Improving Dehydration Efficiency and Quality in Highbush Blueberries via Combined Pulsed Microwave Pretreatment and Osmotic Dehydration
Abstract
1. Introduction
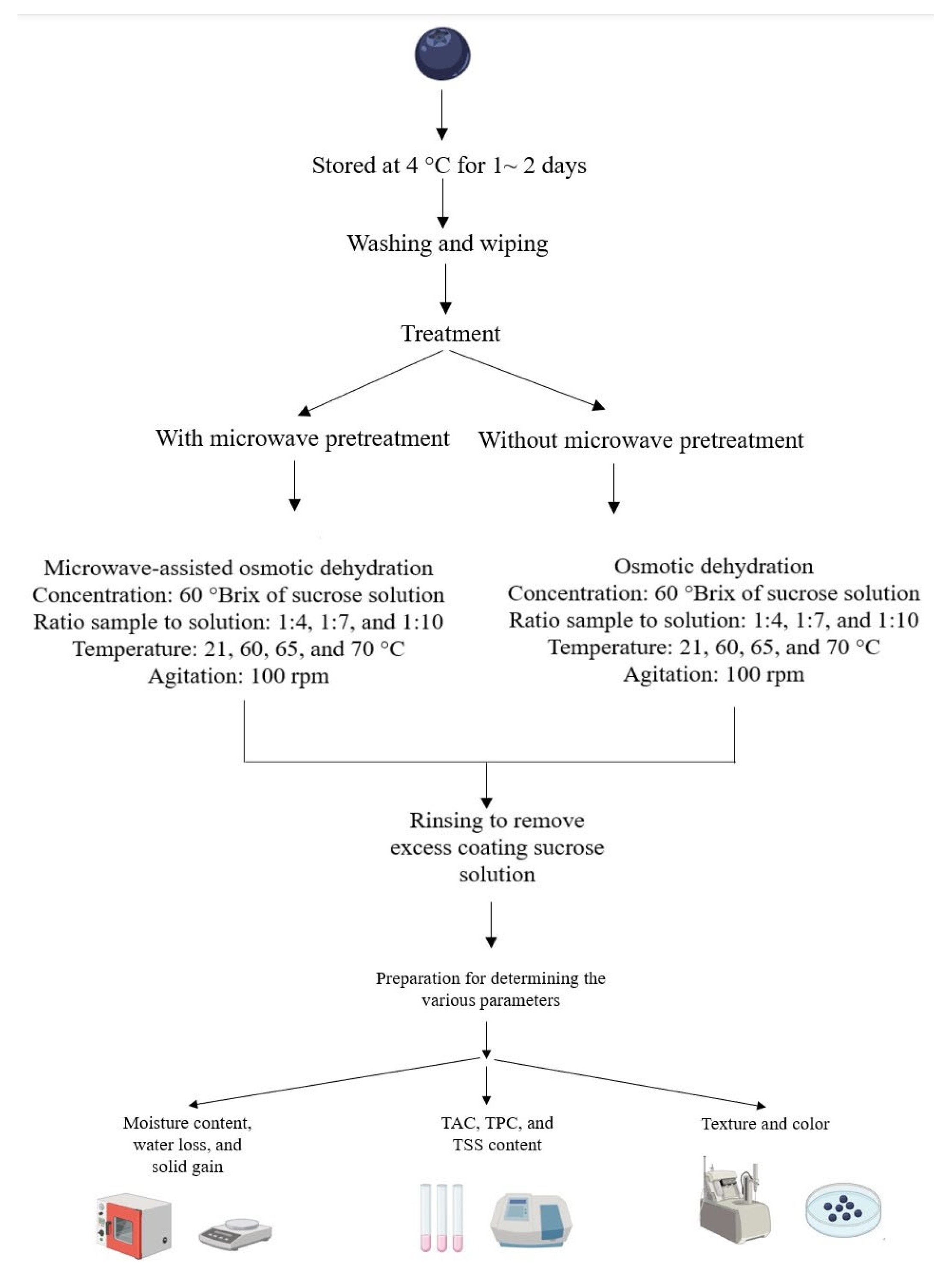
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Microwave Pretreatment
2.4. Osmotic Solution Preparation and Dehydration Procedure
2.5. Calculation of Process Efficiency Parameters
2.6. Physical and Chemical Quality Properties
2.6.1. Color Analysis
2.6.2. Texture Analysis
2.6.3. Blueberry Extraction
2.6.4. Total Anthocyanin Content (TAC)
2.6.5. Total Phenolic Content (TPC)
2.7. Statistical Analysis
3. Results and Discussions
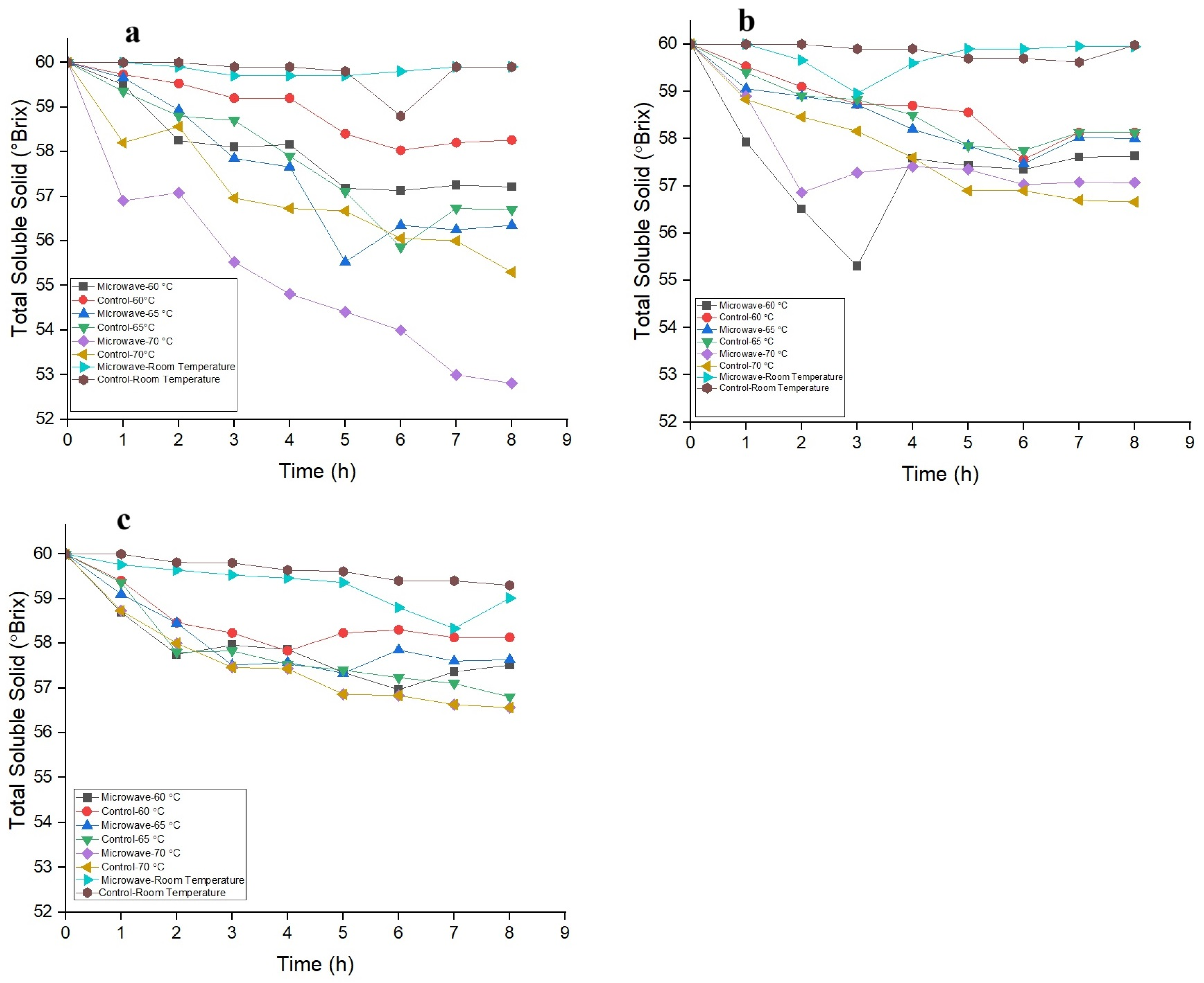
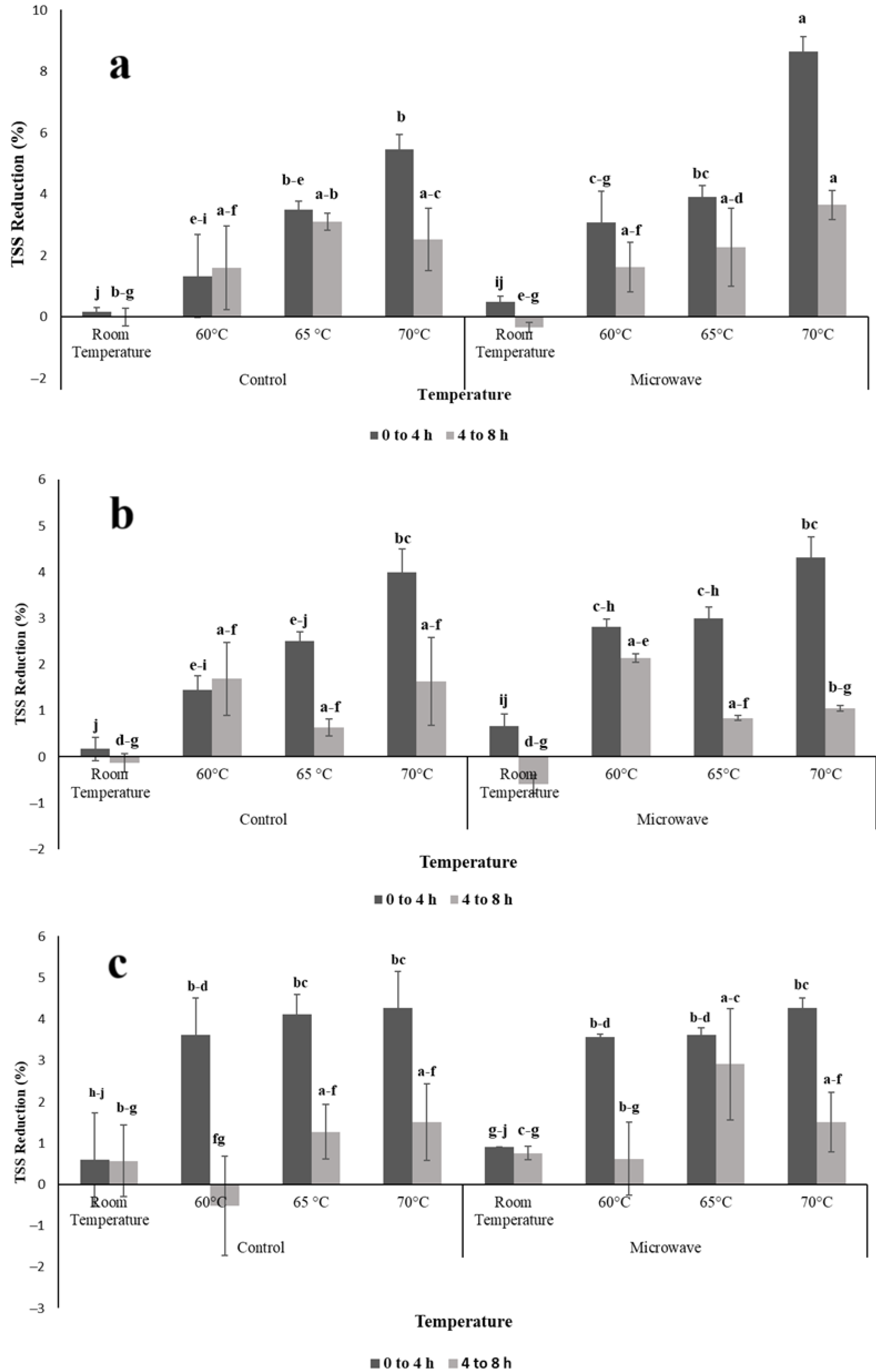
3.1. Effects of OD Parameters and Pretreatments on TSS Content of Osmotic Agent
3.2. Optimum Processing Time
3.3. Moisture Content and Total Soluble Solid of Osmotically Dehydrated Blueberry
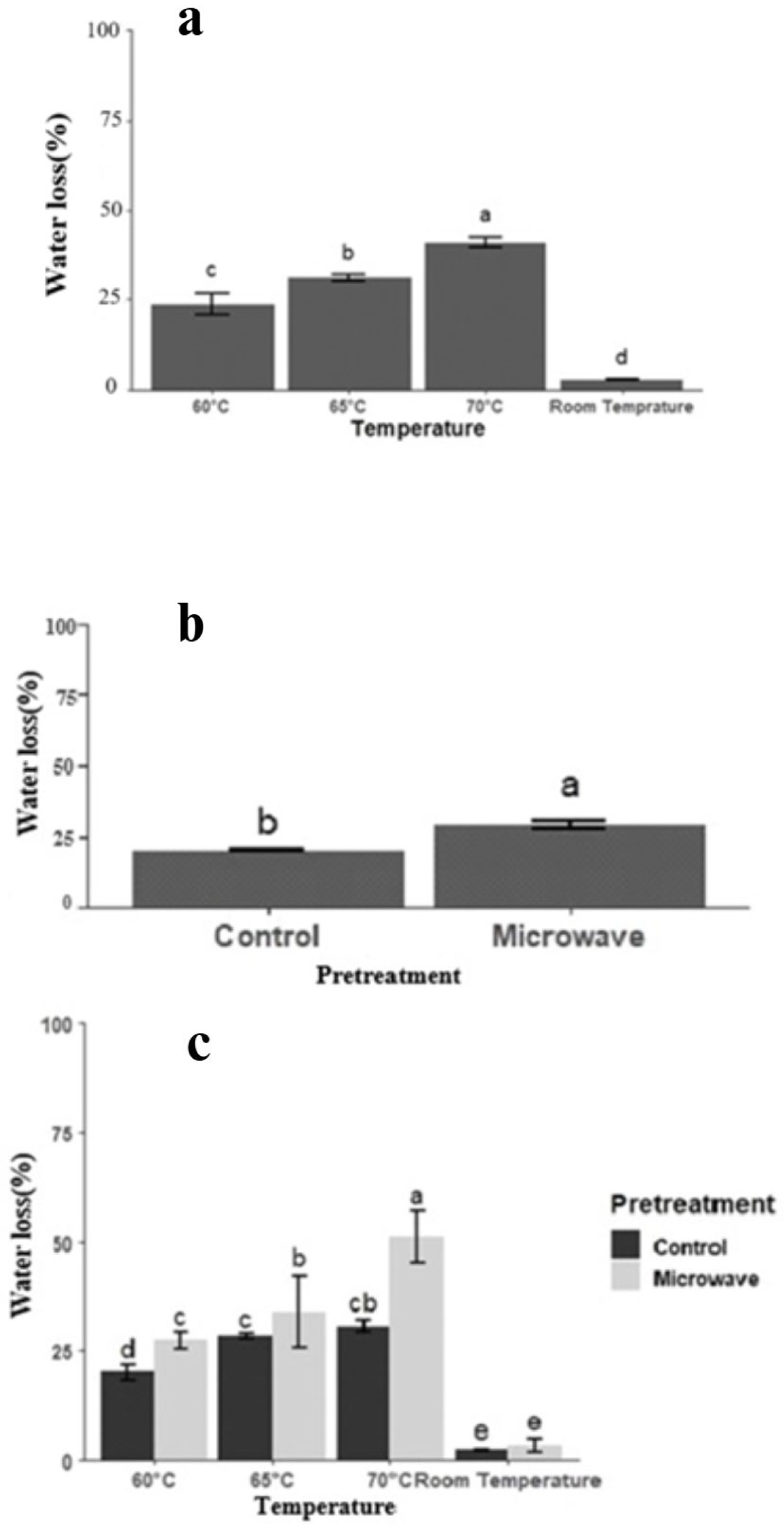
3.4. Water Loss, Solid Gain, and Quality Aspects of Osmotically Dehydrated Blueberry
3.4.1. Water Loss and Solid Gain Aspect
3.4.2. Quality Aspects
Changes in Total Anthocyanin and Phenolic Content (TAC, TPC)
Physical Properties (Texture, Color)

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashwan, A.K.; Osman, A.I.; Karim, N.; Mo, J.; Chen, W. Unveiling the Mechanisms of the Development of Blueberries-Based Functional Foods: An Updated and Comprehensive Review. Food Rev. Int. 2024, 40, 1913–1940. [Google Scholar] [CrossRef]
- Qi, J.; Sun, S.; Zhang, L.; Zhu, Y.; Zhou, H.; Gan, X.; Li, B.; Chen, Y.; Li, W.; Li, T.; et al. Seasonal Variation of Antioxidant Bioactive Compounds in Southern Highbush Blueberry Leaves and Non-Destructive Quality Prediction in Situ by a Portable near-Infrared Spectrometer. Food Chem. 2024, 457, 139925. [Google Scholar] [CrossRef]
- Li, C.; Pan, Y.; Yang, X.; Cai, D.; Pan, W.; Anwar, A.; Song, S.; Chen, R. Heterogeneous Fruiting Phenomenon of Southern Highbush Blueberry Differentiated by Branches under Efficient Cultivation Mode. Hortic. Environ. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Azevedo, C.; Benevenuto, J.; Mengist, M.F.; Luby, C.; Pottorff, M.; Casorzo, G.I.P.; Mackey, T.; Lila, M.A.; Giongo, L.; et al. Inference of the Genetic Basis of Fruit Texture in Highbush Blueberries Using Genome-Wide Association Analyses. Hortic. Res. 2024, 11, uhae233. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Tryzno-Gendek, E.; Kot, A.; Kamińska-Dwórznicka, A.; Nowak, D. Pre-Treatment Impact on Freeze-Drying Process and Properties of Dried Blueberries. Processes 2025, 13, 537. [Google Scholar] [CrossRef]
- Celli, G.B.; Dibazar, R.; Ghanem, A.; Brooks, M.S.-L. Degradation Kinetics of Anthocyanins in Freeze-Dried Microencapsulates from Lowbush Blueberries (Vaccinium angustifolium Aiton) and Prediction of Shelf-Life. Dry. Technol. 2016, 34, 1175–1184. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioproc. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, T.Z.; Fan, X.; Xu, Y. Osmotic Dehydration of Blueberries Pretreated with Pulsed Electric Fields: Effects on Dehydration Kinetics, and Microbiological and Nutritional Qualities. Dry. Technol. 2017, 35, 1543–1551. [Google Scholar] [CrossRef]
- Kumar Dash, K.; Sundarsingh, A.; BhagyaRaj, G.V.S.; Kumar Pandey, V.; Kovács, B.; Mukarram, S.A. Modelling of Ultrasonic Assisted Osmotic Dehydration of Cape Gooseberry Using Adaptive Neuro-Fuzzy Inference System (ANFIS). Ultrason. Sonochem. 2023, 96, 106425. [Google Scholar] [CrossRef]
- Yang, H.; Min, S.; Lee, S.Y.; Yang, J.; Lee, M.; Park, S.; Eun, J.; Chung, Y. Influence of Salt Concentration on Kimchi Cabbage (Brassica rapa L. Ssp. Pekinensis) Mass Transfer Kinetics and Textural and Microstructural Properties during Osmotic Dehydration. J. Food Sci. 2023, 88, 1610–1622. [Google Scholar] [CrossRef]
- Sharif, I.; Adewale, P.; Dalli, S.S.; Rakshit, S. Microwave Pretreatment and Optimization of Osmotic Dehydration of Wild Blueberries Using Response Surface Methodology. Food Chem. 2018, 269, 300–310. [Google Scholar] [CrossRef]
- Kucner, A.; Klewicki, R.; Sójka, M. The Influence of Selected Osmotic Dehydration and Pretreatment Parameters on Dry Matter and Polyphenol Content in Highbush Blueberry (Vaccinium corymbosum L.) Fruits. Food Bioproc. Technol. 2013, 6, 2031–2047. [Google Scholar] [CrossRef]
- Nuñez-Mancilla, Y.; Pérez-Won, M.; Uribe, E.; Vega-Gálvez, A.; Di Scala, K. Osmotic Dehydration under High Hydrostatic Pressure: Effects on Antioxidant Activity, Total Phenolics Compounds, Vitamin C and Colour of Strawberry (Fragaria vesca). LWT Food Sci. Technol. 2013, 52, 151–156. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Influence of Osmotic Treatment Assisted by Ultrasound on the Physico-Chemical Characteristics of Blueberries (Vaccinium myrtillus L.). Ultrasonics 2021, 110, 106298. [Google Scholar] [CrossRef]
- Barman, N.; Badwaik, L.S. Effect of Ultrasound and Centrifugal Force on Carambola (Averrhoa carambola L.) Slices during Osmotic Dehydration. Ultrason. Sonochem. 2017, 34, 37–44. [Google Scholar] [CrossRef]
- Amami, E.; Fersi, A.; Vorobiev, E.; Kechaou, N. Osmotic Dehydration of Carrot Tissue Enhanced by Pulsed Electric Field, Salt and Centrifugal Force. J. Food Eng. 2007, 83, 605–613. [Google Scholar] [CrossRef]
- Moreno, J.; Gonzales, M.; Zúñiga, P.; Petzold, G.; Mella, K.; Muñoz, O. Ohmic Heating and Pulsed Vacuum Effect on Dehydration Processes and Polyphenol Component Retention of Osmodehydrated Blueberries (Cv. Tifblue). Innov. Food Sci. Emerg. Technol. 2016, 36, 112–119. [Google Scholar] [CrossRef]
- Arballo, J.R.; Campañone, L.A. Application of Microwave Processing in the Food Industry. In Emerging Thermal Processes in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 165–226. [Google Scholar]
- Norouzi, S.; Orsat, V.; Yeasmen, N.; Dumont, M.-J. Osmotic Dehydration of Waxy Skinned Berries—A Review. Dry. Technol. 2024, 42, 1270–1294. [Google Scholar] [CrossRef]
- Zielinska, M.; Zielinska, D. Effects of Freezing, Convective and Microwave-Vacuum Drying on the Content of Bioactive Compounds and Color of Cranberries. LWT 2019, 104, 202–209. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.V. Osmotic Dehydration of Fruits and Vegetables: A Review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M. Effect of Microwave-Vacuum, Ultrasonication, and Freezing on Mass Transfer Kinetics and Diffusivity during Osmotic Dehydration of Cranberries. Dry. Technol. 2018, 36, 1158–1169. [Google Scholar] [CrossRef]
- Norouzi, S.; Fadavi, A.; Darvishi, H. The Ohmic and Conventional Heating Methods in Concentration of Sour Cherry Juice: Quality and Engineering Factors. J. Food Eng. 2021, 291, 110242. [Google Scholar] [CrossRef]
- Rosner, B. A Generalization of the Paired T-Test. Appl. Stat. 1982, 31, 9. [Google Scholar] [CrossRef]
- Zia, M.P.; Alibas, I. Influence of the Drying Methods on Color, Vitamin C, Anthocyanin, Phenolic Compounds, Antioxidant Activity, and in Vitro Bioaccessibility of Blueberry Fruits. Food Biosci. 2021, 42, 101179. [Google Scholar] [CrossRef]
- Vitali, D.; Dragojević, I.V.; Šebečić, B. Effects of Incorporation of Integral Raw Materials and Dietary Fibre on the Selected Nutritional and Functional Properties of Biscuits. Food Chem. 2009, 114, 1462–1469. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Li, S.; Wang, Y.; Fan, L.; Zhou, J. Effects of High Power Pulsed Microwave on the Enhanced Color and Flavor of Aged Blueberry Wine. Food Sci. Biotechnol. 2024, 33, 33–45. [Google Scholar] [CrossRef]
- Islam, M.Z.; Das, S.; Monalisa, K.; Sayem, A.S.M. Influence of Osmotic Dehydration on Mass Transfer Kinetics and Quality Retention of Ripe Papaya (Carica papaya L.) during Drying. AgriEngineering 2019, 1, 220–234. [Google Scholar] [CrossRef]
- Abrahão, F.R.; Corrêa, J.L.G. Osmotic Dehydration: More than Water Loss and Solid Gain. Crit. Rev. Food Sci. Nutr. 2023, 63, 2970–2989. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of Osmotic Dehydration Pre-Treatment on Oven Drying and Freeze Drying Performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Goula, A.M.; Kokolaki, M.; Daftsiou, E. Use of Ultrasound for Osmotic Dehydration. The Case of Potatoes. Food Bioprod. Process. 2017, 105, 157–170. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Ramaswamy, H.S. Microwave-Osmotic Dehydration of Apples Under Continuous Flow Medium Spray Conditions: Comparison with Other Methods. Dry. Technol. 2009, 28, 49–56. [Google Scholar] [CrossRef]
- Manzoor, A.; Khan, M.A.; Mujeebu, M.A.; Shiekh, R.A. Comparative Study of Microwave Assisted and Conventional Osmotic Dehydration of Apple Cubes at a Constant Temperature. J. Agric. Food Res. 2021, 5, 100176. [Google Scholar] [CrossRef]
- An, K.; Fu, M.; Zhang, H.; Tang, D.; Xu, Y.; Xiao, G. Effect of Ethyl Oleate Pretreatment on Blueberry (Vaccinium corymbosum L.): Drying Kinetics, Antioxidant Activity, and Structure of Wax Layer. J. Food Sci. Technol. 2019, 56, 783–791. [Google Scholar] [CrossRef]
- Bélafi-Bakó, K.; Cserjési, P.; Beszédes, S.; Csanádi, Z.; Hodúr, C. Berry Pectins: Microwave-Assisted Extraction and Rheological Properties. Food Bioproc. Technol. 2012, 5, 1100–1105. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A.; Gręda, K.J. Effect of Pre-Treatment Conditions on Content and Activity of Water and Colour of Freeze-Dried Pumpkin. LWT Food Sci. Technol. 2014, 59, 1075–1081. [Google Scholar] [CrossRef]
- Ando, Y.; Okunishi, T.; Okadome, H. Influences of Blanching and Freezing Pretreatments on Moisture Diffusivity and Quality Attributes of Pumpkin Slices During Convective Air-Drying. Food Bioproc. Technol. 2019, 12, 1821–1831. [Google Scholar] [CrossRef]
- Layeghinia, N.; Karimi, S.; Abbasi, H.; Silavi, K. Effect of Osmotic Dehydration on Qualitative and Nutritional Characteristics and Kinetics of Microwave Drying of Iranian Quince Slices. J. Agric. Food Res. 2025, 20, 101749. [Google Scholar] [CrossRef]
- Eyembe, L.E.; Nde, D.B.; Jean, S. Osmotic Dehydration Kinetics of Plantain (Musa paradisiaca) Using a Low-Calorie Sugar. Discov. Food 2024, 4, 172. [Google Scholar] [CrossRef]
- Stojanovic, J.; Silva, J.L. Influence of Osmotic Concentration, Continuous High Frequency Ultrasound and Dehydration on Antioxidants, Colour and Chemical Properties of Rabbiteye Blueberries. Food Chem. 2007, 101, 898–906. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of Temperature and PH on Stability of Anthocyanin Obtained from Blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chou, C.-C. Effect of Heat Treatment on Total Phenolic and Anthocyanin Contents as Well as Antioxidant Activity of the Extract from Aspergillus Awamori-Fermented Black Soybeans, a Healthy Food Ingredient. Int. J. Food Sci. Nutr. 2009, 60, 627–636. [Google Scholar] [CrossRef]
- Yue, X.; Xu, Z. Changes of Anthocyanins, Anthocyanidins, and Antioxidant Activity in Bilberry Extract during Dry Heating. J. Food Sci. 2008, 73, C494–C499. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blueberry Products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Dimopoulos, G.; Taoukis, P. Combined Effect of Pulsed Electric Field and Osmotic Dehydration Pre-Treatments on Mass Transfer and Quality of Air Dried Goji Berry. Innov. Food Sci. Emerg. Technol. 2018, 49, 106–115. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Xu, B.; Tchabo, W.; Tahir, H.E.; Mustapha, A.T.; Ma, H. Effects of Ultrasound, Osmotic Dehydration, and Osmosonication Pretreatments on Bioactive Compounds, Chemical Characterization, Enzyme Inactivation, Color, and Antioxidant Activity of Dried Ginger Slices. J. Food Biochem. 2019, 43, e12832. [Google Scholar] [CrossRef]
- Chaguri, L.; Sanchez, M.S.; Flammia, V.P.; Tadini, C.C. Green Banana (Musa cavendishii) Osmotic Dehydration by Non-Caloric Solutions: Modeling, Physical-Chemical Properties, Color, and Texture. Food Bioproc. Technol. 2017, 10, 615–629. [Google Scholar] [CrossRef]
- Zielinska, M.; Zielinska, D.; Markowski, M. The Effect of Microwave-Vacuum Pretreatment on the Drying Kinetics, Color and the Content of Bioactive Compounds in Osmo-Microwave-Vacuum Dried Cranberries (Vaccinium macrocarpon). Food Bioproc. Technol. 2018, 11, 585–602. [Google Scholar] [CrossRef]
- Kutlu, N. Optimization of Ohmic Heating-Assisted Osmotic Dehydration as a Pretreatment for Microwave Drying of Quince. Food Sci. Technol. Int. 2022, 28, 60–71. [Google Scholar] [CrossRef]

| Treatment | t-Value | |
|---|---|---|
| Moisture Content (%) | TSS content (°Brix) | |
| Room temperature × 1:4 × Control | 0.72 ns | 3.24 ns |
| 60 °C × 1:4 × Control | 2.13 ns | 2.94 ns |
| 65 °C × 1:4 × Control | 19.95 ** | 7.45 * |
| 70 °C × 1:4 × Control | 6.44 * | 35.92 ** |
| Room temperature × 1:4 × MW | 1.69 ns | 1.49 ns |
| 60 °C × 1:4 × MW | 3.69 ns | 74 ** |
| 65 °C × 1:4 × MW | 2.98 ns | 2.97 ns |
| 70 °C × 1:4 × MW | 4.98 * | 10.33 ** |
| Room temperature × 1:7 × Control | 3.66 ns | 3.42 ns |
| 60 °C × 1:7 × Control | 18.58 ** | 16.25 ** |
| 65 °C × 1:7 × Control | 7.68 * | 1.8 ns |
| 70 °C × 1:7 × Control | 3.81 ns | 5.5 * |
| Room temperature × 1:7 × MW | 14.12 ** | 5.55 * |
| 60 °C × 1:7 × MW | 2.47 ns | 3.59 ns |
| 65 °C × 1:7 × MW | 7.7 * | 1.59 ns |
| 70 °C × 1:7 × MW | 17.9 ** | 4.01 ns |
| Room temperature × 1:10 × Control | 1.51 ns | 2.8 ns |
| 60 °C × 1:10 × Control | 1.68 ns | 6.18 * |
| 65 °C × 1:10 × Control | 6.22 * | 10.29 ** |
| 70 °C × 1:10 × Control | 5.77 * | 6.7 * |
| Room temperature × 1:10 × MW | 3.59 ns | 1.4 ns |
| 60 °C × 1:10 × MW | 4.26 ns | 2.61 ns |
| 65 °C × 1:10 × MW | 9.74 * | 3.92 ns |
| 70 °C × 1:10 × MW | 6.91 * | 101.67 ** |
| Source of Variation | df a | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| Water Loss | Solid Gain | Texture | Total Anthocyanin Content | Total Phenolic Content | ΔE | ||
| Temperature | 3 | 4618.97 ** | 138.64 ** | 0.18 ns | 1543.89 ** | 1563.07 * | 72.11 ** |
| Ratio | 2 | 38.67 ns | 13.14 ** | 0.22 ns | 1540.51 ** | 10660.56 ** | 85.62 ** |
| Pretreatment | 1 | 1330.82 ** | 0.18 ns | 0.23 ns | 740.09 ** | 2009.99 * | 76.54 ** |
| Temperature × Ratio | 6 | 21.19 ns | 5.41 ** | 0.06 ns | 1041.76 ** | 4739.91 ** | 32.37 ** |
| Temperature × Pretreatment | 3 | 308.24 ** | 50.81 ** | 0.19 ns | 569.64 ** | 967.26 ns | 122.82 ** |
| Ratio × Pretreatment | 2 | 13.80 ns | 14.86 ** | 0.33 ns | 420.00 ** | 1003.92 ns | 32.83 * |
| Temperature × Ratio × Pretreatment | 6 | 33.49 ns | 2.58 ** | 0.11 ns | 158.95 ** | 4535.67 ** | 57.37 ** |
| Error | 48 | 19.34 | 0.20 | 0.20 | 5.48 | 446.09 | 6.52 |
| Coefficient of Variation (%) | 17.85 | 10.34 | 0.45 | 5.56 | 6.86 | 33.14 | |
| Osmotic Dehydration Parameter | Color Parameters | |||
|---|---|---|---|---|
| a | b | L | ||
| Temperature | Room temperature | 0.53 ns | 1.49 ns | 5.40 ** |
| 60 °C | 5.72 ** | 0.56 ns | 0.81 ns | |
| 65 °C | 1.01 ns | 3.52 ** | 0.21 ns | |
| 70 °C | 2.85 * | 1.15 ns | 2.71 * | |
| Ratio | 1:4 | 9.78 ** | 3.11 ns | 0.93 ns |
| 1:7 | 2.89 ** | 0.91 ns | 2.59 * | |
| 1:10 | 1.26 ns | 1.08 ns | 2.15 * | |
| Pretreatment | Control | 3.00 ** | 3.99 ** | 2.11 * |
| Microwave | 3.93 ** | 1.00 ns | 2.73 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norouzi, S.; Orsat, V.; Dumont, M.-J. Improving Dehydration Efficiency and Quality in Highbush Blueberries via Combined Pulsed Microwave Pretreatment and Osmotic Dehydration. Agriculture 2025, 15, 1602. https://doi.org/10.3390/agriculture15151602
Norouzi S, Orsat V, Dumont M-J. Improving Dehydration Efficiency and Quality in Highbush Blueberries via Combined Pulsed Microwave Pretreatment and Osmotic Dehydration. Agriculture. 2025; 15(15):1602. https://doi.org/10.3390/agriculture15151602
Chicago/Turabian StyleNorouzi, Shokoofeh, Valérie Orsat, and Marie-Josée Dumont. 2025. "Improving Dehydration Efficiency and Quality in Highbush Blueberries via Combined Pulsed Microwave Pretreatment and Osmotic Dehydration" Agriculture 15, no. 15: 1602. https://doi.org/10.3390/agriculture15151602
APA StyleNorouzi, S., Orsat, V., & Dumont, M.-J. (2025). Improving Dehydration Efficiency and Quality in Highbush Blueberries via Combined Pulsed Microwave Pretreatment and Osmotic Dehydration. Agriculture, 15(15), 1602. https://doi.org/10.3390/agriculture15151602






