Deciphering Heat Stress Mechanisms and Developing Mitigation Strategies in Dairy Cattle: A Multi-Omics Perspective
Abstract
1. Introduction
2. Multidimensional Analysis of Physiological Responses to HS in Dairy Cows
2.1. Impact on Production Performance
2.2. Immune Barrier Collapse
2.3. Endocrine Alterations
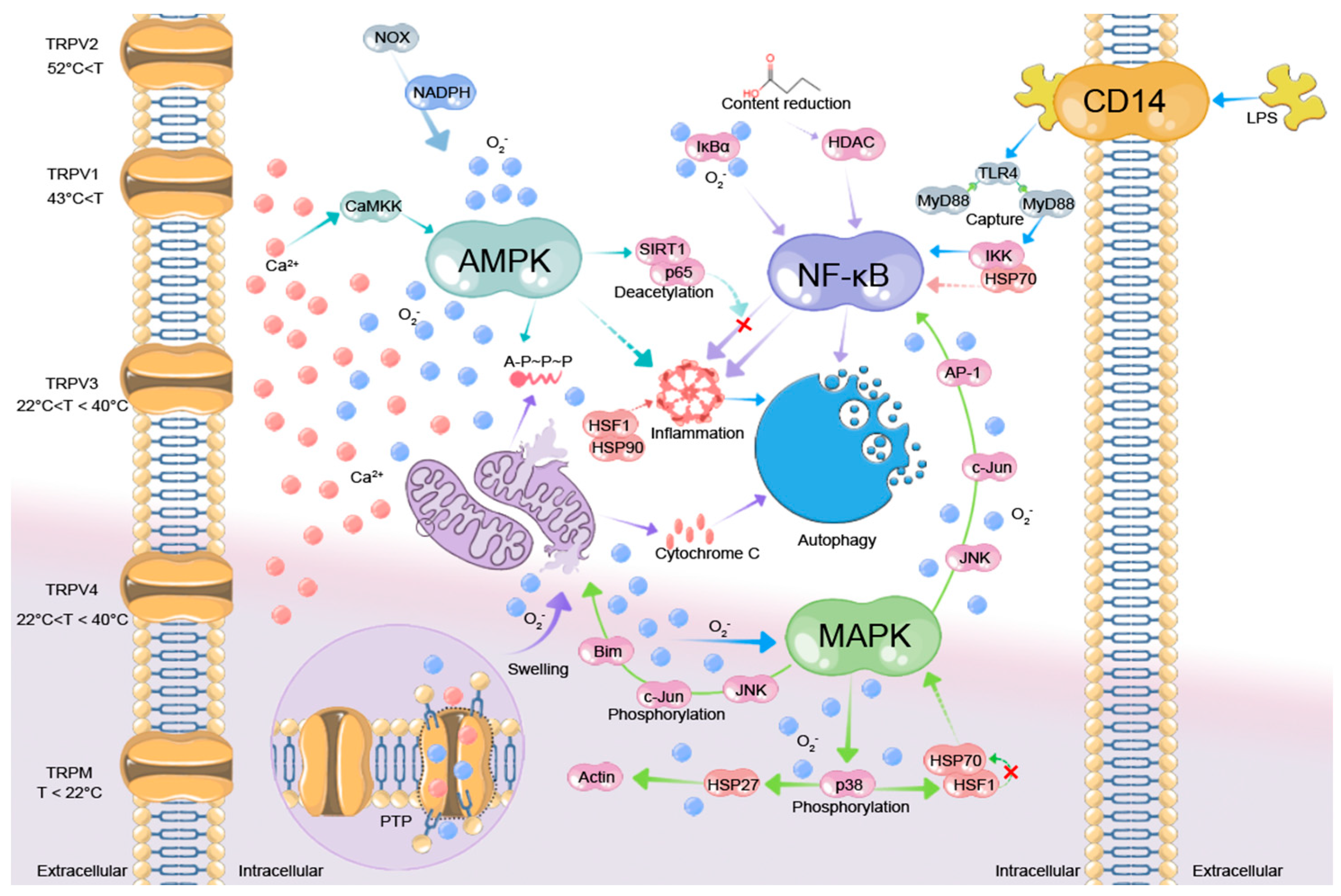
3. Mechanisms Underlying HS Generation from a Multi-Omics Perspective
3.1. Investigating Cellular Physiological Alterations Through Thermosensation Mechanisms
3.2. The Impact of Physiological Changes on Signal Transduction
4. Multi-Omics-Driven Stress Resistance Strategies
4.1. Modifying HSP Gene Expression in Heat-Stressed Dairy Cows Using CRISPR-Cas Technology
4.2. Modulating Heat Stress-Induced Gastrointestinal Microbiota Shifts via Quorum Sensing Molecules
4.3. Intelligent Breeding and Digitalized Management
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic Hormone |
| AI | Artificial Intelligence |
| A1 | Annexin 1 |
| ATAC-seq | Assay for Transposase-Accessible Chromatin using sequencing |
| BHB | β-hydroxybutyrate |
| BLG | β-lactoglobulin |
| CaMKKβ | Ca2+/Calmodulin-Dependent Kinase Kinase β |
| CD21+ | Cluster of Differentiation 21 positive |
| CRH | Corticotropin-Releasing Hormone |
| ChIP-seq | Chromatin ImmunoPrecipitation sequencing |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR-associated protein 9 |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 |
| Cytc | Cytochrome c |
| DIA | Data-Independent Acquisition |
| DMI | Dry Matter Intake |
| DNA | Deoxyribonucleic Acid |
| DNMT3B | DNA (Cytosine-5)-Methyltransferase 3 Beta |
| DS | Drooling Scores |
| EI-MN-PB | Environmental Intelligence–Molecular Network–Precision Breeding |
| FSH | Follicle Stimulating Hormone |
| FOXO3a | Forkhead Box O3a |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GO | Gene Ontology |
| GLUT4 | Glucose Transporter Type 4 |
| GPx | Glutathione Peroxidase |
| GnRH | Gonadotropin-Releasing Hormone |
| GWAS | Genome-Wide Association Study |
| gRNA | Guide RNA |
| HPA | Hypothalamo–Pituitary–Adrenal |
| HPO | Hypothalamic–Pituitary–Ovarian |
| HDAC | Histone Deacetylase |
| HSEs | Heat Shock Elements |
| HSF1 | Heat Shock Factor 1 |
| HSP | Heat Shock Protein |
| HSP27 | Heat Shock Protein 27 |
| HSP70 | Heat Shock Protein 70 |
| HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A |
| HS | Heat Stress |
| IKK | IκB Kinase |
| IL-6 | Interleukin-6 |
| IMS | Ion Mobility Spectrometry |
| IoT | Internet of Things |
| IP3 | Inositol Trisphosphate |
| IκBα | Inhibitor of Nuclear Factor Kappa B Alpha |
| JNK | c-Jun N-terminal Kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LH | Luteinizing Hormone |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-Activated Protein Kinase |
| MCU | Mitochondrial Calcium Un |
| MDA | Malondialdehyde |
| MKP-1 | MAPK Phosphatases-1 |
| MnSOD | Manganese Superoxide Dismutase |
| MS | Mass Spectrometry |
| MyD88 | Myeloid differentiation primary response 88 |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NEB | Negative Energy Balance |
| NGS | Next-Generation Sequencing |
| NMR | Nuclear Magnetic Resonance |
| NOX | NADPH Oxidase |
| Npc1/1 | Niemann-Pick Disease, Type C1 |
| NF-κB | Nuclear Factor-Kappa B |
| QS | Quorum Sensing |
| P38 | Mitogen-Activated Protein Kinase p38 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| ROS | Reactive Oxygen Species |
| RNA | Ribonucleic Acid |
| RNA-seq | RNA Sequencing |
| RR | Respiratory Rate |
| RT | Rectal Temperature |
| SCFAs | Short-Chain Fatty Acids |
| scRNA-seq | Single-Cell RNA Sequencing |
| SOD | Superoxide Dismutase |
| STAT6 | Signal Transducer and Activator of Transcription 6 |
| STEM | Short Time-series Expression Miner |
| TCA | Tricarboxylic Acid Cycle |
| THI | Temperature Humidity Index |
| Th | T Helper Cell |
| THZ | Thermal Hazard Zone |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TRP | Transient Receptor Potential |
| TRPM | Transient Receptor Potential Melastatin |
| TRPV | Transient Receptor Potential Vanilloid |
| WES | Whole Exome Sequencing |
| WGS | Whole Genome Sequencing |
| WssGWAS | Weighted Single-Step Genome-Wide Association Study |
References
- Rounce, D.R.; Hock, R.; Maussion, F.; Hugonnet, R.; Kochtitzky, W.; Huss, M.; Berthier, E.; Brinkerhoff, D.; Compagno, L.; Copland, L.; et al. Global glacier change in the 21st century: Every increase in temperature matters. Science 2023, 379, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef] [PubMed]
- Slayi, M.; Zhou, L.; Jaja, I.F. Strategies, challenges, and outcomes of heat stress resilience in sub-saharan african community-based cattle feedlots: A systematic review. Front. Vet. Sci. 2024, 11, 1455917. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Nutritional strategies for managing the heat-stressed dairy cow. J. Dairy Sci. 1997, 77, 21. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat stress: A serious disruptor of the reproductive physiology of dairy cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, Q.; Tao, J. Blood metabolomic studies of heat stress cow with GC-MS. Acta Vet. Zootech. Sin. 2015, 46, 1356–1362. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Luo, H.; Mi, S.; Liu, W.; Chu, Q.; Wang, Y. Knowledge map ping analysis on heat stress genetic mechanisms research of dairy cattle. Acta Vet. Zootech. Sin. 2021, 52, 1141–1153. [Google Scholar] [CrossRef]
- Min, L.; Zhao, S.; Tian, H.; Zhou, X.; Zhang, Y.; Li, S.; Yang, H.; Zheng, N.; Wang, J. Metabolic responses and “omics” technologies for elucidating the effects of heat stress in dairy cows. Int. J. Biometeorol. 2016, 61, 1149–1158. [Google Scholar] [CrossRef]
- Magana, J.; Gavojdian, D.; Menachem, Y.; Lazebnik, T.; Zamansky, A.; Adams-Progar, A. Machine learning approaches to predict and detect early-onset of digital dermatitis in dairy cows using sensor data. Front. Vet. Sci. 2023, 10, 1295430. [Google Scholar] [CrossRef] [PubMed]
- Worku, D.; Hussen, J.; De Matteis, G.; Schusser, B.; Alhussien, M.N. Candidate genes associated with heat stress and breeding strategies to relieve its effects in dairy cattle: A deeper insight into the genetic architecture and immune response to heat stress. Front. Vet. Sci. 2023, 10, 1151241. [Google Scholar] [CrossRef] [PubMed]
- Nuffield Council on Bioethics. Genome editing—An ethical review. Jahrb. Wiss. Eth. 2017, 22, 293–340. [Google Scholar] [CrossRef]
- Vautard, R.; Barnes, C.R.; Philip, S.; Kew, S.; Pinto, I.; Otto, F.E.L. Heat extremes linearly shift with global warming, with frequency doubling per decade since 1979. Environ. Res. Lett. 2024, 19, 094033. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Sousa, F.C.d.; da Silva, A.L.; Schultz, É.B.; Valderrama Londoño, R.I.; de Souza, P.A.R. Heat stress in dairy cows: Impacts, identification, and mitigation strategies—A review. Animals 2025, 15, 249. [Google Scholar] [CrossRef]
- Țogoe, D.; Mincă, N.A. The impact of heat stress on the physiological, productive, and reproductive status of dairy cows. Agriculture 2024, 14, 1241. [Google Scholar] [CrossRef]
- Hao, L.Y.; Wang, J.; Sun, P.; Bu, D.P. The effect of heat stress on the metabolism of dairy cows: Updates & review. Austin J. Nutr. Metab. 2016, 3, 1036. [Google Scholar]
- Chen, X.; Li, C.; Fang, T.; Yao, J.; Gu, X. Effects of heat stress on endocrine, thermoregulatory, and lactation capacity in heat-tolerant and -sensitive dry cows. Front. Vet. Sci. 2024, 11, 1405263. [Google Scholar] [CrossRef]
- Sguizzato, A.L.L.; Marcondes, M.I.; Dijkstra, J.; Valadares Filho, S.d.C.; Campos, M.M.; Machado, F.S.; Silva, B.C.; Rotta, P.P. Energy requirements for pregnant dairy cows. PLoS ONE 2020, 15, e0235619. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Gao, J.; Qi, Z. Effects of heat stress on rumen microbiota and its relationship with performance of dairy cows. Chin. J. Anim. Nutr. 2019, 31, 4458–4463. [Google Scholar]
- Marquez-Acevedo, A.S.; Hood, W.; Collier, R.; Skibiel, A. Mitochondrial response to heat stress and its implications on dairy cattle bioenergetics, metabolism, and production. J. Dairy Sci. 2023, 106, 7295–7309. [Google Scholar] [CrossRef] [PubMed]
- Bharati, J.; Dangi, S.S.; Chouhan, V.S.; Mishra, S.R.; Bharti, M.K.; Verma, V.; Shankar, O.; Yadav, V.P.; Das, K.; Paul, A.; et al. Expression dynamics of HSP70 during chronic heat stress in Tharparkar cattle. Int. J. Biometeorol. 2017, 61, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Roufayel, R.; Johnston, D.S.; Mosser, D.D. The elimination of miR-23a in heat-stressed cells promotes NOXA-Induced cell death and is prevented by HSP70. Cell Death Dis. 2014, 5, e1546. [Google Scholar] [CrossRef]
- Luo, H.; Hu, L.; Brito, L.F.; Dou, J.; Sammad, A.; Chang, Y.; Ma, L.; Guo, G.; Liu, L.; Zhai, L.; et al. Weighted single-step GWAS and RNA sequencing reveals key candidate genes associated with physiological indicators of heat stress in Holstein cattle. J. Anim. Sci. Biotechnol. 2022, 13, 108. [Google Scholar] [CrossRef]
- Joo, S.; Lee, S.J.; Park, D.S.; Kim, D.H.; Gu, B.; Park, Y.; Rim, C.; Kim, M.; Kim, E. Changes in blood metabolites and immune cells in Holstein and Jersey dairy cows by heat stress. Animals 2021, 11, 974. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, R.; Wu, Y.; Zhang, D.; Xu, H.; Zhang, Y.; Qi, Z. Effect of Seasonal Thermal stress on oxidative status, immune response and stress hormones of lactating dairy cows. Anim. Nutr. 2021, 7, 216–223. [Google Scholar] [CrossRef]
- Mishra, S.R. Behavioural, physiological, neuro-endocrine and molecular responses of cattle against heat stress: An updated review. Trop. Anim. Health Prod. 2021, 53, 400. [Google Scholar] [CrossRef]
- Catozzi, C.; Ávila, G.; Zamarian, V.; Pravettoni, D.; Sala, G.; Ceciliani, F.; Lacetera, N.; Lecchi, C. In-vitro effect of heat stress on bovine monocytes lifespan and polarization. Immunobiology 2020, 225, 151888. [Google Scholar] [CrossRef]
- Feher, J. The Adrenal Cortex; Feher, J., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 810–819. [Google Scholar]
- Cavalcanti, D.M.H.; Lotufo, C.M.C.; Borelli, P.; Ferreira, Z.S.; Markus, R.P.; Farsky, S.H.P. Endogenous glucocorticoids control neutrophil mobilization from bone marrow to blood and tissues in non-inflammatory conditions. Br. J. Pharmacol. 2007, 152, 1291–1300. [Google Scholar] [CrossRef]
- Tejaswi, V.; Balachander, B.; Samad, H.A.; Sarkar, M.; Maurya, V.P.; Singh, G. Assessment of heat stress induced alterations in polymorphonuclear (PMN) cell activity in native and crossbred cows. J. Appl. Anim. Res. 2020, 48, 549–552. [Google Scholar] [CrossRef]
- Ricci, E.; Ronchetti, S.; Gabrielli, E.; Pericolini, E.; Gentili, M.; Roselletti, E.; Vecchiarelli, A.; Riccardi, C. GILZ restrains neutrophil activation by inhibiting the MAPK pathway. J. Leukoc. Biol. 2019, 105, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Maciuszek, M.; Klak, K.; Rydz, L.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. Cortisol metabolism in carp macrophages: A role for macrophage-derived cortisol in M1/M2 polarization. Int. J. Mol. Sci. 2020, 21, 8954. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Ronchi, B.; Kuzminsky, G.; Nardone, A. Lymphocyte functions in dairy cows in hot environment. Int. J. Biometeorol. 2005, 50, 105–110. [Google Scholar] [CrossRef]
- Park, D.S.; Gu, B.-H.; Park, Y.J.; Joo, S.S.; Lee, S.-S.; Kim, S.-H.; Kim, E.T.; Kim, D.H.; Lee, S.S.; Lee, S.J.; et al. Dynamic changes in blood immune cell composition and function in Holstein and jersey steers in response to heat stress. Cell Stress Chaperon. 2021, 26, 705–720. [Google Scholar] [CrossRef]
- Murata, H.; Kunii, H.; Kusama, K.; Sakurai, T.; Bai, H.; Kawahara, M.; Takahashi, M. Heat stress induces oxidative stress and activates the KEAP1-NFE2L2-ARE pathway in bovine endometrial epithelial cells. Biol. Reprod. 2021, 105, 1114–1125. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. The Bach family of transcription factors: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 345–356. [Google Scholar] [CrossRef]
- Marin, D.; Fernandez, G.J.; Hernandez, J.C.; Taborda, N. A systems biology approach unveils different gene expression control mechanisms governing the immune response genetic program in peripheral blood mononuclear cells exposed to SARS-CoV-2. PLoS ONE 2024, 19, e0314754. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Zheng, N.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly-Y, M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 2011, 6, e21205. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-Chain Fatty Acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Liver Physiol. 2017, 4, G559–G571. [Google Scholar] [CrossRef]
- Min, L.; Cheng, J.; Shi, B.; Yang, H.; Zheng, N.; Wang, J. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J. Zhejiang Univ. Sci. B 2015, 16, 541–548. [Google Scholar] [CrossRef]
- Collier, R.J.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H. Invited review: Genes involved in the bovine heat stress response. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef]
- Starkie, R.L.; Hargreaves, M.; Rolland, J.; Febbraio, M.A. Heat stress, cytokines, and the immune response to exercise. Brain Behav. Immun. 2005, 19, 404–412. [Google Scholar] [CrossRef]
- McFadden, J.W.; Rico, J.E. Invited review: Sphingolipid biology in the dairy cow: The emerging role of ceramide. J. Dairy Sci. 2019, 102, 7619–7639. [Google Scholar] [CrossRef]
- Rhoads, M.; Rhoads, R.; Vanbaale, M.; Collier, R.; Sanders, S.; Weber, W.; Crooker, B.; Baumgard, L. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Abbas, Z.; Sammad, A.; Hu, L.; Fang, H.; Xu, Q.; Wang, Y. Glucose metabolism and dynamics of facilitative glucose transporters (GLUTs) under the influence of heat stress in dairy cattle. Metabolites 2020, 10, 312. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Bruce Currie, W. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef] [PubMed]
- Blond, B.; Majkić, M.; Spasojević, J.; Hristov, S.; Radinović, M.; Nikolić, S.; Anđušić, L.; Čukić, A.; Došenović Marinković, M.; Vujanović, B.D.; et al. Influence of heat stress on body surface temperature and blood metabolic, endocrine, and inflammatory parameters and their correlation in cows. Metabolites 2024, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H.; Zhu, H.; Wang, Y. Cellular and molecular adaptation of bovine granulosa cells and oocytes under heat stress. Animals 2020, 10, 110. [Google Scholar] [CrossRef]
- Oakley, A.E.; Breen, K.M.; Clarke, I.J.; Karsch, F.J.; Wagenmaker, E.R.; Tilbrook, A.J. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: Influence of ovarian steroids. Endocrinology 2009, 150, 341–349. [Google Scholar] [CrossRef]
- Gauthier, D. The influence of season and shade on oestrous behaviour, timing of preovulatory LH surge and the pattern of progesterone secretion in FFPN and creole heifers in a tropical climate. Reprod. Nutr. Dev. 1986, 26, 767–775. [Google Scholar] [CrossRef]
- Roth, Z. Influence of heat stress on reproduction in dairy cows—Physiological and practical aspects. J. Anim. Sci. 2020, 98, S80–S87. [Google Scholar] [CrossRef]
- Armstrong, D.T.; Dorrington, J.H. Estrogen biosynthesis in the ovaries and testes. Adv. Sex Horm. Res. 1977, 3, 217–258. [Google Scholar]
- Guzeloglu, A.; Ambrose, J.D.; Kassa, T.; Diaz, T.; Thatcher, M.J.; Thatcher, W.W. Long-term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress. Anim. Reprod. Sci. 2001, 66, 15–34. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2018, 9, 32–38. [Google Scholar] [CrossRef]
- Turk, R.; Rošić, N.; Vince, S.; Perkov, S.; Samardžija, M.; Beer-Ljubić, B.; Belić, M.; Robić, M. The influence of heat stress on energy metabolism in simmental dairy cows during the periparturient period. Vet. Arh. 2020, 90, 1–10. [Google Scholar] [CrossRef]
- Kilany, A.A.; El-Darawany, A.-H.A.; El-Tarabany, A.A.; Al-Marakby, K.M. Effect of folic acid supplements on progesterone profile and blood metabolites of heat-stressed Holstein cows during the early stage of pregnancy. Animals 2022, 12, 1872. [Google Scholar] [CrossRef] [PubMed]
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2010, 34, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Subcellular Biochemistry in Transient Receptor Potential (TRP) Channels; Harris, J.R., Boekema, E.J., Eds.; Springer: Singapore, 2018; pp. 141–165. [Google Scholar]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Saito, S.; Fukuta, N.; Shingai, R.; Tominaga, M. Evolution of vertebrate transient receptor potential vanilloid 3 channels: Opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet. 2011, 7, e1002041. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Dikmen, S.; Hansen, P.J. Is the temperature-humidity index the best indicator of heat stress in lactating cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef]
- Peier, A.; Reeve, A.; Andersson, D.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Yoshioka, T. Role of TRP channels in the induction of heat shock proteins (HSPs) by heating skin. Biophysics 2015, 11, 25–32. [Google Scholar] [CrossRef]
- Lee, S.H.; Duron, H.E.; Chaudhuri, D. Beyond the TCA cycle: New insights into mitochondrial calcium regulation of oxidative phosphorylation. Biochem. Soc. Trans. 2023, 51, 1661–1673. [Google Scholar] [CrossRef]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J. Calcium overload and mitochondrial metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Pumford, N.R.; Bottje, W.; Nakagawa, K.; Miyazawa, T.; Akiba, Y.; Toyomizu, M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. J. Poult. Sci. 2007, 44, 439–445. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef]
- Zhao, Q.; Fujiwara, Y.; Kondo, T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic. Biol. Med. 2006, 40, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lemasters, J.J. Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett. 2002, 512, 1–7. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Vais, H.; Payne, R.; Paudel, U.; Li, C.; Foskett, J.K. Coupled transmembrane mechanisms control MCU-mediated mitochondrial Ca2+ uptake. Proc. Natl. Acad. Sci. USA 2020, 117, 21731–21739. [Google Scholar] [CrossRef]
- Kumar, A.; Majhi, R.; Acharya, T.; Smalla, K.; Gundelfinger, E.; Goswami, C. TRPV4 interacts with mitochondrial proteins and acts as a mitochondrial structure-function regulator. bioRxiv 2018, 330993. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Y.; Chen, Z.; Fan, Y.; Liu, J.; Xu, Y.; Ahmed, Z.; Zhang, J.; Li, F.; Qi, X.; et al. Temperature adaptation patterns in chinese cattle revealed by TRPM2 gene mutation analysis. Anim. Biotechnol. 2024, 35, 2299944. [Google Scholar] [CrossRef]
- Kim, H.S.; Ullevig, S.L.; Zamora, D.; Lee, C.F.; Asmis, R. Redox Regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc. Natl. Acad. Sci. USA 2012, 109, E2803–E2812. [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Lifshits, L.; Seger, R. Nuclear p38: Roles in physiological and pathological processes and regulation of nuclear translocation. Int. J. Mol. Sci. 2020, 21, 6102. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and P38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef]
- D’Angelo, D.; Vecellio Reane, D.; Raffaello, A. Neither too much nor too little: Mitochondrial calcium concentration as a balance between physiological and pathological conditions. Front. Mol. Biosci. 2023, 10, 1336416. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.J.; Sonveaux, P.; Porporato, P.E.; Danhier, P.; Gallez, B.; Batinic-Haberle, I.; Nien, Y.-C.; Schroeder, T.; Dewhirst, M.W. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA 2010, 107, 20477–20482. [Google Scholar] [CrossRef]
- Segal, A.W.; Abo, A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem. Sci. 1993, 18, 43–47. [Google Scholar] [CrossRef]
- Evans, M.J.; Choi, W.-G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Sakagami, H. Molecular mechanisms underlying Ca2+/calmodulin-dependent protein kinase kinase signal transduction. Int. J. Mol. Sci. 2022, 23, 11025. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Kanarek, N.; Ben-Neriah, Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol. Rev. 2012, 246, 77–94. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway de Macario, E.; Campanella, C.; Macario, A.J.L.; Marino Gammazza, A. Functions and therapeutic potential of extracellular Hsp60, Hsp70, and Hsp90 in neuroinflammatory disorders. Appl. Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Lazaro, I.; Oguiza, A.; Recio, C.; Mallavia, B.; Madrigal-Matute, J.; Blanco, J.; Egido, J.; Martin-Ventura, J.-L.; Gomez-Guerrero, C. Targeting HSP90 ameliorates nephropathy and atherosclerosis through suppression of NF-κB and STAT signaling pathways in diabetic mice. Diabetes 2015, 64, 3600–3613. [Google Scholar] [CrossRef] [PubMed]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.-U.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K.; et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef]
- Andreakos, E.; Sacre, S.M.; Smith, C.; Lundberg, A.; Kiriakidis, S.; Stonehouse, T.; Monaco, C.; Feldmann, M.; Foxwell, B.M. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood 2004, 103, 2229–2237. [Google Scholar] [CrossRef]
- Patra, A.K.; Kar, I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J. Anim. Sci. Technol. 2021, 63, 211–247. [Google Scholar] [CrossRef]
- Wen, C.; Li, S.; Wang, J.; Zhu, Y.; Zong, X.; Wang, Y.; Jin, M. Heat stress alters the intestinal microbiota and metabolomic profiles in mice. Front. Microbiol. 2021, 12, 706772. [Google Scholar] [CrossRef]
- Bridgeman, S.; Ellison, G.; Newsholme, P.; Mamotte, C. The HDAC inhibitor butyrate impairs β cell function and activates the disallowed gene hexokinase I. Int. J. Mol. Sci. 2021, 22, 13330. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kukita, A.; Kukita, T.; Shobuike, T.; Nakamura, T.; Kohashi, O. Two histone deacetylase inhibitors, trichostatin a and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood 2003, 101, 3451–3459. [Google Scholar] [CrossRef]
- Zhou, R.; Gong, A.-Y.; Chen, D.; Miller, R.E.; Eischeid, A.N.; Chen, X.-M. Histone deacetylases and NF-kB signaling coordinate expression of CX3CL1 in epithelial cells in response to microbial challenge by suppressing miR-424 and miR-503. PLoS ONE 2013, 8, e65153. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.; Gumilar, K.E.; Li, X.-G.; Tjokroprawiro, B.A.; Lu, C.-H.; Lu, J.; Zhou, M.; Sobol, R.W.; Tan, M. Targeting HSF1 for cancer treatment: Mechanisms and inhibitor development. Theranostics 2023, 13, 2281–2300. [Google Scholar] [CrossRef] [PubMed]
- Fasano, C.; Disciglio, V.; Bertora, S.; Lepore Signorile, M.; Simone, C. FOXO3a from the nucleus to the mitochondria: A round trip in cellular stress response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK-SIRT1-FOXO pathway: The in-depth analysis and breakthrough prospects of oxidative stress-induced diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef]
- Zeng, H.; Xia, H.; Wang, X.; Wang, Y.; Fang, J.; Li, S.; Zhai, Y.; Han, Z. Comprehensive profiling of ceRNA (circRNA-miRNA-mRNA) networks in hypothalamic-pituitary-mammary gland axis of dairy cows under heat stress. Int. J. Mol. Sci. 2023, 24, 888. [Google Scholar] [CrossRef]
- Song, M.; Koo, T. Recent advances in CRISPR technologies for genome editing. Arch. Pharmacal Res. 2021, 44, 537–552. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Chen, J.; Dai, L.; Chen, R.; Ye, J.; Hao, D. A heat shock 70kDa protein MaltHSP70-2 contributes to thermal resistance in Monochamus alternatus (Coleoptera: Cerambycidae): Quantification, localization, and functional analysis. BMC Genom. 2022, 23, 646. [Google Scholar] [CrossRef]
- Nandy, S.; Pathak, B.; Zhao, S.; Srivastava, V. Heat-shock-inducible CRISPR/Cas9 system generates heritable mutations in rice. Plant Direct 2019, 3, e00145. [Google Scholar] [CrossRef]
- Dikmen, S.; Khan, F.A.; Huson, H.J.; Sonstegard, T.S.; Moss, J.I.; Dahl, G.E.; Hansen, P.J. The SLICK hair locus derived from senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5508–5520. [Google Scholar] [CrossRef]
- Sigdel, A.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Whole genome mapping reveals novel genes and pathways involved in milk production under heat stress in US Holstein cows. Front. Genet. 2019, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Chai, J.; Xiong, H.; Li, W.; Huang, T.; Liu, Y.; Suo, X.; Zhang, N.; Li, X.; Jiang, S.; et al. Association analysis of HSP70A1A haplotypes with heat tolerance in Chinese Holstein cattle. Cell Stress Chaperon. 2013, 18, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Turman, M.A.; Rosenfeld, S.L. Heat shock protein 70 overexpression protects LLC-PK1 tubular cells from heat shock but not hypoxia. Kidney Int. 1999, 55, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kisliouk, T.; Cramer, T.; Meiri, N. Methyl CpG level at distal part of heat-shock protein promoter HSP70 exhibits epigenetic memory for heat stress by modulating recruitment of POU2F1-associated nucleosome remodeling deacetylase (NuRD) complex. J. Neurochem. 2017, 141, 358–372. [Google Scholar] [CrossRef]
- Ho, T.-C.; Kim, H.S.; Chen, Y.; Li, Y.; LaMere, M.W.; Chen, C.; Wang, H.; Gong, J.; Palumbo, C.D.; Ashton, J.M.; et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci. Adv. 2021, 7, eabg3217. [Google Scholar] [CrossRef]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef]
- Wu, R.; Li, X.; Ma, N.; Jin, X.; Yuan, X.; Qu, C.; Tang, H.; Liu, Z.; Zhang, Z. Bacterial quorum sensing molecules promote allergic airway inflammation by activating the retinoic acid response. iScience 2020, 23, 101288. [Google Scholar] [CrossRef]
- Hu, R.; Yang, T.; Ai, Q.; Shi, Y.; Ji, Y.; Sun, Q.; Tong, B.; Chen, J.; Wang, Z. Autoinducer-2 promotes the colonization of Lactobacillus Rhamnosus GG to improve the intestinal barrier function in a neonatal mouse model of antibiotic-induced intestinal dysbiosis. J. Transl. Med. 2024, 22, 177. [Google Scholar] [CrossRef]
- Fu, C.; Ge, J.; Qu, M.; Ouyang, K.; Qiu, Q. Effects of 4-hydroxy-2,5-dimethyl-3(2H)-furanone supplementation on growth performance, serum antioxidant capacity, rumen fermentation characteristics, rumen bacterial quorum sensing, and microbial community in Hu sheep. Anim. Biosci. 2025, 38, 1422–1434. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Dai, J.; Qu, M.; Ouyang, K.; Qiu, Q. Heated drinking water in winter improves growth performance of male Hu sheep by modulating rumen quorum sensing and metabolites, and enhancing serum antioxidant capacity. Anim. Biosci. 2025. ahead of print. Available online: https://www.animbiosci.org/journal/view.php?number=25456 (accessed on 6 July 2025). [CrossRef]
- Moura-Alves, P.; Faé, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Meng, F.; Ge, Z.; Zhou, Y.; Fan, Z.; Du, J. Bioinspired peptide/polyamino acid assemblies as quorum sensing inhibitors for the treatment of bacterial infections. J. Mater. Chem. B 2024, 12, 11596–11610. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, G.; Yang, Y.; Wang, N.; Zhang, Y.; Su, Y.; Zhao, F.; Wu, J.; Wang, L.; Lin, Y.; et al. Nanomaterials regulate bacterial quorum sensing: Applications, mechanisms, and optimization strategies. Adv. Sci. 2024, 11, 2306070. [Google Scholar] [CrossRef] [PubMed]
- Zenger, K.R.; Khatkar, M.S.; Jones, D.B.; Khalilisamani, N.; Jerry, D.R.; Raadsma, H.W. Genomic selection in aquaculture: Application, limitations and opportunities with special reference to marine shrimp and pearl oysters. Front. Genet. 2019, 9, 693. [Google Scholar] [CrossRef]
- Scherer, N.; Fässler, D.; Borisov, O.; Cheng, Y.; Schlosser, P.; Wuttke, M.; Haug, S.; Li, Y.; Telkämper, F.; Patil, S.; et al. Coupling metabolomics and exome sequencing reveals graded effects of rare damaging heterozygous variants on gene function and human traits. Nat. Genet. 2025, 57, 193–205. [Google Scholar] [CrossRef]
- Christensen, O.F.; Börner, V.; Varona, L.; Legarra, A. Genetic evaluation including intermediate omics features. Genetics 2021, 219, iyab130. [Google Scholar] [CrossRef]
- Cui, H.; Tejada-Lapuerta, A.; Brbić, M.; Saez-Rodriguez, J.; Cristea, S.; Goodarzi, H.; Lotfollahi, M.; Theis, F.J.; Wang, B. Towards multimodal foundation models in molecular cell biology. Nature 2025, 640, 623–633. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, L. AI-driven multi-omics integration for multi-scale predictive modeling of causal genotype-environment-phenotype relationships. Comput. Struct. Biotechnol. J. 2025, 27, 265–277. [Google Scholar] [CrossRef]
- Sharma, A.; Kosasih, E.; Zhang, J.; Brintrup, A.; Calinescu, A. Digital twins: State of the art theory and practice, challenges, and open research questions. J. Ind. Inf. Integr. 2022, 30, 100383. [Google Scholar] [CrossRef]
- Han, X.; Lin, Z.; Clark, C.; Vucetic, B.; Lomax, S. AI based digital twin model for cattle caring. Sensors 2022, 22, 7118. [Google Scholar] [CrossRef]
- Abdulai, A.-R.; Tetteh Quarshie, P.; Duncan, E.; Fraser, E. Is agricultural digitization a reality among smallholder farmers in Africa? Unpacking farmers’ lived realities of engagement with digital tools and services in rural Northern Ghana. Agric. Food Secur. 2023, 12, 11. [Google Scholar] [CrossRef]
- Global Gene Editing Regulation Tracker. Available online: https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org/ (accessed on 11 March 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Li, L.; Ouyang, K.; Qu, M.; Qiu, Q. Deciphering Heat Stress Mechanisms and Developing Mitigation Strategies in Dairy Cattle: A Multi-Omics Perspective. Agriculture 2025, 15, 1477. https://doi.org/10.3390/agriculture15141477
Xiong Z, Li L, Ouyang K, Qu M, Qiu Q. Deciphering Heat Stress Mechanisms and Developing Mitigation Strategies in Dairy Cattle: A Multi-Omics Perspective. Agriculture. 2025; 15(14):1477. https://doi.org/10.3390/agriculture15141477
Chicago/Turabian StyleXiong, Zhiyi, Lin Li, Kehui Ouyang, Mingren Qu, and Qinghua Qiu. 2025. "Deciphering Heat Stress Mechanisms and Developing Mitigation Strategies in Dairy Cattle: A Multi-Omics Perspective" Agriculture 15, no. 14: 1477. https://doi.org/10.3390/agriculture15141477
APA StyleXiong, Z., Li, L., Ouyang, K., Qu, M., & Qiu, Q. (2025). Deciphering Heat Stress Mechanisms and Developing Mitigation Strategies in Dairy Cattle: A Multi-Omics Perspective. Agriculture, 15(14), 1477. https://doi.org/10.3390/agriculture15141477





