Greenhouse Evaluation of Conventional and Biorational Insecticides for Managing the Invasive Thrips parvispinus (Karny) (Thysanoptera: Thripidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plants
2.2. Thrips Parvispinus Rearing and Egg Cohort
2.3. Prophylactic Spray Applications
2.4. Curative Spray Applications
2.5. Statistical Analyses
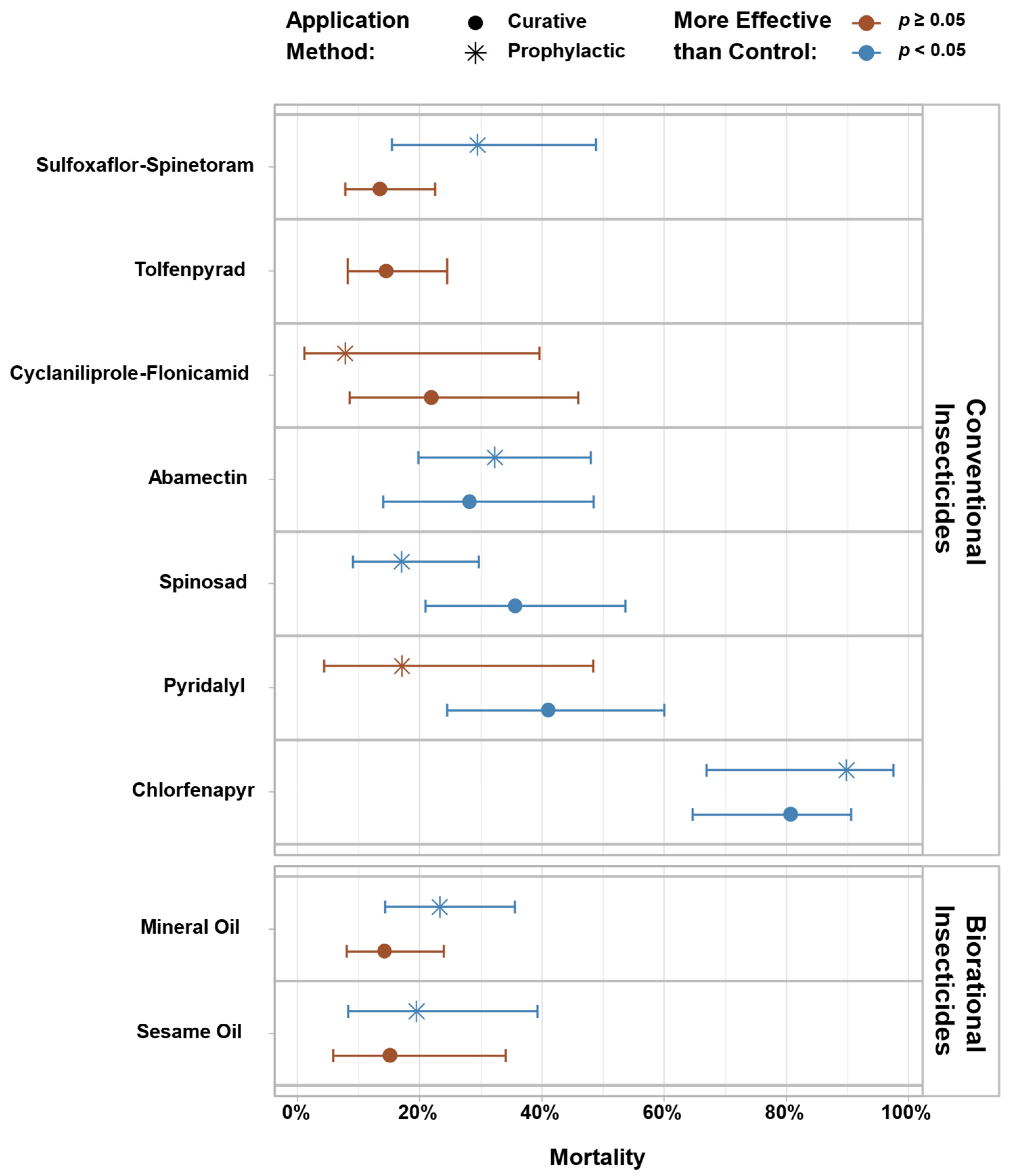
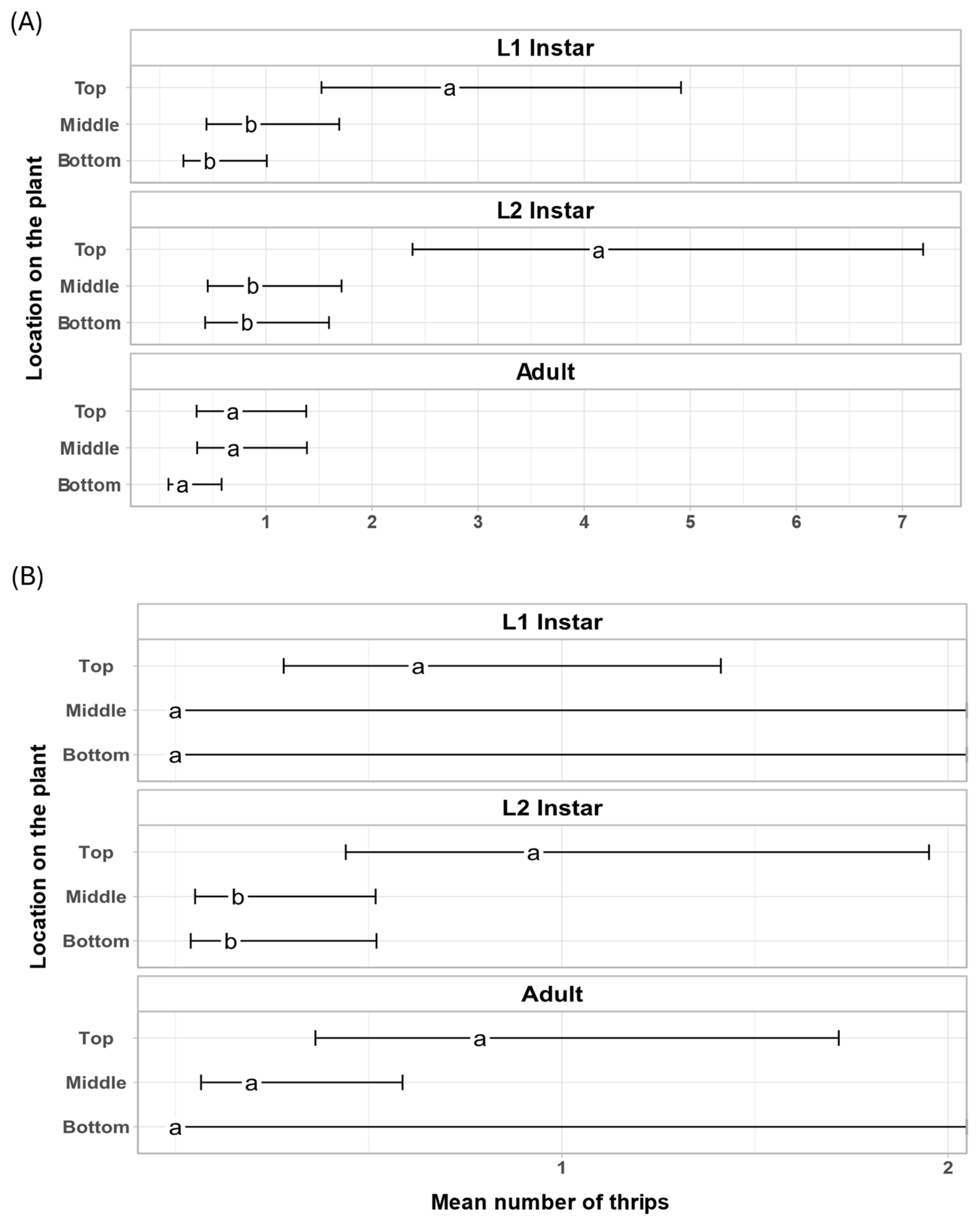
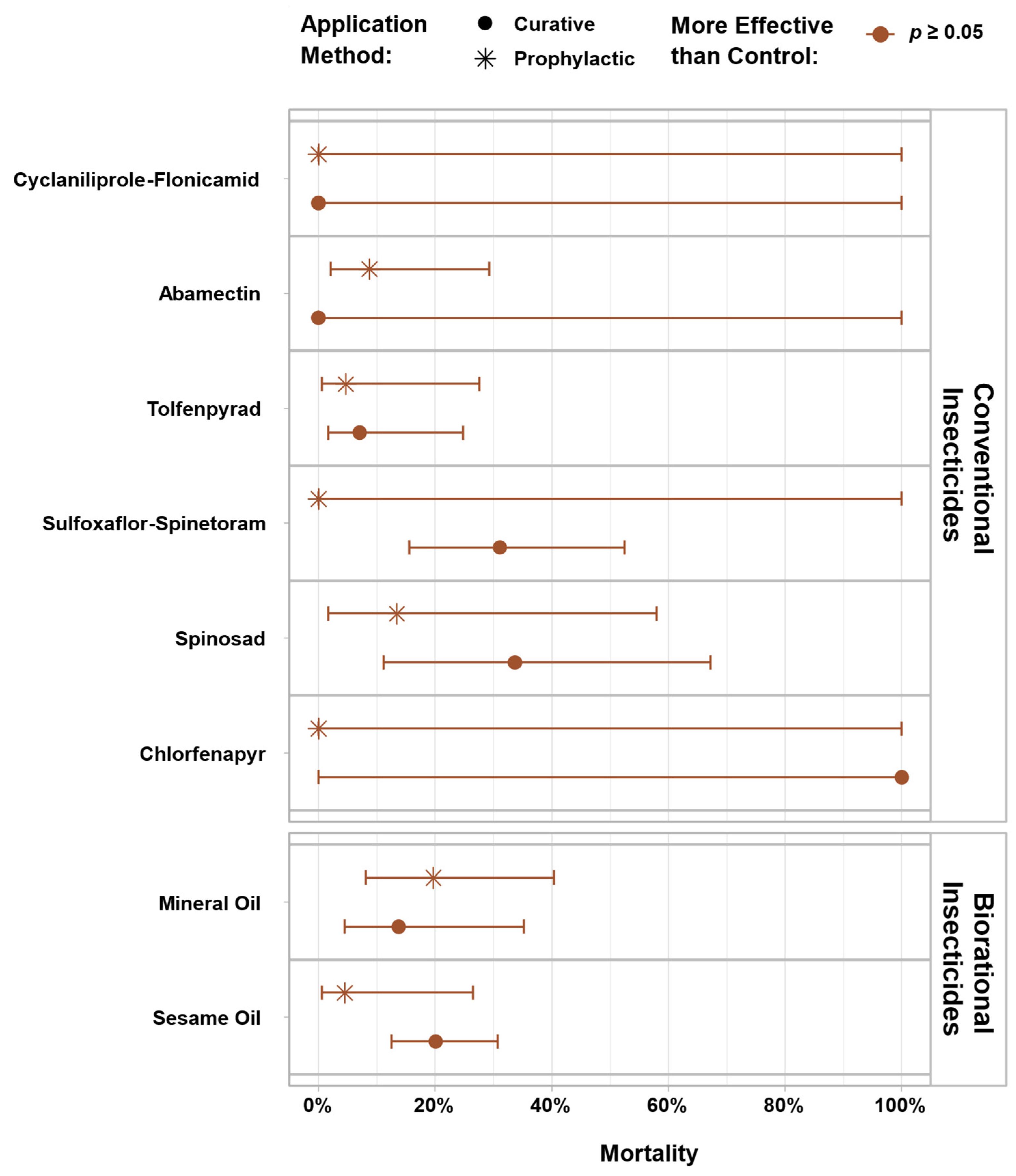
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes, C.P. Thysanoptera (Hexapoda) of the Philippine Islands. Raffles Bull. Zool. 1994, 42, 107–507. [Google Scholar]
- Johari, A.; Herlinda, S.; Pujiastuti, Y.; Irsan, C.; Sartiami, D. Morphological and Genetic Variation of Thrips Parvispinus (Thysanoptera: Thripidae) in Chili Plantation (Capsicum annuum L.) in the Lowland and Highland of Jambi Province, Indonesia. Am. J. Biosci. 2014, 2, 17–21. [Google Scholar]
- Sridhar, V.; Chandana, P.S.; Rachana, R. Global Status of Thrips parvispinus (Karny, 1922), an Invasive Pest. J. Res. PJTSAU 2021, 49, 1–11. [Google Scholar]
- Thorat, S.; Sisodiya, D.; Gangwar, R. Invasive Thrips, Thrips parvispinus (Karny) an Invasive Threat: A Review. Environ. Ecol. 2022, 40, 2170–2175. [Google Scholar]
- Mound, L.A.; Collins, D.W. A South East Asian Pest Species Newly Recorded from Europe: Thrips parvispinus (Thysanoptera: Thripidae), Its Confused Identity and Potential Quarantine Significance. Eur. J. Entomol. 2000, 97, 197–200. [Google Scholar] [CrossRef]
- Murai, T.; Watanabe, H.; Toriumi, W.; Adati, T.; Okajima, S. Damage to Vegetable Crops by Thrips parvispinus Karny (Thysanoptera: Thripidae) and Preliminary Studies on Biology and Control. J. Insect Sci. 2009, 10, 166. [Google Scholar]
- Sugano, J.; Hamasaki, R.; Villalobos, E.; Chou, M.; Wright, M.; Fukuda, S.; Swift, S.; Ferreira, S.; Tsuda, D.; Diaz-Lyke, M. Damage to Papaya Caused by Thrips parvispinus (Karny); University of Hawaii: Honolulu, HI, USA, 2015. [Google Scholar]
- Soto-Adames, F. Thrips parvispinus (Karny); Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 2020. [Google Scholar]
- EPPO Global Database. Thrips Parvispinus (THRIPV): Distribution. 2025. Available online: https://gd.eppo.int/taxon/THRIPV/distribution (accessed on 10 January 2025).
- United States Department of Agriculture National Agricultural Statistics Service. USDA-NASS Southern Region News Release Floriculture Production & Sales; United States Department of Agriculture National Agricultural Statistics Service: Washington, DC, USA, 2021.
- Ahmed, M.Z.; Revynthi, A.M.; Mckenzie, C.; Osborne, L.S. Thrips parvispinus (Karny), an Emerging Invasive Regulated Pest in the United States; IFAS Extension: Apopka, FL, USA, 2023. [Google Scholar]
- United States Department of Agriculture National Agricultural Statistics Service. USDA-NASS Florida Agricultural Facts; United States Department of Agriculture National Agricultural Statistics Service: Washington, DC, USA, 2022.
- Veeranna, D.; Reddy, R.U.; Moguloju, M.; Padmaja, G. Report on Heavy Infestation and Damage by Invasive Thrips Species, Thrips parvispinus (Karny) on Chilli in Telangana State of India. Pharma Innov. 2022, 11, 3845–3848. [Google Scholar]
- Hutasoit, R.; Triwidodo, H.; Anwar, R. Biology and Demographic Statistic of Thrips Parvispinus Karny (Thysanoptera: Thripidae) in Chili Pepper (Capsicum annuum Linnaeus). Indones. J. Entomol. 2017, 14, 107–116. [Google Scholar] [CrossRef]
- Gao, Y.; Lei, Z.; Reitz, S.R. Western Flower Thrips Resistance to Insecticides: Detection, Mechanisms and Management Strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef]
- Bogran, C.E.; Ludwig, S.; Metz, B. Using Oils as Pesticides. Texas Farmer Collection. Agrilife Extension Texas A&M System. 2006. Available online: https://hdl.handle.net/1969.1/86885 (accessed on 10 January 2025).
- Buteler, M.; Stadler, T. A Review on the Mode of Action and Current Use of Petroleum Distilled Spray Oils. In Pesticides in the Modern World-Pesticides Use and Management; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Food and Agriculture Organization of the United Nations Guidelines on Prevention and Management of Pesticide Resistance; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012.
- Ataide, L.M.; Vargas, G.; Velazquez-Hernandez, Y.; Reyes-Arauz, I.; Villamarin, P.; Canon, M.A.; Yang, X.; Riley, S.S.; Revynthi, A.M. Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions. Insects 2024, 15, 48. [Google Scholar] [CrossRef]
- Insecticide Resistance Action Committee, The Irac Mode of Action Classification Online. Available online: https://irac-online.org/mode-of-action/classification-online/ (accessed on 7 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’ (2.0.0), R Package Version; Posit: Boston, MA, USA, 2023; 1.
- Brooks, M.; Bolker, B.; Kristensen, K.; Maechler, M.; Magnusson, A.; McGillycuddy, M.; Skaug, H.; Nielsen, A.; Berg, C.; van Bentham, K. glmmTMB: Generalized Linear Mixed Models Using Template Model Builder. R Package. 2022. [Computer Software]. Available online: https://cran.r-project.org/package=glmmTMB (accessed on 10 January 2025).
- Bolker, B.M. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 1-4008-4090-2. [Google Scholar]
- Stroup, W.W. Rethinking the Analysis of Non-normal Data in Plant and Soil Science. Agron. J. 2015, 107, 811–827. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package. 2022. [Computer Software]. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 10 January 2025).
- Zhang, Z.; Wu, Q.; Li, X.; Zhang, Y.; Xu, B.; Zhu, G. Life History of Western Flower Thrips, Frankliniella occidentalis (Thysan., Thripae), on Five Different Vegetable Leaves. J. Appl. Entomol. 2007, 131, 347–354. [Google Scholar] [CrossRef]
- De Kogel, W.; Bosco, D.; Van Der Hoek, M.; Mollema, C. Effect of Host Plant on Body Size of Frankliniella occidentalis (Thysanoptera: Thripidae) and Its Correlation with Reproductive Capacity. EJE 2013, 96, 365–368. [Google Scholar]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and Ecological Consequences of Plant Defence Induction and Suppression in Herbivore Communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.C. Plant Defence against Herbivory and Insect Adaptations. AoB Plants 2018, 10, ply037. [Google Scholar] [CrossRef]
- Gaskin, R.; Steele, K.; Forster, W. Characterising Plant Surfaces for Spray Adhesion and Retention. New Zealand Plant Prot. 2005, 58, 179–183. [Google Scholar] [CrossRef]
- Tobin, P.C.; Berec, L.; Liebhold, A.M. Exploiting Allee Effects for Managing Biological Invasions. Ecol. Lett. 2011, 14, 615–624. [Google Scholar] [CrossRef]
- Allee, W.C. Animal Aggregations. Q. Rev. Biol. 1927, 2, 367–398. [Google Scholar] [CrossRef]
- Allee, W.C.; Bowen, E.S. Studies in Animal Aggregations: Mass Protection against Colloidal Silver among Goldfishes. J. Exp. Zool. 1932, 61, 185–207. [Google Scholar] [CrossRef]
- Courchamp, F.; Berec, L.; Gascoigne, J. Allee Effects in Ecology and Conservation; OUP Oxford: Oxford, UK, 2008; ISBN 0-19-152466-2. [Google Scholar]
- Kramer, A.M.; Dennis, B.; Liebhold, A.M.; Drake, J.M. The Evidence for Allee Effects. Popul. Ecol. 2009, 51, 341–354. [Google Scholar] [CrossRef]
- Branco, M.; Dokhelar, T.; Brockerhoff, E.G.; Liebhold, A.M.; Jactel, H. Widespread Experimental Evidence of Allee Effects in Insects: A Meta-Analysis. Entomol. Gen. 2024, 44, 765–778. [Google Scholar] [CrossRef]
- Bhuyain, M.M.H.; Lim, U.T. Relative Susceptibility to Pesticides and Environmental Conditions of Frankliniella Intonsa and F. occidentalis (Thysanoptera: Thripidae), an Underlying Reason for Their Asymmetrical Occurrence. PLoS ONE 2020, 15, e0237876. [Google Scholar] [CrossRef] [PubMed]
- Herron, G.A.; Rophail, J. First Detection of Chlorfenapyr (Secure®) Resistance in Two-Spotted Spider Mite (Acari: Tetranychidae) from Nectarines in an Australian Orchard. Exp. Appl. Acarol. 2003, 31, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Eger, J.; Stavisky, J.; Funderburk, J. Comparative Toxicity of Spinosad to Frankliniella Spp. (Thysanoptera: Thripidae), with Notes on a Bioassay Technique. Fla. Entomol. 1998, 81, 547–551. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Sadof, C.S. Effects of Spinosad and Acephate on Western Flower Thrips inside and Outside a Greenhouse. HortTechnology 2000, 10, 359–362. [Google Scholar] [CrossRef]
- Herron, G.; James, T. Insecticide Resistance in Australian Populations of Western Flower Thrips, “Frankliniella Occidentalis” (Pergande) (Thysanoptcra: Thripidae). Gen. Appl. Entomol. J. Entomol. Soc. N. S. Wales 2007, 36, 1–5. [Google Scholar]
- Bielza, P. Insecticide Resistance Management Strategies against the Western Flower Thrips, Frankliniella occidentalis. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 1131–1138. [Google Scholar] [CrossRef]
- Kirst, H.A. The Spinosyn Family of Insecticides: Realizing the Potential of Natural Products Research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef]
- Bret, B.; Larson, L.; Schoonover, J.; Sparks, T.; Thompson, G. Biological Properties of Spinosad. Down Earth 1997, 52, 6–13. [Google Scholar]
- Tillman, P.G.; Mulrooney, J.E. Effect of Selected Insecticides on the Natural Enemies Coleomegilla Maculata and Hippodamia Convergens (Coleoptera: Coccinellidae), Geocoris Punctipes (Hemiptera: Lygaeidae), and Bracon Mellitor, Cardiochiles Nigriceps, and Cotesia Marginiventris (Hymenoptera: Braconidae) in Cotton. J. Econ. Entomol. 2000, 93, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Biological and Integrated Control. IOBC-WPRS Pesticide Side Effect Database; The International organisation for Biological Control (IOBC): Zürich, Switzerland, 2023. [Google Scholar]
- Lasota, J.A.; Dybas, R.A. Abamectin as a Pesticide for Agricultural Use. Acta Leiden 1990, 59, 217–225. [Google Scholar] [PubMed]
- Ishaaya, I.; Kontsedalov, S.; Horowitz, A.R. Emamectin, a Novel Insecticide for Controlling Field Crop Pests. Pest Manag. Sci. Former. Pestic. Sci. 2002, 58, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Duso, C.; Malagnini, V.; Pozzebon, A.; Buzzetti, F.M.; Tirello, P. A Method to Assess the Effects of Pesticides on the Predatory Mite Phytoseiulus Persimilis (Acari Phytoseiidae) in the Laboratory. Biocontrol Sci. Technol. 2008, 18, 1027–1040. [Google Scholar] [CrossRef]
- Alhewairini, S.; Al-Azzazy, M. Side Effects of Abamectin and Hexythiazox on Seven Predatory Mites. Braz. J. Biol. 2021, 83, e251442. [Google Scholar] [CrossRef]
- Assis, C.P.O.; Gondim, M.G.C.; Siqueira, H.A.A. Synergism to Acaricides in Resistant Neoseiulus californicus (Acari: Phytoseiidae), a Predator of Tetranychus urticae (Acari: Tetranychidae). Crop Prot. 2018, 106, 139–145. [Google Scholar] [CrossRef]
- Immaraju, J.A.; Paine, T.D.; Bethke, J.A.; Robb, K.L.; Newman, J.P. Western Flower Thrips (Thysanoptera: Thripidae) Resistance to Insecticides in Coastal California Greenhouses. J. Econ. Entomol. 1992, 85, 9–14. [Google Scholar] [CrossRef]
- Döker, İ.; Revynthi, A.M.; Mannion, C.; Carrillo, D. First Report of Acaricide Resistance in Tetranychus urticae (Acari: Tetranychidae) from South Florida1. Syst. Appl. Acarol. 2020, 25, 1209–1214. [Google Scholar]
- Revynthi, A.M.; Cruz, L.F.; Canon, M.A.; Crane, J.H.; Kendra, P.E.; Mannion, C.; Carrillo, D. Evaluation of Abamectin as a Potential Chemical Control for the Lychee Erinose Mite (Acari: Eriophyidae), a New Invasive Pest in Florida. Fla. Entomol. 2022, 105, 1–5. [Google Scholar] [CrossRef]
- Morse, J.G.; Hoddle, M.S. Invasion Biology of Thrips. Annu. Rev. Entomol. 2006, 51, 67–89. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion Biology, Ecology, and Management of Western Flower Thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.; Coy-Barrera, E. Overview of Updated Control Tactics for Western Flower Thrips. Insects 2023, 14, 649. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential Oil Compounds for Thrips Control—A Review. Nat. Prod. Commun. 2008, 3, 1934578X0800300726. [Google Scholar] [CrossRef]
- Ataide, L.M.S.; Velazquez-Hernandez, Y.; Reyes-Arauz, I.; Villamarin, P.; Canon, M.A.; Revynthi, A.M. Potential of Dip Treatments to Disinfest Cuttings of the Invasive Thrips parvispinus (Thysanoptera: Thripidae). J. Econ. Entomol. 2025, 118, toae265. [Google Scholar] [CrossRef]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Robb, K.L.; Parrella, M.P. IPM of Western Flower Thrips. In Thrips Biology and Management; Springer: Berlin/Heidelberg, Germany, 1995; pp. 365–370. [Google Scholar]
| Active Ingredient(s) | Trade Name | EPA Registration | Insecticide Group | Rate * | Rate in 1L Solution | Site ** | |
|---|---|---|---|---|---|---|---|
| Conventional Insecticides | Spinosad | Conserve SC | 62719-291 | 5 | 1.20 mL/ha | 0.78 mL | G, N, L |
| Tolfenpyrad | Hachi-Hachi SC | 71711-31-67690 | 21A | 323.1 mL/ha | 2.11 mL | G, N, S, L | |
| Chlorfenapyr | Piston TR | 91234-19 | 13 | 119.7 mL/ha | 0.78 mL | G | |
| Cyclaniliprole–Flonicamid | Pradia | 71512-33-59807 | 28–29 | 209.4 mL/ha | 1.37 mL | G, N, S | |
| Abamectin | Timectin 0.15 EC | 84229-1 | 6 | 95.7 mL/ha | 0.63 mL | S, G, N | |
| Sulfoxaflor–Spinetoram | Xxpire | 62719-676 | 4C-5 | 31.5 g/ha | 206 mg | G, N | |
| Pyridalyl | Overture 35 WP | 59639-125 | Unclassified | 91.8 g/ha | 599 mg | G | |
| Biorational Insecticides | Sesame oil | Bee Safe 3-in-1 | FIFRA 25 (b) exempt | Unclassified | 35.9 mL/ha | 23.02 mL | S, G, N, L |
| Mineral oil | Ultra-fine | 86330-11 | Unclassified | 3% | 30 mL | G, N, L, I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataide, L.M.S.; Vargas, G.; Velazquez-Hernandez, Y.; De Giosa, M.; Reyes-Arauz, I.; Villamarin, P.; Canon, M.A.; Riley, S.S.; Revynthi, A.M. Greenhouse Evaluation of Conventional and Biorational Insecticides for Managing the Invasive Thrips parvispinus (Karny) (Thysanoptera: Thripidae). Agriculture 2025, 15, 1451. https://doi.org/10.3390/agriculture15131451
Ataide LMS, Vargas G, Velazquez-Hernandez Y, De Giosa M, Reyes-Arauz I, Villamarin P, Canon MA, Riley SS, Revynthi AM. Greenhouse Evaluation of Conventional and Biorational Insecticides for Managing the Invasive Thrips parvispinus (Karny) (Thysanoptera: Thripidae). Agriculture. 2025; 15(13):1451. https://doi.org/10.3390/agriculture15131451
Chicago/Turabian StyleAtaide, Livia M. S., German Vargas, Yisell Velazquez-Hernandez, Marcello De Giosa, Isamar Reyes-Arauz, Paola Villamarin, Maria A. Canon, Simon S. Riley, and Alexandra M. Revynthi. 2025. "Greenhouse Evaluation of Conventional and Biorational Insecticides for Managing the Invasive Thrips parvispinus (Karny) (Thysanoptera: Thripidae)" Agriculture 15, no. 13: 1451. https://doi.org/10.3390/agriculture15131451
APA StyleAtaide, L. M. S., Vargas, G., Velazquez-Hernandez, Y., De Giosa, M., Reyes-Arauz, I., Villamarin, P., Canon, M. A., Riley, S. S., & Revynthi, A. M. (2025). Greenhouse Evaluation of Conventional and Biorational Insecticides for Managing the Invasive Thrips parvispinus (Karny) (Thysanoptera: Thripidae). Agriculture, 15(13), 1451. https://doi.org/10.3390/agriculture15131451





