | 0.34 | 0.30 |
| MACM (t ha−1) | Spherical | 0.20 | 1.08 | 31,826.72 | 0.81 | 0.56 | 0.84 | 0.60 |
| DAP (Kg ha−1) | Spherical | 0.15 | 0.15 | 31,826.72 | 0.00 | 0.74 | 0.37 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe, K.; Hermoza, N.; Mejia, S.; Romero-Chavez, L.E.; Ottos, E.; Arce, A.; Solórzano Acosta, R. Spatial Analysis of Soil Acidity and Available Phosphorus in Coffee-Growing Areas of Pichanaqui: Implications for Liming and Site-Specific Fertilization. Agriculture 2025, 15, 1632. https://doi.org/10.3390/agriculture15151632
Quispe K, Hermoza N, Mejia S, Romero-Chavez LE, Ottos E, Arce A, Solórzano Acosta R. Spatial Analysis of Soil Acidity and Available Phosphorus in Coffee-Growing Areas of Pichanaqui: Implications for Liming and Site-Specific Fertilization. Agriculture. 2025; 15(15):1632. https://doi.org/10.3390/agriculture15151632
Chicago/Turabian StyleQuispe, Kenyi, Nilton Hermoza, Sharon Mejia, Lorena Estefani Romero-Chavez, Elvis Ottos, Andrés Arce, and Richard Solórzano Acosta. 2025. "Spatial Analysis of Soil Acidity and Available Phosphorus in Coffee-Growing Areas of Pichanaqui: Implications for Liming and Site-Specific Fertilization" Agriculture 15, no. 15: 1632. https://doi.org/10.3390/agriculture15151632
APA StyleQuispe, K., Hermoza, N., Mejia, S., Romero-Chavez, L. E., Ottos, E., Arce, A., & Solórzano Acosta, R. (2025). Spatial Analysis of Soil Acidity and Available Phosphorus in Coffee-Growing Areas of Pichanaqui: Implications for Liming and Site-Specific Fertilization. Agriculture, 15(15), 1632. https://doi.org/10.3390/agriculture15151632






