Harnessing Microbial Agents to Improve Soil Health and Rice Yield Under Straw Return in Rice–Wheat Agroecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Model of the Experiment
2.3. Parameters and Measurement Techniques
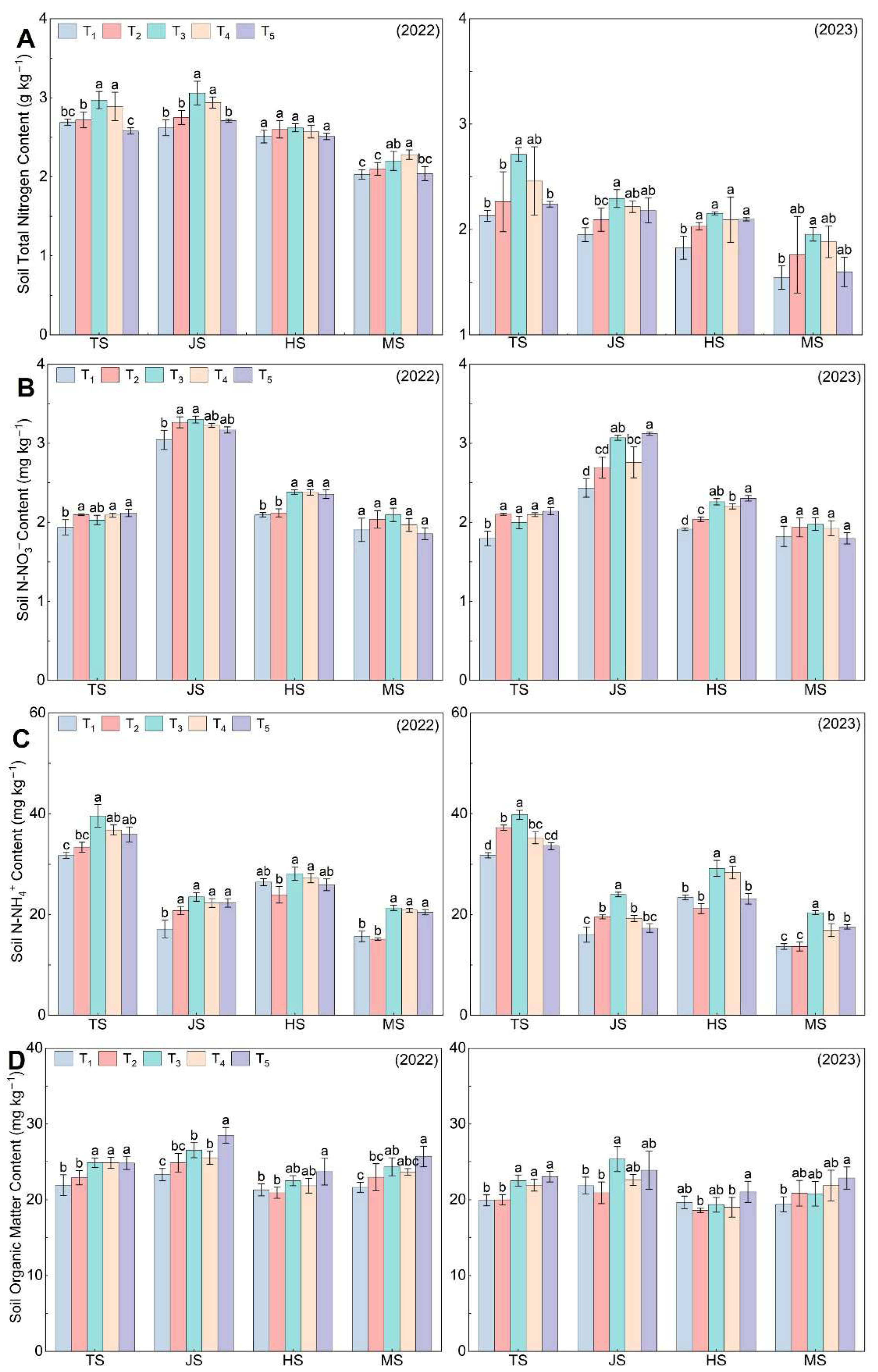
2.3.1. Soil Nutrients
2.3.2. Soil Fungal Community Structure
2.3.3. Dry Matter Accumulation and Yield Determination
2.4. Calculation of Physiological Indices
2.5. Statistical Analysis of Results
3. Result
3.1. Rice Yield and Dry Matter Accumulation
3.2. Dry Matter Transport and Crop Growth During Grain Filling
3.3. Chemical Characteristics of Soil
3.4. Fungal Microbial Composition
3.4.1. Rarefaction Curves
3.4.2. Soil Microbial Diversity Analysis
3.4.3. Soil Fungal Community Composition
3.5. Correlations Between Soil Fungi and Rice/Soil Parameters
4. Discussion
4.1. Effects of Rice–Wheat Rotation, Wheat Straw Return, and Combined Application of Microbial Agents on Rice Growth and Soil Environment
4.2. Improving Rice Yield and Soil Quality Through Wheat Straw Incorporation and Microbial Inoculants in a Rice–Wheat Rotation System
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhang, F.; Zhang, K.; Liao, P.; Xu, Q. Effect of Agricultural Management Practices on Rice Yield and Greenhouse Gas Emissions in the Rice–Wheat Rotation System in China. Sci. Total Environ. 2024, 916, 170307. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current Status and Environment Impact of Direct Straw Return in China’s Cropland—A Review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Gong, Y.; Hu, T.; Lal, R.; Zheng, J.; Justine, M.F.; Azhar, M.; Che, M.; Zhang, H. Effect of Slope, Rainfall Intensity and Mulch on Erosion and Infiltration under Simulated Rain on Purple Soil of South-Western Sichuan Province, China. Water 2016, 8, 528. [Google Scholar] [CrossRef]
- Dai, Z.G.; Lu, J.W.; Li, X.K.; Lu, M.X.; Yang, W.B.; Gao, X.Z. Experiment on Nutrient Release Characteristics of Straw Returned to the Field from Different Crops. Trans. Chin. Soc. Agric. Eng. 2010, 26, 272–276. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, L.; Wei, X.; Li, T.; Tang, Y.; Zeng, X.; Lei, Z.; Wen, J.; Su, S. Promoted Decomposition in Straw Return to Double-Cropped Rice Fields Controls Soil Acidity, Increases Soil Fertility and Improves Rice Yield. Chem. Eng. J. 2025, 509, 161309. [Google Scholar] [CrossRef]
- Kandeler, E. Chapter 7—Physiological and Biochemical Methods for Studying Soil Biota and Their Functions. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 187–222. ISBN 978-0-12-415955-6. [Google Scholar]
- Gałązka, A.; Jankiewicz, U.; Orzechowski, S. The Role of Ligninolytic Enzymes in Sustainable Agriculture: Applications and Challenges. Agronomy 2025, 15, 451. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Soni, P.; Jain, R.; Chauhan, A.; Kumar, R.; Gunsola, D.; Chattaraj, S.; de los Santos-Villalobos, S.; Mitra, D.; Gaur, A. Microbial Metabolites: A Sustainable Solution for Agricultural Challenges. Pedosphere, 2025; in press. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yang, X.; Du, R.; Chen, Y.; Wang, S.; Qiu, R. Biosorption Mechanisms Involved in Immobilization of Soil Pb by Bacillus subtilis DBM in a Multi-Metal-Contaminated Soil. J. Environ. Sci. 2014, 26, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, P.; Mohan Kumar, S.P.; Jyothi Lakshmi, M.; Upreti, K.K. Growth Stimulation and Induction of Systemic Resistance in Tomato against Early and Late Blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- de Lima, B.C.; Moro, A.L.; Santos, A.C.P.; Bonifacio, A.; Araujo, A.S.F.; de Araujo, F.F. Bacillus Subtilis Ameliorates Water Stress Tolerance in Maize and Common Bean. J. Plant Interact. 2019, 14, 432–439. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, H.; Pare, P. Sustained Growth Promotion in Arabidopsis with Long-Term Exposure to the Beneficial Soil Bacterium Bacillus Subtilis (GB03). Plant Signal. Behav. 2009, 4, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Zhang, D.; Guan, C.; Xu, J.; Li, S.; Shen, G.; Luo, Y.; Li, Y. Safe Antifungal Lipopeptides Derived from Bacillus marinus B-9987 against Grey Mold Caused by Botrytis cinerea. J. Integr. Agric. 2017, 16, 1999–2008. [Google Scholar] [CrossRef]
- de Oliveira, H.P.; de Melo, R.O.; Cavalcante, V.S.; Monteiro, T.S.A.; de Freitas, L.G.; Lambers, H.; Valadares, S.V. Phosphate Fertilizers Coated with Phosphate-Solubilising Trichoderma harzianum Increase Phosphorus Uptake and Growth of Zea mays. Plant Soil. 2025, 508, 613–624. [Google Scholar] [CrossRef]
- Al-Zuhairi, F.F.A.; Al-Aareji, J.M.A.; Al-Taae, A.K. Effect of Rootstock and Bio-Fertilizers on Some Mineral Concentrations in the Leaves of Local Lemon (Citrus limon L.) Transplants and Available Nutrients in the Media. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 12049. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The Effect of Trichoderma harzianum in Mitigating Low Temperature Stress in Tomato (Solanum lycopersicum L.) Plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological Functions of Trichoderma Spp. for Agriculture Applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Zheng, K.; Cheng, J.; Xia, J.; Liu, G.; Xu, L. Effects of Soil Bulk Density and Moisture Content on the Physico-Mechanical Properties of Paddy Soil in Plough Layer. Water 2021, 13, 2290. [Google Scholar] [CrossRef]
- Lu, H.F. The Effects of Combined Application of Bacillus Subtilis and Yeast on the Soil Habitat and Physiology and Biochemistry of Rice Irrigated with Reclaimed Water. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2020. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Wang, C.; Ma, Y.M.; Wang, C.Y.; Li, Z.X.; Luo, J.S.; Peng, Z.L.; Liu, R.H.J.; Huang, X.H.; Cao, Y.; Peng, Z.B.; et al. The effects of planting methods and nitrogen application rates on nutrient absorption characteristics and root vitality of hybrid indica rice. Acta Agron. Sin. 2024, 50, 3069–3082. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, P.; Yang, Z.; Liu, S.; Li, Y.; Li, L.; Wang, T.; Siddique, K.H.M. The Responses of Crop Yield and Greenhouse Gas Emissions to Straw Returning from Staple Crops: A Meta-Analysis. Agriculture 2025, 15, 408. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, H.; Zhang, W.; Li, W.; Su, H.; Wu, S.; Xu, Q.; Li, Y.; Yao, H. Straw Return Enhances Grain Yield and Quality of Three Main Crops: Evidence from a Meta-Analysis. Front. Plant Sci. 2024, 15, 1433220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.H.; Qin, Y.H.; Dan, Y.B.; Chen, H.; Hao, X.S.; Tian, X.H. The effects of combined straw returning and reduced nitrogen fertilizer application on dry matter accumulation, nitrogen transport and yield of red clover rice. Acta Agron. Sin. 2023, 50, 756–770. [Google Scholar]
- Wu, J.; Wang, R.; Lu, G.D.; Long, B.; Yang, F.X.; Chen, H.N.; Huang, H. The effects of different straw returning methods in Huanghuang on rice yield and soil nutrients. Acta Agric. Boreali-Sin. 2019, 34, 177–183. [Google Scholar] [CrossRef]
- Zhao, B.; Dong, W.; Chen, Z.; Zhao, X.; Cai, Z.; Feng, J.; Li, S.; Sun, X. Microbial Inoculation Accelerates Rice Straw Decomposition by Reshaping Structure and Function of Lignocellulose-Degrading Microbial Consortia in Paddy Fields. Bioresour. Technol. 2024, 413, 131545. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.E.S.; Filla, V.A.; da Silva, J.P.M.; Barbosa Júnior, M.R.; de Oliveira-Paiva, C.A.; Coelho, A.P.; Lemos, L.B. Application of Bacillus spp. Phosphate-Solubilizing Bacteria Improves Common Bean Production Compared to Conventional Fertilization. Plants 2023, 12, 3827. [Google Scholar] [CrossRef] [PubMed]
- Rais, A.; Shakeel, M.; Malik, K.; Hafeez, F.Y.; Yasmin, H.; Mumtaz, S.; Hassan, M.N. Antagonistic Bacillus spp. Reduce Blast Incidence on Rice and Increase Grain Yield under Field Conditions. Microbiol. Res. 2018, 208, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Lü, X.P.; Bai, J.P.; Qiao, Y.; Paré, P.W.; Wang, S.M.; Zhang, J.L.; Wu, Y.N.; Pang, X.P.; Xu, W.B.; et al. Beneficial Soil Bacterium Bacillus Subtilis (GB03) Augments Salt Tolerance of White Clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Zhang, X.J.; Ma, L.L.; Shi, J.J.; Jia, W.; Qi, Y.Z.; Yin, B.Z.; Zhen, W.C. The influence of microbial agents on the growth of wheat and the prevention effect of soil-borne diseases. Crop Mag. 2017, 155–160. [Google Scholar] [CrossRef]
- Lu, J.; Liu, B.Z.; Zhou, C.Y.; Wang, C.A.; Zhang, W.X. The Influence of Bio-Organic Bacterial Fertilizer on Rice Yield and Rice Quality. Bull. Chin. Agric. Sci. 2009, 25, 146–150. [Google Scholar]
- Qu, H.Y.; Liu, L.M.; Wu, C. The degradation effect of Trichoderma liquid fermentation on straw. Jiangsu Agric. Sci. 2014, 42, 283–285. [Google Scholar] [CrossRef]
- Zhao, J.T.; Shi, Y.P.; Cao, M.J.; Ren, J.J.; Wang, Y.Q.; Chen, Y.P.; Wang, Y.R.; Chen, G. The influence of slow and controlled release fertilizers combined with straw returning to the field on rice yield and nitrogen utilization rate. Zhejiang Agric. Sci. 2025, 66, 590–594. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, J.; Wu, J.F.; Ni, G.R.; Xie, K.L.; Zhou, C.H.; Rong, Q.L.; Zhao, X.M. The influence of returning straw and its biochar to the field on nutrient absorption, distribution and yield of rice. J. Jiangxi Agric. Univ. 2023, 45, 1118–1128. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Q.; Zhou, J.; Wang, M.; Liang, Y.; Sun, B.; Chu, H.; Yang, Y. The Scale Dependence of Fungal Community Distribution in Paddy Soil Driven by Stochastic and Deterministic Processes. Fungal Ecol. 2019, 42, 100856. [Google Scholar] [CrossRef]
- Salamanca-Fonseca, M.; Sanchez, A.; Corrales, A.; Kauserud, H.; Thoen, E.; Krabberød, A.K.; Skrede, I. Beyond Seasonal and Host Factors: Ecosystem Dynamics Drive Palm-Associated Root Fungal Communities at a Local Scale. Plant Soil. 2025, 1–15. [Google Scholar] [CrossRef]
- Man, B.Y.; Xiang, X.; Luo, Y.; Mao, X.T.; Zhang, C.; Sun, B.H.; Wang, X. Characteristics of soil fungal communities under typical vegetation types in Huangshan Mountain and their influencing factors. Mycosystema 2021, 40, 2735–2751. [Google Scholar] [CrossRef]
- Wan, R.Y.; Ma, H.J.; Jiang, B.; Yang, L.R.; Zhou, D.P.; He, M.Z.; Yang, G.R. Composition of soil fungal communities in tea gardens and their influencing factors. Chin. Agric. Sci. Bull. 2021, 37, 88. [Google Scholar] [CrossRef]
- Xia, M.; Li, P.; Liu, J.; Qin, W.; Dai, Q.; Wu, M.; Li, Z.; Li, D.; Liu, M. Long-Term Fertilization Promotes the Microbial-Mediated Transformation of Soil Dissolved Organic Matter. Commun. Earth Environ. 2025, 6, 114. [Google Scholar] [CrossRef]
- Luo, J.R.; Song, W.J.; Liu, W.; Zhang, D.H.; Jiang, X.; Lu, B.L. Effects of Straw Returning Combined with Nitrogen Fertilizer Application on Rice Growth, Soil Properties, and Microbial Diversity in the Early Stage. J. Agric. Biotechnol. 2023, 31, 2019–2034. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.Y.; Li, Z.; Li, B.; Li, Y.; Ao, J.C. Effects of applying different beneficial microbial agents on the rhizosphere soil microecology of continuously cropped flue-cured tobacco. Southwest China J. Agric. Sci. 2024, 37, 612–621. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.K.M.; Cai, L. Cross-Kingdom Synthetic Microbiota Supports Tomato Suppression of Fusarium Wilt Disease. Nat. Commun. 2022, 13, 7890. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ma, Y.; Wu, Z.; Yang, Y.; Yuan, X.; Chen, K.; Luo, Y.; He, Z.; Huang, X.; Deng, P.; et al. Enhancing Rice Ecological Production: Synergistic Effects of Wheat-Straw Decomposition and Microbial Agents on Soil Health and Yield. Front. Plant Sci. 2024, 15, 1368184. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Z.; Borjigin, Q.; Gao, J.L.; Yu, X.F.; Hu, S.P.; Li, R.P. Effects of Different Straw Return Methods on Soil Properties and Yield Potential of Maize. Sci. Rep. 2024, 14, 28682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Jiao, X.; Jiang, H.; Liu, Y.; Wang, X.; Ma, C. The Fate and Challenges of the Main Nutrients in Returned Straw: A Basic Review. Agronomy 2024, 14, 698. [Google Scholar] [CrossRef]
- Lin, S.; Quan, G.; Fu, X.J.; Liu, Z.Z.; Lü, X.H. Effects of straw returning on soil physicochemical properties and yield and quality of japonica rice in coastal rice areas. North. Rice 2023, 53, 29–32. [Google Scholar] [CrossRef]
- Akwakwa, G.H.; Xiaoyan, W. Impact of Rice–Wheat Straw Incorporation and Varying Nitrogen Fertilizer Rates on Soil Physicochemical Properties and Wheat Grain Yield. Agronomy 2023, 13, 2363. [Google Scholar] [CrossRef]
- Peng, X.L.; Dong, Q.; Zhang, C.; Li, P.F.; Li, B.L.; Liu, Z.L.; Yu, C.L. Effects of straw incorporation rate on soil reductive substances and rice growth under different soil conditions. Chin. J. Rice Sci. 2024, 38, 198–210. [Google Scholar] [CrossRef]
- Solanki, K.; Choudhary, S.K.; Singh, A.; Maurya, S.; Shukla, S.; Yadav, S.; Anshuman, K.; Singh, R.D. Response of Combined Application of Nutrient Levels with Microbial Strains on Crop Growth, Nodulation and Yield of Soybean. Int. J. Environ. Clim. Change 2023, 13, 919–924. [Google Scholar] [CrossRef]
- Sahu, R.K.; Kumar, S.; Thakur, R.; Mitra, N.G. Effects of Bioinoculants on Total Chlorophyll Content, Yield of Soybean and Fertility Status of a Vertisols. J. Exp. Agric. Int. 2023, 45, 266–272. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of Nitrogen Application Rate on the Photosynthetic Pigment, Leaf Fluorescence Characteristics, and Yield of Indica Hybrid Rice and Their Interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, H.B.; Prabha, R. (Eds.) Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 2: Functional Applications; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-81-322-2643-7. [Google Scholar]
- Liu, K.; Zhou, S.; Li, S.; Wang, J.; Wang, W.; Zhang, W.; Zhang, H.; Gu, J.; Yang, J.; Liu, L. Differences and Mechanisms of Post-Anthesis Dry Matter Accumulation in Rice Varieties with Different Yield Levels. Crop Environ. 2022, 1, 262–272. [Google Scholar] [CrossRef]
- Smith, C.W.; Dilday, R.H. (Eds.) Rice: Origin, History, Technology, and Production; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-0-471-34516-9. [Google Scholar]




| Treatment | Straw Return | Microbial Agents (Bacillus subtilis/Trichoderma harzianum) |
|---|---|---|
| T1 | None | None |
| T2 | Straw return | None |
| T3 | Straw return | Bacillus subtilis/Trichoderma harzianum = 1:1 |
| T4 | Straw return | Bacillus subtilis/Trichoderma harzianum = 3:1 |
| T5 | Straw return | Bacillus subtilis/Trichoderma harzianum = 1:3 |
| Year | Treatment | Grain Yield (kg hm−2) | Heading Stage | Maturing Stage | ||||
|---|---|---|---|---|---|---|---|---|
| Stem (kg hm−2) | Leaf (kg hm−2) | Spike (kg hm−2) | Stem (kg hm−2) | Leaf (kg hm−2) | Spike (kg hm−2) | |||
| 2022 | T1 | 9270.37 c | 6603.60 d | 2877.00 d | 3175.80 b | 4495.27 b | 2064.80 c | 9788.80 d |
| T2 | 9623.47 c | 6809.87 c | 3530.40 c | 3397.20 a | 4557.00 b | 2452.80 b | 10,902.60 c | |
| T3 | 10,860.02 a | 7733.27 a | 4027.53 a | 3230.40 b | 4897.00 a | 2691.07 a | 12,353.13 a | |
| T4 | 10,256.13 b | 7209.53 b | 3655.47 b | 3304.93 ab | 4804.67 a | 2477.80 b | 11,532.00 b | |
| T5 | 10,232.76 b | 7284.00 b | 3516.20 c | 3240.93 b | 4828.13 a | 2439.00 b | 10,659.93 c | |
| F value | 18.16 ** | 55.24 ** | 51.99 ** | 3.75 * | 13.76 ** | 45.82 ** | 53.35 ** | |
| 2023 | T1 | 9482.05 c | 6837.07 c | 2595.47 d | 3214.13 b | 4735.13 a | 2055.67 c | 9190.87 c |
| T2 | 10,436.26 b | 6856.20 c | 2980.87 c | 3353.87 a | 4737.60 a | 2283.60 c | 9336.87 c | |
| T3 | 11,474.18 a | 7850.20 a | 3836.87 a | 3139.60 b | 4843.33 a | 2793.60 a | 11,272.80 a | |
| T4 | 10,638.25 b | 7755.93 a | 3356.47 b | 3332.33 b | 4825.33 a | 2554.87 b | 10,965.60 a | |
| T5 | 10,511.69 b | 7250.20 b | 3220.20 b | 3198.60 a | 4874.07 a | 2499.00 b | 10,023.23 b | |
| F value | 60.88 ** | 34.39 ** | 59.14 ** | 8.29 ** | 1.19 | 38.34 ** | 55.83 ** | |
| Year | Treatment | MTL (%) | MCL (%) | MTSS (%) | MCSS (%) | ADM (kg hm−2) | GRP (g m−2 d−1) |
|---|---|---|---|---|---|---|---|
| 2022 | T1 | 28.22 b | 8.30 b | 31.92 b | 21.54 b | 3692.47 c | 12.31 c |
| T2 | 30.51 ab | 9.89 a | 33.08 b | 20.67 b | 4174.93 bc | 13.92 bc | |
| T3 | 33.17 a | 10.84 a | 36.67 a | 22.96 a | 4950.00 a | 16.50 a | |
| T4 | 32.22 a | 10.21 a | 33.36 b | 20.85 b | 4644.53 ab | 15.48 ab | |
| T5 | 30.61 ab | 10.11 a | 33.71 b | 23.04 a | 3885.93 c | 12.95 c | |
| F value | 4.99 * | 4.79 * | 9.88 ** | 6.77 ** | 11.39 ** | 11.41 ** | |
| 2023 | T1 | 20.79 c | 5.87 c | 30.92 b | 23.07 b | 3315.87 b | 11.05 b |
| T2 | 23.39 bc | 7.49 b | 30.72 b | 22.54 b | 3186.27 b | 10.62 b | |
| T3 | 27.19 a | 9.26 a | 38.30 a | 26.68 a | 4083.07 a | 13.61 a | |
| T4 | 23.85 b | 7.31 b | 37.78 a | 26.72 a | 3901.07 a | 13.00 a | |
| T5 | 22.40 bc | 7.20 b | 32.77 b | 23.70 b | 3727.29 ab | 12.43 ab | |
| F value | 7.95 ** | 12.09 ** | 32.92 ** | 8.40 ** | 5.04 * | 5.05 * |
| Year | Treatment | Jointing Stage | Heading Stage | ||||
|---|---|---|---|---|---|---|---|
| Chao1 | Shannon | Coverage | Chao1 | Shannon | Coverage | ||
| 2022 | T1 | 389.16 c | 5.43 ab | 0.9999 a | 405.17 d | 4.63 c | 1.00 a |
| T2 | 429.59 b | 5.19 abc | 0.9996 a | 404.88 d | 5.26 b | 1.00 a | |
| T3 | 486.53 a | 5.59 a | 0.9996 a | 592.11 a | 6.11 a | 1.00 a | |
| T4 | 362.07 c | 5.01 bc | 0.9997 a | 543.55 b | 5.22 b | 1.00 a | |
| T5 | 369.23 c | 4.85 c | 0.9997 a | 492.24 c | 5.22 b | 1.00 a | |
| F value | 19.86 ** | 5.43 * | 1.67 | 60.87 ** | 21.46 ** | 0.21 | |
| 2023 | T1 | 178.80 b | 4.26 c | 1.00 a | 129.75 b | 3.94 bc | 1.00 a |
| T2 | 192.37 a | 4.73 a | 1.00 a | 135.63 b | 3.87 c | 1.00 a | |
| T3 | 181.69 ab | 4.55 b | 1.00 a | 154.84 a | 4.48 a | 1.00 a | |
| T4 | 176.45 b | 4.82 a | 1.00 a | 146.63 a | 4.20 ab | 1.00 a | |
| T5 | 179.54 b | 4.88 a | 1.00 a | 103.69 c | 4.28 a | 1.00 a | |
| F value | 3.14 | 27.88 ** | 1.17 | 35.81 ** | 7.21 ** | 2.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wen, Y.; Liu, R.; Peng, Z.; Luo, G.; Wang, C.; Wang, Z.; Yang, Z.; Chen, Z.; Ma, J.; et al. Harnessing Microbial Agents to Improve Soil Health and Rice Yield Under Straw Return in Rice–Wheat Agroecosystems. Agriculture 2025, 15, 1538. https://doi.org/10.3390/agriculture15141538
Ma Y, Wen Y, Liu R, Peng Z, Luo G, Wang C, Wang Z, Yang Z, Chen Z, Ma J, et al. Harnessing Microbial Agents to Improve Soil Health and Rice Yield Under Straw Return in Rice–Wheat Agroecosystems. Agriculture. 2025; 15(14):1538. https://doi.org/10.3390/agriculture15141538
Chicago/Turabian StyleMa, Yangming, Yanfang Wen, Ruhongji Liu, Zhenglan Peng, Guanzhou Luo, Cheng Wang, Zhonglin Wang, Zhiyuan Yang, Zongkui Chen, Jun Ma, and et al. 2025. "Harnessing Microbial Agents to Improve Soil Health and Rice Yield Under Straw Return in Rice–Wheat Agroecosystems" Agriculture 15, no. 14: 1538. https://doi.org/10.3390/agriculture15141538
APA StyleMa, Y., Wen, Y., Liu, R., Peng, Z., Luo, G., Wang, C., Wang, Z., Yang, Z., Chen, Z., Ma, J., & Sun, Y. (2025). Harnessing Microbial Agents to Improve Soil Health and Rice Yield Under Straw Return in Rice–Wheat Agroecosystems. Agriculture, 15(14), 1538. https://doi.org/10.3390/agriculture15141538








