Climate-Induced Heat Stress Responses on Indigenous Varieties and Elite Hybrids of Mango (Mangifera indica L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Sampling
2.2. Meteorological and Phenology Data Recording
2.3. Total Heat Accumulation (THA)
2.4. Varieties and Hybrids
2.5. Estimation of Percent Incidence of Sunburn Symptoms
2.6. Biochemical Characterization
2.6.1. Assessment of Maturity Index
2.6.2. Estimation of Total Acidity
2.6.3. Quantification of Phenolic Compounds Using High-Pressure Liquid Chromatography (HPLC)
2.6.4. Aroma Profiling
2.6.5. Volatile Organic Compound Profiling Using Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.6.6. Statistical Analysis
3. Results
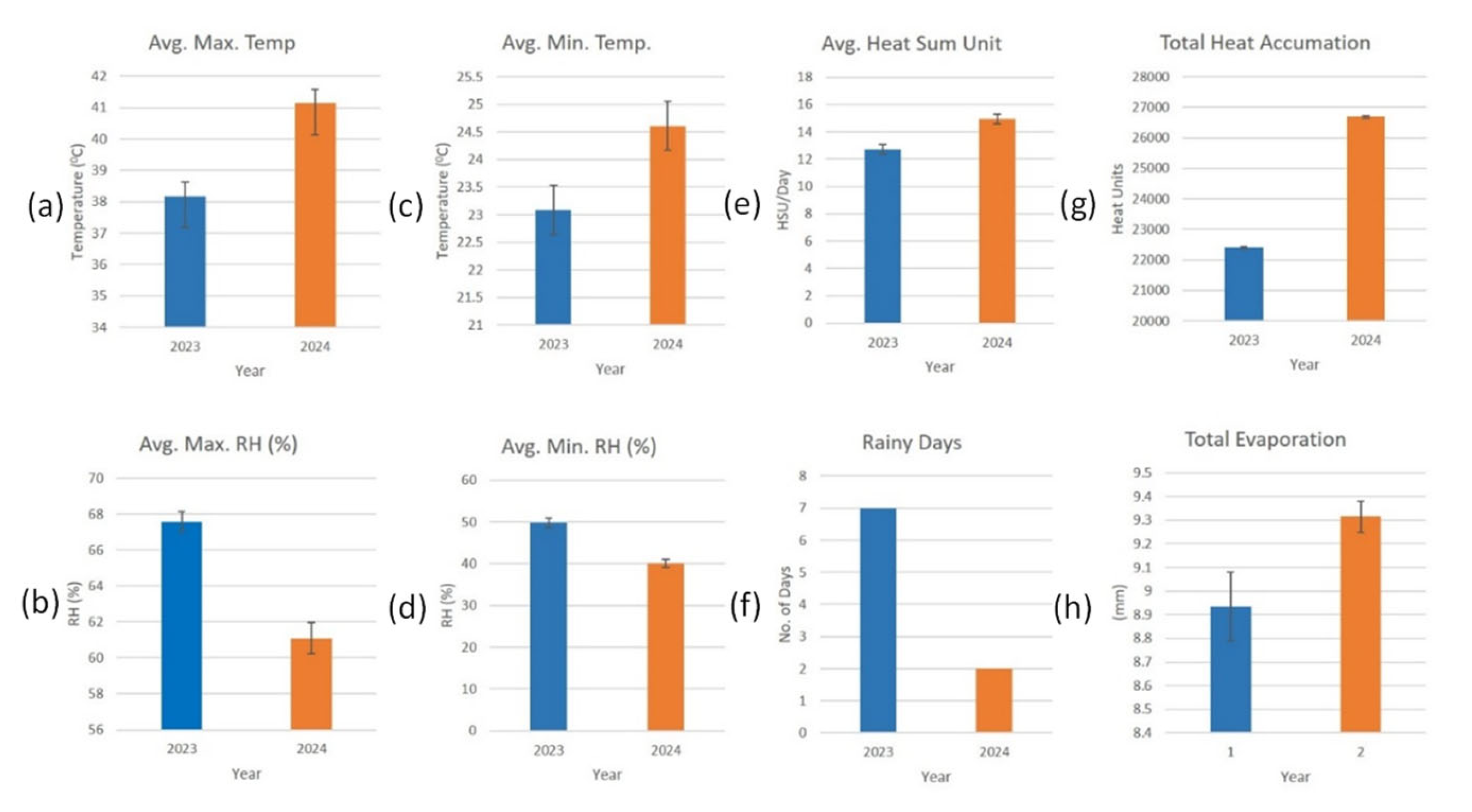
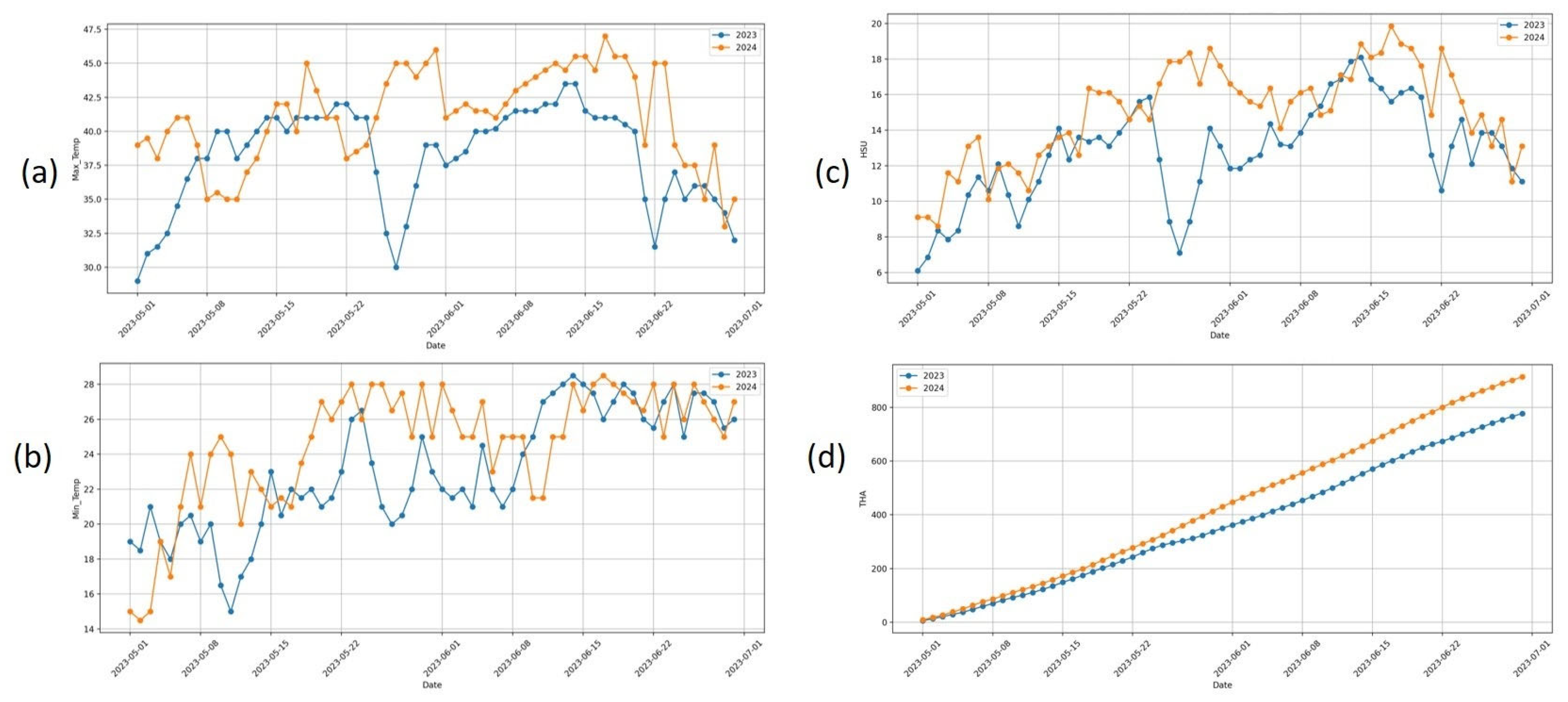
3.1. Comparative Evaluation of Meteorological Parameters During 2023 and 2024
3.2. Impact of Excessive Heat on the Phenology of Mango
3.3. Incidence of the Symptoms
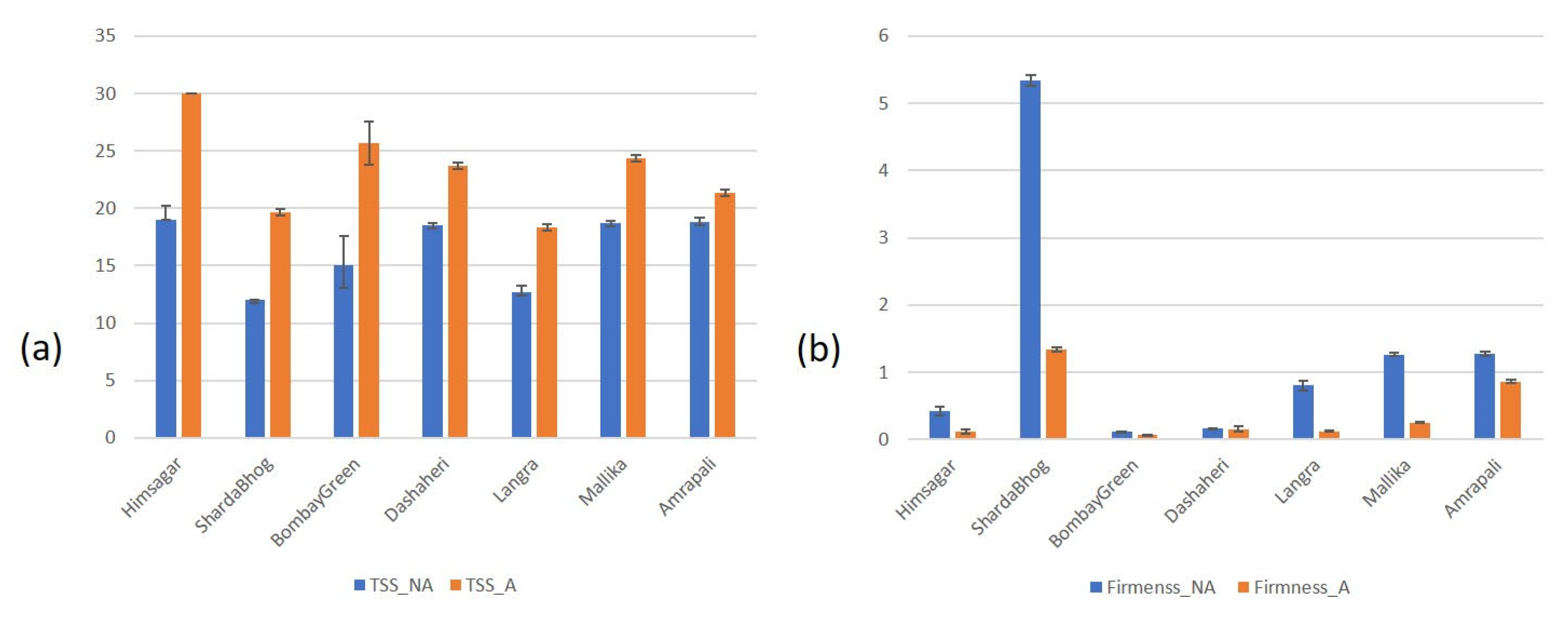
3.4. TSS and Firmness in Affected vs. Non-Affected Tissue
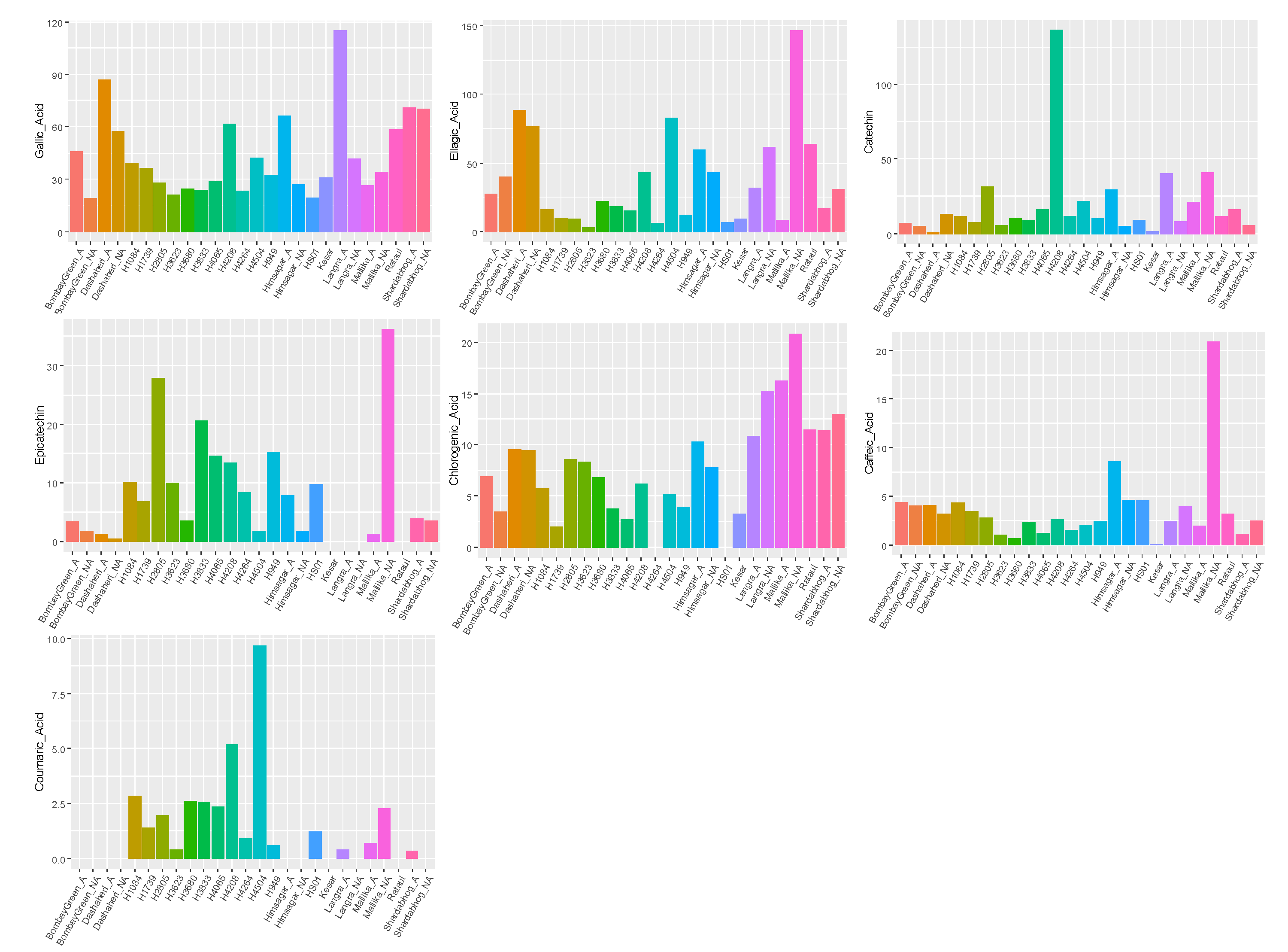
3.5. Change in Phenolic Compound Content
3.6. Change in Aroma Profile of Severely Affected Genotypes
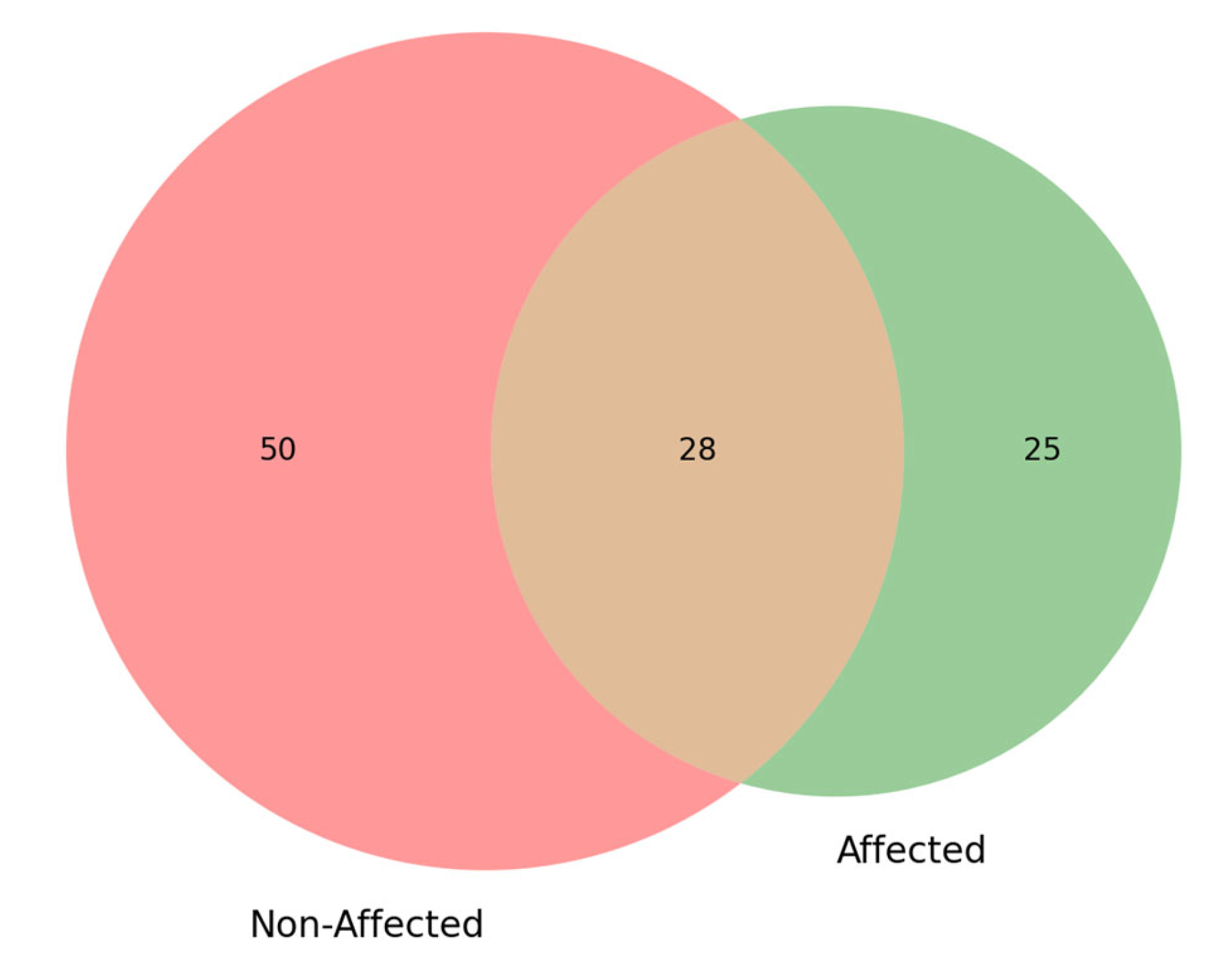
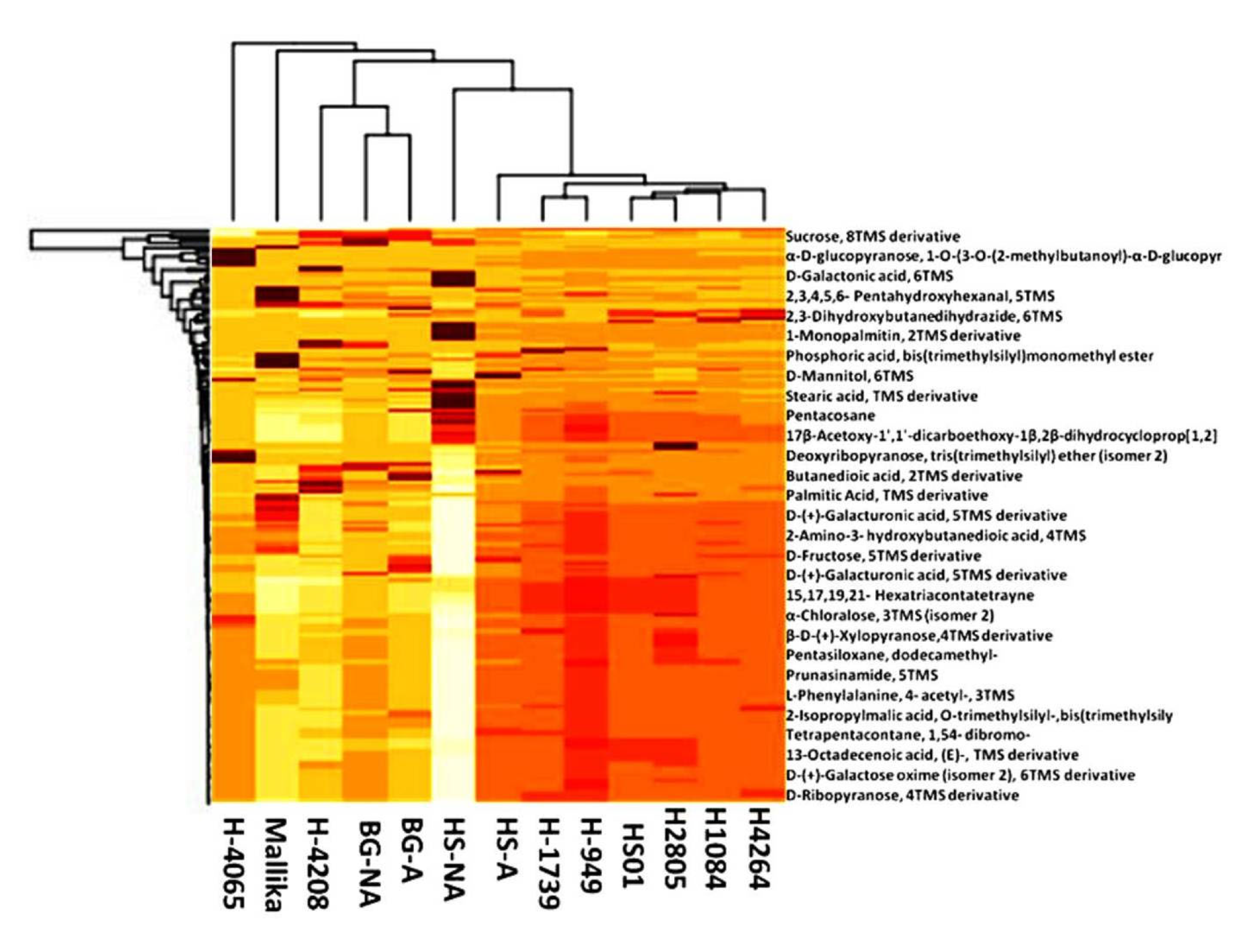
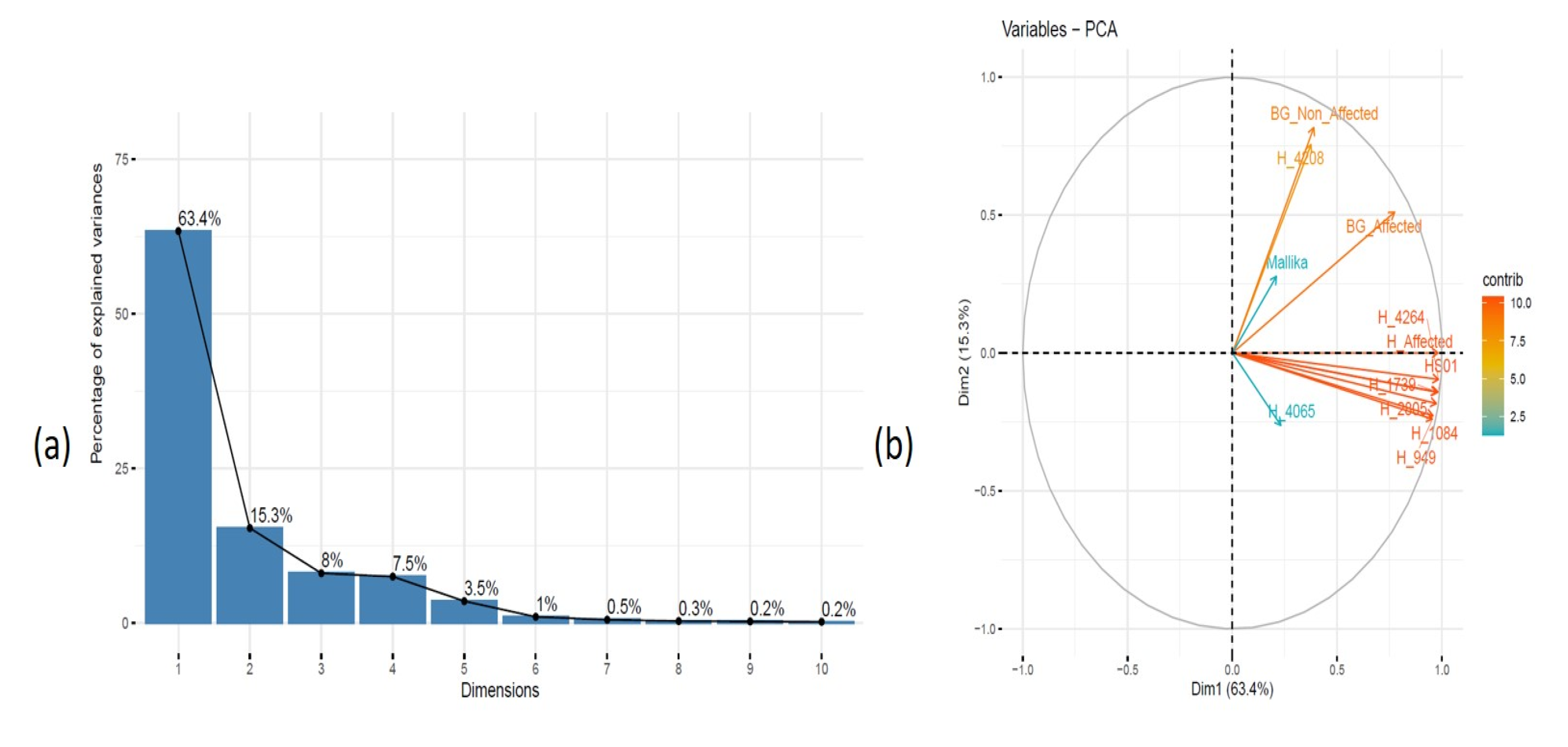
3.7. Differentially Expressed Compound Detection via GC-MS Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBCH | BiologischeBundesanstalt, Bundessortenamt und Chemische Industrie |
| GC-MS | Gas Chromatography-Mass Spectrometry |
References
- El Khayat, M.; Halwani, D.A.; Hneiny, L.; Alameddine, I.; Haidar, M.A.; Habib, R.R. Impacts of climate change and heat stress on farmworkers’ health: A scoping review. Front. Public Health 2022, 10, 782811. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Scientific Research: Wuhan, China, 2019; Volume 1, pp. 93–174. [Google Scholar]
- Kumari, M.; Singh, A.K.; Dubey, K. Biotic and abiotic stress management in scientific cultivation of Mango (Mangifera indica L.) for sustainable yield and quality with environmental safety. J. Pharmacogn. Phytochem. 2020, 9, 392–396. [Google Scholar]
- Vanalli, C.; Casagrandi, R.; Gatto, M.; Bevacqua, D. Shifts in the thermal niche of fruit trees under climate change: The case of peach cultivation in France. Agric. For. Meteorol. 2021, 300, 108327. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 27 December 2024).
- Laxman, R.H.; Annapoornamma, C.J.; Biradar, G. Mango. In Abiotic Stress Physiology of Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2016; pp. 169–181. [Google Scholar]
- Zhang, H.; Liu, Z.; Luo, R.; Sun, Y.; Yang, C.; Li, X.; Gao, A.; Pu, J. Genome-wide characterization, identification and expression profile of MYB transcription factor gene family during abiotic and biotic stresses in mango (Mangifera indica). Plants 2022, 11, 3141. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Muthamil, S.; Shilpha, J.; Venkatramanan, V.; Priya, A.; Kim, J.; Shin, Y.; Chen, J.T.; Baskar, V.; Park, K.; et al. Molecular insights into abiotic stresses in mango. Plants 2023, 12, 1939. [Google Scholar] [CrossRef]
- Manzoor, M.A.; Xu, Y.; Xu, J.; Wang, Y.; Sun, W.; Liu, X.; Wang, L.; Wang, J.; Liu, R.; Whiting, M.D.; et al. Fruit crop abiotic stress management: A comprehensive review of plant hormone-mediated responses. Fruit Res. 2023, 3, 1–18. [Google Scholar] [CrossRef]
- Kumar Dwivedi, S.; Mishra, D.; Kumar Gupta, A.; Dayal, V.; Kumar, D.; Kumar Singh, S.; Saroj, P.L.; Soni, S.K. Jelly seed disorder in mango: A comprehensive review of current status and future directions. Appl. Fruit Sci. 2024, 66, 1659–1668. [Google Scholar] [CrossRef]
- Oak, P.; Deshpande, A.; Giri, A.; Gupta, V. Metabolomic dynamics reveals oxidative stress in spongy tissue disorder during ripening of Mangifera indica L. fruit. Metabolites 2019, 9, 255. [Google Scholar] [CrossRef]
- Léchaudel, M.; Lopez-Lauri, F.; Vidal, V.; Sallanon, H.; Joas, J. Response of the physiological parameters of mango fruit (transpiration, water relations, and antioxidant system) to its light and temperature environment. J. Plant Physiol. 2013, 170, 567–576. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2015, 37, 1718. [Google Scholar] [CrossRef]
- Sharma, M.; Negi, S.; Kumar, P.; Srivastava, D.K.; Choudhary, M.K.; Irfan, M. Fruit ripening under heat stress: The intriguing role of ethylene-mediated signaling. Plant Sci. 2023, 335, 111820. [Google Scholar] [CrossRef] [PubMed]
- Khanum, Z.; Tiznado-Hernández, M.E.; Ali, A.; Musharraf, S.G.; Shakeel, M.; Khan, I.A. Adaptation mechanism of mango fruit (Mangifera indica L. cv. Chaunsa White) to heat suggests modulation in several metabolic pathways. RSC Adv. 2020, 10, 35531–35544. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Saini, M.K. Postharvest vapour heat treatment as a phytosanitary measure influences the aroma volatiles profile of mango fruit. Food Chem. 2014, 164, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Piao, S.; Fu, Y.H.; Gao, M.; Peñuelas, J.; Janssens, I.A. Climatic warming increases spatial synchrony in spring vegetation phenology across the Northern Hemisphere. Geophys. Res. Lett. 2019, 46, 1641–1650. [Google Scholar] [CrossRef]
- Kumar, U.; Hansen, E.M.; Thomsen, I.K.; Vogeler, I. Performance of APSIM to simulate the dynamics of winter wheat growth, phenology, and nitrogen uptake from early growth stages to maturity in Northern Europe. Plants 2023, 12, 986. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Lorite, I.J.; Cabezas-Luque, J.M.; Arquero, O.; Gabaldón-Leal, C.; Santos, C.; Rodríguez, A.; Ruiz-Ramos, M.; Lovera, M. The role of phenology in the climate change impacts and adaptation strategies for tree crops: A case study on almond orchards in Southern Europe. Agric. For. Meteorol. 2020, 294, 108142. [Google Scholar] [CrossRef]
- Pelletier, V.; Pepin, S.; Gallichand, J.; Caron, J. Reducing cranberry heat stress and midday depression with evaporative cooling. Sci. Hortic. 2016, 198, 445–453. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.H. Salicylic acid-induced heat or cold tolerance in relation to Ca²⁺ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Almeida, T.; Pinto, G.; Correia, B.; Gonçalves, S.; Meijon, M.; Escandón, M. In-depth analysis of the Quercus suber metabolome under drought stress and recovery reveals potential key metabolic players. Plant Sci. 2020, 299, 110606. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.T.; Xu, Y. Unraveling proteomic adaptations to moderate heat stress in Arabidopsis thaliana: Insights for developing thermotolerant crops. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.C.; Santos, C.; Silva, A.M.; Dias, M.C. Modulation of phenolic and lipophilic compounds of olive fruits in response to combined drought and heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.K.; Maurya, N.K.; Goswami, S.; Bardhan, K.; Singh, S.K.; Prakash, J.; Pradhan, S.; Kumar, A.; Chinnusamy, V.; Kumar, P.; et al. Physio-biochemical and molecular stress regulators and their crosstalk for low-temperature stress responses in fruit crops: A review. Front. Plant Sci. 2022, 13, 1022167. [Google Scholar] [CrossRef]
- Rachappanavar, V.; Padiyal, A.; Sharma, J.K.; Gupta, S.K. Plant hormone-mediated stress regulation responses in fruit crops—A review. Sci. Hortic. 2022, 304, 111302. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The role of polyphenols in abiotic stress tolerance and their antioxidant properties to scavenge reactive oxygen species and free radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plant phenolics in abiotic stress tolerance: An overview. Plant Signal. Mol. 2019, 157–168. [Google Scholar] [CrossRef]
- Chalker-Scott, L.; Fuchigami, L.H. The role of phenolic compounds in plant stress responses. In Low Temperature Stress Physiology in Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–80. [Google Scholar]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Rajan, S.; Tiwari, D.; Singh, V.K.; Saxena, P.; Singh, S.; Reddy, Y.T.N.; Upreti, K.K.; Burondkar, M.M.; Bhagwan, A.; Kennedy, R. Application of extended BBCH scale for phenological studies in mango (Mangifera indica L.). J. Appl. Hortic. 2011, 13, 108–114. [Google Scholar] [CrossRef]
- Oppenheimer, C. The acclimatization of new tropical and sub-tropical fruit trees in Palestine. Bull. Agric. Res. Stat. Rehovot 1947, 44, 184–189. [Google Scholar]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- WMO Confirms 2024 as Warmest Year on Record at About 1.55°C Above Pre-Industrial Level. Available online: https://wmo.int/news/media-centre/wmo-confirms-2024-warmest-year-record-about-155degc-above-pre-industrial-level (accessed on 14 January 2025).
- Meier, M.; Vitasse, Y.; Bugmann, H.; Bigler, C. Phenological shifts induced by climate change amplify drought for broad-leaved trees at low elevations in Switzerland. Agric. For. Meteorol. 2021, 307, 108485. [Google Scholar] [CrossRef]
- Gordo, O.; Sanz, J.J. Phenology and climate change: A long-term study in a Mediterranean locality. Oecologia 2005, 146, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, Y.; Soni, S.K.; Bajpai, A.; Tiwari, S.; Rastogi, L.; Trivedi, M. Patterns of shifting phenostages and flowering time in mango (Mangifera indica L.) due to climate change. Preprints, 2023. [Google Scholar] [CrossRef]
- Makhmale, S.; Bhutada, P.; Yadav, L.; Yadav, B.K. Impact of climate change on phenology of mango—The case study. Ecol. Environ. Conserv. 2016, 22, S127–S132. [Google Scholar]
- Oliveira, M.B.; Figueiredo, M.G.F.; Pereira, M.C.T.; do Carmo Mouco, M.A.; Ribeiro, L.M.; Mercadante-Simões, M.O. Structural and cytological aspects of mango floral induction using paclobutrazol. Sci. Hortic. 2020, 262, 109057. [Google Scholar] [CrossRef]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the relevance of high temperature and elevated CO₂ in plant secondary metabolites (PSMs) production. Heliyon 2021, 7, e07709. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- RA, D. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Some physiological changes in strawberry (Fragaria × ananassa ‘Camarosa’) plants under heat stress. J.Hortic. Sci. Biotechnol. 2003, 78, 894–898. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Khan, N.A.; Umar, S. Regulatory role of proline in heat stress tolerance: Modulation by salicylic acid. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 437–448. [Google Scholar]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.O. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Exp. Environ. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Heena; Kaushal, S.; Kaur, V.; Panwar, H.; Sharma, P.; Jangra, R. Isolation of quinic acid from dropped Citrus reticulata Blanco fruits: Its derivatization, antibacterial potential, docking studies, and ADMET profiling. Front. Chem. 2024, 12, 1372560. [Google Scholar] [CrossRef]
- Arya, A.; Al-Obaidi, M.M.J.; Shahid, N.; Noordin, M.I.B.; Looi, C.Y.; Wong, W.F.; Khaing, S.L.; Mustafa, M.R. Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney, and pancreas tissues of STZ-induced diabetic rats: A mechanistic study. Food Chem. Toxicol. 2014, 71, 183–196. [Google Scholar] [CrossRef]
- Krämer, M.; Bongaerts, J.; Bovenberg, R.; Kremer, S.; Müller, U.; Orf, S.; Wubbolts, M.; Raeven, L. Metabolic engineering for microbial production of shikimic acid. Metab. Eng. 2003, 5, 277–283. [Google Scholar] [CrossRef]
- Karaman, M.; Tesanovic, K.; Gorjanovic, S.; Pastor, F.T.; Simonovic, M.; Glumac, M.; Pejin, B. Polarography as a technique of choice for the evaluation of total antioxidant activity: The case study of selected Coprinus comatus extracts and quinic acid, their antidiabetic ingredient. Nat. Prod. Res. 2021, 35, 1711–1716. [Google Scholar] [CrossRef]
- Pero, R.W.; Lund, H.; Leanderson, T. Antioxidant metabolism induced by quinic acid: Increased urinary excretion of tryptophan and nicotinamide. Antioxid. Metab. 2008, 23, 335–346. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; El-Otmani, M.; Agustí, M. Examining the impact of dry climates temperature on citrus fruit internal ripening. Sci. Hortic. 2024, 337, 113501. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, L.; Liu, Z.; Zhou, J. CAS1-synthesized β-Caryophyllene enhances broad-spectrum stress resistance in tomatoes. Plant Physiol. Biochem. 2025, 222, 109726. [Google Scholar] [CrossRef] [PubMed]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; García-Sánchez, F. Physiological, nutritional and metabolomic responses of tomato plants after the foliar application of amino acids aspartic acid, glutamic acid, and alanine. Front. Plant Sci. 2021, 11, 2138. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Zheng, W.; Tan, J.; Li, Z.; Wu, M.; Hong, S.-B.; Zhao, Y.; Zhu, Z.; Zang, Y. Glutamic acid and poly-γ-glutamic acid enhanced the heat resistance of Chinese cabbage (Brassica rapa L. ssp. pekinensis) by improving carotenoid biosynthesis, photosynthesis, and ROS signaling. Int. J. Mol. Sci. 2022, 23, 11671. [Google Scholar] [CrossRef]
- Ulukapi, K.; Nasircilar, A.G. The role of exogenous glutamine on germination, plant development, and transcriptional expression of some stress-related genes in onion under salt stress. Folia Hortic. 2024, 35, 1. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef]
- Babaei, M.; Shabani, L.; Hashemi-Shahraki, S. Improving the effects of salt stress by β-carotene and gallic acid using increased antioxidant activity and regulating ion uptake in Lepidium sativum. L. Bot. Stud. 2022, 63, 22. [Google Scholar]
- Saidi, I.; Guesmi, F.; Kharbech, O.; Hfaiedh, N.; Djebali, W. Gallic acid improves the antioxidant ability against cadmium toxicity: Impact on leaf lipid composition of sunflower (Helianthus annuus) seedlings. Ecotoxicol. Environ. Saf. 2021, 210, 111906. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. Improvement of cold stress resistance via free radical scavenging ability and promoted water status and photosynthetic capacity of gallic acid in soybean leaves. J. Soil Sci. Plant Nutr. 2017, 17, 366–384. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.; Cai, Y.; Wakisaka, M.; Yang, Z.; Yin, Y.; Fang, W.; Xu, Y.; Omura, T.; Yu, R.; Zheng, A.L.T. Mitigation of oxidative stress damage caused by abiotic stress to improve biomass yield of microalgae: A review. Sci. Total Environ. 2023, 896, 165200. [Google Scholar] [CrossRef]
- Xu, S.; Hossain, M.; Lau, B.B.Y.; Quynh, T.; Rawal, A.; Aldous, L. Total quantification and extraction of shikimic acid from star anise (Illicium verum) using solid-state NMR and cellulose-dissolving aqueous hydroxide solutions. Sustain. Chem. Pharm. 2017, 5, 115–121. [Google Scholar] [CrossRef]
- Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. Enhancing the enrichment of pharmacophore-based target prediction for the polypharmacological profiles of drugs. J. Chem. Inf. Model. 2016, 56, 1175–1183. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushwaha, A.K.; Thukkaram, D.; Rastogi, D.; Singh, N.S.; Beer, K.; Debnath, P.; Dayal, V.; Yadav, A.; Das, S.S.; Bajpai, A.; et al. Climate-Induced Heat Stress Responses on Indigenous Varieties and Elite Hybrids of Mango (Mangifera indica L.). Agriculture 2025, 15, 1619. https://doi.org/10.3390/agriculture15151619
Kushwaha AK, Thukkaram D, Rastogi D, Singh NS, Beer K, Debnath P, Dayal V, Yadav A, Das SS, Bajpai A, et al. Climate-Induced Heat Stress Responses on Indigenous Varieties and Elite Hybrids of Mango (Mangifera indica L.). Agriculture. 2025; 15(15):1619. https://doi.org/10.3390/agriculture15151619
Chicago/Turabian StyleKushwaha, Amar Kant, Damodaran Thukkaram, Dheerendra Rastogi, Ningthoujam Samarendra Singh, Karma Beer, Prasenjit Debnath, Vishambhar Dayal, Ashish Yadav, Swosti Suvadarsini Das, Anju Bajpai, and et al. 2025. "Climate-Induced Heat Stress Responses on Indigenous Varieties and Elite Hybrids of Mango (Mangifera indica L.)" Agriculture 15, no. 15: 1619. https://doi.org/10.3390/agriculture15151619
APA StyleKushwaha, A. K., Thukkaram, D., Rastogi, D., Singh, N. S., Beer, K., Debnath, P., Dayal, V., Yadav, A., Das, S. S., Bajpai, A., & Manoharan, M. (2025). Climate-Induced Heat Stress Responses on Indigenous Varieties and Elite Hybrids of Mango (Mangifera indica L.). Agriculture, 15(15), 1619. https://doi.org/10.3390/agriculture15151619





