Relative Growth Rate and Specific Absorption Rate of Nutrients in Lactuca sativa L. Under Secondary Paper Sludge Application and Soil Contamination with Lead
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Plant Treatment
2.2. Plant Growth Measurement
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Plant Growth Parameters
3.2. Growth Rate Parameters
| Parameter | 0 mg Pb(NO3)2 kg−1 | 50 mg Pb(NO3)2 kg−1 | 250 mg Pb(NO3)2 kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 0% | 20% | 40% | 0% | 20% | 40% | |
| Shoot dry weight, g | |||||||||
| 17 days after sowing | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a |
| 21 days after sowing | 0.03 ± 0.01 b | 0.03 ± 0.02 ab | 0.06 ± 0.02 a | 0.03 ± 0.01 b | 0.06 ± 0.02 a | 0.03 ± 0.00 b | 0.03 ± 0.01 b | 0.05 ± 0.02 a | 0.05 ± 0.02 a |
| 34 days after sowing | 0.56 ± 0.06 b | 1.04 ± 0.19 a | 0.83 ± 0.1 a | 0.29 ± 0.03 c | 0.52 ± 0.06 b | 0.71 ± 0.09 a | 0.34 ± 0.06 c | 0.41 ± 0.04 bc | 0.66 ± 0.09 ab |
| 46 days after sowing | 0.97 ± 0.24 b | 1.33 ± 0.15 ab | 1.62 ± 0.11 a | 1.46 ± 0.24 a | 1.47 ± 0.07 a | 1.37 ± 0.08 a | 1.06 ± 0.06 b | 1.11 ± 0.16 b | 1.59 ± 0.18 a |
| Root dry weight, g | |||||||||
| 17 days after sowing | 0.002 ± 0.000 b | 0.003 ± 0.001 ab | 0.004 ± 0.001 a | 0.003 ± 0.002 ab | 0.003 ± 0.002 a | 0.002 ± 0.000 a | 0.002 ± 0.000 b | 0.003 ± 0.001 ab | 0.004 ± 0.001 a |
| 21 days after sowing | 0.006 ± 0.001 a | 0.007 ± 0.003 a | 0.011 ± 0.003 a | 0.008 ± 0.005 a | 0.011 ± 0.005 a | 0.007 ± 0.010 a | 0.010 ± 0.002 a | 0.012 ± 0.005 a | 0.010 ± 0.003 a |
| 34 days after sowing | 0.192 ± 0.027 b | 0.306 ± 0.130 a | 0.280 ± 0.045 a | 0.058 ± 0.014 c | 0.147 ± 0.029 b | 0.167 ± 0.023 b | 0.071 ± 0.021 c | 0.114 ± 0.014 b | 0.163 ± 0.027 b |
| 46 days after sowing | 0.510 ± 0.131 bcd | 0.606 ± 0.066 ab | 0.676 ± 0.065 a | 0.447 ± 0.035 cd | 0.524 ± 0.086 b | 0.502 ± 0.001 c | 0.405 ± 0.154 c | 0.410 ± 0.057 d | 0.515 ± 0.077 bc |
| Leaf area, cm2 | |||||||||
| 21 days after sowing | 53 ± 19 a | 23 ± 11 ab | 29 ± 9 ab | 18 ± 4 b | 26 ± 10 ab | 21 ± 3 ab | 18 ± 3 b | 26 ± 10 ab | 27 ± 6 ab |
| 34 days after sowing | 114 ± 7 bc | 124 ± 37 ab | 136 ± 20 ab | 110 ± 48 abc | 113 ± 25 b | 87 ± 8 cd | 78 ± 18 d | 81 ± 12 d | 152 ± 8 a |
| 46 days after sowing | 288 ± 39 ab | 302 ± 20 ab | 320 ± 13 a | 345 ± 37 a | 335 ± 11 a | 300 ± 16 ab | 267 ± 24 b | 419 ± 120 a | 323 ± 40 ab |
| SLA, m2 kg−1 | |||||||||
| 21 days after sowing | 81 ± 5 a | 68 ± 7 b | 52 ± 6 c | 52 ± 4 c | 44 ± 2 d | 64 ± 5 b | 52 ± 4 c | 53 ± 3 c | 55 ± 4 bc |
| 34 days after sowing | 20 ± 4 c | 22 ± 3 c | 26 ± 4 bc | 39 ± 4 a | 21 ± 3 c | 26 ± 2 b | 23 ± 3 bc | 20 ± 2 c | 23 ± 1 bc |
| 46 days after sowing | 28 ± 3 a | 23 ± 3 bc | 20 ± 1 c | 24 ± 2 bc | 23 ± 3 bc | 22 ± 1 c | 25 ± 2 ab | 21 ± 2 c | 20 ± 1 c |
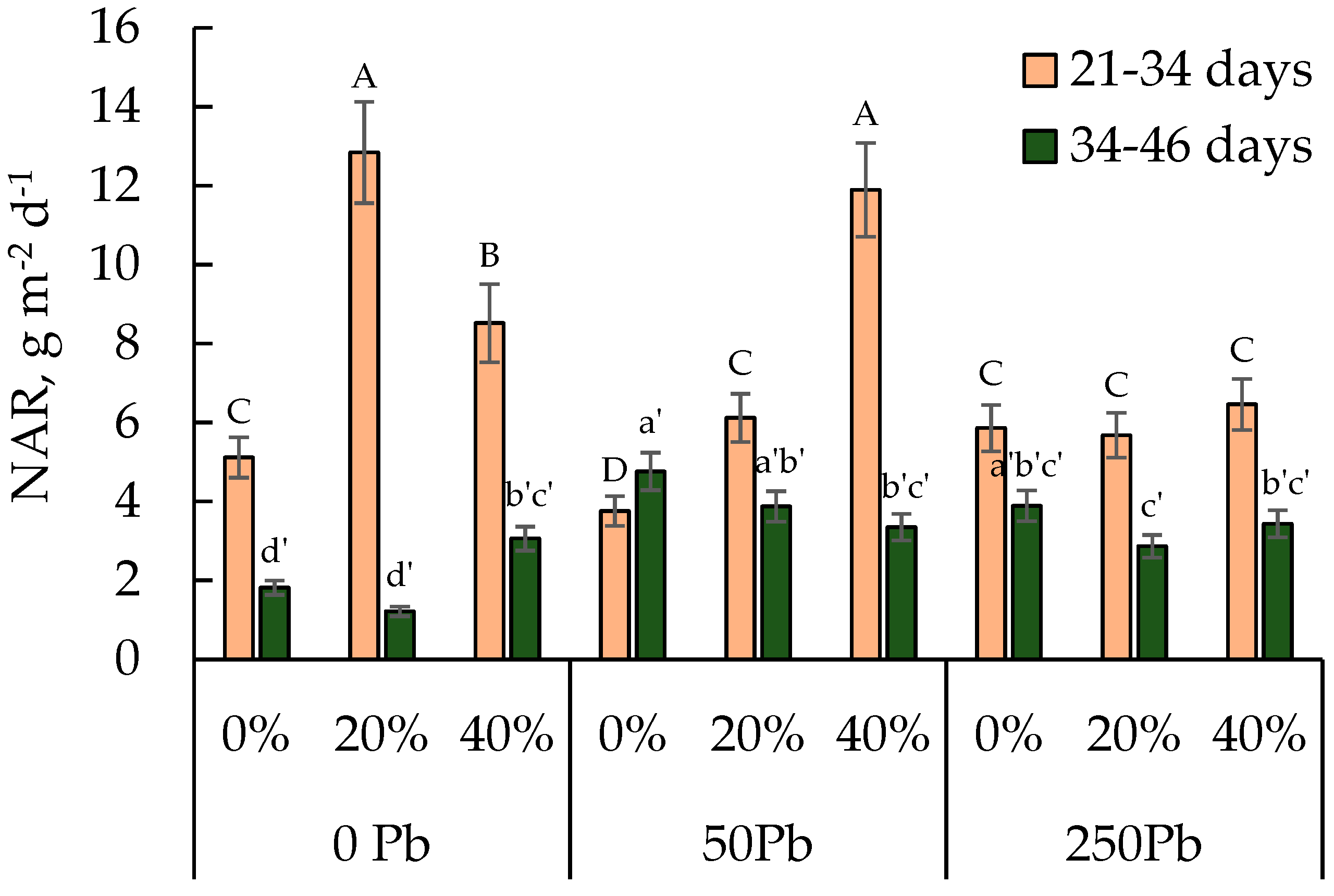
3.3. Calculated Net Assimilation Rate
3.4. Pb Content in Shoots and Roots of Seedling
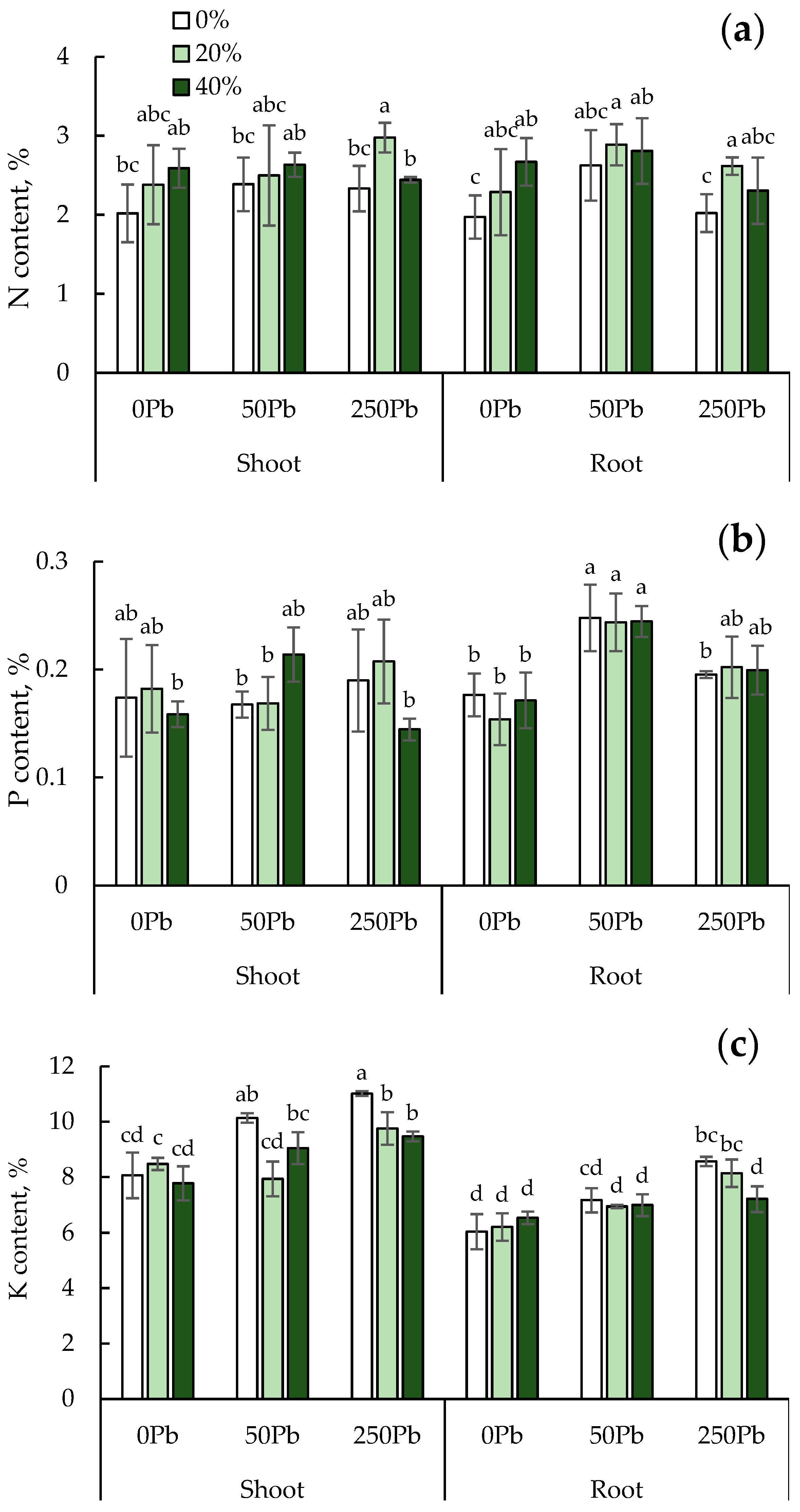
3.5. Macroelement Content in Shoots and Roots of L. sativa Seedling
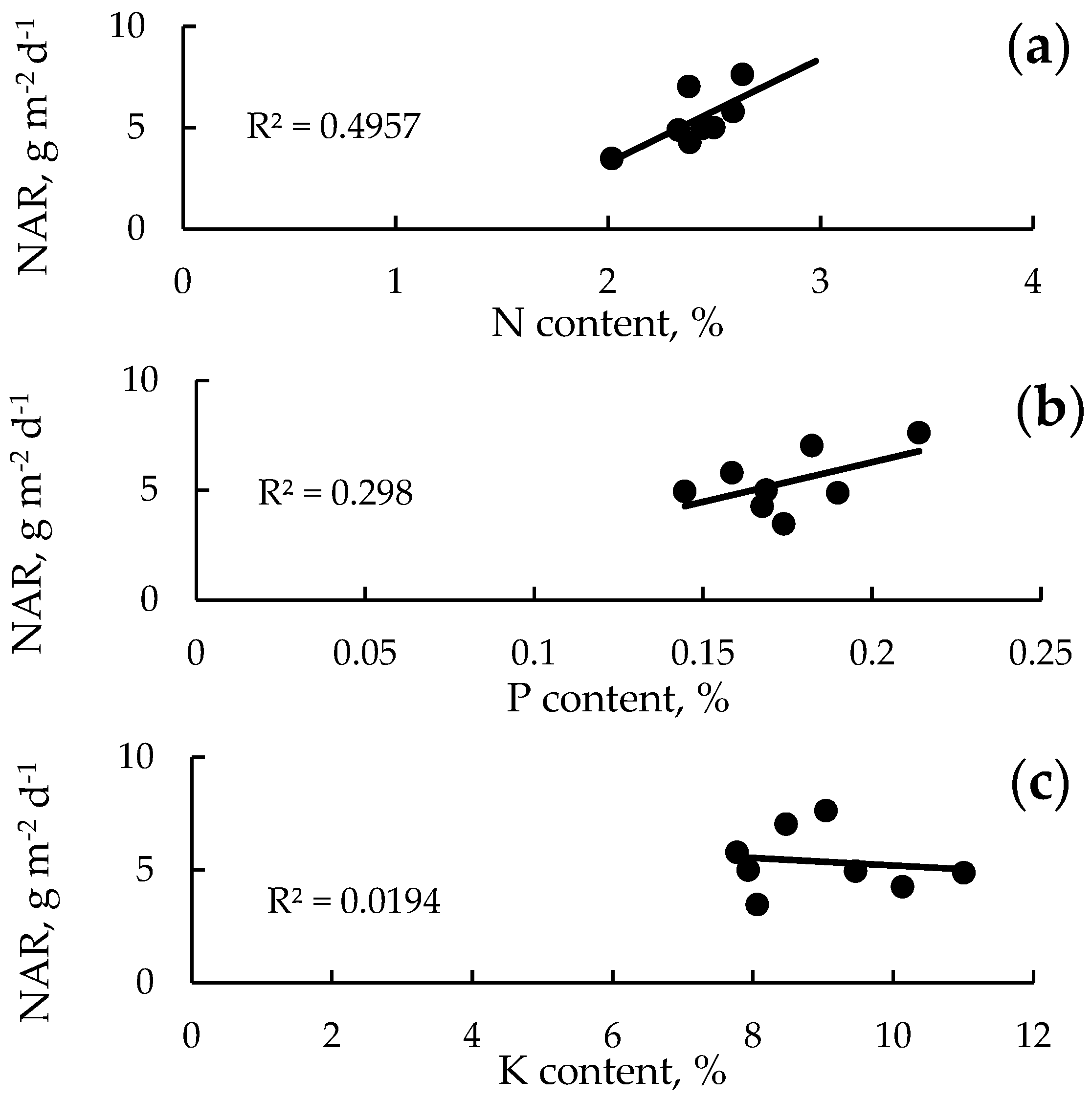
3.6. Relationship Between NAR and Macronutrient Content
3.7. Specific Absorption Rate of Nitrogen, Phosphorus, and Potassium
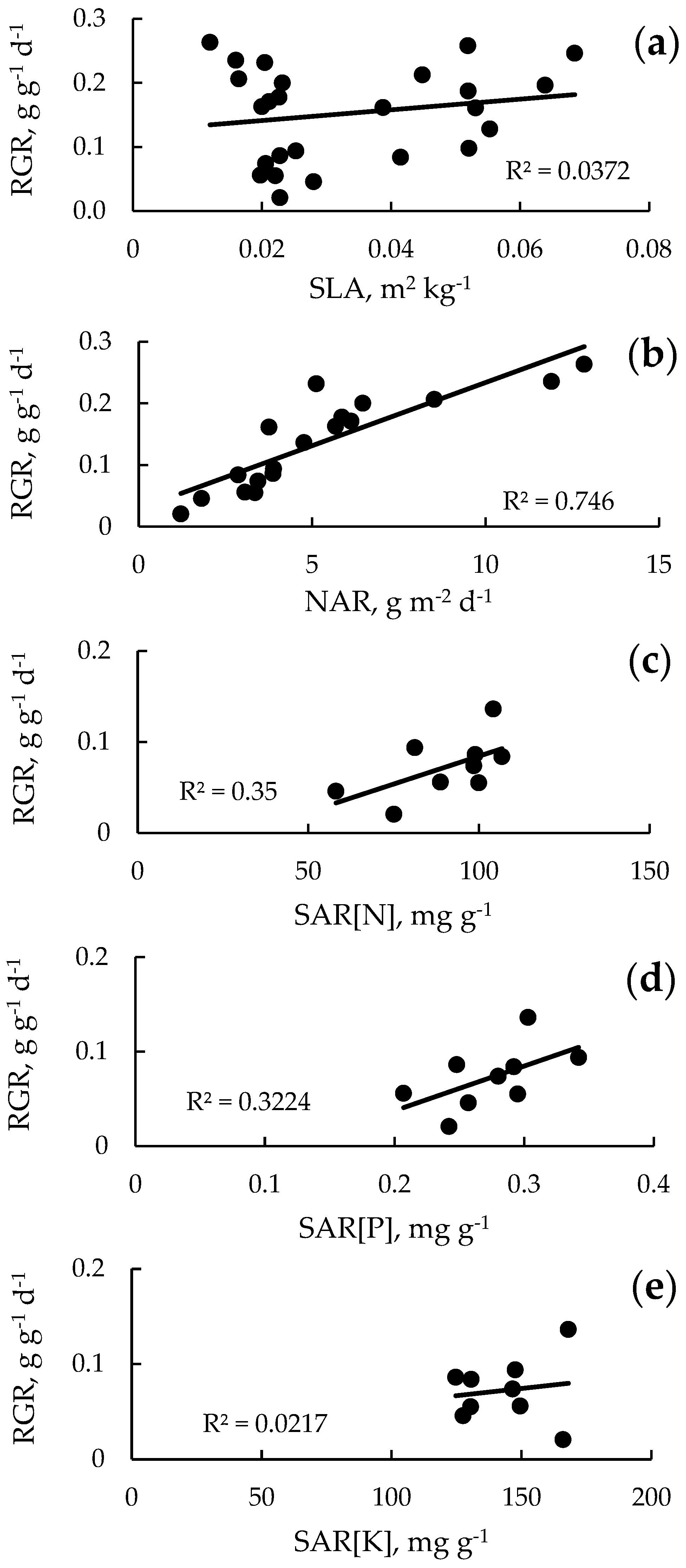
3.8. Relationship Between Relative Growth Rate (RGR) and Specific Leaf Area (SLA), Net Assimilation Rate (NAR), and Specific Absorption Rate (SAR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGR | Absolute growth rate |
| RGR | Relative growth rate |
| NAR | Net assimilation rate |
| SLA | Specific leaf area |
| SAR | Specific absorption rate |
References
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus biochar application enhances Ca uptake of upland rice, soil available P, exchangeable K, yield, and N use efficiency of sugarcane in a crop rotation system. J. Plant Nutr. Soil Sci. 2020, 183, 58–68. [Google Scholar] [CrossRef]
- Ikkonen, E.; Chazhengina, S.; Butilkina, M.; Sidorova, V. Physiological response of onion (Allium cepa L.) seedlings to shungite application under two soil water regimes. Acta Physiol. Plant. 2021, 43, 76. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and application of lignosulfonates and sulfonated Lignin. ChemSusChem Rev. 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Yurkevich, M.; Kurbatov, A.; Ikkonen, E. Effect of Secondary Paper Sludge on Physiological Traits of Lactuca sativa L. under Heavy-Metal Stress. Plants 2024, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Elliott, A. A review of secondary sludge reduction technologies for the pulp and paper industry. Water Res. 2006, 40, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Management of Pulp and Paper Mill Waste, 1st ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2015; p. 197. [Google Scholar]
- Turner, T.; Wheeler, R.; Oliver, I.W. Evaluating land application of pulp and paper mill sludge: A review. J. Environ. Manag. 2022, 317, 115439. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.R.; Cabral, F.; Loґpez-Pineiro, A. Short-term effects on soil properties and wheat production from secondary paper sludge application on two Mediterranean agricultural soils. Bioresour. Technol. 2008, 99, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.; Muir, I.; Richardson, S.; Hickman, G.; Chambers, B. Land Spreading on Agricultural Land: Nature and Impact of Paper Wastes Applied in England & Wales, 1st ed.; Environment Agency: Bristol, UK, 2005; p. 66. [Google Scholar]
- Butylkina, M.; Ikkonen, E. Physical Properties of Retisol under Secondary Pulp and Paper Sludge Application. Land 2023, 12, 2022. [Google Scholar] [CrossRef]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhy, Z.L.; Wang, F.; Zhang, X.; Li, B.; Liu, Z.X.; Wu, X.X.; Ge, S.F.; Jiang, Y.M. Role of calcium as a possible regulator of growth and nitrate nitrogen metabolism in apple dwarf rootstock seedlings. Sci. Hortic. 2021, 276, 109740. [Google Scholar] [CrossRef]
- Ingestad, T. Nitrogen stress in birch seedlings. 1I. N, K P, Ca and Mg nutrition. Physiol. Plant. 1979, 45, 149–157. [Google Scholar] [CrossRef]
- Hunt, R. Relative growth rates. In Basic Growth Analysis; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Broadley, M.R.; Seginer, I.; Burns, A.; Escobar-Gutiérrez, A.J.; Burns, I.G.; White, P.J. The nitrogen and nitrate economy of butterhead lettuce (Lactuca sativa var capitata L). J. Exp. Bot. 2003, 54, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Xu, F.; Chen, G.; Huang, X.; Wang, S.; Du, L.; Ding, G. Evaluation of the Effects of Nitrogen, Phosphorus, and Potassium Applications on the Growth, Yield, and Quality of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 2477. [Google Scholar] [CrossRef]
- Ondrasek, G.; Shepherd, J.; Rathod, S.; Dharavath, R.; Rashid, M.I.; Brtnicky, M.; Shahid, M.S.; Horvatineca, J.; Rengelgh, Z. Metal contamination—A global environmental issue: Sources, implications & advances in mitigation. RSC Adv. 2025, 15, 3904–3927. [Google Scholar] [PubMed]
- Sahito, Z.A.; Zehra, A.; Yu, S.; Chen, S.; He, Z.; Yang, X. Chinese sapindaceous tree species (Sapindus mukorosii) exhibits lead tolerance and long-term phytoremediation potential for moderately contaminated soils. Chemosphere 2023, 338, 139376. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, M.S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.; McBratney, A.B. A review of the contamination of soil with lead II. Spatial distribution and risk assessment of soil lead. Environ. Int. 2001, 27, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Capelo, A.; Santos, C.; Loureiro, S.; Pedro, M.A. Phytotoxicity of lead on Lactuca sativa: Effects On growth, mineral nutrition, photosynthetic activity and oxidant metabolism. Fresenius Environ. Bull. 2012, 21, 450–459. [Google Scholar]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and paper mill sludge management practices: What are the challenges to assess the impacts on greenhouse gas emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef]
- Cabral, F.; Vasconcelos, E.; Goss, M.J.; Cordovil, C.M. The value, use, and environmental impacts of pulp-mill sludge additions to forest and agricultural lands in Europe. Environ. Rev. 1998, 6, 55–64. [Google Scholar] [CrossRef]
- Camberato, J.J.; Gagnon, B.; Angers, D.A.; Chantigny, M.H.; Pan, W.L. Pulp and paper mill by-products as soil amendments and plant nutrient sources. Can. J. Soil Sci. 2006, 86, 641–653. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Conde-Cid, M.; Arias-Estévez, M.; Fernández-Calviño, D.; Alonso-Vega, F. Specific Adsorption of Heavy Metals in Soils: Individual and Competitive Experiments. Agronomy 2020, 10, 1113. [Google Scholar] [CrossRef]
- Ikkonen, E.; Chazhengina, S.; Jurkevich, M. Photosynthetic Nutrient and Water Use Efficiency of Cucumis sativus under Contrasting Soil Nutrient and Lignosulfonate Levels. Plants 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Halsey, K.H.; Milligan, A.J.; Behrenfeld, M.J. Physiological optimization underlies growth rate-independent chlorophyll-specific gross and net primary production. Photosynth. Res. 2010, 103, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: Relationship with daily irradiance. Funct. Ecol. 2002, 16, 682–689. [Google Scholar] [CrossRef]
- Loveys, B.R.; Scheurwater, I.; Pons, T.L.; Fitter, A.H.; Atkin, O.K. Growth temperature influences the underlying components of relative growth rate: An investigation using inherently fast- and slow-growing plant species. Plant Cell Environ. 2002, 25, 975–988. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar]
- Atkin, O.K.; Schortemeyer, M.; McFarlane, N.; Evans, J.R. Variation in the components of relative growth rate in 10 Acacia species from contrasting environments. Plant Cell Environ. 1998, 21, 1007–1017. [Google Scholar] [CrossRef]
- Rand, R.H. Tree size frequency distributions, plant density, age and community disturbance. Ecol. Lett. 2003, 6, 405–411. [Google Scholar] [CrossRef]
- Poorter, H.; Pothmann, P. Growth and carbon economy of a fast- and slow- growing grass species as dependent on ontogeny. New Phytol. 1992, 120, 159–166. [Google Scholar] [CrossRef]
- Villar, R.; Marañón, T.; Quero, J.L.; Lambers, H. Variation in relative growth rate of 20 Aegilops species (Poaceae) in the field: The importance of net assimilation rate or specific leaf area depends on the time scale. Plant Soil 2005, 272, 11–27. [Google Scholar] [CrossRef]
- Shipley, B. Plasticity in relative growth rate and its components following a change in irradiance. Plant Cell Environ. 2000, 23, 1207–1216. [Google Scholar] [CrossRef]
- Gent, M.P. Factors Affecting Relative Growth Rate of Lettuce and Spinach in Hydroponics in a Greenhouse. HortScience 2017, 52, 1742–1747. [Google Scholar] [CrossRef]
- Osone, Y.; Ishida, A.; Tateno, M. Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New Phytol. 2008, 179, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Veenendaal, E.; Swaine, M.; Lecha, R.; Walsh, M.; Abebrese, I.; Owusu-Afriyie, K. Responses of West African forest tree seedlings to irradiance and soil fertility. Funct. Ecol. 1996, 10, 501–511. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Cao, T.; Ni, L.; Li, W.; Zhu, G. Relationships between relative growth rate and its components across 11 submersed macrophytes. J. Freshw. Ecol. 2012, 27, 471–480. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998; p. 540. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Mishra, S. Micronutrients and their diverse role in agricultural crops: Advances and future prospective. Acta Physiol. Plant. 2015, 37, 139. [Google Scholar] [CrossRef]
- Popescu, S.M.; Zheljazkov, V.D.; Astatkie, T.; Burducea, M.; Termeer, W.C. Immobilization of Pb in Contaminated Soils with the Combination Use of Diammonium Phosphate with Organic and Inorganic Amendments. Horticulturae 2023, 9, 278. [Google Scholar] [CrossRef]
- Cao, X.D.; Ammar, L.W.; Bing, M.; Yongliang, L.Y. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shi, F.; Chen, H.; Zhang, Y.; Guo, Y.; Mao, R. Relationship between relative growth rate and C:N:P stoichiometry for the marsh herbaceous plants under water-level stress conditions. Glob. Ecol. Conserv. 2021, 25, e01416. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Relative Growth Rate of Leaf Biomass and Leaf Nitrogen Content in Several Mediterranean Woody Species. Plant Ecol. 2003, 168, 321–332. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Cross-species relationships between seedling relative growth rate, nitrogen productivity and root vs leaf function in 28 Australian woody species: Seedling growth relationships in woody species. Funct. Ecol. 2000, 14, 97–107. [Google Scholar] [CrossRef]
- Si, P.; Thurling, N. A greater relative growth rate of Brassica rapa L. at low temperatures increases biomass at anthesis. Aust. J. Agric. Res. 2001, 52, 645–652. [Google Scholar] [CrossRef]
- Feng, R.; Wang, S.; Ma, J.; Wang, N.; Wang, X.; Ren, F.; Li, H.; Liang, D.; Hu, J.; Li, X.; et al. Nutrient Additions Regulate Height Growth Rate but Not Biomass Growth Rate of Alpine Plants Through the Contrasting Effect of Total and Available Nitrogen. Plants 2025, 14, 1143. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.W.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]


| Parameter | 0 mg Pb(NO3)2 kg−1 | 50 mg Pb(NO3)2 kg−1 | 250 mg Pb(NO3)2 kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 0% | 20% | 40% | 0% | 20% | 40% | |
| Pb content, g kg−1 | |||||||||
| In shoosts | |||||||||
| 34 days after sowing | 0.8 ± 0.0 c | 0.7 ± 0.0 c | 0.5 ± 0.0 c | 1.5 ± 0.1 b | 1.4 ± 0.2 b | 1.3 ± 0.3 b | 2.5 ± 0.4 a | 3.4 ± 1.0 a | 1.9 ± 0.5 ab |
| 46 days after sowing | 0.4 ± 0.1 d | 0.8 ± 0.1 dc | 0.5 ± 0.1 d | 2.6 ± 0.3 b | 0.9 ± 0.2 c | 1.2 ± 0.2 c | 4.7 ± 1.2 a | 5.1 ± 0.5 a | 4.0 ± 0.6 a |
| In roots | |||||||||
| 34 days after sowing | 1.7 ± 0.6 d | 1.4 ± 0.3 d | 0.9 ± 0.2 d | 6.2 ± 1.0 c | 5.6 ± 0.8 c | 5.7 ± 0.5 c | 83.8 ± 10.1 a | 62.3 ± 4.8 b | 65.8 ± 4.8 b |
| 46 days after sowing | 1.2 ± 0.0 d | 0.9 ± 0.1 d | 1.1 ± 0.3 d | 9.6 ± 0.6 c | 8.4 ± 2.6 c | 8.2 ± 6.9 c | 55.7 ± 11.5 a | 38.4 ± 1.7 b | 34.9 ± 9.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikkonen, E.; Yurkevich, M. Relative Growth Rate and Specific Absorption Rate of Nutrients in Lactuca sativa L. Under Secondary Paper Sludge Application and Soil Contamination with Lead. Agriculture 2025, 15, 1541. https://doi.org/10.3390/agriculture15141541
Ikkonen E, Yurkevich M. Relative Growth Rate and Specific Absorption Rate of Nutrients in Lactuca sativa L. Under Secondary Paper Sludge Application and Soil Contamination with Lead. Agriculture. 2025; 15(14):1541. https://doi.org/10.3390/agriculture15141541
Chicago/Turabian StyleIkkonen, Elena, and Marija Yurkevich. 2025. "Relative Growth Rate and Specific Absorption Rate of Nutrients in Lactuca sativa L. Under Secondary Paper Sludge Application and Soil Contamination with Lead" Agriculture 15, no. 14: 1541. https://doi.org/10.3390/agriculture15141541
APA StyleIkkonen, E., & Yurkevich, M. (2025). Relative Growth Rate and Specific Absorption Rate of Nutrients in Lactuca sativa L. Under Secondary Paper Sludge Application and Soil Contamination with Lead. Agriculture, 15(14), 1541. https://doi.org/10.3390/agriculture15141541






