Effects of Grape Pomace on Sow Blood, Colostrum and Milk Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding
2.2. Analysis of Feeds and Their Nutrient Composition
2.3. Sampling and Analysis of Blood, Colostrum, and Milk Samples
2.4. Statistical Analysis
3. Results
3.1. Biochemical Profile
3.2. Immunoglobulin Concentration in Colostrum and Milk of Sows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DGP | The experimental group of sows that received a supplement of dried grape pomace in the amount of 1% of the diet |
| CON | The control group of sows |
| 7d a.p. | 7th day antepartum |
| 1d p.p. | 1st day postpartum |
| d0 | Time of birth of the first piglet, equals time of colostrum sampling |
| d1, d2, d3, d7, d28 | Days after parturition, equals days of milk sampling |
| GAE | Equivalent gallic acid |
| CAE | Equivalent caffeic acid |
| QE | Equivalent quercetin |
| TEAC | Trolox equivalent antioxidant capacity |
| ALB | Albumin |
| CHOL | Total cholesterol |
| TRIGS | Triglycerides |
| GLU | Glucose |
| TP | Total protein |
| DBIL | Direst bilirubin |
| CREAT | Creatinine |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| GGT | γ-glutamyl transferase |
| Ca | Calcium |
| Mg | Magnesium |
| P | Phosphorus |
| BHB | β-hydroxybutyrate |
| TAS | Total antioxidant status |
| GLOB | Globulin |
| A/G ratio | Albumin/globulin ratio |
| IgG | Immunoglobulin G |
| IgA | Immunoglobulin A |
| IgM | Immunoglobulin M |
References
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crop. Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Hanušovský, O.; Gálik, B.; Bíro, D.; Šimko, M.; Juráček, M.; Rolinec, M.; Zábranský, Ľ.; Philipp, C.; Puntigam, R.; Slama, J.A.; et al. The nutritional potential of grape by-products from the area of Slovakia and Austria. Emir. J. Food Agric. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Rivera, O.M.P.; Leos, M.D.S.; Solis, V.E.; Domínguez, J.M. Recent trends on the valorization of winemaking industry wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100415. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed. Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Ivanišová, E.; Terentjeva, M.; Kántor, A.; Frančáková, H.; Kačániová, M. Phytochemical and antioxidant profile of different varietes of grape from the Small Carpathians wine region of Slovakia. Erwerbs-Obstbau 2019, 61, 53–59. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Immunoglobulins in Mammary Secretions. In Advanced Dairy Chemistry, 1st ed.; Sawyer, L., Ed.; Springer: Boston, MA, USA, 2013; pp. 275–294. [Google Scholar]
- Wiegert, J.G.; Knauer, M.T. Sow functional teat number impacts colostrum intake and piglet throughput. J. Anim. Sci. 2018, 96 (Suppl. 2), 51–52. [Google Scholar] [CrossRef]
- Merlot, E.; Pastorelli, H.; Prunier, A.; Père, M.C.; Louveau, I.; Lefaucheur, L.; Perruchot, M.H.; Meunier-Salaün, M.C.; Gardan-Salmon, D.; Gondret, F.; et al. Sow environment during gestation: Part I. Influence on maternal physiology and lacteal secretions in relation with neonatal survival. Animal 2019, 13, 1432–1439. [Google Scholar] [CrossRef]
- KilBride, A.L.; Mendl, M.; Statham, P.; Held, S.; Harris, M.; Cooper, S.; Green, L.E. A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England. Prev. Veter.-Med. 2012, 104, 281–291. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Theil, P.K. Colostrum and milk production. In The Gestating and Lactating Sow, 1st ed.; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 173–192. [Google Scholar]
- Butler, J.E.; Kehrli, M.E., Jr. Immunoglobulins and immunocytes in the mammary gland and its secretions. In Mucosal Immunology, 1st ed.; Mestecky, J., Lamm, M., Strober, W., Bienenstock, J., McGhee, J.R., Mayer, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1764–1793. [Google Scholar]
- Rooke, J.A.; Bland, I.M. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 2002, 78, 13–23. [Google Scholar] [CrossRef]
- Blecha, F. Immunological aspects: Comparison with other species. In The Lactating Sow, 1st ed.; Verstegen, M.W.A., Moughan, P.J., Schrama, J.W., Eds.; Wageningen Pers: Wageningen, The Netherlands, 1998; pp. 23–44. [Google Scholar]
- Markowska-Daniel, I.; Pomorska-Mol, M. Shifts in immunoglobulins levels in the porcine mammary secretions during whole lactation period. Bull. Vet. Inst. Pulawy 2010, 54, 345–349. [Google Scholar]
- Nuntapaitoon, M.; Juthamanee, P.; Theil, P.K.; Tummaruk, P. Impact of sow parity on yield and composition of colostrum and milk in Danish Landrace× Yorkshire crossbred sows. Prev. Veter.-Med. 2020, 181, 105085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kim, H.J.; Cho, J.H.; Chen, Y.J.; Yoo, J.S.; Min, B.J.; Wang, Y.; Kim, I.H. Effects of phytogenic substances on growth performance, digestibility of nutrients, faecal noxious gas content, blood and milk characteristics and reproduction in sows and litter performance. J. Anim. Feed Sci. 2008, 17, 50. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, G.; Kebreab, E.; Yu, Q.; Li, J.; Zhang, X.; He, H.; Fang, R.; Dai, Q. Effects of dietary grape seed polyphenols supplementation during late gestation and lactation on antioxidant status in serum and immunoglobulin content in colostrum of multiparous sows. J. Anim. Sci. 2019, 97, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, A.J.; Maes, D.G.; Mateusen, B.; Deprez, P.; Janssens, G.P.; De Lange, L.; Counotte, G. Serum biochemical reference values for gestating and lactating sows. Veter.-J. 2007, 174, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Alsop, J.E.; Hurnik, D.; Bildfell, R.J. Porcine ketosis: A case report and literature summary. J. Swine Health Prod. 1994, 2, 5–8. [Google Scholar]
- Lipiński, K.; Antoszkiewicz, Z.; Mazur-Kuśnirek, M.; Korniewicz, D.; Kotlarczyk, S. The effect of polyphenols on the performance and antioxidant status of sows and piglets. Ital. J. Anim. Sci. 2019, 18, 174–181. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 5 June 2025).
- Mixtajová, E.; Gálik, B.; Bíro, D.; Juráček, M.; Šimko, M.; Hanušovský, O.; Kolláthová, R.; Rolinec, M. Hematological profiles of new-born piglets and sows fed with diet containing grape pomace. J. Central Eur. Agric. 2022, 23, 274–282. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Methods of Analysis AOAC, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Vašeková, P.; Juráček, M.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Kolláthová, R.; Ivanišová, E. Bioactive compounds and fatty acid profile of grape pomace. Acta Fytotech. Zootech. 2020, 23, 230–235. [Google Scholar] [CrossRef]
- Heath, M.F.; Evans, R.J.; Gresham, A.C.J. Blood biochemical reference ranges for sows under modern management conditions. Br. Veter.-J. 1991, 147, 331–333. [Google Scholar] [CrossRef]
- Friendship, R.; Lumsden, J.H.; Mcmillan, I.; Wilson, M.R. Hematology and biochemistry reference values for Ontario swine. Can. J. Comparat. Med. 1984, 48, 390. [Google Scholar]
- Pietrzak, E.; Grela, E.R. The effects of adding lucerne protein concentrate to diets on the reproductive traits and blood metabolic profiles of sows and piglets. J. Anim. Feed Sci. 2015, 24, 216–225. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Geudeke, M.J.; Van Rossem, H.; Kroon, M.C.; Counotte, C.H.M. Haematology and biochemistry reference values for sows kept under modern management conditions. Veter.-Q. 1994, 16, 127–130. [Google Scholar] [CrossRef]
- Dupak, R.; Kovac, J.; Kalafova, A.; Kovacik, A.; Tokarova, K.; Hascik, P.; Simonova, N.; Kacaniova, M.; Mellen, M.; Capcarova, M. Supplementation of grape pomace in broiler chickens diets and its effect on body weight, lipid profile, antioxidant status and serum biochemistry. Biologia 2021, 76, 2511–2518. [Google Scholar] [CrossRef]
- Žvorc, Z.; Mrljak, V.; Sušić, V.; Pompe Gotal, J. Haematological and biochemical parameters during pregnancy and lactation in sows. Vet. Arhiv 2006, 76, 245–253. [Google Scholar]
- Perri, A.M.; O’Sullivan, T.L.; Harding, J.C.; Friendship, R.M. The use of serum beta-hydroxybutyrate to determine whether nursery pigs selected on the basis of clinical signs are anorexic. Can. Vet. J. 2016, 57, 1143. [Google Scholar]
- Flis, M.; Sobotka, W.; Antoszkiewicz, Z.; Lipiński, K.; Zduńczyk, Z. The effect of grain polyphenols and the addition of vitamin E to diets enriched with α-linolenic acid on the antioxidant status of pigs. J. Anim. Feed. Sci. 2010, 19, 539–553. [Google Scholar] [CrossRef][Green Version]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red grape pomace rich in polyphenols diet increases the antioxidant status in key organs-kidneys, liver, and spleen of piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef]
- Kolláthová, R.; Galik, B.; Halo, M.; Kováčik, A.; Hanušovský, O.; Biro, D.; Rolince, M.; Juráček, M.; Šimko, M. The effects of dried grape pomace supplementation on biochemical blood serum indicators and digestibility of nutrients in horses. Czech J. Anim. Sci. 2020, 65, 58–65. [Google Scholar] [CrossRef]
- Segura, M.; Martínez-Miró, S.; López, M.J.; Madrid, J.; Hernández, F. Effect of parity on reproductive performance and composition of sow colostrum during first 24 h postpartum. Animals 2020, 10, 1853. [Google Scholar] [CrossRef]
- Foisnet, A.; Farmer, C.; David, C.; Quesnel, H. Relationships between colostrum production by primiparous sows and sow physiology around parturition. J. Anim. Sci. 2010, 88, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Rolinec, M.; Bíro, D.; Šťastný, P.; Gálik, B.; Šimko, M.; Juráček, M. Immunoglobulins in colostrum of sows with porcine reproductive and respiratory syndrome-PRRS. J. Cent. Eur. Agric. 2012, 13, 303–311. [Google Scholar] [CrossRef]
- Hurley, W.L. Composition of sow colostrum and milk. In The Gestating and Lactating Sow, 1st ed.; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 193–229. [Google Scholar]
- Kielland, C.; Rootwelt, V.; Reksen, O.; Framstad, T. The association between immunoglobulin G in sow colostrum and piglet plasma. J. Anim. Sci. 2015, 93, 4453–4462. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef]
- Sun, H.Y.; Kim, I.H. Coated omega-3 fatty acid from linseed oil positively affect sow immunoglobulin G concentration and pre-weaning performance of piglet. Anim. Feed. Sci. Technol. 2020, 269, 114676. [Google Scholar] [CrossRef]
| Control Group | Dried Grape Pomace Group | |
|---|---|---|
| Ingredients of diet (%) | ||
| Barley grain | 33.0 | 32.69 |
| Maize grain | 33.0 | 32.69 |
| Soybean meal | 15.8 | 15.6 |
| Wheat grain | 7.0 | 6.93 |
| Rapeseed meal | 3.0 | 2.97 |
| Vitamin mineral premix 1 | 3.0 | 2.97 |
| Sunflower oil | 2.6 | 2.57 |
| PKK energy 2 | 1.5 | 1.49 |
| Abrocel RC 3 | 1.0 | 0.99 |
| Neutox 4 | 0.1 | 0.1 |
| Dried grape pomace powder | 0 | 1.0 |
| Nutritional characteristics | ||
| Dry matter (g/kg) | 893 | 892 |
| Crude protein (g/kg) | 174 | 173 |
| Ether extract (g/kg) | 20.8 | 21.3 |
| Crude fiber (g/kg) | 46.7 | 48 |
| Ash (g/kg) | 56.2 | 56 |
| Nitrogen free extract (g/kg) | 595 | 594 |
| Starch (g/kg) | 408 | 404 |
| Total sugar (g/kg) | 41.3 | 42.6 |
| Non-fiber saccharides (g/kg) | 508 | 505 |
| Net energy (MJ/kg) | 9.29 | 9.37 |
| Ca (g/kg) | 8.78 | 8.73 |
| P (g/kg) | 6.24 | 6.21 |
| Mg (g/kg) | 2.59 | 2.58 |
| Na (g/kg) | 2.97 | 2.94 |
| K (g/kg) | 9.37 | 9.4 |
| Cu (mg/kg) | 25.9 | 25.7 |
| Fe (mg/kg) | 354 | 351 |
| Mn (mg/kg) | 84.7 | 84 |
| Threonine (g/kg) | 6.18 | 6.15 |
| Lysine (g/kg) | 9.37 | 9.31 |
| Cysteine (g/kg) | 2.01 | 1.99 |
| Methionine (g/kg) | 1.74 | 1.72 |
| Group | 7d a.p. | 1d p.p. | |
|---|---|---|---|
| ALT (U/L) | DGP | 35.2 ± 3.63 | 23.7 ± 3.56 |
| CON | 41.0 ± 3.35 | 31.3 ± 1.52 | |
| AST (U/L) | DGP | 25.0 ± 3.11 | 21.7 ± 2.12 a |
| CON | 19.9 ± 0.68 | 34.3 ± 5.07 b | |
| ALP (U/L) | DGP | 21.9 ± 2.83 | 26.9 ±3.87 |
| CON | 22.4 ± 1.84 | 22.1 ± 2.41 | |
| GGT (U/L) | DGP | 34.4 ± 1.49 | 42.7 ± 1.44 |
| CON | 38.4 ± 1.62 | 42.2 ± 1.96 | |
| CREAT (μmol/L) | DGP | 202.8 ± 8.0 | 172.8 ± 8.78 a |
| CON | 197.1 ± 3.95 | 197.4 ± 2.76 b | |
| DBIL (μmol/L) | DGP | 1.4 ± 0.09 | 0.7 ± 0.15 |
| CON | 1.1 ± 0.13 | 0.5 ± 0.06 | |
| TP (g/L) | DGP | 67.6 ± 1.81 | 73.0 ± 1.31 a |
| CON | 63.2 ± 1.74 | 64.4 ± 1.30 b | |
| ALB (g/L) | DGP | 40.2 ± 0.96 | 41.3 ± 1.28 |
| CON | 40.6 ± 1.19 | 41.3 ± 0.63 | |
| GLOB (g/L) | DGP | 27.4 ± 2.67 | 31.7 ± 2.30 a |
| CON | 22.6 ± 2.63 | 23.0 ± 1.69 b | |
| A/G ratio | DGP | 1.7 ± 0.19 | 1.4 ± 0.16 a |
| CON | 2.1 ± 0.24 | 1.9 ± 0.17 b | |
| UREA (mmol/L) | DGP | 8.0 ± 0.25 | 9.5 ± 0.58 |
| CON | 7.9 ± 0.20 | 10.7 ± 0.52 | |
| CHOL (mmol/L) | DGP | 1.5 ± 0.04 | 1.7 ± 0.07 |
| CON | 1.6 ± 0.03 | 1.7 ± 0.06 | |
| TRIG (mmol/L) | DGP | 0.8 ± 0.05 | 0.5 ± 0.10 |
| CON | 0.7 ± 0.05 | 0.3 ± 0.04 | |
| GLUC (mmol/L) | DGP | 4.4 ± 0.20 | 4.1 ± 0.17 |
| CON | 4.1 ± 0.10 | 4.1 ± 0.06 | |
| Ca (mmol/L) | DGP | 2.5 ± 0.07 a | 2.7 ± 0.08 |
| CON | 2.6 ± 0.05 b | 2.5 ± 0.03 | |
| Mg (mmol/L) | DGP | 1.1 ± 0.02 | 1.0 ± 0.04 |
| CON | 1.1 ± 0.02 | 1.0 ± 0.02 | |
| P (mmol/L) | DGP | 1.9 ± 0.07 | 2.3 ± 0.06 |
| CON | 2.0 ± 0.05 | 2.4 ± 0.06 | |
| BHB (mmol/L) | DGP | 0.009 ± 0.002 | 0.008 ± 0.002 |
| CON | 0.008 ± 0.001 | 0.005 ± 0.002 | |
| TAS (mmol/L) | DGP | 0.646 ± 0.015 | 0.684 ± 0.022 |
| CON | 0.609 ± 0.024 | 0.575 ± 0.016 | |
| IgG (mg/mL) | DGP | 11.1 ± 0.76 | 10.2 ± 0.35 |
| CON | 10.3 ± 0.21 | 12.3 ± 2.13 | |
| IgA (mg/mL) | DGP | 1.0 ± 0.24 | 1.2 ± 0.20 |
| CON | 1.3 ± 0.24 | 1.7 ± 0.38 | |
| IgM (mg/mL) | DGP | 5.6 ± 0.64 | 6.0 ± 0.68 |
| CON | 7.0 ± 1.22 | 6.8 ± 1.47 |
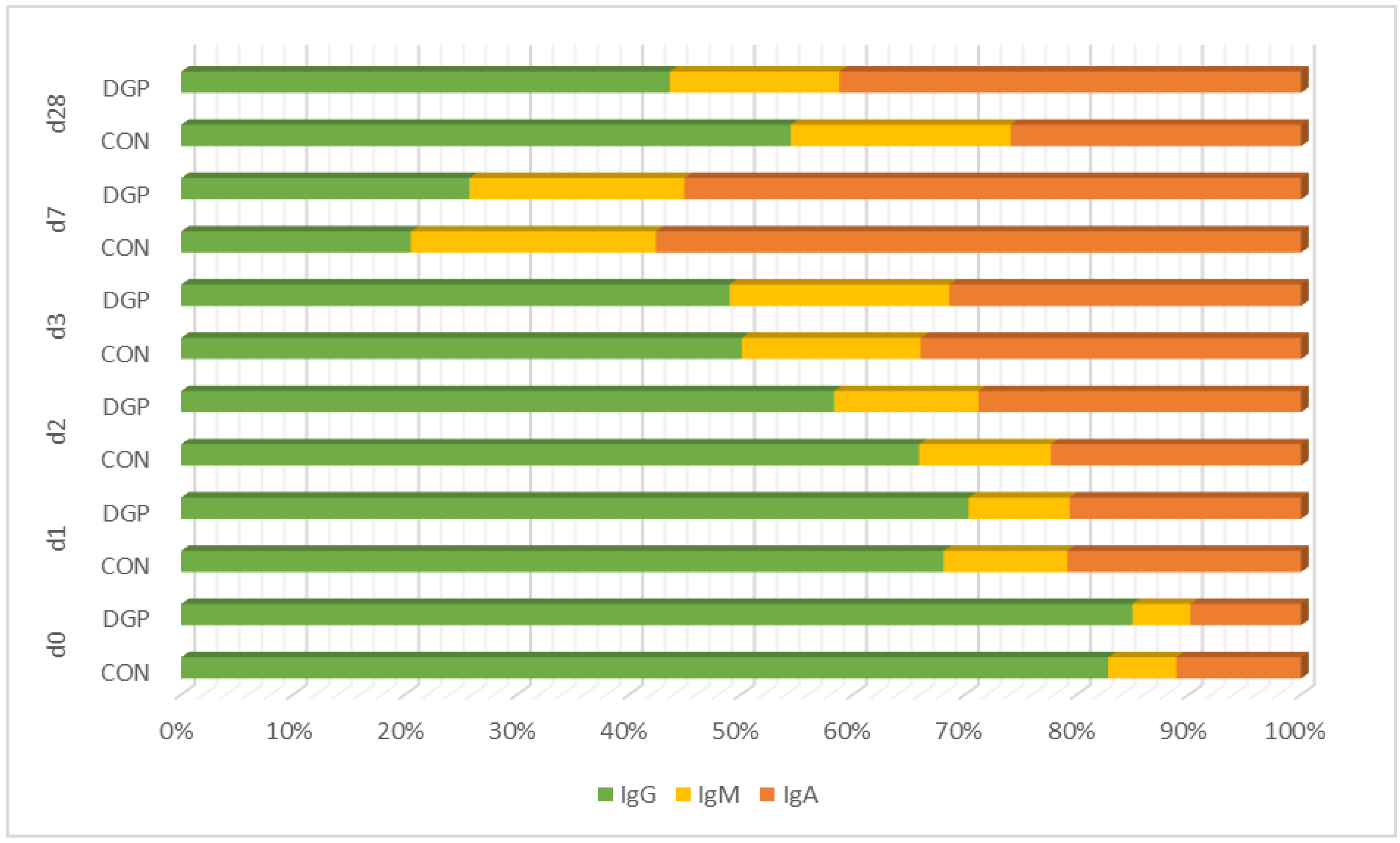
| Group | d0 | d1 | d2 | d3 | d7 | d28 | |
|---|---|---|---|---|---|---|---|
| IgA (mg/mL) | DGP | 12.9 ± 3.45 | 6.1 ± 1.42 | 7.0 ± 2.85 | 3.4 ± 0.59 | 4.0 ± 0.46 | 3.8 ± 1.09 |
| CON | 13.6 ± 2.83 | 5.6 ± 1.45 | 4.8 ± 1.90 | 3.7 ± 0.72 | 4.2 ± 0.97 | 1.8 ± 0.40 | |
| IgM (mg/mL) | DGP | 6.8 ± 1.00 | 2.6 ± 0.28 | 3.1 ±0.34 | 2.1 ± 0.49 | 1.4 ± 0.08 | 1.4 ± 0.19 |
| CON | 7.4 ± 0.54 | 3.0 ± 0.58 | 2.5 ± 0.29 | 1.7 ± 0.26 | 1.6 ± 0.25 | 1.4 ± 0.20 | |
| IgG (mg/mL) | DGP | 111.3 ± 7.2 | 20.7 ± 3.49 | 14.2 ± 4.99 | 5.3 ± 0.48 | 1.9 ± 0.19 | 4.0 ± 1.25 |
| CON | 100.7 ± 2.2 | 18.3 ± 5.00 | 14.2 ± 5.42 | 5.4 ± 0.54 | 1.5 ± 0.29 | 3.8 ± 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolinec, M.; Mixtajová, E.; Gálik, B.; Hanušovský, O.; Šimko, M.; Schubertová, Z.; Kováčik, A.; Vargová, R.; Madajová, V.; Juráček, M. Effects of Grape Pomace on Sow Blood, Colostrum and Milk Parameters. Agriculture 2025, 15, 1443. https://doi.org/10.3390/agriculture15131443
Rolinec M, Mixtajová E, Gálik B, Hanušovský O, Šimko M, Schubertová Z, Kováčik A, Vargová R, Madajová V, Juráček M. Effects of Grape Pomace on Sow Blood, Colostrum and Milk Parameters. Agriculture. 2025; 15(13):1443. https://doi.org/10.3390/agriculture15131443
Chicago/Turabian StyleRolinec, Michal, Eva Mixtajová, Branislav Gálik, Ondrej Hanušovský, Milan Šimko, Zuzana Schubertová, Anton Kováčik, Renata Vargová, Viera Madajová, and Miroslav Juráček. 2025. "Effects of Grape Pomace on Sow Blood, Colostrum and Milk Parameters" Agriculture 15, no. 13: 1443. https://doi.org/10.3390/agriculture15131443
APA StyleRolinec, M., Mixtajová, E., Gálik, B., Hanušovský, O., Šimko, M., Schubertová, Z., Kováčik, A., Vargová, R., Madajová, V., & Juráček, M. (2025). Effects of Grape Pomace on Sow Blood, Colostrum and Milk Parameters. Agriculture, 15(13), 1443. https://doi.org/10.3390/agriculture15131443








