Degradation of Biodegradable Mulch-Derived Microplastics and Their Effects on Bacterial Communities and Radish Growth in Three Vegetable-Cultivated Purple Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Soils and MPs
2.2. Experimental Design
2.3. Plant Harvest and Determination
2.4. Soil Properties and Bacterial Community Determination
2.5. MPs Extraction and Characterization
2.6. Statistical Analysis
3. Results
3.1. MP Degradation in Three Purple Soils
3.2. Effects of MPs on Soil Properties and Enzyme Activities
3.3. Effects of MPs on Soil Bacterial Community
3.3.1. Alpha and Beta Diversity
3.3.2. Soil Bacterial Community Composition
3.4. Effects of MPs on Radish Seedling Growth and Nutrient Contents
4. Discussion
4.1. The Degradation of MPs in Different Purple Soils
4.2. The Changes in Soil Properties in Different Purple Soils
4.3. The Responses of the Bacterial Community in Different Purple Soils
4.4. The Responses of Radish Growth in Different Purple Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDMs | Biodegradable mulch films |
| PE-MPs | Polyethylene microplastics |
| Bio-MPs | Biodegradable microplastics |
| MP | Microplastic |
| PBAT | Polybutylene adipate terephthalate |
| PLA | Polylactic acid |
| AS | Acidic purple soil |
| NS | Neutral purple soil |
| CS | Calcareous purple soil |
References
- Adamczewska-Sowinska, K.; Bykowy, J.; Jaworska, J. Effect of biodegradable mulch and different synthetic mulches on growth and yield of field-grown small-fruited tomato (Lycopersicon esculentum Mill.). Agriculture 2025, 15, 212. [Google Scholar] [CrossRef]
- Qiang, L.; Hu, H.; Li, G.; Xu, J.; Cheng, J.; Wang, J.; Zhang, R. Plastic mulching, and occurrence, incorporation, degradation, and impacts of polyethylene microplastics in agroecosystems. Ecotox. Environ. Saf. 2023, 263, 115274. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, H.; Wen, Y.; Song, X.; Wang, X.; Zhang, Z. Research progress on degradation of biodegradable micro-nano plastics and its toxic effect mechanism on soil ecosystem. Environ. Res. 2024, 262, 119979. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reverón, R.; Alvarez-Méndez, S.J.; Kropp, R.M.; Perdomo-González, A.; Hernández-Borges, J.; Díaz-Peña, F.J. Microplastics in agricultural systems: Analytical methodologies and effects on soil quality and crop yield. Agriculture 2022, 12, 1162. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, S.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.; Jones, D.L.; Chadwick, D.R.; et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef]
- Deng, Y.X.; Zeng, Z.J.; Feng, W.Y.; Liu, J.; Yang, F. Characteristics and migration dynamics of microplastics in agricultural soils. Agriculture 2024, 14, 157. [Google Scholar] [CrossRef]
- Wang, K.; Flury, M.; Sun, S.; Cai, J.; Zhang, A.; Li, Q.; Jiang, R. In-field degradation of polybutylene adipate-co-terephthalate (PBAT) films, microplastic formation, and impacts on soil health. Environ. Res. 2025, 272, 121086. [Google Scholar] [CrossRef]
- Wang, K.; Min, W.; Flury, M.; Gunina, A.; Lv, J.; Li, Q.; Jiang, R. Impact of long-term conventional and biodegradable film mulching on microplastic abundance, soil structure and organic carbon in a cotton field. Environ. Pollut. 2024, 356, 124367. [Google Scholar] [CrossRef]
- Graf, M.; Greenfield, L.M.; Reay, M.K.; Bargiela, R.; Golyshin, P.N.; Evershed, R.P.; Lloyd, C.E.M.; Williams, G.B.; Chadwick, D.R.; Jones, D.L. Field-based assessment of the effect of conventional and biodegradable plastic mulch film on nitrogen partitioning, soil microbial diversity, and maize biomass. Appl. Soil Ecol. 2024, 202, 105595. [Google Scholar] [CrossRef]
- Zhou, A.; Ji, Q.; Kong, X.; Zhu, F.; Meng, H.; Li, S.; He, H. Response of soil property and microbial community to biodegradable microplastics, conventional microplastics and straw residue. Appl. Soil Ecol. 2024, 196, 105302. [Google Scholar] [CrossRef]
- Chu, J.; Zhou, J.; Wang, Y.; Jones, D.L.; Ge, J.; Yang, Y.; Brown, R.W.; Zang, H.; Zeng, Z. Field application of biodegradable microplastics has no significant effect on plant and soil health in the short term. Environ. Pollut. 2023, 316, 120556. [Google Scholar] [CrossRef]
- Reay, M.K.; Greenfield, L.M.; Graf, M.; Lloyd, C.E.M.; Evershed, R.P.; Chadwick, D.R.; Jones, D.L. LDPE and biodegradable PLA-PBAT plastics differentially affect plant-soil nitrogen partitioning and dynamics in a Hordeum vulgare mesocosm. J. Hazard. Mater. 2023, 447, 130825. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Chadwick, D.R.; Zang, H.D.; Graf, M.; Liu, X.J.; Wang, K.; Greenfield, L.M.; Jones, D.L. Bioplastic (PHBV) addition to soil alters microbial community structure and negatively affects plant-microbial metabolic functioning in maize. J. Hazard. Mater. 2023, 441, 129959. [Google Scholar] [CrossRef]
- Fan, H.; Hong, X.; Wang, H.; Gao, F.; Su, Z.; Yao, H. Biodegradable microplastics affect tomato (Solanum lycopersicum L.) growth by interfering rhizosphere key phylotypes. J. Hazard. Mater. 2025, 487, 137208. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Teng, Y.; Wang, X.; Ren, W.J.; Wang, X.M.; Luo, Y.M.; Zhang, H.M.; Christie, P. Soil type driven change in microbial community affects Poly(butylene adipate-co-terephthalate) degradation potential. Environ. Sci. Technol. 2021, 55, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Gioacchini, P.; Montecchio, D.; Rapisarda, S.; Ciavatta, C.; Marzadori, C. Biodegradable plastics: Effects on functionality and fertility of two different soils. Appl. Soil Ecol. 2022, 169, 104216. [Google Scholar] [CrossRef]
- Rauscher, A.; Meyer, N.; Jakobs, A.; Bartnick, R.; Lueders, T.; Lehndorff, E. Biodegradable microplastic increases CO2 emission and alters microbial biomass and bacterial community composition in different soil types. Appl. Soil Ecol. 2023, 182, 104714. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Shang, J.; Wang, X. Microplastic additions alter soil organic matter stability and bacterial community under varying temperature in two contrasting soils. Sci. Total Environ. 2022, 838, 156471. [Google Scholar] [CrossRef]
- Špela, Ž.; Damjana, D.; Matej, H.; Matic, N.; Marina, P. Soil water repellency of two disturbed soils contaminated with different agricultural microplastics tested under controlled laboratory conditions. Geoderma 2025, 453, 117124. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Liu, L.; Zheng, Y.; Xie, D. High negative surface charge increases the acidification risk of purple soil in China. Catena. 2021, 196, 104819. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, T.; Du, B. Effect of organic matter and calcium carbonate on behaviors of cadmium adsorption–desorption on/from purple paddy soils. Chemosphere 2014, 99, 41–48. [Google Scholar] [CrossRef] [PubMed]
- EN 17033; Plastics-Biodegradable Mulch Films for Use in Agriculture and Horticulture—Requirements and Test Methods. European Standard, European Committee for Standardization: Brussels, Belgium, 2018.
- Lu, R.K. Analytical Methods in Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 274–323. [Google Scholar]
- Guan, S.Y. Soil Enzymes and Their Research Methods; Agriculture Press: Beijing, China, 1986; pp. 12–315. [Google Scholar]
- Li, S.T.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.Y.; Jones, D.L.; Wang, J.K. Macro- and microplastic accumulation in soil after 32 years of plastic film mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 472:2013; Plastics-Vocabulary. British Standards Institution (BSI): London, UK, 2013.
- Zhang, Y.; Gao, W.; Mo, A.; Jiang, J.; He, D. Degradation of polylactic acid/polybutylene adipate films in different ratios and the response of bacterial community in soil environments. Environ. Pollut. 2022, 313, 120167. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cao, Y.; Fan, L.; Xie, Y.; Zhou, X.; Ren, Q.; Yang, X.; Gao, X.; Feng, Y. Degradation characteristics of polybutylene adipate terephthalic acid (PBAT) and its effect on soil physicochemical properties: A comparative study with several polyethylene (PE) mulch films. J. Hazard. Mater. 2023, 456, 131661. [Google Scholar] [CrossRef]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Nomadolo, N.; Dada, O.E.; Swanepoel, A.; Mokhena, T.; Muniyasamy, S. A Comparative Study on the Aerobic Biodegradation of the Biopolymer Blends of Poly(butylene succinate), Poly(butylene adipate terephthalate) and Poly(lactic acid). Polymers 2022, 14, 1894. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef]
- Wang, F.; Sun, J.; Han, L.; Liu, W.; Ding, Y. Microplastics regulate soil microbial activities: Evidence from catalase, dehydrogenase, and fluorescein diacetate hydrolase. Environ. Res. 2024, 263, 120064. [Google Scholar] [CrossRef]
- Gao, B.; Gao, F.; Zhang, X.; Li, Y.; Yao, H. Effects of different sizes of microplastic particles on soil respiration, enzyme activities, microbial communities, and seed germination. Sci. Total Environ. 2024, 933, 173100. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Liu, J.; Wu, H. Discrepant responses of bacterial community and enzyme activities to conventional and biodegradable microplastics in paddy soil. Sci. Total Environ. 2024, 909, 168513. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Wang, F.; Chen, Q.a.; Huang, H.; Wang, J.; Ma, C.; Sun, K.; Rillig, M.C.; Kuzyakov, Y.; et al. Polylactic acid microplastics induced negative priming and improved carbon sequestration via microbial processes in different paddy soils. Soil. Biol. Biochem. 2025, 201, 109653. [Google Scholar] [CrossRef]
- Wu, P.; Li, Z.S.; Gao, J.; Zhao, Y.P.; Wang, H.; Qin, H.M.; Gu, Q.; Wei, R.; Liu, W.D.; Han, X. Characterization of a PBAT degradation carboxylesterase from Thermobacillus composti KWC4. Catalysts 2023, 13, 340. [Google Scholar] [CrossRef]
- Huo, Y.; Dijkstra, F.A.; Possell, M.; Singh, B. Mineralisation and priming effects of a biodegradable plastic mulch film in soils: Influence of soil type, temperature and plastic particle size. Soil. Biol. Biochem. 2024, 189, 109257. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, X.; Zhang, Z. Effects of soil properties and land use patterns on the distribution of microplastics: A case study in southwest China. J. Environ. Manag. 2024, 356, 120598. [Google Scholar] [CrossRef]
- Xiang, Y.; Peñuelas, J.; Sardans, J.; Liu, Y.; Yao, B.; Li, Y. Effects of microplastics exposure on soil inorganic nitrogen: A comprehensive synthesis. J. Hazard. Mater. 2023, 460, 132514. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Peng, Y.; Zhang, Z.; Fan, Z.; Wang, J.; Wang, X. Microbes drive metabolism, community diversity, and interactions in response to microplastic-induced nutrient imbalance. Sci. Total Environ. 2023, 877, 162885. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Li, W.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F.; Xing, B. Response of peanut plant and soil N-fixing bacterial communities to conventional and biodegradable microplastics. J. Hazard. Mater. 2023, 459, 132142. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Liu, L.; Zou, G.; Zuo, Q.; Li, C.; Gu, J.; Kang, L.; Ma, M.; Liang, K.; Liu, D.; Du, L. Soil bacterial community and metabolism showed a more sensitive response to PBAT biodegradable mulch residues than that of LDPE mulch residues. J. Hazard. Mater. 2022, 438, 129507. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Wang, Y.; Zhang, Y.; Xu, T.; Zhang, Y.; Xi, J.; Hou, L.; Li, L.; Zhang, Z.; et al. Negative effects of poly(butylene adipate-co-terephthalate) microplastics on Arabidopsis and its root-associated microbiome. J. Hazard. Mater. 2022, 437, 129294. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gu, H.; Sun, X.; Wang, Y.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Wang, G. Metagenomic exploration of microbial and enzymatic traits involved in microplastic biodegradation. Chemosphere 2024, 348, 140762. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Harkes, P.; van Steenbrugge, J.J.M.; Geissen, V. Effects of microplastics on common bean rhizosphere bacterial communities. Appl. Soil Ecol. 2023, 181, 104649. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. J. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Wang, J.; Zhang, K.; Yang, Z.; Li, J.; Liu, X.; Wu, P.; Lin, Y.; Shangguan, Z.; et al. Temporal dynamics of rhizosphere bacterial community in the Robinia pseudoacacia–Mesorhizobium loti symbiotic system for remediation of cadmium-contaminated soils. Appl. Soil Ecol. 2024, 198, 105375. [Google Scholar] [CrossRef]
- Feng, L.J.; Jia, R.; Zeng, Z.; Yang, G.F.; Xu, X.Y. Simultaneous nitrification-denitrification and microbial community profile in an oxygen-limiting intermittent aeration SBBR with biodegradable carriers. Biodegradation 2018, 29, 473–486. [Google Scholar]
- Hu, X.; Gu, H.; Sun, X.; Wang, Y.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Wang, G. Distinct influence of conventional and biodegradable microplastics on microbe-driving nitrogen cycling processes in soils and plastispheres as evaluated by metagenomic analysis. J. Hazard. Mater. 2023, 451, 131097. [Google Scholar] [CrossRef]
- Zantis, L.J.; Adamczyk, S.; Velmala, S.M.; Adamczyk, B.; Vijver, M.G.; Peijnenburg, W.; Bosker, T. Comparing the impact of microplastics derived from a biodegradable and a conventional plastic mulch on plant performance. Sci. Total Environ. 2024, 935, 173265. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Phytotoxicity assessment of biodegradable and non-biodegradable plastics using seed germination and early growth tests. Chemosphere 2022, 289, 133132. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Huerta Lwanga, E.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Wang, L.; Zhang, C.; Zhang, Y. Are biodegradable mulch films a sustainable solution to microplastic mulch film pollution? A biogeochemical perspective. J. Hazard. Mater. 2023, 459, 132024. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, Y.; Li, H.; Dong, H.; Li, B.; Guo, Y.; Wang, Y.; Guo, X.; Yin, T.; Liu, X.; et al. Responses of lettuce (Lactuca sativa L.) growth and soil properties to conventional non-biodegradable and new biodegradable microplastics. Environ. Pollut. 2024, 341, 122897. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhang, C.; Zhang, R.; Li, Q.; Liu, H.; Wang, X.-X. Effects of microplastics derived from biodegradable mulch film on different plant species growth and soil properties. Sci. Total Environ. 2024, 948, 174899. [Google Scholar] [CrossRef] [PubMed]

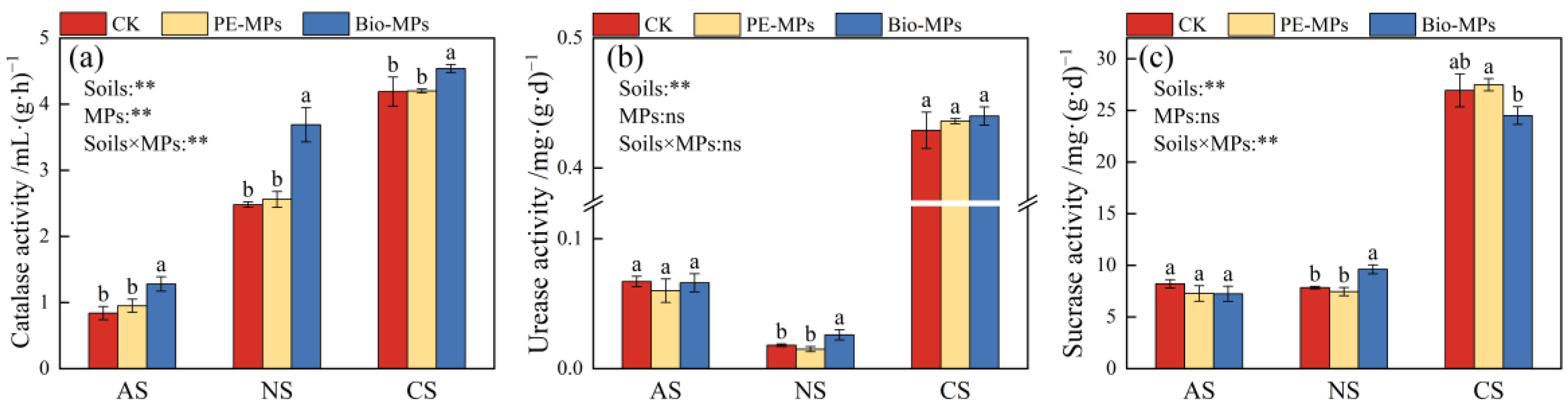
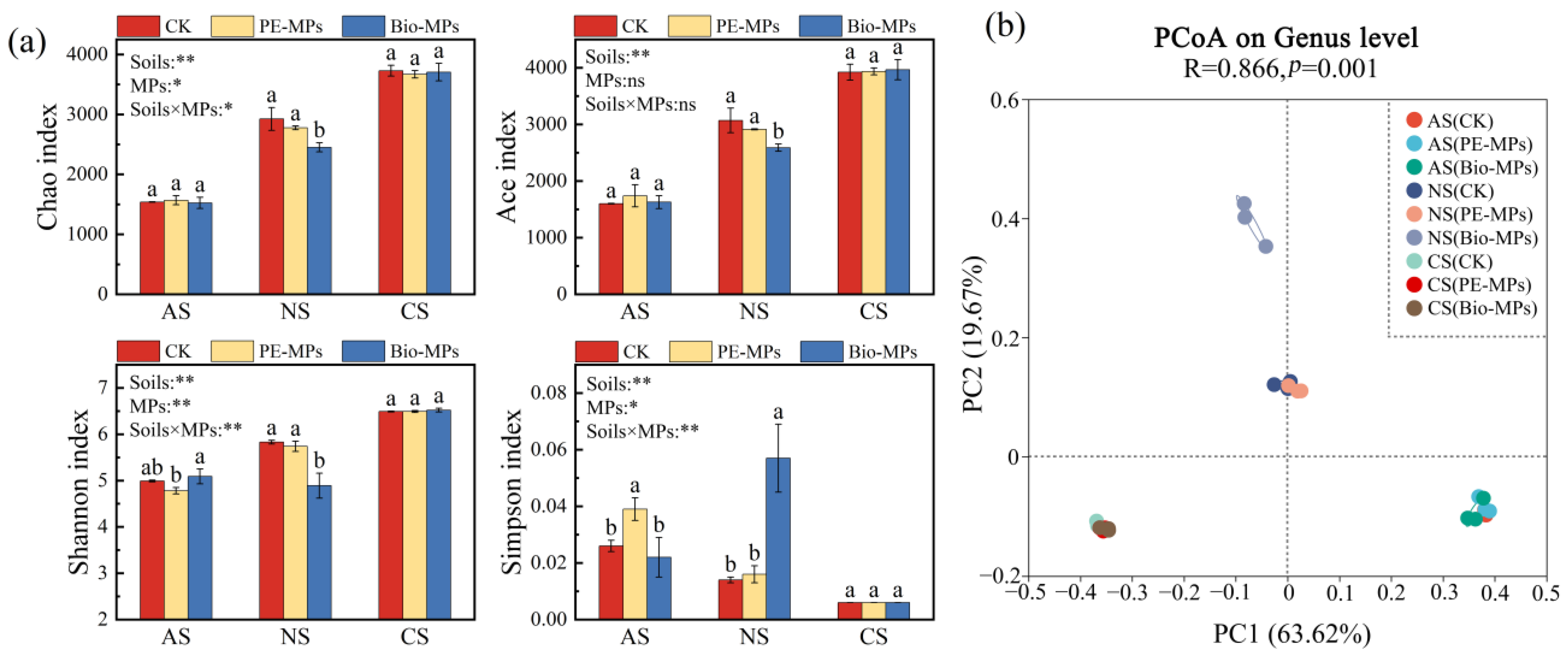
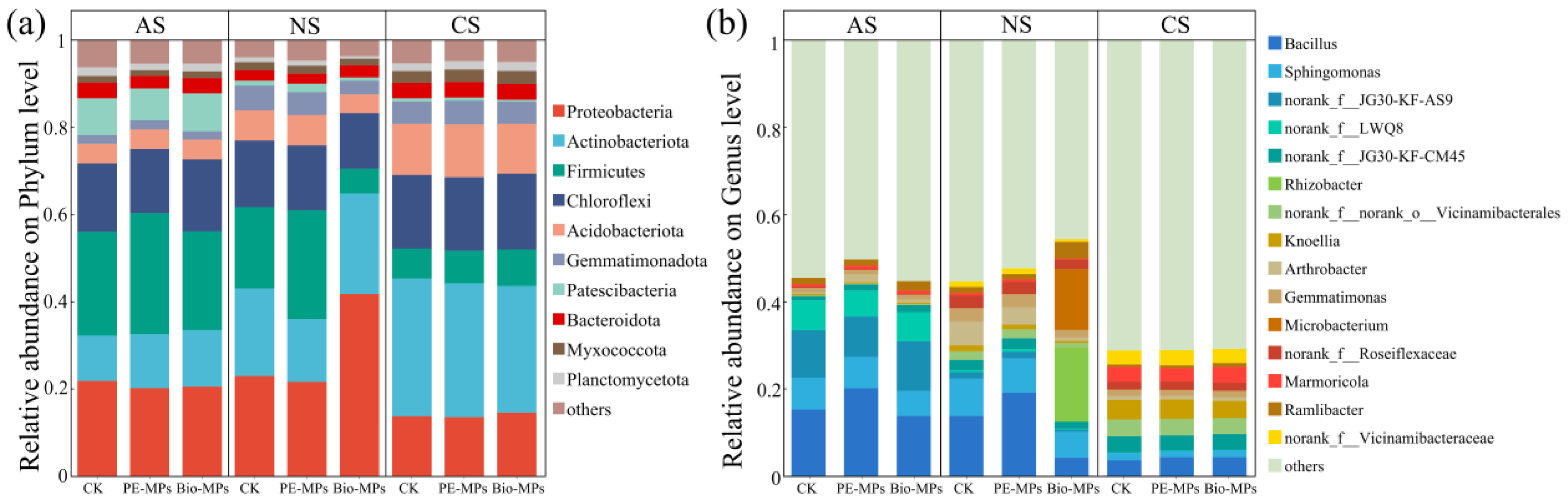

| Soils | MPs | pH | SOM (g⋅kg−1) | NH4+-N (mg⋅kg−1) | NO3−-N (mg⋅kg−1) | AP (mg⋅kg−1) | AK (mg⋅kg−1) |
|---|---|---|---|---|---|---|---|
| AS | CK | 5.38 ± 0.05 d | 16.25 ± 0.07 c | 57.46 ± 4.16 a | 5.17 ± 0.66 f | 288.47 ± 10.55 b | 474.25 ± 5.22 c |
| PE-MPs | 5.32 ± 0.05 d | 16.26 ± 0.05 c | 57.01 ± 2.29 a | 8.28 ± 1.42 e | 296.87 ± 2.42 b | 463.88 ± 13.15 c | |
| Bio-MPs | 5.43 ± 0.05 d | 16.77 ± 0.14 c | 52.40 ± 2.29 a | 11.29 ± 0.22 d | 331.46 ± 3.96 a | 468.24 ± 13.04 c | |
| NS | CK | 6.41 ± 0.03 c | 20.07 ± 0.17 b | 1.00 ± 0.09 b | 80.50 ± 3.85 b | 289.76 ± 2.38 b | 666.60 ± 1.31 a |
| PE-MPs | 6.50 ± 0.02 c | 19.40 ± 0.27 b | 1.12 ± 0.15 b | 75.25 ± 1.75 b | 278.77 ± 5.56 b | 643.65 ± 20.65 ab | |
| Bio-MPs | 7.30 ± 0.13 b | 20.87 ± 0.40 b | 0.94 ± 0.13 b | 32.58 ± 4.07 c | 322.73 ± 7.90 a | 617.67 ± 12.01 b | |
| CS | CK | 8.00 ± 0.04 a | 27.16 ± 0.05 a | 0.32 ± 0.05 c | 122.73 ± 12.51 a | 105.83 ± 1.83 c | 369.37 ± 4.06 d |
| PE-MPs | 8.00 ± 0.04 a | 27.06 ± 0.07 a | 0.32 ± 0.02 c | 113.41 ± 8.99 a | 93.87 ± 9.13 c | 353.47 ± 4.87 d | |
| Bio-MPs | 7.96 ± 0.01 a | 27.27 ± 0.14 a | 0.24 ± 0.02 c | 117.93 ± 9.35 a | 93.22 ± 6.00 c | 349.68 ± 11.92 d | |
| Soils | ** | ** | ** | ** | ** | ** | |
| MPs | ** | ns | ns | ** | ** | ** | |
| Soils × MPs | ** | ns | ns | ** | ** | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, R.; Liu, Z.; Mu, Y.; Chen, J.; Zhao, X. Degradation of Biodegradable Mulch-Derived Microplastics and Their Effects on Bacterial Communities and Radish Growth in Three Vegetable-Cultivated Purple Soils. Agriculture 2025, 15, 1512. https://doi.org/10.3390/agriculture15141512
Ao R, Liu Z, Mu Y, Chen J, Zhao X. Degradation of Biodegradable Mulch-Derived Microplastics and Their Effects on Bacterial Communities and Radish Growth in Three Vegetable-Cultivated Purple Soils. Agriculture. 2025; 15(14):1512. https://doi.org/10.3390/agriculture15141512
Chicago/Turabian StyleAo, Ruixue, Zexian Liu, Yue Mu, Jiaxin Chen, and Xiulan Zhao. 2025. "Degradation of Biodegradable Mulch-Derived Microplastics and Their Effects on Bacterial Communities and Radish Growth in Three Vegetable-Cultivated Purple Soils" Agriculture 15, no. 14: 1512. https://doi.org/10.3390/agriculture15141512
APA StyleAo, R., Liu, Z., Mu, Y., Chen, J., & Zhao, X. (2025). Degradation of Biodegradable Mulch-Derived Microplastics and Their Effects on Bacterial Communities and Radish Growth in Three Vegetable-Cultivated Purple Soils. Agriculture, 15(14), 1512. https://doi.org/10.3390/agriculture15141512





