Effects of Refrigerated Storage on the Physicochemical, Color and Rheological Properties of Selected Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Varietal Identification of the Tested Honeys
2.1.2. Determination of the Sugar Profile of Honeys
2.2. Tests Carried out During Storage of Honeys
2.2.1. Determination of Moisture and Extract Content
2.2.2. Evaluation of pH and Free Acidity
2.2.3. Determination of Electrical Conductivity of Honeys
2.2.4. Color Analysis
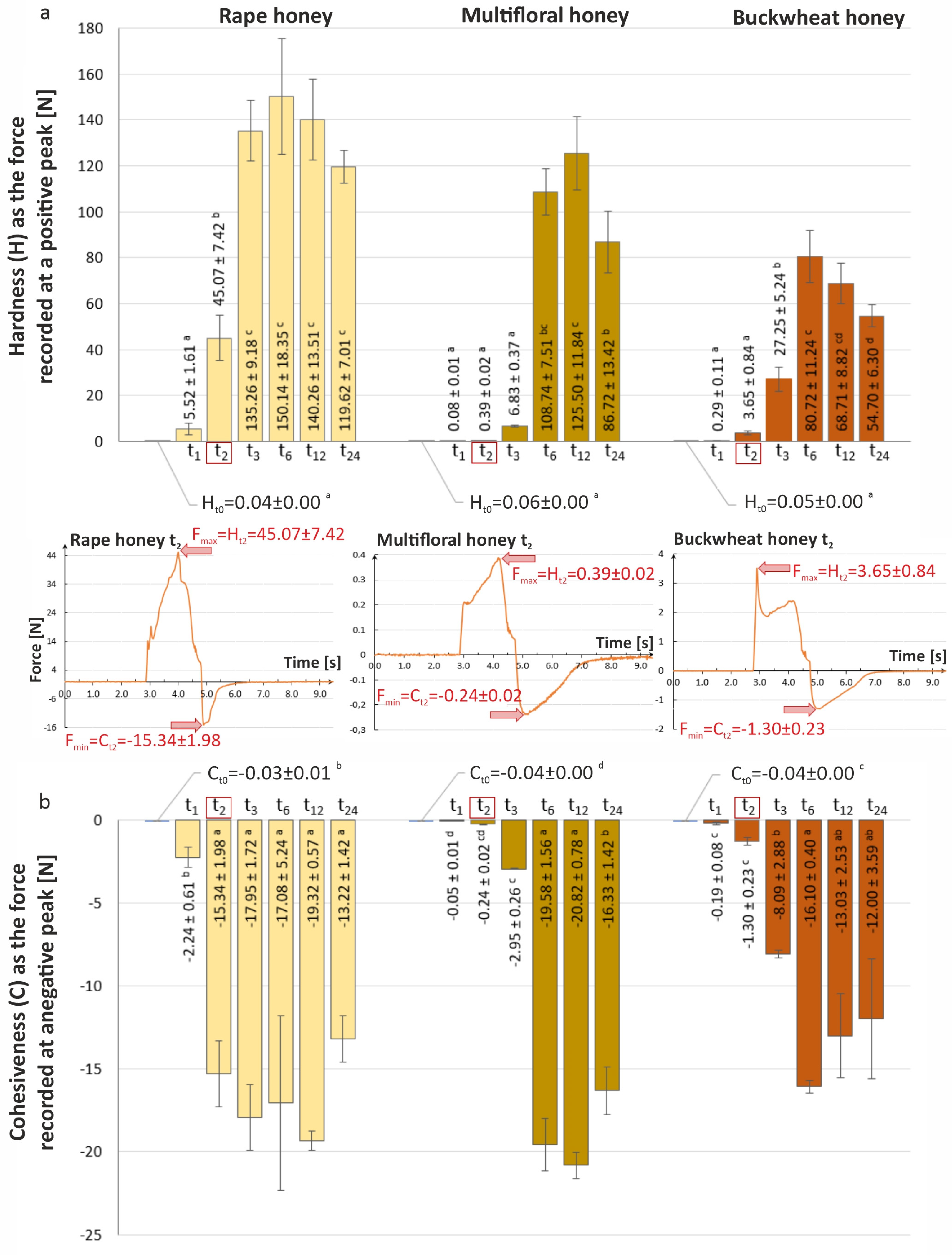
2.2.5. Texture Analysis
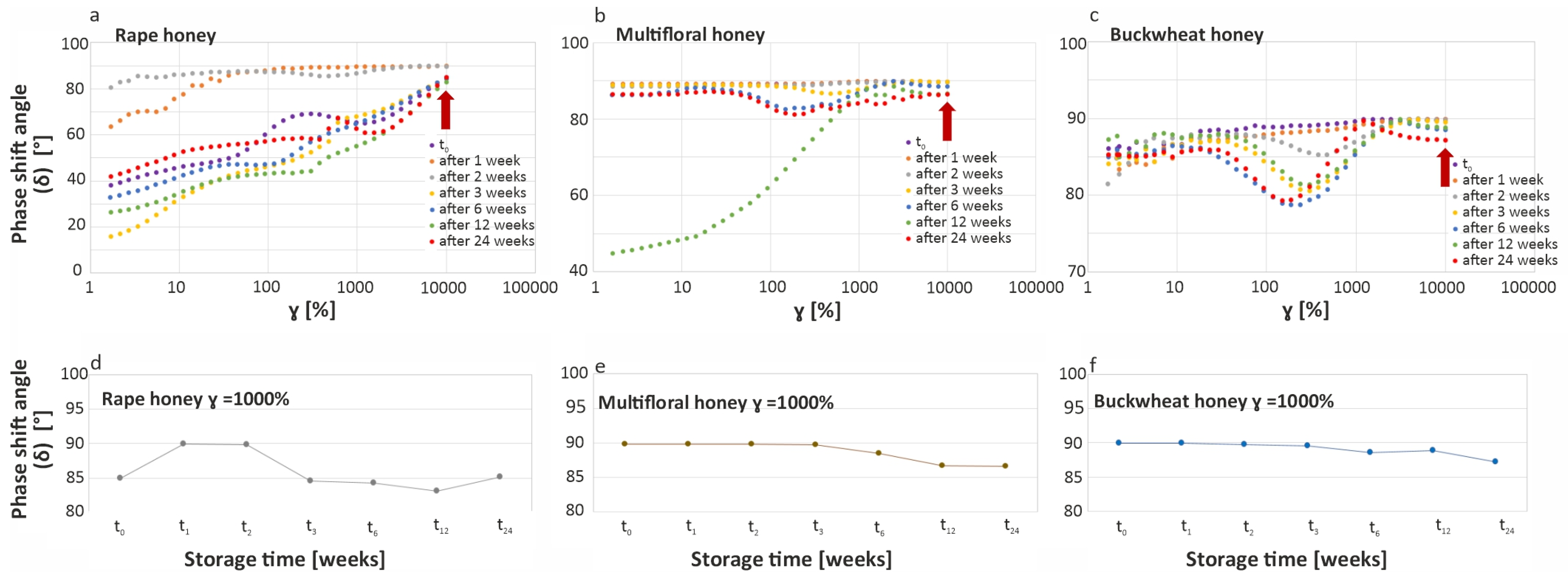
2.2.6. Rheological Measurements
2.2.7. Size and Structure of Crystals
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Physicochemical Changes of the Tested Honey Varieties During Storage
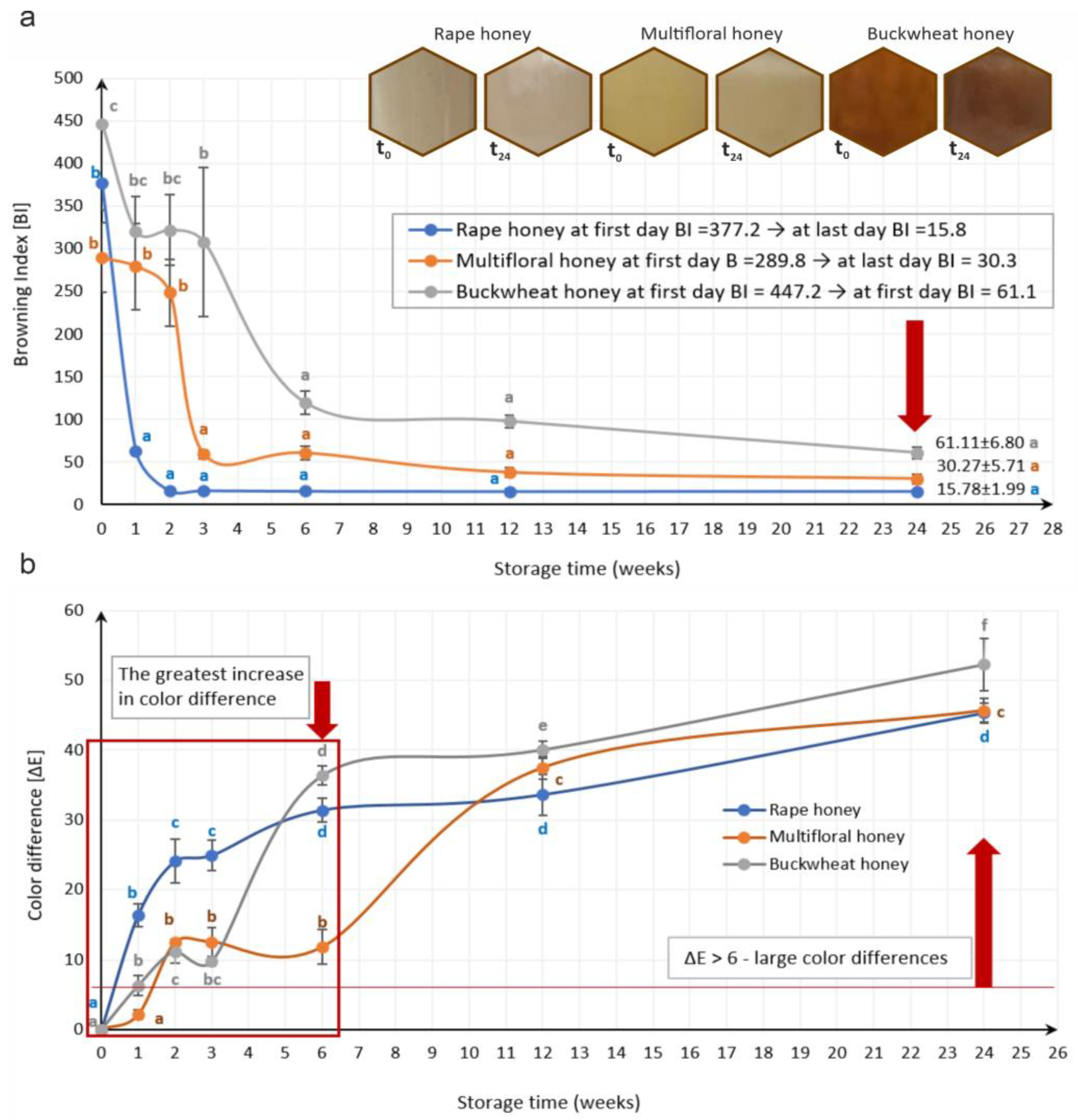
3.2. Analysis of Color Changes of Tested Honey Varieties During Storage
3.3. Characteristics of Honey Crystallization Process
3.4. Analysis of Texture Changes of Tested Honey Varieties During Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tomaszewska-Gras, J.; Bakier, S.; Goderska, K.; Mansfel, K. Differential scanning calorimetry for determining the thermodynamic properties of selected honeys. J. Apic. Sci. 2015, 59, 109–118. [Google Scholar] [CrossRef]
- Bakier, S.; Miastkowski, K.; Bakoniuk, J.R. Rheological properties of some honeys in liquefied and crystallised states. J. Agric. Sci. 2016, 60, 153–166. [Google Scholar] [CrossRef]
- Suriwong, V.; Jaturonglumlert, S.; Varith, J.; Narkprasom, K.; Tanongkankit, Y.; Nitatwichit, C.; Thaisamak, P. Thai creamed honey: Enthalpy of crystal melting and texture profile under different storage conditions. Int. Food Res. J. 2021, 28, 936–944. [Google Scholar] [CrossRef]
- Dettori, A.; Tappi, S.; Piana, L.; Dalla, R.M.; Rocculi, P. Kinetic of induced honey crystallization and related evolution of structural and physical properties. LWT 2018, 95, 333–338. [Google Scholar] [CrossRef]
- Lupano, C.E. DSC study of honey granulation stored at various temperatures. Food Res. Int. 1997, 30, 683–688. [Google Scholar] [CrossRef]
- Conforti, P.A.; Lupano, C.E.; Malacalza, N.H.; Arias, V.; Castells, C.B. Crystallization of honey at −20 °C. Int. J. Food Prop. 2006, 9, 99–107. [Google Scholar] [CrossRef]
- Venir, E.; Spaziani, M.; Maltini, E. Crystallization in Tarassaco Italian honey studied by DSC. Food Chem. 2010, 122, 410–415. [Google Scholar] [CrossRef]
- Cohen, I.; Weihs, D. Rheology and micro rheology of natural and reduced-calorie Israeli honeys as a model for high-viscosity Newtonian liquids. J. Food Eng. 2010, 100, 366–371. [Google Scholar] [CrossRef]
- Kabbani, D.; Sepulcre, F.; Wedekind, J. Ultrasound-assisted liquefaction of rosemary honey: Influence on rheology and crystal content. J. Food Eng. 2011, 107, 173–178. [Google Scholar] [CrossRef]
- Schiassi, M.C.E.V.; Souza, V.R.D.; Alves, N.A.; Lago, A.M.T.; Silva, S.H.; Carvalho, G.R.; de Resende, J.V.; Queiroz, F. Effect of botanical origin on stability and crystallization of honey during storage. Br. Food J. 2021, 124, 2689–2704. [Google Scholar] [CrossRef]
- Cavia, M.M.; Fernández-Muiño, M.A.; Alonso-Torre, S.R.; Huidobro, J.F.; Sancho, M.T. Evolution of acidity of honeys from continental climates: Influence of induced granulation. Food Chem. 2007, 100, 1728–1733. [Google Scholar] [CrossRef]
- Bakier, S. Crystalline structure characteristics of some polish honeys. Agric. Eng. 2007, 5, 13–21. [Google Scholar]
- Rybak-Chmielewska, H.; Szczęsna, T. Storage Conditions and Honey Quality. BASIC Issues of Honey Quality; Press Institute of Horticulture and Floriculture, Apiculture Department: Puławy, Poland, 1996; Volume 24. [Google Scholar]
- Kędzia, B.; Hołderna-Kędzia, E. Honey, Composition and Biological Properties; Rzeczpospolita Publishing House SA: Warsaw, Poland, 2008; pp. 187–206. (In Polish) [Google Scholar]
- Manikis, I.; Thrasyvoulou, A. The relation of physicochemical characteristics of honey and the crystallization sensitive parameters. Apiacta 2001, 36, 106–112. [Google Scholar]
- Costa, L.C.V.; Kaspchak, E.; Queiroz, M.B.; Almeida, M.M.D.; Quast, E.; Quast, L.B. Influence of temperature and homogenization on honey crystallization. Braz. J. Food Res. 2015, 18, 155–161. [Google Scholar] [CrossRef]
- Zamora, M.C.; Chirife, J. Determination of water activity change due to crystallization in honeys from Argentina. Food Control 2006, 17, 59–64. [Google Scholar] [CrossRef]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of physico-chemical properties and antioxidant capacities of bioactive honey produced from Australian grown Agastache rugosa and its correlation with colour and poly-phenol content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef]
- Ji, P.; Liu, X.; Yang, C.; Wu, F.; Sun, J.; Cao, W.; Zhao, H. Natural crystallization properties of honey and seed crystals-induced crystallization process for honey performance enhancing. Food Chem. 2023, 405, 134972. [Google Scholar] [CrossRef]
- Krishnan, R.; Mohammed, T.; Kumar, G.S.; Sh, A. Honey crystallization: Mechanism, evaluation and application. Pharm. Innov. 2021, 10, 222–231. [Google Scholar] [CrossRef]
- Martínez, R.A.; Schnezov, N.; Brumovsky, L.A.; Román, A.B.P. Influence of temperature and packaging type on quality parameters and antimicrobial properties during Yateí honey storage. Food Sci. Technol. 2018, 38, 196–202. [Google Scholar] [CrossRef]
- Pidek, A.; Brzozowski, P. Crystallization of honey. Beekeeping 2004, 9, 18–19. (In Polish) [Google Scholar]
- Pospiech, M.; Javůrkova, Z.; Hrabec, P.; Starha, P.; Ljasovska, S.; Bednar, J.; Tremlova, B. Identification of pollen taxa by different microscopy techniques. PLoS ONE 2021, 16, e0256808. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Polish Minister of Agriculture and Rural Development of January 14 (2009) on Methods of Analysis Related to the Assessment of Honey (Dz.U. Journal of Laws of 2009, No. 17, Item 94). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20090170094/O/D20090094.pdf (accessed on 10 October 2024). (In Polish)
- PalDat—Palynological Database. Available online: www.paldat.org (accessed on 5 October 2024).
- Cabrera, M.; Santander, E. Physicochemical and sensory analysis of honeys from eastern Formosa province (Argentina) and its relationship with their botanical origin. Food Chem. Adv. 2022, 1, 100026. [Google Scholar] [CrossRef]
- Bhandari, B.; D’Arcy, B.; Kelly, C. Rheology and crystallization kinetics of honey: Present status. Int. J. Food Prop. 1999, 2, 217–226. [Google Scholar] [CrossRef]
- Gleiter, R.; Horn, H.; Isengard, H. Influence of type and state of crystallisation on the water activity of honey. Food Chem. 2006, 96, 441–445. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernandez-Gonzalez, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Naik, R.R.; Gandhi, N.S.; Thakur, M.; Nanda, V. Analysis of crystallization phenomenon in Indian honey using molecular dynamics simulations and artificial neural network. Food Chem. 2019, 300, 125182. [Google Scholar] [CrossRef]
- Baloš, M.Z.; Popov, N.; Jakšić, S.; Mihaljev, Z.; Pelić, M.; Ratajac, R.; Pelić, D.L. Sunflower Honey—Evaluation of Quality and Stability during Storage. Foods 2023, 12, 2585. [Google Scholar] [CrossRef]
- Directive 2014/63/EU of the European Parliament and of the Council of 15 May (2014) Amending Council Directive 2001/110/EC Relating to Honey. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2014:164:FULL (accessed on 10 October 2024).
- Codex Alimentarius Commission. Codex Standard for Honey; FAO: Rome, Italy, 2001. [Google Scholar]
- PN-88/A-77626; Bee Honey. Polish Standard, Polish Standardization Committee: Warsaw, Poland, 1988. (In Polish)
- AOAC. AOAC Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 2000. [Google Scholar]
- Bogdanov, S.; Martin, P.; Lullman, C. Harmonised Methods of the European Honey Commission. (FAM, Bee Department, Bern 3003, Switzerland). Apidologie 2009, 159, 1–63. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 10 October 2024).
- Regulation of the Polish Minister of Agriculture and Rural Development of May 29 (2015) Amending the Regulation on Specific Requirements for the Commercial Quality of Honey (Dz.U. Journal of Laws of 2015 No. 850). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20230002513/O/D20232513.pdf (accessed on 10 October 2024). (In Polish)
- Cano, C.B.; Felsner, M.L.; Matos, J.R.; Bruns, R.E.; Whatanabe, H.M.; Almeida-Muradian, L.B. Comparison of methods for determining moisture content of citrus and eucalyptus Brazilian honeys by refractometry. J. Food Compos. Anal. 2001, 14, 101–109. [Google Scholar] [CrossRef]
- Zarei, M.; Fazlara, A.; Alijani, N. Evaluation of the changes in physicochemical and antioxidant properties of honey during storage. Funct. Food Health Dis. 2019, 9, 593–605. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I. The impact of different heating temperatures on physicochemical, color attributes, and antioxidant activity parameters of Greek honeys. J. Food Proc. Eng. 2018, 41, e12668. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gonzaga, L.V.; Biluca, F.C.; Schulza, M.; Vitali, L.; Micke, G.A.; Costa, A.C.O.; Fetta, R. Stability of Brazilian Apis mellifera L. honey during prolonged storage: Physicochemical parameters and bioactive compounds. LWT 2020, 129, 109521. [Google Scholar] [CrossRef]
- Julika, W.N.; Ajit, A.; Naila, A.; Sulaiman, A.Z. The effect of storage condition on physicochemical properties of some stingless bee honey collected in Malaysia local market. Mater. Today Proc. 2022, 57, 1396–1402. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Piepiórka-Stepuk, J.; Wojtasik-Kalinowska, I.; Sterczyńska, M.; Mierzejewska, S.; Stachnik, M.; Jakubowski, M. The effect of heat treatment on bioactive compounds and color of selected pumpkin cultivars. LWT 2023, 175, 114469. [Google Scholar] [CrossRef]
- Gonzales, A.P.; Burin, L.; del Pilar Buera, M. Color changes during storage of honeys in relation to their composition and initial color. Food Res. Intern. 1999, 32, 185–191. [Google Scholar] [CrossRef]
- Chaikham, P.; Kemsawasd, V.; Apichartsrangkoon, A. Effects of conventional and ultrasound treatments on physicochemical properties and antioxidant capacity of floral honeys from Northern Thailand. Food Biosci. 2016, 15, 19–26. [Google Scholar] [CrossRef]
- Tappi, S.; Glicerina, V.; Ragni, L.; Dettori, A.; Romani, S.; Rocculi, P. Physical and structural properties of honey crystallized by static and dynamic processes. J. Food Eng. 2021, 292, 110316. [Google Scholar] [CrossRef]
- Dolik, K.; Kubiak, M.; Seńcio, M. Texture measurement analyzer, TMS-Pro—Principle of operation and application in food product testing. PAK 2010, 6, 636–639. (In Polish) [Google Scholar]
- Faustino, C.; Pinheiro, L. Analytical Rheology of Honey: A State-of-the-Art Review. Foods 2021, 10, 1709. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lin, C.H.; Wu, F.Y.; Chen, H.H. Rheological properties of crystallized honey prepared by a new type of nuclei. J. Food Process Eng. 2009, 32, 512–527. [Google Scholar] [CrossRef]
- Piana, M.L.; Cianciabella, M.; Daniele, G.M.; Badiani, A.; Rocculi, P.; Tappi, S.; Gatti, E.; Marcazzan, G.L.; Magli, M.; Medoro, C.; et al. Influence of the physical state of two monofloral honeys on sensory properties and consumer satisfaction. Foods 2023, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Ucurum, O.; Tosunoglu, H.; Takma, C.; Birlik, P.M.; Berber, M.; Kolayli, S. Distinctive properties of the pine, oak, chestnut and multifloral blossom and honeydew honeys. Eur. Food Res. Technol. 2024, 250, 1765–1774. [Google Scholar] [CrossRef]
- Fuentes, M.O.; Alizadeh, K.; Bucarey, S.A.; Castaneza, Z.E.; Vásquez-Quitral, P. Analysis of organic molecules, physicochemical parameters, and pollen as indicators for authenticity, botanical origin, type and quality of honey samples examined. Int. J. Food Prop. 2020, 23, 2242–2256. [Google Scholar] [CrossRef]
- Tao, Z.; Raffel, R.A.; Souid, A.K.; Goodisman, J. Kinetic studies on enzyme catalyzed reactions: Oxidation of glucose, decomposition of hydrogen peroxide and their combination. Biophys. J. 2009, 96, 2977–2988. [Google Scholar] [CrossRef]
- Belay, A.; Haki, G.D.; Birringer, M.; Borck, H.; Addi, A.; Baye, K.; Melaku, S. Rheology and botanical origin of Ethiopian monofloral honey. LWT 2017, 75, 393–401. [Google Scholar] [CrossRef]
- Belay, A.; Haki, G.D.; Birringer, M.; Borck, H.; Lee, Y.C.; Kim, K.T.; Baye, K.; Melaku, S. Enzyme activity, amino acid profiles and hydroxymethylfurfural content in Ethiopian monofloral honey. J. Food Sci. Technol. 2017, 54, 2769–2778. [Google Scholar] [CrossRef]
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Singh, I.; Singh, S. Honey moisture reduction and its quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Miłek, J. Studying and Modeling of Deactivation of Catalase. Ph.D. Thesis, West Pomeranian University of Technology, Szczecin, Poland, 2011. (In Polish). [Google Scholar]
- Zarei, M.; Fazlara, A.; Tulabifard, N. Effect of thermal treatment on physicochemical and antioxidant properties of honey. Heliyon 2019, 5, e01894. [Google Scholar] [CrossRef]
- Iglesias, M.T.; Martín-Álvarez, P.J.; Polo, M.C.; de Lorenzo, C.; González, M.; Pueyo, E. Changes in the Free Amino Acid Contents of Honeys During Storage at Ambient Temperature. J. Agric. Food Chem. 2006, 54, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Chakir, A.; Romane, A.; Marcazzan, G.L.; Ferrazzi, P. Physicochemical properties of some honeys produced from different plants in Morocco. Arab. J. Chem. 2016, 9, S946–S954. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and classification of greek pine honeys according to their geographical origin based on volatiles. Physicochemical Parameters and Chemometrics. Food Chem. 2014, 146, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, B.; Tomaka, K.; Uram-Dudek, A.; Paradowska, K. Physicochemical analysis of honeys from the Podkarpacie region. Post. Fitoter. 2020, 21, 219–227. [Google Scholar] [CrossRef]
- Kücük, M.; Kolail, S.; Karaoglu, S.; Ulusoy, E.; Baltac, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- da Silva Cruz, L.F.; Lemos, P.V.F.; de Souza Santos, T.; Tavares, P.P.L.G.; Nascimento, R.Q.; Almeida, L.M.R.; de Souza, C.O.; Druzian, J.I. Storage conditions significantly influence the stability of stingless bee (Melipona scutellaris) honey. J. Apic. Res. 2023, 62, 530–541. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Wołosiak, R. Physicochemical parameters of quality of floral honey. Bromatol. Toxicol. Chem. 2015, 3, 440–444. (In Polish) [Google Scholar]
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Aljuhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E. Determination of physicochemical properties of multifloral honeys stored in different containers. J. Food Process. Preserv. 2018, 42, e13379. [Google Scholar] [CrossRef]
- Vijayakumar, K.T.; Bhat, N.S.; Neethu, T.; Nayimabanu, T.; Kumar, H.L.N. Periodical changes in quality parameters of honey during storage and processing. Int. J. Chem. Stud. 2021, 9, 19–24. [Google Scholar] [CrossRef]
- Khan, K.A.; Ghramh, H.A.; Babiker, M.; Ahmad, Z.; El-Niweiri, M.A.A.; Ibrahim, E.H.; Brima, E.I.; Mohammed, M.E.A. Tolerance of Ziziphus and Acacia honeys to one-year storage conditions and altitude. J. King Saud Univ. Sci. 2021, 33, 101577. [Google Scholar] [CrossRef]
- Cabrera, M.; Perez, M.; Gallez, L.; Andrada, A.; Balbarrey, G. Colour, antioxidante capacity, phenolic and flavonoid content of honey from the Hu- mid Chaco Region Argentina. Revista Internacional de Botánica Experimental. Phyton-Int. J. Exp. Bot. 2017, 86, 4–11. Available online: http://www.revistaphyton.fund-romuloraggio (accessed on 10 October 2024).
- Piotraszewska-Pająk, A.; Gliszczyńska-Świgło, A. Directions of colour changes of nectar honeys depending on honey type and storage conditions. J. Apic. Sci. 2015, 59, 51–61. [Google Scholar] [CrossRef]
- Quintero-Lira, A.; Ángeles-Santos, A.; Aguirre-Álvarez, G.; Reyes-Munguía, A.; Almaraz-Buendía, I.; Campos-Montiel, R.G. Effects of liquefying crystallized honey by ultrasound on crystal size, 5-hydroxymethylfurfural, colour, phenolic compounds and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 619–626. [Google Scholar] [CrossRef]
- Popek, S.; Figiel, K. Determining the colour of varietal honeys using the L* a* b* system. Crac. Rev. Econ. Manag. 2007, 743, 179–187. Available online: https://r.uek.krakow.pl/jspui/bitstream/123456789/356/1/137138903.pdf (accessed on 10 October 2024). (In Polish).
- Turkut, G.M.; Degirmenci, A.; Yildiz, O.; Can, Z.; Cavrar, S.; Karahalil, F.Y.; Kolayli, S. Investigating 5-hydroxymethylfurfural formation kinetic and antioxidant activity in heat treated honey from different floral sources. J. Food Meas. Charact. 2018, 12, 2358–2365. [Google Scholar] [CrossRef]
- Wilczyńska, A. The effect of processing on honey quality—Changes of colour parameters and HMF content under heating and storage. Sci. J. Poznań Univ. Econ. 2011, 196, 91–98. Available online: https://bazekon.uek.krakow.pl/zeszyty/171207637 (accessed on 10 October 2024).
- Berk, B.; Grunin, L.; Oztop, M.H. Anon-conventional TD-NMRapproach to monitor honey crystallization melting. J. Food Eng. 2021, 292, 110292. [Google Scholar] [CrossRef]
- Suriwong, V.; Jaturonglumlert, S.; Varith, J.; Narkprasom, K.; Nitatwichit, C. Crystallisation behaviour of sunflower longan honey with glucose addition by absorbance measurement. Int. Food Res. J. 2020, 27, 727–734. [Google Scholar]
- Juszczak, L.; Fortuna, T. Rheology of selected polish honeys. J. Food Eng. 2006, 75, 43–49. [Google Scholar] [CrossRef]
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. Effect of moisture content on the viscosity of honey at different temperatures. J. Food Eng. 2006, 72, 372–377. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Skałecki, P.; Litwińczuk, A. Characterisation of viscosity colour 5-hydroxymethylfurfural content diastase activity in raw rape honey (Brassica napus) at different temperatures. J. Food Sci. Technol. 2016, 53, 2092–2098. Available online: https://pubmed.ncbi.nlm.nih.gov/27413239/ (accessed on 10 October 2024). [CrossRef] [PubMed]
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Skałecki, P. Effect of freezing and room temperatures storage for 18 months on quality of raw rapeseed honey (Brassica napus). J. Sci. Technol. 2016, 53, 3349–3355. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Wolanciuk, A.; Florek, M.; Skałecki, P.; Litwińczuk, A. Hydroxymethylfurfural content, diastase activity and colour of multifloral honeys in relation to origin and storage time. J. Cent. Eur. Agric. 2017, 18, 657–668. [Google Scholar] [CrossRef]
- Popek, S. Identyfication of honey types. Nahrung/Food 2003, 47, 39–40. [Google Scholar] [CrossRef]
- Popek, S.; Halagarda, M.; Kursa, K. A new model to identify botanical origin of Polish honeys based on the physicochemical parametres and chemometric analysis. LWT 2017, 77, 482–487. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Marrone, M.; Bruner-Montero, G.; Gaitán, K.; de Núñez, L.; Otero-Palacio, R.; Fernández-Marín, H. Assessment of the quality, chemometric and pollen diversity of Apis mellifera honey from different seasonal harvests. Foods 2023, 12, 3656. [Google Scholar] [CrossRef]
- Fagúndez, G.A.; Blettler, D.C.; Gallo, M.A. Pollen assemblage variability of Apis mellifera honeys (Diamante, Entre Ríos, Argentina). Grana 2022, 61, 366–380. [Google Scholar] [CrossRef]
- Yoo, B. Effect of temperature on dynamic rheology of Korean honeys. J. Food Eng. 2004, 65, 459–463. [Google Scholar] [CrossRef]
- Kumar, J.S.; Mandal, M. Rheology and thermal properties of marketed Indian honey. Nutr. Food Sci. 2009, 39, 111–117. [Google Scholar] [CrossRef]
- Witczak, M.; Juszczak, L.; Gałkowska, D. Non-Newtonian behaviour of heather honey. J. Food Eng. 2011, 104, 532–537. [Google Scholar] [CrossRef]
- Tikhonov, A.I.; Bondarenko, L.A.; Jarnych, T.G.; Szpyczak, O.S.; Kowal, W.M.; Skrypnik–Tichonow, R.I. Natural Honey in Medicine and Pharmacy (Origin, Properties, Application, Medicinal Preparations); Sądecki Bartnik Beekeeping Farm: Stróże, Poland, 2017. (In Polish) [Google Scholar]
- Ciursa, P.; Oroian, M. Voltammetric e-tongue for honey adulteration detection. Sensors 2021, 21, 5059. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, H.; Åkerlind, U. Chapter 6. Crystal Growth in Liquids and Melts. In Solidification and Crystallization Processing in Metals and Alloys; Wiley Online Library: Tokyo, Japan, 2012. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Banded spherulitic growth in a liquid crystal. J. Cryst. Growth. 2000, 217, 332–343. [Google Scholar] [CrossRef]
- Souda, R. Dewetting of thin amorphous solid water films and Liquid-Cubic ice coexistence in droplets studied using Infrared-Absorption and Secondary-Ion-Mass spectroscopy. J. Phys. Chem. 2008, 112, 11976–11980. [Google Scholar] [CrossRef]
- Misyura, S. The Anomalously High Rate of Crystallization, Controlled by Crystal Forms under the Conditions of a Limited Liquid Volume. Cryst. Growth Des. 2018, 18, 1327–1338. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tropin, T.V. Theory of crystal nucleation of glass-forming liquids: Some new developments. Int. J. Appl. Glass Sci. 2021, 13, 171–198. [Google Scholar] [CrossRef]
- Inoue, M.; Yoshino, K.; Moritake, H.; Toda, K. Influence of Liquid-Crystal directors in an electric field on elastic wave propagating in Liquid-Crystal cell. Jpn. J. Appl. Phys. 2001, 40, 5798. [Google Scholar] [CrossRef]
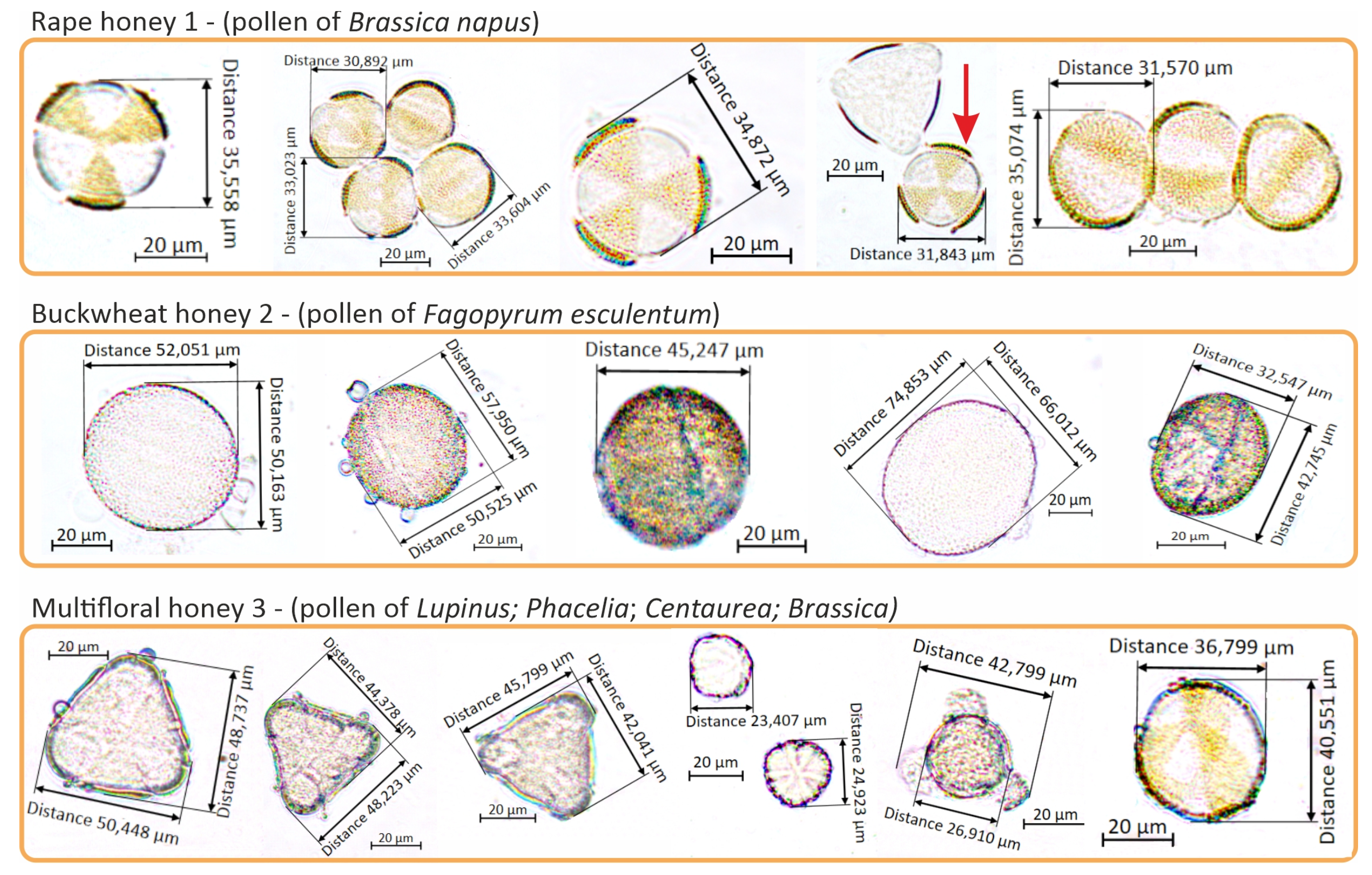


| Sugar Profile | Unit | Rape Honey 1 | Buckwheat Honey 2 | Multifloral Honey 3 |
|---|---|---|---|---|
| Fructose (F) | g/100 g | 34.30 ± 6.90 | 34.70 ± 6.90 | 36.40 ± 7.30 |
| Galactose (Ga) | g/100 g | <0.10 (0.10 ± 0.02) | <0.10 (0.10 ± 0.02) | <0.10 (0.10 ± 0.02) |
| Glucose (G) | g/100 g | 37.50 ± 7.50 | 34.30 ± 6.90 | 35.50 ± 7.10 |
| Maltose (M) | g/100 g | 0.23 ± 0.05 | 0.53 ± 0.11 | 0.58 ± 0.12 |
| Saccharose (S) | g/100 g | <0.10 (0.10 ± 0.02) | <0.10 (0.10 ± 0.02) | <0.10 (0.10 ± 0.02) |
| Sugars sum | g/100 g | 72.23 ± 14.49 | 69.73 ± 13.95 | 72.68 ± 14.56 |
| Water (W) | % | 17.1 ± 0.00 | 18.00 ± 0.10 | 17.50 ± 0.10 |
| F/G | - | 0.92 | 1.01 | 1.02 |
| G/W | - | 2.19 | 1,91 | 2.03 |
| (G − W)/F | - | 0.59 | 0.47 | 0.49 |
| Storage Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Honey | t0 | t1 | t2 | t3 | t6 | t12 | t24 | Error | |
| Moisture (g/100 g w/w) | Rape | 17.10 ± 0.00 a | 17.23 ± 0.12 ab | 17.33 ± 0.15 ab | 17.33 ± 0.06 ab | 17.43 ± 0.12 b | 17.90 ± 0.20 c | 18.07 ± 0.06 c↑ | 0.013 |
| Multifloral | 17.53 ± 0.06 a | 17.57 ± 0.12 a | 17.60 ± 0.10 a | 17.67 ± 0.06 a | 17.73 ± 0.06 ab | 17.67 ± 0.15 a | 17.93 ± 0.06 b↑ | 0.009 | |
| Buckwheat ND | 18.00 ± 0.10 a | 18.20 ± 0.10 a | 18.23 ± 0.06 a | 18.27 ± 0.06 a | 18.33 ± 0.21 a | 18.23 ± 0.23 a | 18.33 ± 0.06 a↑ | 0.018 | |
| Extract (%) | Rape | 81.40 ± 0.00 c | 81.27 ± 0.12 bc | 81.13 ± 0.12 bc | 81.17 ± 0.06 bc | 81.07 ± 0.12 b | 80.60 ± 0.20 a | 80.43 ± 0.06 a↓ | 0.013 |
| Multifloral | 80.97 ± 0.06 b | 80.93 ± 0.12 b | 80.90 ± 0.10 b | 80.83 ± 0.06 b | 80.77 ± 0.06 ab | 80.83 ± 0.15 b | 80.57 ± 0.06 a↓ | 0.009 | |
| Buckwheat ND | 80.50 ± 0.10 a | 80.30 ± 0.10 a | 80.27 ± 0.06 a | 80.23 ± 0.06 a | 80.17 ± 0.21 a | 80.27 ± 0.23 a | 80.17 ± 0.06 a↓ | 0.018 | |
| Acidity free (meq/kg) | Rape | 10.67 ± 0.58 a | 10.90 ± 0.92 a | 12.27 ± 1.19 ab | 15.97 ± 1.23 c↑ | 14.43 ± 1.50 bc | 11.13 ± 0.21 a | 11.90 ± 0.26 a | 0.769 |
| Multifloral | 21.27 ± 0.86 a | 26.37 ± 0.57 c↑ | 24.33 ± 1.27 bc | 21.70 ± 0.95 ab | 20.23 ± 0.40 a | 21.53 ± 1.71 ab | 22.50 ± 1.08 ab | 1.119 | |
| Buckwheat ND | 40.70 ± 0.26 a | 40.53 ± 0.35 a | 42.33 ± 1.57 a↑ | 42.23 ± 0.51 a | 41.73 ± 0.35 a | 40.70 ± 0.50 a | 41.00 ± 0.26 a | 0.480 | |
| pH (-) | Rape | 3.71 ± 0.14 ab | 3.93 ± 0.13 c | 3.95 ± 0.09 bc | 3.84 ± 0.07 abc | 3.77 ± 0.08 ab | 3.79 ± 0.04 ab | 3.58 ± 0.10 a↓ | 0.010 |
| Multifloral | 3.58 ± 0.08 b | 3.69 ± 0.07 bc | 3.87 ± 0.14 cd | 3.91 ± 0.09 cd | 3.99 ± 0.02 d | 3.32 ± 0.04 a | 3.21 ± 0.07 a↓ | 0.006 | |
| Buckwheat ND | 3.83 ± 0.02 a | 3.75 ± 0.05 a | 3.72 ± 0.02 a | 3.78 ± 0.06 a | 3.85 ± 0.04 a | 3.72 ± 0.06 a | 3.71 ± 0.10 a↓ | 0.003 | |
| Conductivity (mS/cm) | Rape | 0.196 ± 0.000 a | 0.193 ± 0.001 a | 0.193 ± 0.002 a | 0.193 ± 0.002 a | 0.196 ± 0.002 a | 0.197 ± 0.002 a | 0.203 ± 0.001 b↑ | 0.000 |
| Multifloral | 0.338 ± 0.005 a | 0.340 ± 0.001 ab | 0.341 ± 0.003 abc | 0.344 ± 0.002 abc | 0.347 ± 0.003 bc | 0.348 ± 0.002 c | 0.360 ± 0.001 d↑ | 0.000 | |
| Buckwheat | 0.465 ± 0.001 ab | 0.470 ± 0.002 bc | 0.471 ± 0.002 c | 0.466 ± 0.002 a | 0.464 ± 0.003 a | 0.479 ± 0.002 d | 0.483 ± 0.001 d↑ | 0.000 | |
| Color Components in Chromatic Space CIE L*a*b* and Cylindrical Space Delta C*H* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks of Storage at 8 ± 1 °C | |||||||||
| t0 | t1 | t2 | t3 | t6 | t12 | t24 | |||
| Type of honey | Rape | CIE L* | 18.92 ± 0.58 a | 17.29 ± 0.7 a | 33.59 ± 4.34 b | 35.37 ± 2.27 b | 45.12 ± 5.20 c | 47.82 ± 1.03 c | 61.78 ± 1.21 d |
| CIE a* | 4.30 ± 0.46 c | −0.26 ± 0.12 b | −0.41 ± 0.36 b | −0.48 ± 0.18 b | −0.52 ± 0.16 b | −0.59 ± 0.05 b | −1.32 ± 0.20 a | ||
| CIE b* | 23.73 ± 2.93 d | 8.16 ± 2.57 bc | 5.37 ± 0.78 a | 5.85 ± 0.81 ab | 7.19 ± 3.27 ab | 7.49 ± 0.41 ab | 10.17 ± 1.47 c | ||
| Chroma C* | 24.12 ± 0.98 d | 8.17 ± 2.57 bc | 5.40 ± 0.37 a | 5.88 ± 0.47 ab | 7.21 ± 0.77 ab | 7.51 ± 0.17 ab | 10.26 ± 0.94 c | ||
| Hue H * [°] | 79.71 ± 1.13 a | 271.91 ± 0.95 b | 274.33 ± 3.81 bc | 274.67 ± 1.39 bc | 274.09 ± 1.13 bc | 274.52 ± 0.33 bc | 277.52 ± 1.77 c | ||
| Multifloral | CIE L* | 10.38 ± 0.59 a | 9.21 ± 0.51 a | 9.77 ± 0.79 a | 22.46 ± 1.80 b | 21.72 ± 2.53 b | 47.53 ± 1.42 c | 55.70 ± 1.25 d | |
| CIE a* | 2.40 ± 0.41 cd | 2.54 ± 0.28 d | 1.84 ± 0.23 c | −0.38 ± 0.27 b | −0.45 ± 0.29 b | −0.60 ± 0.21 b | −1.27 ± 0.30 a | ||
| CIE b* | 11.74 ± 0.64 a | 10.19 ± 0.80 a | 10.31 ± 0.48 a | 10.45 ± 1.37 a | 10.39 ± 1.18 a | 15.67 ± 1.22 b | 15.82 ± 2.16 b | ||
| Chroma C* | 11.98 ± 0.62 a | 10.50 ± 0.83 a | 10.48 ± 0.47 a | 10.45 ± 1.37 a | 10.40 ± 1.18 a | 15.69 ± 1.22 b | 15.89 ± 2.15 b | ||
| Hue H* [°] | 78.43 ± 2.09 ab | 76.03 ± 0.88 a | 79.87 ± 1.40 b | 272.03 ± 1.17 c | 272.44 ± 1.56 c | 274.70 ± 0.85 c | 272.20 ± 1.50 c | ||
| Buckwheat | CIE L* | 4.02 ± 0.29 a | 8.01 ± 0.79 b | 11.92 ± 1.41 c | 10.79 ± 0.90 bc | 35.01 ± 1.43 d | 39.09 ± 0.64 e | 53.28 ± 3.94 f | |
| CIE a* | 3.96 ± 1.10 bc | 1.59 ± 0.28 a | 1.55 ± 0.33 a | 4.35 ± 1.31 bc | 4.77 ± 0.14 c | 3.17 ± 0.52 b | 5.24 ± 0.59 c | ||
| CIE b* | 5.35 ± 0.58 a | 9.47 ± 0.66 b | 13.85 ± 0.35 c | 12.17 ± 1.14 c | 24.26 ± 1.36 d | 24.63 ± 1.64 d | 22.69 ± 1.14 d | ||
| Chroma C* | 6.72 ± 0.66 a | 9.61 ± 0.62 b | 13.94 ± 0.33 c | 12.99 ± 0.85 c | 24.73 ± 1.31 d | 24.84 ± 1.58 d | 23.28 ± 1.17 d | ||
| Hue H* [°] | 53.87 ± 8.83 a | 80.40 ± 2.18 c | 83.62 ± 1.44 c | 70.19 ± 6.83 b | 78.84 ± 0.87 bc | 82.59 ± 1.51 c | 77.00 ± 1.29 bc | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piepiórka-Stepuk, J.; Sterczyńska, M.; Stachnik, M.; Pawłowski, P. Effects of Refrigerated Storage on the Physicochemical, Color and Rheological Properties of Selected Honey. Agriculture 2025, 15, 1476. https://doi.org/10.3390/agriculture15141476
Piepiórka-Stepuk J, Sterczyńska M, Stachnik M, Pawłowski P. Effects of Refrigerated Storage on the Physicochemical, Color and Rheological Properties of Selected Honey. Agriculture. 2025; 15(14):1476. https://doi.org/10.3390/agriculture15141476
Chicago/Turabian StylePiepiórka-Stepuk, Joanna, Monika Sterczyńska, Marta Stachnik, and Piotr Pawłowski. 2025. "Effects of Refrigerated Storage on the Physicochemical, Color and Rheological Properties of Selected Honey" Agriculture 15, no. 14: 1476. https://doi.org/10.3390/agriculture15141476
APA StylePiepiórka-Stepuk, J., Sterczyńska, M., Stachnik, M., & Pawłowski, P. (2025). Effects of Refrigerated Storage on the Physicochemical, Color and Rheological Properties of Selected Honey. Agriculture, 15(14), 1476. https://doi.org/10.3390/agriculture15141476








