Effects on Powdery Mildew and the Mutualistic Fungal Endophyte Epichloë gansuensis When Host Achnatherum inebrians Plants Are Sprayed with Different Fungicides
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Disease Investigation
2.3. Determination of Chlorophyll Content
2.4. Growth and Biomass of Plants and Epichloë Endophyte Retention Rate Detection
2.5. Statistical Analyses
3. Results
3.1. The Percentage of Diseased Leaves
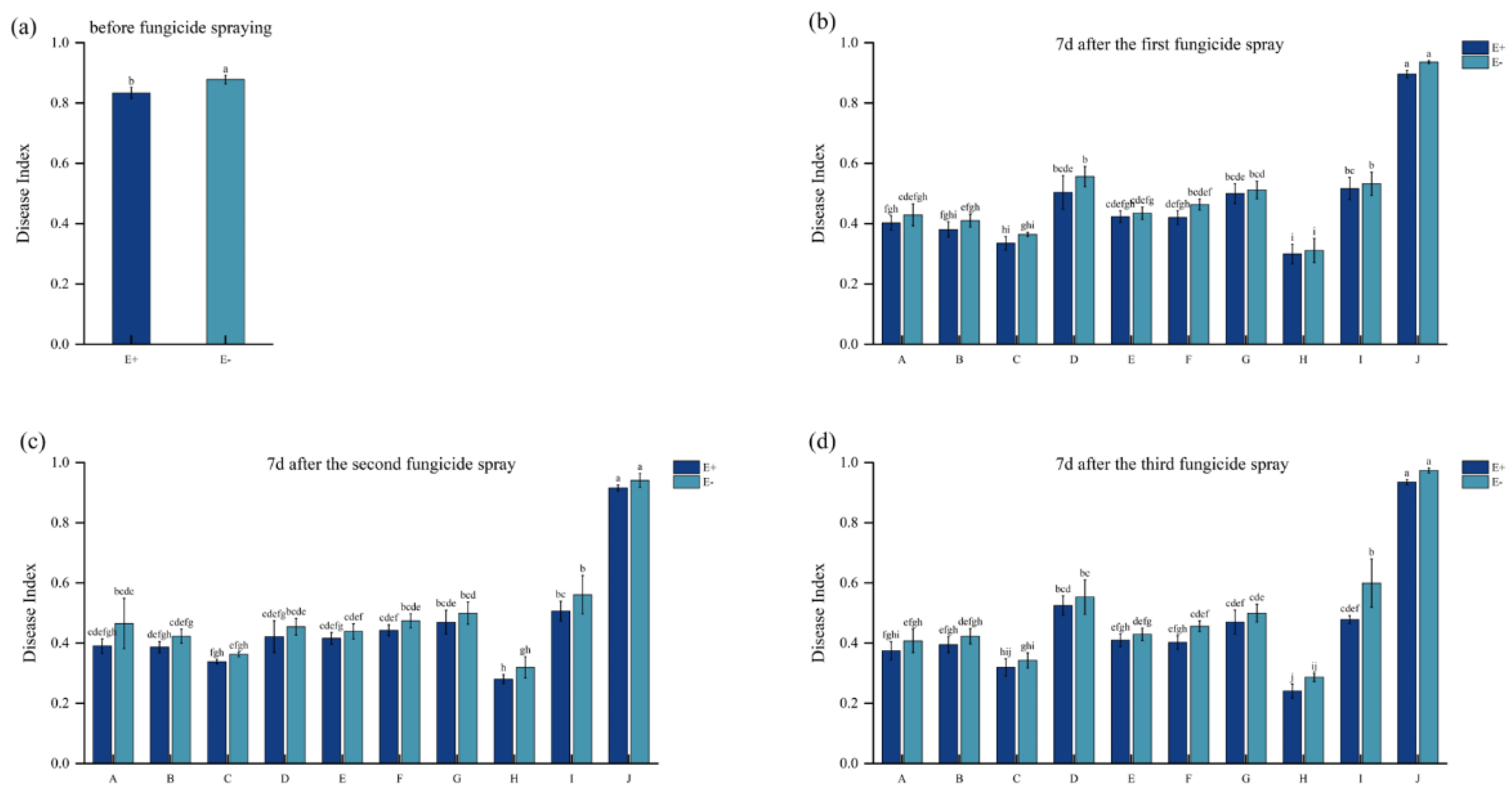
3.2. Disease Index of A. inebrians Under Different Fungicides
3.3. Effect of Different Fungicides on the Control of Powdery Mildew
3.4. Chlorophyll Content
3.5. Plant Growth and Biomass
3.6. Epichloë Endophyte Retention
4. Discussion
4.1. How Fungicides Affect Powdery Mildew and Thus Promote E+/E− Plant Growth
4.2. How Fungicides Affect the Epichloë Endophyte in Host Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, J.Z. Several poisonous weeds in grassland of northwest. Anim. Husb. Vet. Med. 1954, 2, 56–60. [Google Scholar]
- Shi, Z.C. Important Poisonous Plants of China Grassland; China Agriculture Press: Beijing, China, 1997; pp. 166–176. [Google Scholar]
- Liu, Z.P. Animal Toxicology; China Agricultural Press: Beijing, China, 2006; pp. 152–154. [Google Scholar]
- Nan, Z.B.; Li, C.J. Neotyphodium in native grasses in China and observations on endophyte/host interactions. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000. [Google Scholar]
- Li, C.J.; Nan, Z.B.; Paul, V.; Dapprich, P.; Liu, Y. A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon 2004, 90, 141–147. [Google Scholar]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Young, C.A.; Sugawara, K. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.B.; Krauss, J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005, 8, 450–456. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Schmid, J. Growth of Epichloë/Neotyphodium and p-endophytes in leaves of Lolium and Festuca grasses. Mycol. Res. 2002, 106, 93–106. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Spiering, M.J.; Scott, V.; Lane, G.A.; Christensen, M.J.; Schmid, J. In planta regulation of extension of an endophytic fungus and maintenance of high metabolic rates in its mycelium in the absence of apical extension. Appl. Environ. Microbiol. 2001, 67, 5377–5383. [Google Scholar] [CrossRef]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef]
- Zhao, Z.R.; Kou, M.Z.; Zhong, R.; Xia, C.; Christensen, M.J.; Zhang, X.X. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by Epichloë gansuensis in Achnatherum inebrians under different soil moisture availability. J. Fungi 2021, 7, 640. [Google Scholar] [CrossRef]
- Zhong, R.; Bastías, D.A.; Zhang, X.X.; Li, C.J.; Nan, Z.B. Vertically transmitted Epichloë systemic endophyte enhances drought tolerance of Achnatherum inebrians host plants through promoting photosynthesis and biomass accumulation. J. Fungi 2022, 8, 512. [Google Scholar] [CrossRef]
- Wang, J.F.; Tian, P.; Christensen, M.J.; Zhang, X.X.; Li, C.J.; Nan, Z.B. Effect of Epichloë gansuensis endophyte on the activity of enzymes of nitrogen metabolism, nitrogen use efficiency and photosynthetic ability of Achnatherum inebrians under various NaCl concentrations. Plant Soil 2019, 435, 57–68. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fan, X.M.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regul. 2010, 60, 91–97. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte. Sci. China Life Sci. 2012, 55, 793–799. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, X.X.; Christensen, M.J.; Nan, Z.B.; Li, C.J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, X.X.; Feng, Y.; Christensen, M.J.; Nan, Z.B. An Epichloë endophyte improves photosynthetic ability and dry matter production of its host Achnatherum inebrians infected by Blumeria graminis under various soil water conditions. Fungal Ecol. 2016, 22, 26–34. [Google Scholar] [CrossRef]
- Kou, M.Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.B.; Zhang, X.X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, S.B.; Zhang, F.; Zhao, Z.R.; Christensen, M.J.; Nan, Z.B.; Zhang, X.X. Transcriptomic Analyses Reveals molecular regulation of photosynthesis by Epichloë endophyte in Achnatherum inebrians under Blumeria graminis infection. J. Fungi 2022, 8, 1201. [Google Scholar] [CrossRef]
- Braun, U. The current systematics and taxonomy of the powdery mildews (Erysiphales): An overview. Mycoscience 2011, 52, 210–212. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; De Vicente, A.; Torés, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef]
- Inuma, T.; Khodaparast, S.A.; Takamatsu, S. Multilocus phylogenetic analyses within Blumeria graminis, a powdery mildew fungus of cereals. Mol. Phylogenet. Evol. 2007, 44, 741–751. [Google Scholar] [CrossRef]
- Glawe, D.A. The powdery mildews: A review of the world’s most familiar (yet poorly known) plant pathogens. Annu. Rev. Phytopathol. 2008, 46, 27–51. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Xing, L.; Wang, X.; Wang, W.; Sun, Y.; Qian, C. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 7727–7732. [Google Scholar] [CrossRef] [PubMed]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Li, N.N.; Zhao, Y.F.; Xia, C.; Zhong, R.; Zhang, X.X. Effects of thiophanate methyl on seed borne Epichloë fungal endophyte of Achnatherum inebrians. J. Pratacult. Sci. 2016, 33, 1306–1314, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.Y.; Feng, W.; Yang, Y.H.; Hou, C.C.; Wang, C.Y.; Guo, T.C. Relationship of physiological indexes and yield loss to severity level of wheat powdery mildew. J. Triticeae Crops 2012, 32, 1192–1198. [Google Scholar]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgenssen, L.N. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Li, C.J.; Nan, Z.B.; Liu, Y. Methodolgy of endophyte detection of drunken horse grass (Achnatherum inebrians). Edible Fungi China 2008, 27 (Suppl. 1), 16–19. [Google Scholar]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Multifunctional fungal plant symbionts: New tools to enhance plant growth and productivity. New Phytol. 2011, 189, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K.; Raj, S.U. Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol. 2003, 21, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Junaid, J.M.; Dar, N.A.; Bhat, T.A.; Bhat, A.H.; Bhat, M.A. Commercial biocontrol agents and their mechanism of action in the management of plant pathogens. J. Anim. Plant Sci. 2013, 1, 39–57. [Google Scholar]
- Nimali, I.N.; Brooks, S.; Lumyong, S.D.K. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Ye, Q.; Zhong, Z.; Chao, S.; Liu, L.; Chen, M.; Feng, X.; Wu, H. Antifungal effect of Bacillus velezensis ZN-S10 against plant pathogen Colletotrichum changpingense and its inhibition mechanism. Int. J. Mol. Sci. 2023, 24, 16694. [Google Scholar] [CrossRef]
- Gong, A.D.; Li, H.P.; Yuan, Q.S. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Ptak, M.; Peypoux, F.; Michel, G. Pore-forming properties of iturin A, a lipopeptide antibiotic. Biochim. Biophys. Acta 1985, 815, 405–409. [Google Scholar] [CrossRef]
- Romero, D.; De Vicente, A.; Zeriouh, H.; Cazorla, F.M.; Fernández-Ortuño, D.; Torés, J.A. Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon. Plant Pathol. 2007, 56, 976–986. [Google Scholar] [CrossRef]
- Xie, D.; Cai, X.; Yang, C.; Xie, L.; Qin, G.; Zhang, M. Studies on the control effect of Bacillus subtilis on wheat powdery mildew. Pest Manag. Sci. 2021, 77, 4375–4382. [Google Scholar] [CrossRef]
- Ma, X.; Yang, X.; Zeng, F.; Yang, L.; Yu, D.; Ni, H. Physcion, a natural anthraquinone derivative, enhances the gene expression of leaf-specific thionin of barley against Blumeria graminis. Pest Manag. Sci. 2010, 66, 718–724. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, S.; Yang, S.; Peng, Y.; Ren, S.; Wen, B.; Chen, N. Physcion and physcion 8-O-β-glucopyranoside: A review of their pharmacology, toxicities and pharmacokinetics. Chem. Biol. Interact. 2019, 310, 108722. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Marsell, A.; Riederer, M. Direct Effects of Physcion, Chrysophanol, Emodin, and Pachybasin on Germination and Appressorium Formation of the Barley (Hordeum vulgare L.) Powdery Mildew Fungus Blumeria graminis f. sp. hordei (DC.) Speer. J. Agric. Food Chem. 2018, 66, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, H. The role of thionins in plant protection. Crit. Rev. Plant Sci. 1994, 13, 1–16. [Google Scholar] [CrossRef]
- Sequeira, L. Mechanisms of induced resistance in plants. Annu. Rev. Microbiol. 1993, 37, 51–79. [Google Scholar] [CrossRef]
- Schneider, S.; Ulrich, W.R. Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic inducers. Physiol. Mol. Plant Pathol. 1994, 45, 291–304. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Shen, S.G.; Xu, L.L. Inhibition mechanism of osthole to plant fungus pathogens. Chin. J. Pestic. Sci. 2004, 6, 28–32, (In Chinese with English Abstract). [Google Scholar]
- Shi, Z.; Shen, S.; Zhou, W.; Wang, F.; Fan, Y. Fusarium graminearum growth inhibition due to glucose starvation caused by Osthol. Int. J. Mol. Sci. 2008, 9, 371–382. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, F.; Zhou, W.; Zhang, P.; Fan, Y.J. Application of osthole induces a resistance response against powdery mildew in pumpkin leaves. Int. J. Mol. Sci. 2007, 8, 1001–1012. [Google Scholar] [CrossRef]
- Li, C.X.; Zhou, W.; Ji, M.S.; Shi, Z.Q. Effect of osthole on the invasion of Sphaerotheca fuliginea. Chin. J. Pestic. Sci. 2007, 9, 49–53, (In Chinese with English Abstract). [Google Scholar]
- Fu, Y.; Wang, C.B.; Ye, F. The application of Sophora flavescens Ait alkaloids in China. Pestic. Sci. Admin. 2005, 26, 30–33. [Google Scholar]
- Pan, J.L. Antibacterial Mechanism of Matrine on Pecan Dry Rot Pathogenic Fungus Botryosphaeria Dothidea; Northeast Forestry University: Harbin, China, 2018. [Google Scholar]
- Sun, Y.; Chen, Y.; Liu, T.; Wang, Y.; Wang, Y.; Han, L.; Feng, J. Evaluating the efficacy of osthole and matrine for control of Sorghum purple spot. J. Plant Dis. Prot. 2021, 128, 1263–1268. [Google Scholar] [CrossRef]
- Kleczewski, N.M.; Butts-Willmsmeyer, C.; Scanlan, C. Assessing the curative and protective impacts of select fungicides for control of powdery mildew of wheat. Plant Dis. 2020, 104, 1195–1200. [Google Scholar] [CrossRef]
- Oliver, R.; Hewitt, H.G. Fungicides in Crop Protection, 2nd ed.; CABI International: Wallingford, UK, 2014. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Guo, Z.F.; Pan, R.Z. Triadimefon and its physiological effects on plants. Plant Pathol. 1989, 1, 75. [Google Scholar]
- Zhao, S.X. Degradation Characteristics of Triadimefon Degrading Bacteria SM3 and Its Application in Polluted Soil; Anhui Agricultural University: Hefei, China, 2022. [Google Scholar]
- Heaney, S.P.; Hall, A.A.; Davies, S.A.; Olaya, G. Resistance to fungicides in the Qol-STAR cross-resistance group: Current perspectives. In Proceedings of the BCPC Conference: Pests and Diseases, Volume 2. Proceedings of an International Conference Held at the Brighton Hilton Metropole Hotel, Brighton, UK, 13–16 November 2000; British Crop Protection Council: Bracknell, Berkshire, UK, 2000; pp. 755–762. [Google Scholar]
- Ishii, H.; Fraaije, B.A.; Sugiyama, T.; Noguchi, K.; Nishimura, K.; Takeda, T. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 2001, 91, 1166–1171. [Google Scholar] [CrossRef]
- Chin, K.M.; Chavaillaz, D.; Kaesbohrer, M.; Staub, T.; Felsenstein, F.G. Characterizing resistance risk of Erysiphe graminis f. sp. tritici to strobilurins. Crop Prot. 2001, 20, 87–96. [Google Scholar] [CrossRef]
- Ishii, H.; Yano, K.; Date, H.; Furuta, A.; Sagehashi, Y.; Yamaguchi, T. Molecular characterization and diagnosis of QoI resistance in cucumber and eggplant fungal pathogens. Phytopathology 2007, 97, 1458–1466. [Google Scholar] [CrossRef]





| Table | Fungicide | Dose (g/L) | Active Ingredient (s) | Formulation | FRAC Code |
|---|---|---|---|---|---|
| A | 15% Triadimefon | 1.666 | Triadimefon | Wettable powder | 3 |
| B | 1% Osthole | 1.666 | Osthole | Water emulsions | BM01 |
| C | 0.5% Physcion | 3.334 | Physcion | Hydrotrope | BM01 |
| D | 16% Polyoxin B | 1.428 | Polyoxin | Suspensions | 19 |
| E | 25% Myclobutanil | 1.500 | Myclobutanil | Hydrotrope | 3 |
| F | 0.5% Matrine | 1.000 | Matrine | Water emulsions | BM01 |
| G | 25% Azoxystrobin | 0.666 | Azoxystrobin | Soluble agents | 11 |
| H | 1 × 1011 cfu·g−1 Bacillus subtilis | 1.428 | Bacillus subtilis | Wettable powder | 44/BM02 |
| I | 5% Carvacrol | 1.334 | Carvacrol | Wettable powder | BM01 |
| CK | Untreated (CK) |
| Treatments | The Percentage of Diseased Leaves | Disease Index | Control Efficacy | Chlorophyll Content | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| E | 265.921 | <0.001 | 207.677 | <0.001 | 78.922 | <0.001 | 385.107 | <0.001 |
| F | 105.934 | <0.001 | 104.803 | <0.001 | 20.961 | <0.001 | 145.270 | <0.001 |
| T | 796.205 | <0.001 | 805.787 | <0.001 | 33.320 | <0.001 | 20.757 | <0.001 |
| E × F | 4.098 | <0.001 | 4.536 | <0.001 | 12.998 | <0.001 | 39.222 | <0.001 |
| E × T | 2.713 | 0.045 | 4.656 | 0.003 | 7.714 | 0.001 | 8.215 | <0.001 |
| F × T | 18.575 | <0.001 | 16.421 | <0.001 | 2.905 | <0.001 | 6.211 | <0.001 |
| E × F × T | 3.401 | <0.001 | 2.896 | <0.001 | 3.229 | <0.001 | 2.247 | 0.001 |
| Treatments | df | Plant Heights | Tillers Per Plant | Dry Weight Per Plant | Fresh Weight Per Plant | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| F | 8 | 25.621 | <0.001 | 3.162 | 0.006 | 217.700 | <0.001 | 5.042 | <0.001 |
| E | 1 | 153.270 | <0.001 | 21.246 | <0.001 | 3005.696 | <0.001 | 120.710 | <0.001 |
| F × E | 8 | 1.464 | 0.195 | 0.845 | 0.580 | 90.832 | <0.001 | 2.339 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Cao, K.; Wu, K.; Christensen, M.J.; Cao, J.; Li, Y.; Zhang, X.; Nan, Z. Effects on Powdery Mildew and the Mutualistic Fungal Endophyte Epichloë gansuensis When Host Achnatherum inebrians Plants Are Sprayed with Different Fungicides. Agriculture 2025, 15, 1565. https://doi.org/10.3390/agriculture15141565
Zhu Y, Cao K, Wu K, Christensen MJ, Cao J, Li Y, Zhang X, Nan Z. Effects on Powdery Mildew and the Mutualistic Fungal Endophyte Epichloë gansuensis When Host Achnatherum inebrians Plants Are Sprayed with Different Fungicides. Agriculture. 2025; 15(14):1565. https://doi.org/10.3390/agriculture15141565
Chicago/Turabian StyleZhu, Yue, Keke Cao, Kelin Wu, Michael J. Christensen, Jianxin Cao, Yanzhong Li, Xingxu Zhang, and Zhibiao Nan. 2025. "Effects on Powdery Mildew and the Mutualistic Fungal Endophyte Epichloë gansuensis When Host Achnatherum inebrians Plants Are Sprayed with Different Fungicides" Agriculture 15, no. 14: 1565. https://doi.org/10.3390/agriculture15141565
APA StyleZhu, Y., Cao, K., Wu, K., Christensen, M. J., Cao, J., Li, Y., Zhang, X., & Nan, Z. (2025). Effects on Powdery Mildew and the Mutualistic Fungal Endophyte Epichloë gansuensis When Host Achnatherum inebrians Plants Are Sprayed with Different Fungicides. Agriculture, 15(14), 1565. https://doi.org/10.3390/agriculture15141565








