Bioactive Compounds, Ruminal Fermentation, and Anthelmintic Activity of Specialty Coffee and Spent Coffee Grounds In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Twenty-Four-Hour In Vitro Batch-Culture Experiment
2.2.1. Experimental Design
2.2.2. Inoculum and Substrates
2.2.3. Measurements
2.3. Chemical Analyses
2.3.1. Nutritional Analysis
2.3.2. Analysis of Polyphenols
2.3.3. Ultra-High-Resolution Mass Spectrometry (UHRMS)
2.4. Anthelmintic Activity of Coffee Extract
2.4.1. Aqueous Coffee Extract
2.4.2. Larval Development Test (LDT)
2.5. Statistical Analysis
3. Results
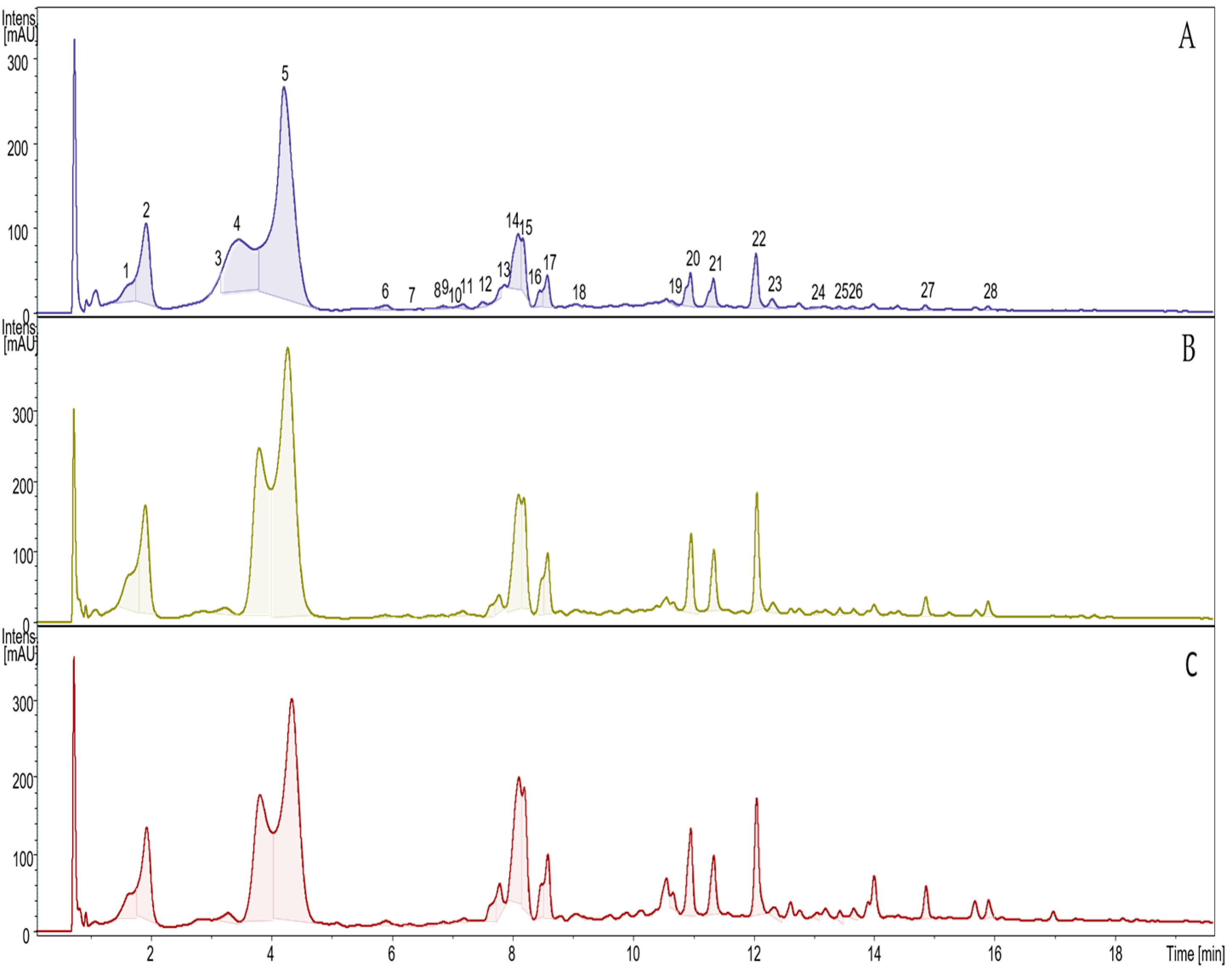
3.1. Bioactive Compounds
3.2. Ruminal Fermentation
3.3. LDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCG | Spent Coffee Ground |
| ETH | Ethiopian Specialty Coffee |
| SCG-ETH | SCGs from Filtered Ethiopian Specialty Coffee |
| SCG-MIX | SCGs from Blended Filtered Specialty Coffee |
| DM | Dry Matter |
| MH | Meadow Hay |
| BG | Barley Grain |
| IVGPT | In Vitro Gas Production Technique |
| IVDMD | In Vitro Dry Matter Digestibility |
| NDF | Neutral Detergent Fiber |
| ADF | Acid Detergent Fiber |
| CP | Crude Protein |
| GIN | Gastrointestinal Nematode |
| LDT | Larval Development Test |
| CGA | Chlorogenic Acid |
| UV | Ultraviolet |
| RT | Retention Time |
References
- Vegro, C.L.R.; de Almeida, L.F. Global coffee market: Socio-economic and cultural dynamics. In Consumer Sci & Strat Market, Coffee Consumption and Industry Strategies in Brazil; de Almeida, L.F., Spers, E.E., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–19. [Google Scholar]
- Várady, M.; Tauchen, J.; Fraňková, A.; Klouček, P.; Popelka, P. Effect of method of processing specialty coffee beans (natural, washed, honey, fermentation, maceration) on bioactive and volatile compounds. LWT 2022, 172, 114245. [Google Scholar] [CrossRef]
- Zarebska, M.; Stanek, N.; Barabosz, K.; Jaszkiewicz, A.; Kulesza, R.; Matejuk, R.; Andrzejewski, D.; Biłos, Ł.; Porada, A. Comparison of chemical compounds and their influence on the taste of coffee depending on green beans storage conditions. Sci. Rep. 2022, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Bolka, M.; Emire, S. Effects of coffee roasting technologies on cup quality and bioactive compounds of specialty coffee beans. Food Sci. Nutr. 2020, 8, 6120–6130. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 8, 23–36. [Google Scholar] [CrossRef]
- Várady, M.; Slusarczyk, S.; Boržíková, J.; Hanková, K.; Vieriková, M.; Marcinčák, S.; Popelka, P. Heavy-metal contents and the impact of roasting on polyphenols, caffeine, and acrylamide in specialty coffee beans. Foods 2021, 10, 1310. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The effect of brewing process parameters on antioxidant activity and caffeine content in infusions of roasted and unroasted arabica coffee beans originated from different countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- Saeli, M.; Capela, M.N.; Piccirillo, C.; Tobaldi, D.M.; Seabra, M.P.; Scalera, F.; Striani, R.; Corcione, C.E.; Campisi, T. Development of energy-saving innovative hydraulic mortars reusing spent coffee ground for applications in construction. J. Clean. Prod. 2023, 399, 136664. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Alzate, C.A.C. Chapter 3-The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 63–92. [Google Scholar] [CrossRef]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso coffee residues: A valuable source of unextracted compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Rim, J.S.; Na, Y.; Lee, S.R. Effects of dietary fermented spent coffee ground on nutrient digestibility and nitrogen utilization in sheep. Asian Australas. J. Anim. Sci. 2018, 31, 363–368. [Google Scholar] [CrossRef]
- Carta, S.; Tsiplakou, E.; Nicolussi, P.; Pulina, G.; Nudda, A. Effects of spent coffee grounds on production traits, haematological parameters, and antioxidant activity of blood and milk in dairy goats. Animal 2022, 16, 100501. [Google Scholar] [CrossRef] [PubMed]
- San Martin, D.; Ibarruri, J.; Luengo, N.; Ferrer, J.; García-Rodríguez, A.; Goiri, I.; Atxaerandio, R.; Medjadbi, M.; Zufía, J.; Sáez de Cámara, E.; et al. Evaluation of Valorisation Strategies to Improve Spent Coffee Grounds’ Nutritional Value as an Ingredient for Ruminants’ Diets. Animals 2023, 13, 1477. [Google Scholar] [CrossRef]
- Hoste, H.; Meza-OCampos, G.; Marchand, S.; Sotiraki, S.; Sarasti, K.; Blomstrand, B.M.; Williams, A.R.; Thamsborg, S.M.; Athanasiadou, S.; Enemark, H.L.; et al. Use of agro-industrial by-products containing tannins for the integrated control of gastrointestinal nematodes in ruminants. Parasite 2022, 29, 10. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.J.; Smith, L.A.; Houdijk, J.G.M.; Athanasiadou, S.; Hutchings, M.R. Ubiquitous parasites drive a 33% increase in methane yield from livestock. Int. J. Parasitol. 2018, 48, 1017–1021. [Google Scholar] [CrossRef]
- European Commission (EC). Council Regulation (EC) 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32009R1099 (accessed on 18 November 2009).
- McDougall, E.I. Studies on ruminant saliva. I. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- Váradyová, Z.; Baran, M.; Zeleňák, I. Comparison of two in vitro fermentation gas production methods using both rumen fluid and faecal inoculum from sheep. Anim. Feed. Sci. Technol. 2005, 123–124, 81–94. [Google Scholar] [CrossRef]
- Mellenberger, R.W.; Satter, L.D.; Millett, M.A.; Baker, A.J. An in vitro technique for estimating digestibility of treated and untreated wood. J. Anim. Sci. 1970, 30, 1005–1011. [Google Scholar] [CrossRef][Green Version]
- Petrič, D.; Mravčáková, D.; Kucková, K.; Čobanová, K.; Kišidayová, S.; Cieslak, A.; Ślusarczyk, S.; Váradyová, Z. Effect of dry medicinal plants (wormwood, chamomile, fumitory and mallow) on in vitro ruminal antioxidant capacity and fermentation patterns of sheep. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Williams, A.G.; Coleman, G.S. The Rumen Protozoa, 1st ed.; Springer: New York, NY, USA, 1992; pp. 1–425. [Google Scholar]
- Horwitz, W. Official Methods of AOAC International, 17th ed.; Association of Official Analytical Chemists (AOAC) International: Gaithersburg, MD, USA; Washington, DC, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Roos, M.H.; Otsen, M.; Hoekstra, R.; Veenstra, J.G.; Lenstra, J.A. Genetic analysis of inbreeding of two strains of the parasitic nematode Haemonchus contortus. Int. J. Parasitol. 2004, 34, 109–115. [Google Scholar] [CrossRef]
- Várady, M.; Bjorn, H.; Nansen, P. In vitro characterization of anthelmintic susceptibility of field isolates of the pig nodular worm Oesophagostomum spp., susceptible or resistant to various anthelmintics. Int. J. Parasitol. 1996, 26, 733–740. [Google Scholar] [CrossRef]
- Hubert, J.; Kerboeuf, D. A new method for culture of larvae used in diagnosis of ruminant gastrointestinal strongylosis: Comparison with fecal cultures. Can. J. Comp. Med. 1984, 48, 63–71. [Google Scholar]
- Dobson, R.J.; Griffiths, D.A.; Donald, A.D.; Waller, P.J. A genetic model describing the evolution of levamisole resistance in Trichostrongylus colubriformis, a nematode parasite of sheep. IMA J. Math. Appl. Med. Biol. 1987, 4, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Oracz, J. Correlation between the stability of chlorogenic acids, antioxidant activity and acrylamide content in coffee beans roasted in different conditions. Int. J. Food Prop. 2015, 18, 290–302. [Google Scholar] [CrossRef]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Chang. 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Bouhzam, I.; Cantero, R.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana-I-Palmer, P.; Puig, R. Extraction of bioactive compounds from spent coffee grounds using ethanol and acetone aqueous solutions. Foods 2023, 12, 4400. [Google Scholar] [CrossRef]
- Shang, Y.F.; Xu, J.L.; Lee, W.J.; Um, B.H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. South Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive compounds and antioxidant activity from spent coffee grounds as a powerful approach for its valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Maiyah, N.; Kerdpiboon, S.; Supapvanich, S.; Kerr, W.L.; Sriprom, P.; Chotigavin, N.; Klaypradit, W.; Puttongsiri, T. Recovering bioactive compounds and antioxidant capacity of medium roasted spent coffee grounds through varied hydrothermal brewing cycles. J. Agric. Food Res. 2025, 20, 101789. [Google Scholar] [CrossRef]
- Várady, M.; Grajzer, M.; Zalewski, I.; Tauchen, J.; Fraňková, A.; Klouček, P.; Popelka, P. Fatty acid composition and sensory properties as descriptors of differentiation of specialty coffees based on spontaneous and induced processing methods. Appl. Food Res. 2024, 4, 100512. [Google Scholar] [CrossRef]
- Wale, K.; Tolessa, K.; Atlabachew, M.; Mehari, B.; Alemayehu, M.; Mengistu, D.A.; Kerisew, B. Level of caffeine, trigonelline and chlorogenic acids in green coffee (Coffea arabica L.) beans from Amhara region, Ethiopia. J. Agric. Food Res. 2024, 16, 101082. [Google Scholar] [CrossRef]
- Várady, M.; Tauchen, J.; Klouček, P.; Popelka, P. Effects of total dissolved solids, extraction yield, grinding, and method of preparation on antioxidant activity in fermented specialty coffee. Fermentation 2022, 8, 375. [Google Scholar] [CrossRef]
- Yust, B.G.; Rao, N.Z.; Schwarzmann, E.T.; Peoples, M.H. Quantification of spent coffee ground extracts by roast and brew method, and their utility in a green synthesis of gold and silver nanoparticles. Molecules 2022, 27, 5124. [Google Scholar] [CrossRef]
- Belviso, S.; Ghirardello, D.; Rantsiou, K.; Giordano, M.; Bertolino, M.; Borgogna, D.; Cavallero, M.C.; Dal Bello, B.; Cena, C.; Rolle, L.; et al. Phytochemical and microbiological stability of spent espresso coffee grounds in capsules. Food Res. Int. 2014, 61, 93–99. [Google Scholar] [CrossRef]
- Brzezińska, R.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E. Spent Coffee Grounds—A Coffee By-Product Abundant in Bioactive Compounds with Antioxidant Properties. Biol. Life Sci. Forum 2023, 26, 106. [Google Scholar] [CrossRef]
- Dang, C.H.; Nguyen, T.D. Physicochemical characterization of Robusta spent coffee ground oil for biodiesel manufacturing. Waste Biomass Valori. 2019, 10, 2703–2712. [Google Scholar] [CrossRef]
- Ibrahim, T.A.; Hassen, A.; Apostolides, Z. The Antimethanogenic Potentials of Plant Extracts: Their Yields and Phytochemical Compositions as Affected by Extractive Solvents. Plants 2022, 11, 3296. [Google Scholar] [CrossRef] [PubMed]
- Batbekh, B.; Ahmed, E.; Hanada, M.; Fukuma, N.; Nishida, T. Assessment of the impact of coffee waste as an alternative feed supplementation on rumen fermentation and methane emissions in an in vitro study. Fermentation 2023, 9, 858. [Google Scholar] [CrossRef]
- Petrič, D.; Komáromyová, M.; Batťányi, D.; Kozłowska, M.; Filipiak, W.; Łukomska, A.; Ślusarczyk, S.; Szumacher-Strabel, M.; Cieślak, A.; Várady, M.; et al. Effect of Sainfoin (Onobrychis viciifolia) Pellets on Rumen Microbiome and Histopathology in Lambs Exposed to Gastrointestinal Nematodes. Agriculture 2022, 12, 301. [Google Scholar] [CrossRef]
- Mikulová, K.; Petrič, D.; Komáromyová, M.; Batťányi, D.; Kozłowska, M.; Cieslak, A.; Ślusarczyk, S.; Várady, M.; Váradyová, Z. Growth Performance and Ruminal Fermentation in Lambs with Endoparasites and In Vitro Effect of Medicinal Plants. Agriculture 2023, 13, 1826. [Google Scholar] [CrossRef]
- Newbold, C.J.; López, S.; Nelson, N.; Ouda, J.O.; Wallace, R.J.; Moss, A.R. Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. Br. J. Nutr. 2005, 94, 27–35. [Google Scholar] [CrossRef]
- Finlay, B.J.; Esteban, G.; Clarke, K.J.; Williams, A.G.; Embley, T.M.; Hirt, R.P. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol. Lett. 1994, 117, 157–161. [Google Scholar] [CrossRef]
- Newbold, C.J.; de la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Ozutsumi, Y.; Tajima, K.; Takenaka, A.; Itabashi, H. The effect of protozoa on the composition of rumen bacteria in cattle using 16S rRNA gene clone libraries. Biosci. Biotechnol. Biochem. 2005, 69, 499–506. [Google Scholar] [CrossRef]
- Goiri, I.; Díaz de Otálora, X.; Ruiz, R.; Rey, J.; Atxaerandio, R.; Lavín, J.L.; San Martin, D.; Orive, M.; Iñarra, B.; Zufia, J.; et al. Spent coffee grounds alter bacterial communities in Latxa dairy ewes. Microorganisms 2020, 8, 1961. [Google Scholar] [CrossRef]
- Medjadbi, M.; Garcia-Rodriguez, A.; Atxaerandio, R.; Charef, S.E.; Picault, C.; Ibarruri, J.; Iñarra, B.; San Martin, D.; Serrano-Pérez, B.; Martin-Alonso, M.J.; et al. Dose-dependent effect of spent coffee grounds on intake, apparent digestibility, fermentation pattern, methane emissions, microbial protein supply, and antioxidant status in Latxa sheep. J. Anim. Sci. 2024, 102, skae351. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, N.D.; Okamoto, T.; Takahashi, J.; Umetsu, K.; Nishida, T. Effect of mixed microbial culture on fermentation of beverage residues and the effect of the fermented beverage residues on in vitro rumen fermentation and methane production. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 349–353. [Google Scholar] [CrossRef]
- Batali, M.E.; Ristenpart, W.D.; Guinard, J.X. Brew temperature, at fixed brew strength and extraction, has little impact on the sensory profile of drip brew coffee. Sci. Rep. 2020, 10, 16450. [Google Scholar] [CrossRef] [PubMed]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef]
- Seo, J.; Jung, J.K.; Seo, S. Evaluation of nutritional and economic feed values of spent coffee grounds and Artemisia princeps residues as a ruminant feed using in vitro ruminal fermentation. PeerJ 2015, 3, e1343. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. from plantation to cup: Changes in bioactive compounds during coffee processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef]
- Váradyová, Z.; Pisarčíková, J.; Babják, M.; Hodges, A.; Mravčáková, D.; Kišidayová, S.; Königová, A.; Vadlejch, J.; Várady, M. Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus contortus. Exp. Parasitol. 2018, 195, 71–77. [Google Scholar] [CrossRef]
- Mravčáková, D.; Váradyová, Z.; Kopčáková, A.; Čobanová, K.; Grešáková, Ľ.; Kišidayová, S.; Babják, M.; Dolinská, M.U.; Dvorožňáková, E.; Königová, A.; et al. Natural chemotherapeutic alternatives for controlling of haemonchosis in sheep. BMC Vet. Res. 2019, 15, 302. [Google Scholar] [CrossRef]
- Ahmed, M.; Laing, M.D.; Nsahlai, I.V. In vitro anthelmintic activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J. Helminthol. 2013, 87, 174–179. [Google Scholar] [CrossRef]
- López-Rodríguez, G.; Zaragoza-Bastida, A.; Reyes-Guerrero, D.E.; Olmedo-Juárez, A.; Valladares-Carranza, B.; Vega-Castillo, L.F.; Rivero-Perez, N. Coffee pulp: A natural alternative for control of resistant nematodes in small ruminants. Pathogens 2023, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Rampurawala, J.; Vedamurthy, A.B.; Joy Hoskeri, H.A. Report on a potent anthelmintic agent-unbaked Coffee Arabica bean extracts. Asian J. Pharm. Clin. Res. 2013, 6, 119–121. [Google Scholar]
- Vargas-Magaña, J.J.; Torres-Acosta, J.F.; Aguilar-Caballero, A.J.; Sandoval-Castro, C.A.; Hoste, H.; Chan-Pérez, J.A. Anthelmintic activity of acetone-water extracts against Haemonchus contortus eggs: Interactions between tannins and other plant secondary compounds. Vet. Parasitol. 2014, 206, 322–327. [Google Scholar] [CrossRef]
- Borges, D.G.L.; Echeverria, J.T.; de Oliveira, T.L.; Heckler, R.P.; de Freitas, M.G.; Damasceno-Junior, G.A.; Carollo, C.A.; Borges, F.A. Discovery of potential ovicidal natural products using metabolomics. PLoS ONE 2019, 14, e0211237. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Jasso Díaz, G.; Hernández, G.T.; Zamilpa, A.; Becerril Pérez, C.M.; Ramírez Bribiesca, J.E.; Hernández Mendo, O.; Sánchez Arroyo, H.; González Cortazar, M.; Mendoza de Gives, P. In vitro assessment of Argemone mexicana, Taraxacum officinale, Ruta chalepensis and Tagetes filifolia against Haemonchus contortus nematode eggs and infective (L3) larvae. Microb. Pathog. 2017, 109, 162–168. [Google Scholar] [CrossRef]

| Substrate | DM 1 | NDF 2 | ADF 3 | CP 4 | N 5 | Ash |
|---|---|---|---|---|---|---|
| (g/kg) | (g/kg DM) | |||||
| BG 6 | 895 | 169 | 75 | 118 | 19.1 | 25.6 |
| MH 7 | 900 | 452 | 287 | 124 | 19.9 | 96.2 |
| ETH 8 | 962 | 463 | 291 | 114 | 18.2 | 42.5 |
| SCG-ETH 9 | 878 | 602 | 374 | 105 | 16.8 | 8.21 |
| SCG-MIX 10 | 908 | 636 | 405 | 109 | 17.5 | 11.3 |
| No. | RT | UV | m/z [M-H] | Formula | MS2 Main ion | MS2 Fragments | Compound | ETH | SCG-ETH | SCG-MIX | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1,80 | 215,325 | 353.0884 | C16H18O9 | 191.0566 | 179,135,161 | cis 3-O-CQA 1 | 0.47 ± 0.01 a | 1.69 ± 0.11 c | 0.87 ± 0.01 b | <0.001 |
| 2 | 1,91 | 277,325 | 353.0875 | C16H18O9 | 191.0566 | 179,135,161 | trans 3-O-CQA 2 | 1.71 ± 0.31 a | 2.66 ± 0.21 b | 2.45 ± 0.41 ab | 0.025 |
| 3 | 3,23 | 215,325 | 353.0878 | C16H18O9 | 179.0548 | 173,191,135,161 | trans 4-O-CQA 3 | 0.01 ± 0.00 a | 0.20 ± 0.01 b | 0.26 ± 0.01 c | <0.001 |
| 4 | 3,80 | 215,325 | 353.0887 | C16H18O9 | 191.0558 | 179,173,135 | cis 5-O-CQA 4 | 3.60 ± 0.71 a | 7.35 ± 1.02 b | 5.44 ± 0.52 ab | 0.003 |
| 5 | 4,27 | 215,325 | 353.0884 | C16H18O9 | 191.0564 | 173,179,135 | trans 5-O-CQA 5 | 9.04 ± 1.05 | 13.1 ± 4.01 | 10.5 ± 3.01 | 0.306 |
| 6 | 5,89 | 215,325 | 335.0766 | C16H16O9 | 161.0244 | 179 | cis 4-O-FQA 6 | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.00 | 0.064 |
| 7 | 6,26 | 215,325 | 353.0874 | C16H18O9 | 191.0563 | 179,173 | cis 4-O-CQA 7 | 0.00 ± 0.00 a | 0.08 ± 0.01 c | 0.03 ± 0.00 b | <0.001 |
| 8 | 6,63 | 215,325 | 337.0923 | C16H18O8 | 173.0457 | 163 | 5-O-pCoCQA 8 | 0.00 ± 0.00 a | 0.04 ± 0.01 b | 0.06 ± 0.00 c | <0.001 |
| 9 | 6,83 | 215,325 | 337.0924 | C16H18O8 | 191.0545 | 173,163 | 4-O-pCoCQA 9 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.422 |
| 10 | 7,06 | 215,325 | 337.0924 | C16H18O8 | 191.0545 | 173,163 | 3-O-pCoCQA 10 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.422 |
| 11 | 7,18 | 215,325 | 335.0768 | C16H16O9 | 161.0245 | 191 | 3-LCQA 11 | 0.04 ± 0.01 a | 0.08 ± 0.01 b | 0.06 ± 0.01 ab | 0.008 |
| 12 | 7,60 | 215,323 | 367.1037 | C17H20O9 | 173.0458 | 193 | 5-O-FQA 12 | 0.05 ± 0.00 a | 0.20 ± 0.02 b | 0.35 ± 0.02 c | <0.001 |
| 13 | 7,78 | 215,323 | 367.1041 | C17H20O9 | 191.0567 | 173,161,134 | 4-O-FQA 13 | 0.06 ± 0.00 a | 0.26 ± 0.21 ab | 0.45 ± 0.01 b | 0.022 |
| 14 | 8,10 | 215,325 | 335.0778 | C16H16O9 | 161.0251 | 179 | ep-3-LCQA 14 | 0.85 ± 0.11 a | 2.33 ± 0.32 b | 2.62 ± 0.71 b | 0.007 |
| 15 | 8,19 | 215,325 | 335.0774 | C16H16O9 | 161.024 | 179 | 3-LCQA 15 | 2.13 ± 0.23 | 1.68 ± 0.41 | 1.64 ± 0.02 | 0.124 |
| 16 | 8,51 | 215,325 | 335.0784 | C16H16O9 | 161.02 | 179 | ep-4-LCQA 16 | 0.76 ± 0.01 b | 0.41 ± 0.03 a | 0.45 ± 0.02 a | <0.001 |
| 17 | 8,58 | 215,325 | 335.0771 | C16H16O9 | 161.019 | 179 | 4-LCQA 17 | 0.34 ± 0.01 a | 0.81 ± 0.02 b | 0.88 ± 0.04 c | <0.001 |
| 18 | 9,30 | 215,325 | 515.1192 | C25H24O12 | 341.0655 | 191 | 3,4-diCQA 18 | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.06 ± 0.00 b | <0.001 |
| 19 | 10,67 | 215,325 | 349.0929 | C17H18O8 | 175.0403 | 160 | 4-LFQA 19 | 0.02 ± 0.01 a | 0.05 ± 0.00 b | 0.19 ± 0.00 c | <0.001 |
| 20 | 10,96 | 215,325 | 515.1199 | C25H24O12 | 353.0869 | 173,179,191,161,135 | 3,4-DiCQA 20 | 0.41 ± 0.01 a | 1.10 ± 0.03 b | 1.20 ± 0.47 b | 0.023 |
| 21 | 11,30 | 215,325 | 515.12 | C25H24O12 | 353.087 | 173,179,191,161,135 | 4,5-DiCQA 21 | 0.36 ± 0.03 a | 0.85 ± 0.04 b | 0.79 ± 0.00 b | <0.001 |
| 22 | 12,06 | 215,325 | 515.1201 | C25H24O12 | 353.0869 | 191,179,173,161,135 | 3,5-DiCQA 22 | 0.62 ± 0.02 a | 1.43 ± 0.08 b | 1.47 ± 0.04 b | <0.001 |
| 23 | 12,32 | 215,325 | 529.1339 | C26H26O13 | 193.0591 | 173,335,161,135 | 5-C3-FQA 23 | 0.10 ± 0.01 a | 0.15 ± 0.07 ab | 0.22 ± 0.03 b | 0.043 |
| 24 | 13,10 | 215,325 | 529.1346 | C26H26O12 | 193.0587 | 367,161,134 | 5-C4-FQA 24 | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.18 ± 0.02 b | <0.001 |
| 25 | 13,40 | 215,325 | 515.1201 | C25H24O12 | 353.0869 | 191,179,173,161,135 | DiCQA 25 | 0.02 ± 0.00 a | 0.07 ± 0.00 b | 0.15 ± 0.02 c | <0.001 |
| 26 | 13,70 | 215,325 | 529.1345 | C26H26O12 | 173.0434 | 367,193,155 | 4-C3FQA 26 | 0.02 ± 0.00 a | 0.05 ± 0.00 b | 0.12 ± 0.01 c | <0.001 |
| 27 | 14,9 | 215,325 | 497.1065 | C25H22O11 | 335.0768 | 161 | 3,5-diLCQA 27 | 0.05 ± 0.00 a | 0.22 ± 0.00 b | 0.37 ± 0.01 c | <0.001 |
| 28 | 15,9 | 215,325 | 497.1075 | C25H22O11 | 335.0757 | 179,161,135 | 5,4-diLCQA 28 | 0.04 ± 0.00 a | 0.17 ± 0.01 b | 0.23 ± 0.01 c | <0.001 |
| Total content: | 20.9 ± 0.09 a | 35.2 ± 0.24 c | 31.2 ± 0.19 b | <0.001 |
| MH-BG 1 | MH-SCG-ETH 2 | BG-SCG-ETH 3 | p | |
|---|---|---|---|---|
| Total gas (mL/g DM) | 289 ± 4.64 b | 266 ± 5.47 a | 269 ± 4.64 a | <0.001 |
| Methane (mmoL/L) | 5.12 ± 0.56 c | 2.98 ± 0.66 a | 3.80 ± 0.15 b | <0.001 |
| Ammonia N (mg/L) | 149 ± 34.6 | 159 ± 29.2 | 143 ± 34.3 | 0.620 |
| IVDMD (g/kg DM) 4 | 534 ± 1.98 a | 545 ± 2.98 b | 543 ± 2.37 b | <0.001 |
| pH | 6.77 ± 0.07 a | 6.68 ± 0.07 a | 6.88 ± 0.10 b | <0.001 |
| Total SCFA (mmoL/L) 5 | 44.6 ± 3.06 | 47.6 ± 6.19 | 47.5 ± 2.14 | 0.233 |
| Acetate (mol%) | 60.9 ± 2.03 | 60.4 ± 2.25 | 59.6 ± 2.18 | 0.425 |
| Propionate (mol%) | 16.8 ± 0.37 a | 18.3 ± 0.81 b | 16.8 ± 0.75 a | <0.001 |
| n-Butyrate (mol%) | 15.6 ± 1.22 b | 13.2 ± 1.32 a | 16.1 ± 1.61 b | 0.005 |
| iso-Butyrate (mol%) | 1.75 ± 0.208 a | 2.29 ± 0.180 b | 2.19 ± 0.291 b | <0.001 |
| n-Valerate (mol%) | 2.73 ± 0.698 | 2.98 ± 0.842 | 2.61 ± 1.420 | 0.742 |
| iso-Valerate (mol%) | 2.26 ± 0.380 | 2.83 ± 0.711 | 2.80 ± 0.720 | 0.115 |
| Acetate:propionate | 3.63 ± 0.171 b | 3.31 ± 0.202 a | 3.55 ± 0.181 b | 0.003 |
| Total protozoa (103/mL) | 108 ± 52.6 b | 66 ± 36.2 a | 61 ± 39.7 a | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leško, M.; Petrič, D.; Várady, M.; Sidoruk, P.; Mikula, R.; Ślusarczyk, S.; Hodurek, P.E.; Komáromyová, M.; Babják, M.; Várady, M.; et al. Bioactive Compounds, Ruminal Fermentation, and Anthelmintic Activity of Specialty Coffee and Spent Coffee Grounds In Vitro. Agriculture 2025, 15, 1515. https://doi.org/10.3390/agriculture15141515
Leško M, Petrič D, Várady M, Sidoruk P, Mikula R, Ślusarczyk S, Hodurek PE, Komáromyová M, Babják M, Várady M, et al. Bioactive Compounds, Ruminal Fermentation, and Anthelmintic Activity of Specialty Coffee and Spent Coffee Grounds In Vitro. Agriculture. 2025; 15(14):1515. https://doi.org/10.3390/agriculture15141515
Chicago/Turabian StyleLeško, Matej, Daniel Petrič, Matúš Várady, Pola Sidoruk, Robert Mikula, Sylwester Ślusarczyk, Paweł Edward Hodurek, Michaela Komáromyová, Michal Babják, Marián Várady, and et al. 2025. "Bioactive Compounds, Ruminal Fermentation, and Anthelmintic Activity of Specialty Coffee and Spent Coffee Grounds In Vitro" Agriculture 15, no. 14: 1515. https://doi.org/10.3390/agriculture15141515
APA StyleLeško, M., Petrič, D., Várady, M., Sidoruk, P., Mikula, R., Ślusarczyk, S., Hodurek, P. E., Komáromyová, M., Babják, M., Várady, M., Patra, A. K., Cieslak, A., & Váradyová, Z. (2025). Bioactive Compounds, Ruminal Fermentation, and Anthelmintic Activity of Specialty Coffee and Spent Coffee Grounds In Vitro. Agriculture, 15(14), 1515. https://doi.org/10.3390/agriculture15141515









