Establishment and Application of Loop-Mediated Isothermal Amplification Assays for Pathogens of Rice Bakanae Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Fungal DNA Extraction
2.3. LAMP Primers Design and Screening
2.4. LAMP Reaction and Product Detection
2.5. Preparation of Pathogen-Infected Rice Seeds
2.6. DNA Extraction from Rice Seeds
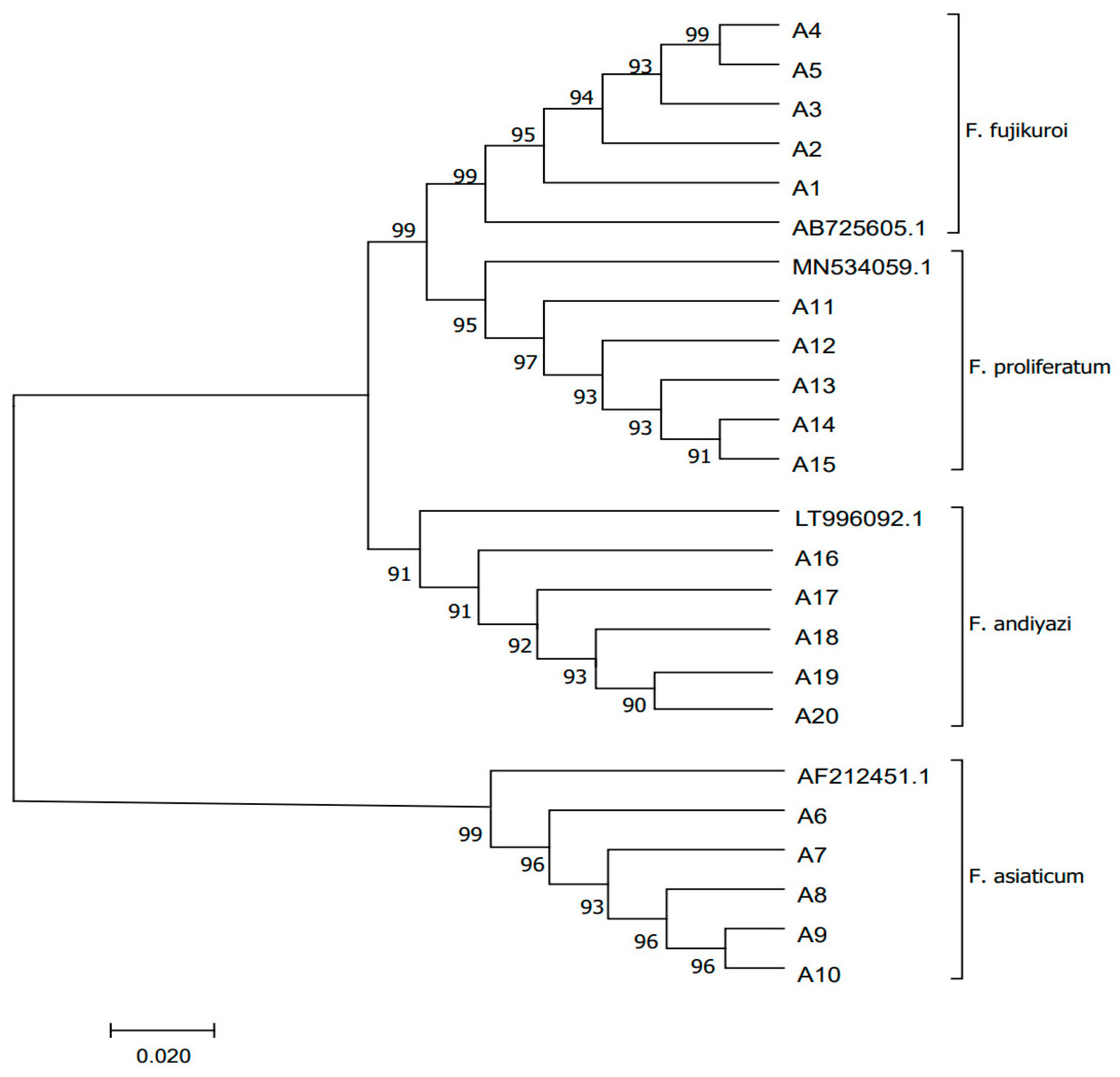
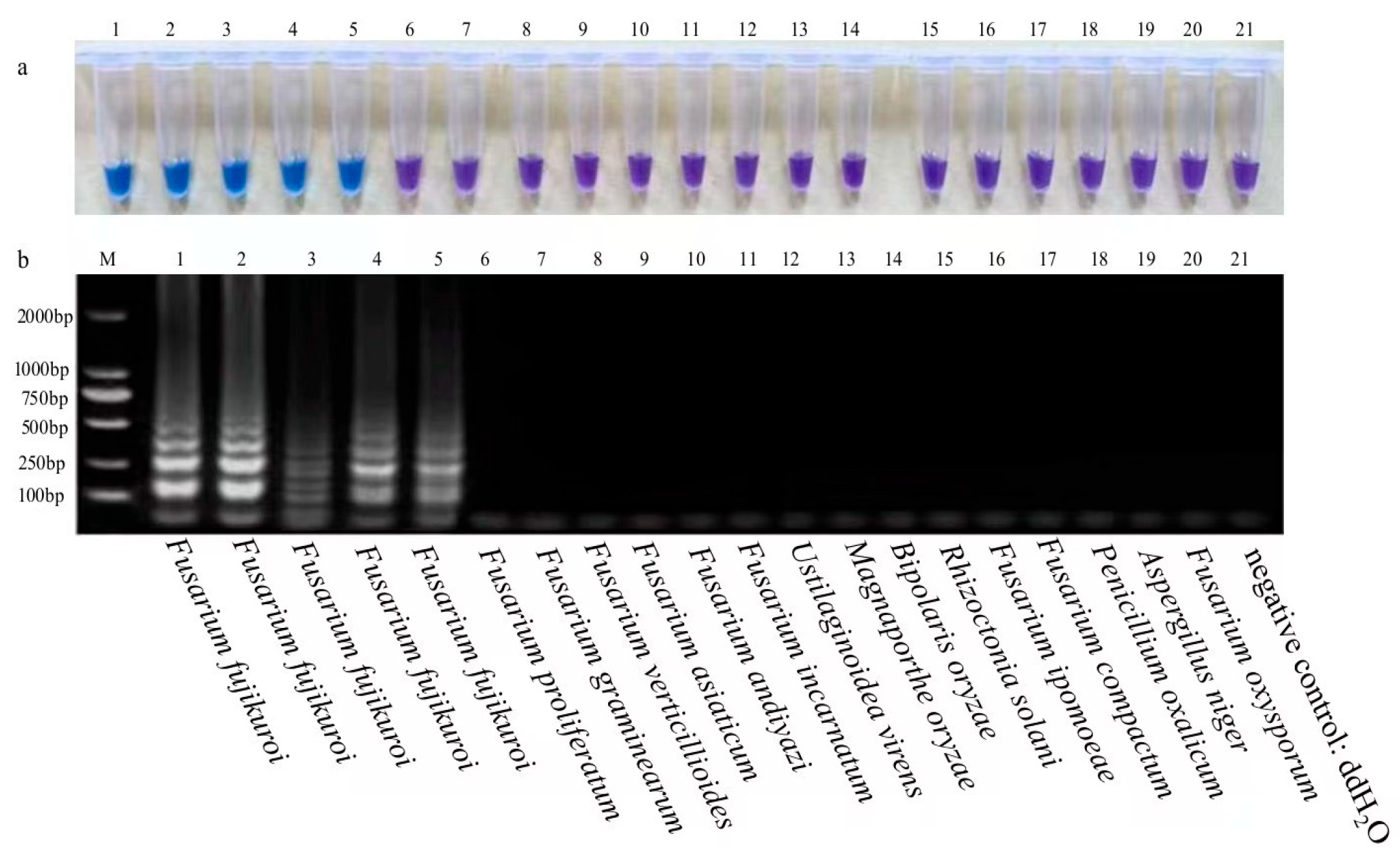
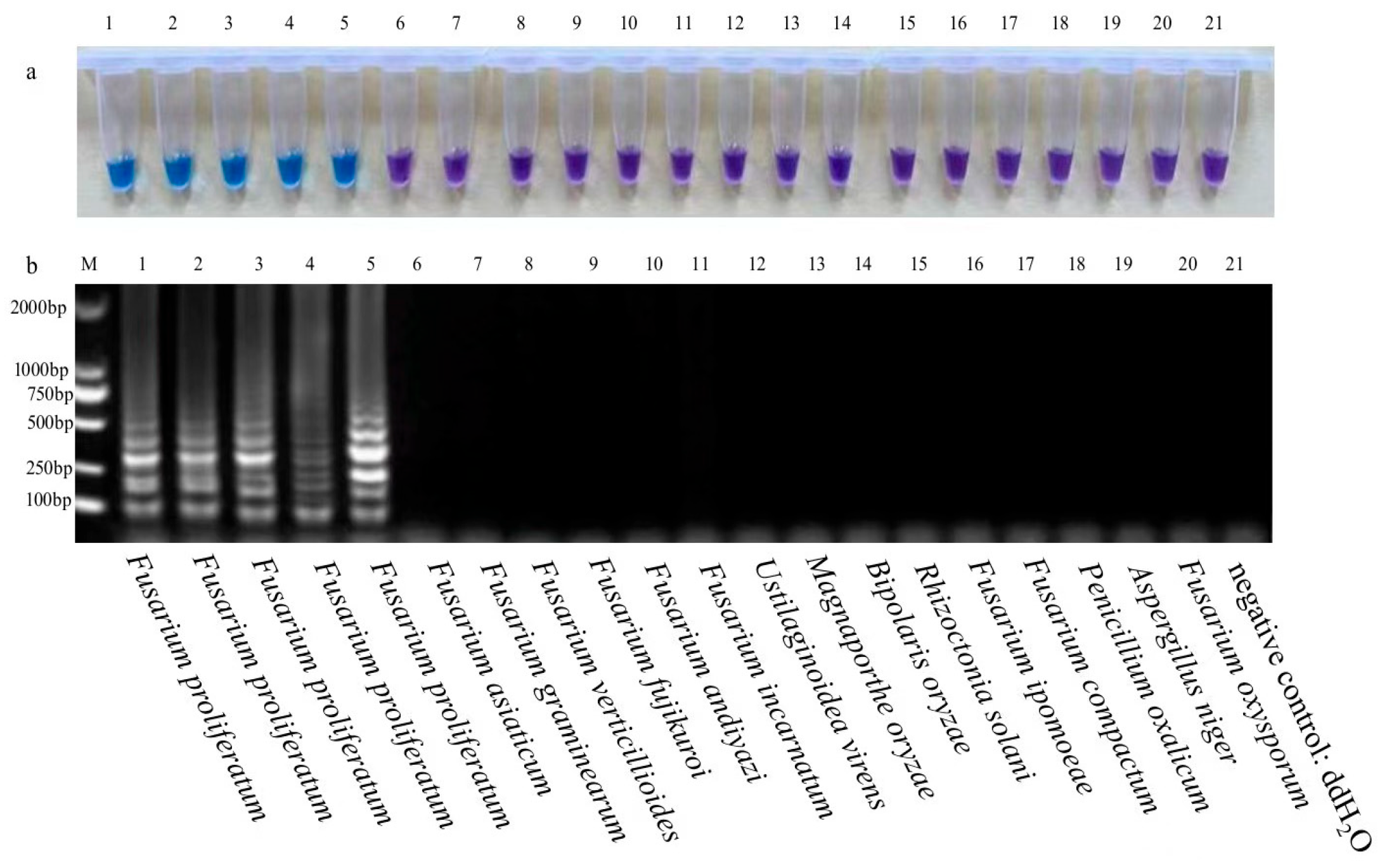
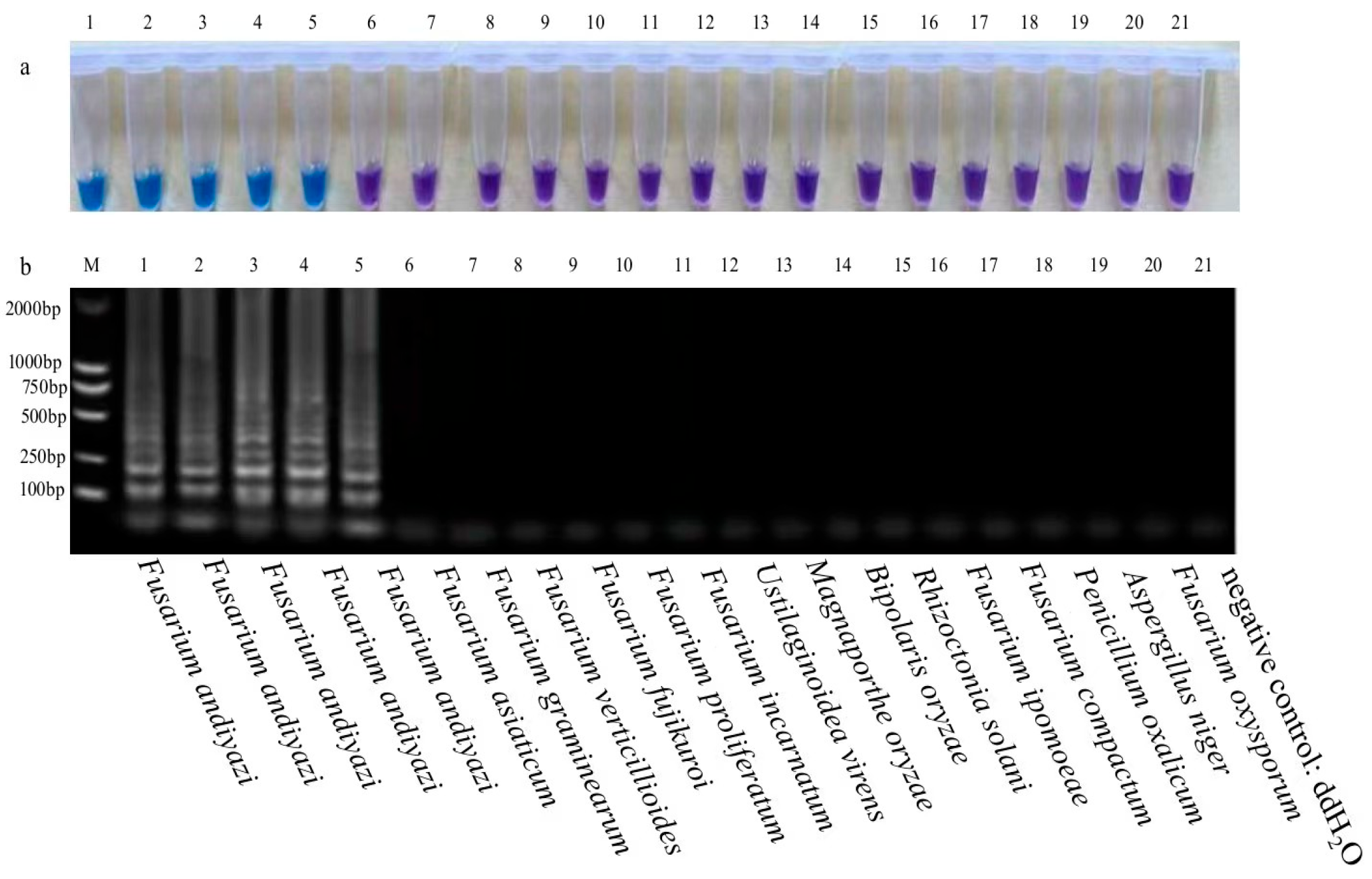
3. Results and Analysis
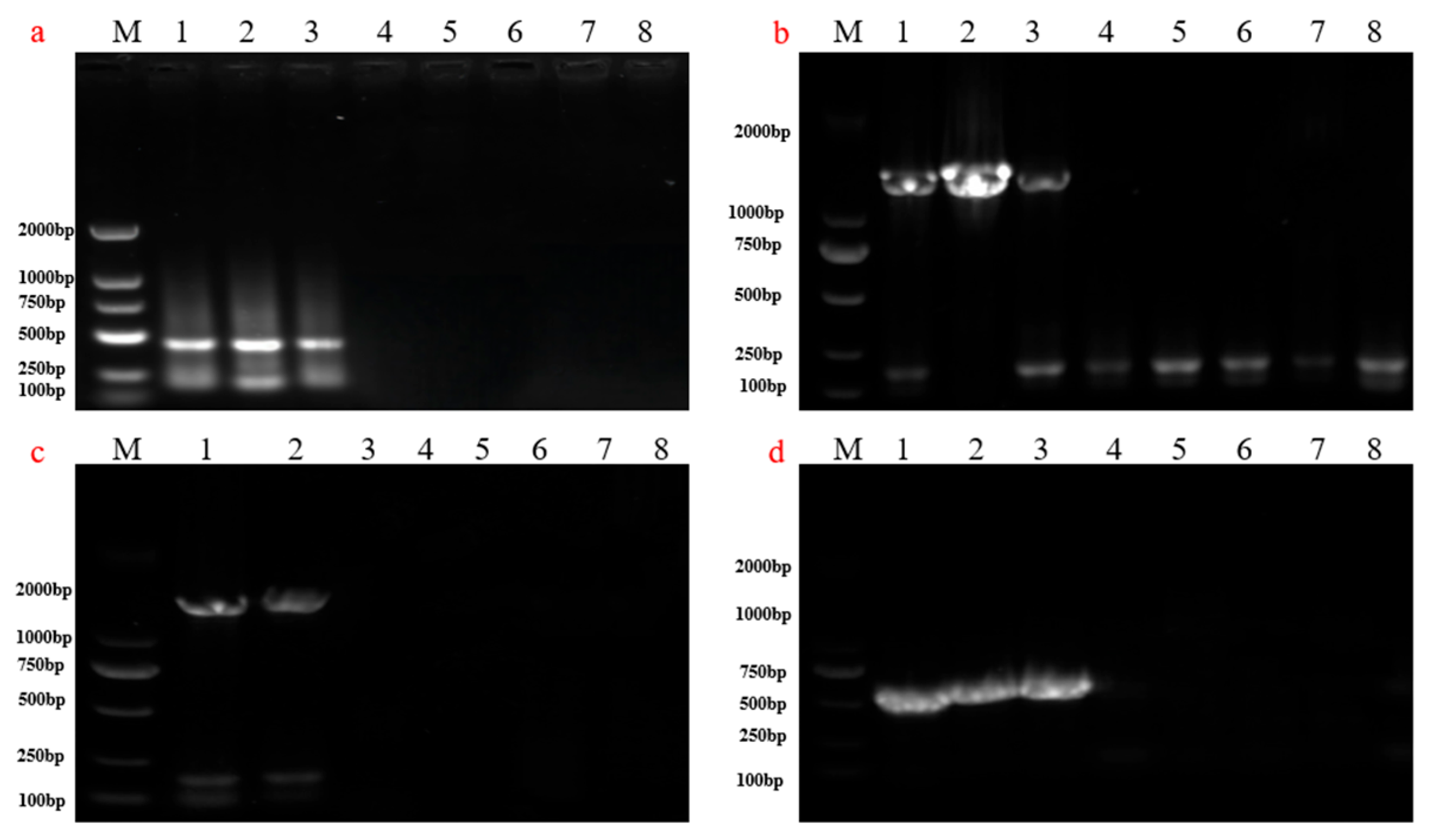
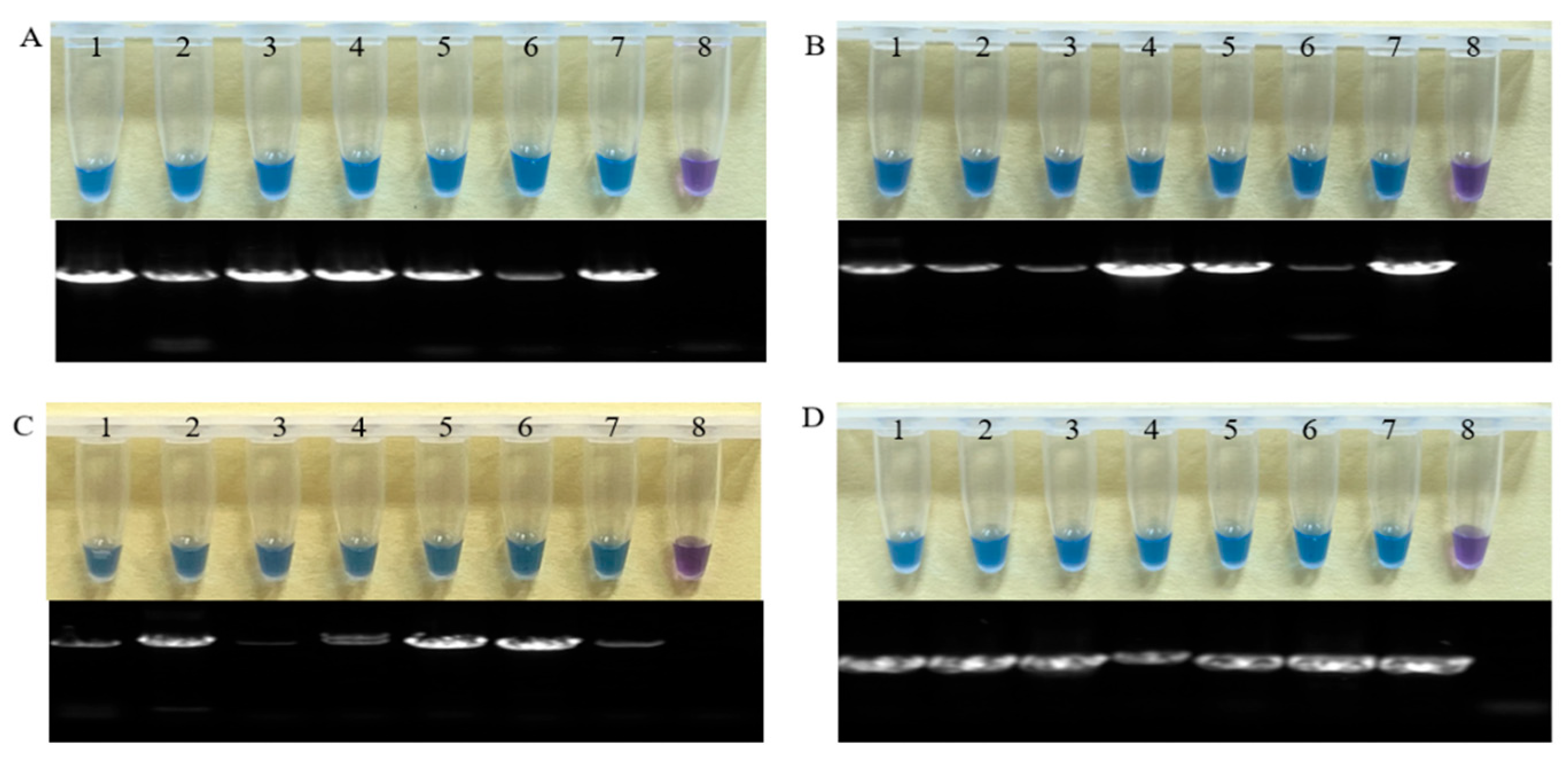
3.1. Specificity of LAMP Assays for RBD
3.2. Sensitivity of the LAMP Assays for RBD
3.2.1. Sensitivity Detection of the Ff-NRPS31-LAMP Assay
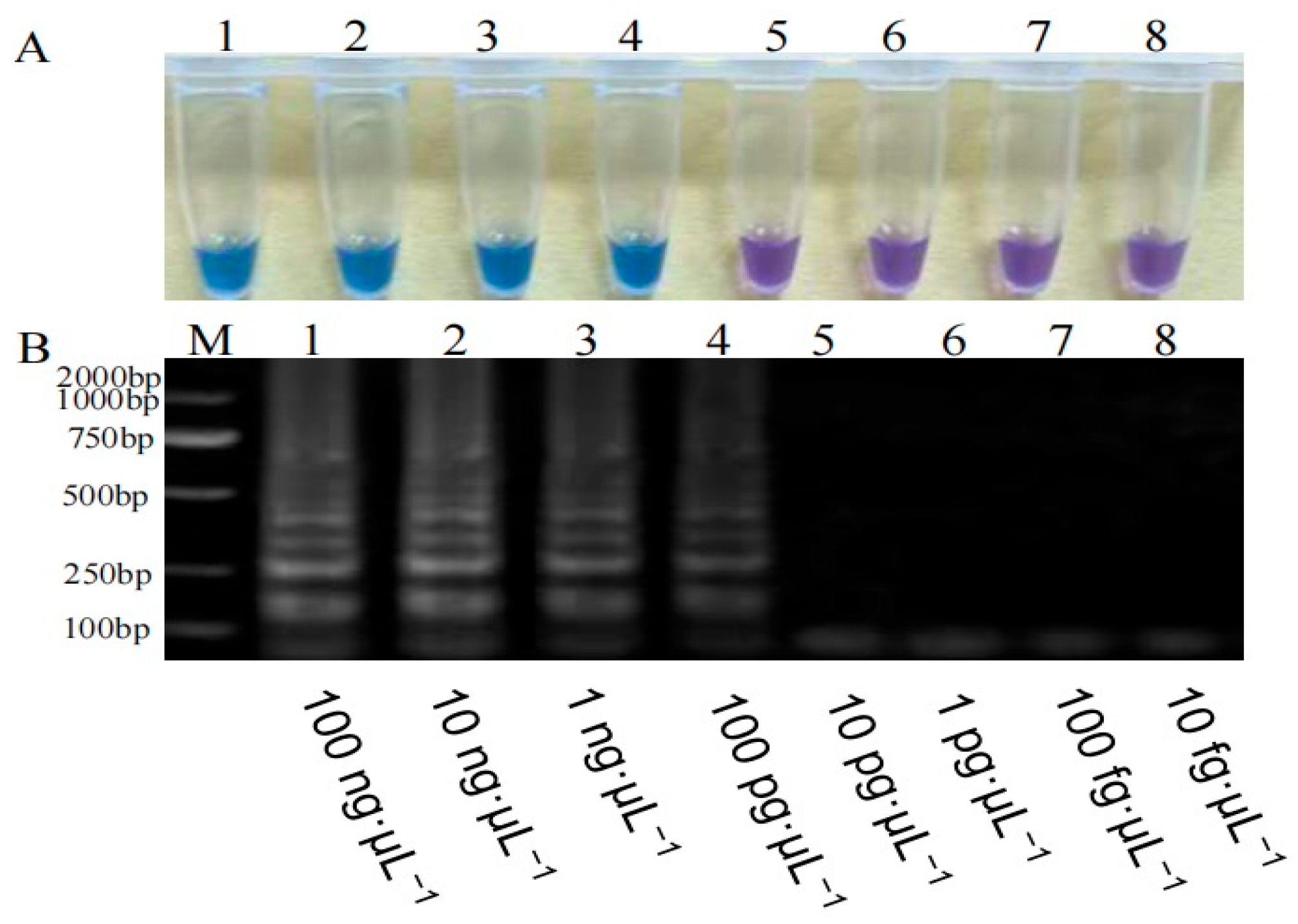
3.2.2. Sensitivity Detection of the Fa-TEF-LAMP Assay
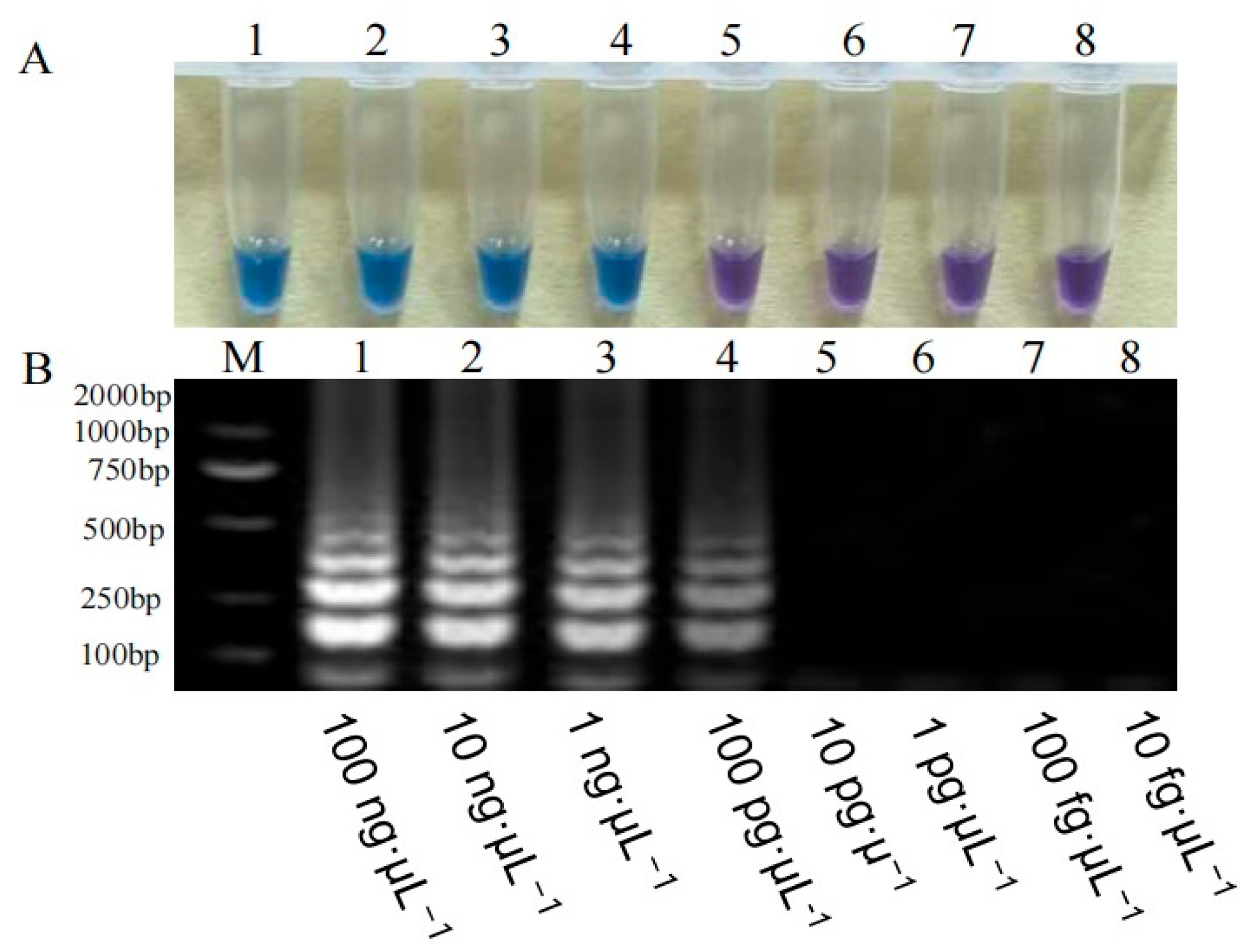
3.2.3. Sensitivity Detection of the Fp-TEF-LAMP Assay

3.2.4. Sensitivity Detection of the Fan-TEF-LAMP Assay
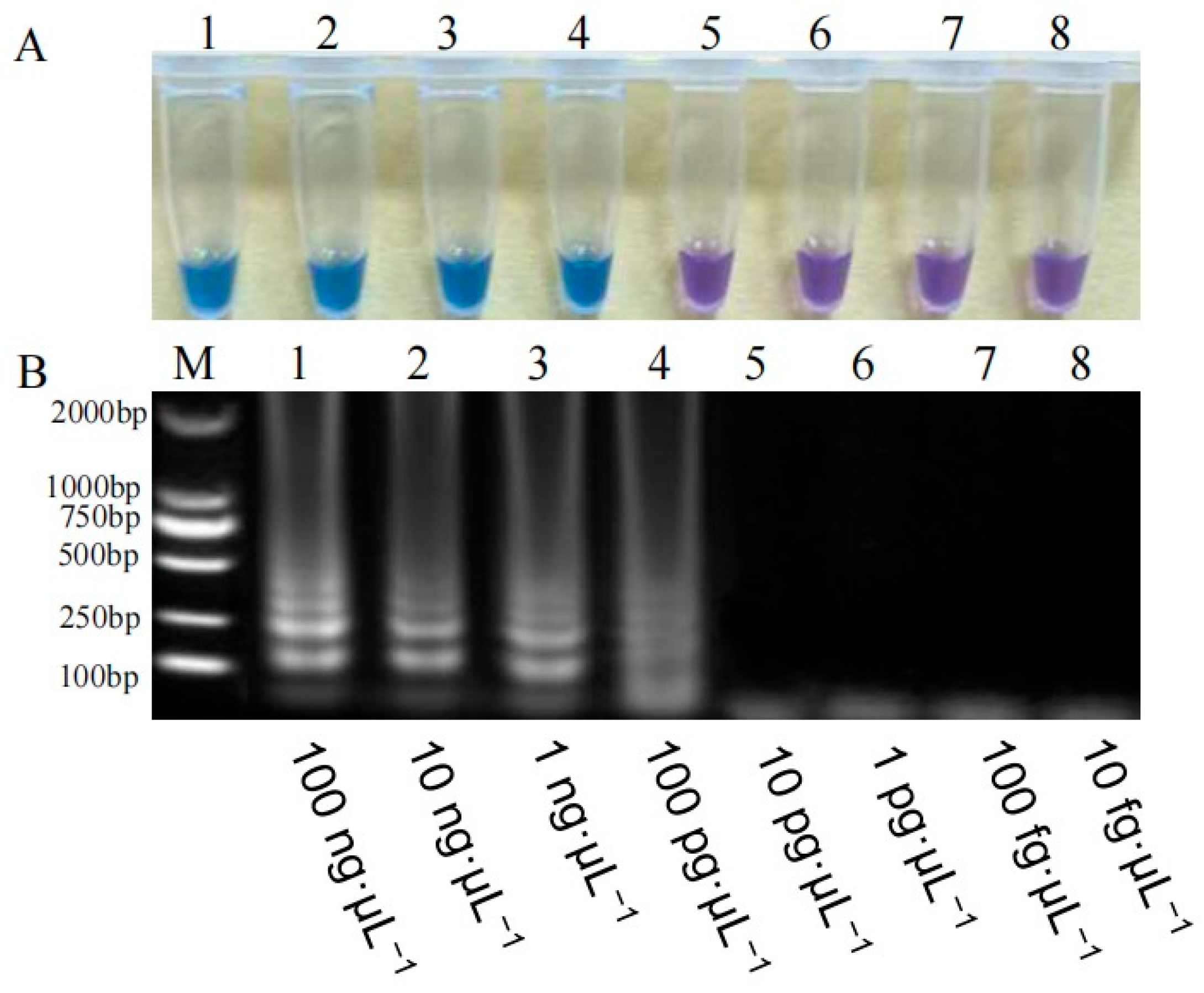
3.2.5. Sensitivity Comparison Between PCR and LAMP Assays for Detecting Rice Bakanae Disease Pathogens
3.3. Detection of Maturity Period of Rice Seeds
| Sample (Cultivar) | Source | Ff-NRPS3-LAMP | Fa-TEF-LAMP | Fp-TEF-LAMP | Fan-TEF-LAMP |
|---|---|---|---|---|---|
| Xinliangyou9328 | Henan | - | - | - | - |
| Xinliangyou1319 | Henan | + | - | - | - |
| Xinliangyou905 | Henan | + | + | - | - |
| Xinliangyoufengnuo | Henan | - | - | - | - |
| Fliangyou6828 | Henan | - | - | + | - |
| Liangyou7166 | Henan | - | - | - | - |
| Liangyou2169 | Henan | + | - | - | - |
| Liangyouwushan | Henan | - | - | - | - |
| Liangyoufenghe | Henan | - | - | - | - |
| Xingengnuo368 | Henan | - | - | - | - |
| Xingengnuo216 | Henan | - | - | - | - |
| Tongyu338 | Jilin | - | - | - | - |
| Tongyu271 | Jilin | + | - | - | - |
| Tonghe885 | Jilin | - | - | - | - |
| Tonghe875 | Jilin | + | - | - | - |
| Zhongkefa5 | Jilin | - | - | - | - |
| Zhongkefa6 | Jilin | - | + | - | - |
| Jijing338 | Jilin | - | - | - | - |
| Tongxi968 | Jilin | - | - | + | - |
| Tongxi969 | Jilin | - | - | - | - |
| Tongxi963 | Jilin | - | - | - | - |
| Tiejing11 | Liaoning | - | - | - | - |
| Tiejing1507 | Liaoning | - | - | - | - |
| Tiejing1603 | Liaoning | - | - | - | - |
| Tiejing1712 | Liaoning | + | + | - | - |
| Tiejing1743 | Liaoning | - | - | - | - |
| Tiejing1808 | Liaoning | + | - | - | - |
| Tiejing1811 | Liaoning | - | - | - | - |
| Tiejingxiang3hao | Liaoning | - | - | - | - |
| Tiejing96 | Liaoning | - | - | - | - |
| Tiejing1947 | Liaoning | + | - | - | - |
| Liaojing1925 | Liaoning | + | - | - | + |
| Liaojingxiang1hao | Liaoning | - | - | - | - |
| Liaojing1499 | Liaoning | - | - | + | - |
| Liaojing1402 | Liaoning | - | - | - | - |
| Liaojing399 | Liaoning | - | - | - | - |
| Liaojing327 | Liaoning | - | - | + | - |
| Tianlongyou2605 | Liaoning | - | - | + | - |
| Tianlongyou 717 | Liaoning | + | - | - | - |
| Beijing705 | Liaoning | - | - | - | - |
| Beijing1702 | Liaoning | - | + | + | - |
| Beijing1501 | Liaoning | - | - | - | + |
| Beijing1604 | Liaoning | + | - | + | + |
| Beijing2 | Liaoning | - | - | - | - |
| Zhonghua11 | Liaoning | - | - | - | - |
| Ribenqing | Liaoning | - | - | - | - |
| Menggudao | Liaoning | - | - | - | - |
| Lijiangxintuanheigu | Liaoning | - | - | - | - |
| Yanfeng47 | Liaoning | - | - | - | - |
| Gangyuan8 | Liaoning | - | - | + | - |
| Beijing143 | Liaoning | - | - | - | - |
| Shennong9816 | Liaoning | + | + | - | + |
| Shennong9903 | Liaoning | - | - | - | - |
| Beijing1hao | Liaoning | - | - | - | - |
| Beijing3hao | Liaoning | - | - | - | - |
3.4. The Detection of Pathogen Seed Carrying
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Jeong, S.; Kim, B.; Kim, S.J.; Kim, H.; Jeong, S.; Yun, G.Y.; Kim, K.Y.; Park, K. Automated detection of rice bakanae disease via drone imagery. Sensors 2022, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Shu, Q. Current insights on rice (Oryza sativa L.) bakanae disease and exploration of its management strategies. J. Zhejiang Univ. Sci. B 2023, 24, 755–778. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, J.E.; Yang, J.W.; Son, H.; Min, K. Application of direct PCR for phylogenetic analysis of Fusarium fujikuroi species complex isolated from rice seeds. Front. Plant Sci. 2023, 13, 1093688. [Google Scholar] [CrossRef] [PubMed]
- Morcia, C.; Tumino, G.; Gasparo, G.; Ceresoli, C.; Fattorini, C.; Ghizzoni, R.; Carnevali, P.; Terzi, V. Moving from qPCR to chip digital PCR assays for tracking of some Fusarium Species causing Fusarium head blight in cereals. Microorganisms 2020, 8, 1307. [Google Scholar] [CrossRef]
- Laha, G.S.; Singh, R.R.; Ladhalakshmi, D.; Sunder, S.; Prasad, M.; Dagar, C.S.; Babu, V.R. Importance and Management of Rice Diseases: A Global Perspective. In Rice Production Worldwide; Springer: Cham, Switzerland, 2017; pp. 303–360. [Google Scholar]
- Ito, S.; Kimura, J. Studies on the bakanae disease of the rice plant. Rep. Hokkaido Agric. Exp. Stn. 1931, 27, 1–95. [Google Scholar]
- Picco, A.M.; Lorenz, E.; Rodolfz, M.; Biloni, M.; Bruneli, A. A multi-location mycological survey on some Italian rice varieties during the crop season. Atti Delle Giornate Fitopatol. 2002, 2, 487–488. [Google Scholar]
- Nguyen, T.T.; Dehne, H.W.; Steiner, U. Histopathological assessment of the infection of maize leaves by Fusarium graminearum, F. proliferatum, and F. verticillioides. Fungal Biol. 2016, 120, 1094–1104. [Google Scholar] [CrossRef]
- Gupta, A.K.; Singh, Y.; Jain, A.K.; Singh, D. Prevalence and incidence of bakanae disease of rice in northern India. J. AgriSearch 2014, 1, 233–237. [Google Scholar]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium Species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Aggarwal, R.; Sharma, S.; Gupta, S.; Rawat, K.; Singh, D.; Singh, A.K.; Krishnan, S.G. Occurrence, identification and pathogenicity of Fusarium species associated with bakanae disease of basmati rice in India. Eur. J. Plant Pathol. 2016, 144, 457–466. [Google Scholar] [CrossRef]
- Dissanayake, M.L.; Tanaka, S.; Ito, S. Fumonisin B(1) production by Fusarium proliferatum strains isolated from Allium fistulosum plants and seeds in Japan. Lett. Appl. Microbiol. 2009, 48, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Malonek, S.; Bömke, C.; Bornberg-Bauer, E.; Rojas, M.C.; Hedden, P.; Hopkins, P.; Tudzynski, B. Distribution of gibberellin biosynthetic genes and gibberellin production in the Gibberella fujikuroi species complex. Phytochemistry 2005, 66, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Manandhar, H.K.; Planter, R.D.; Manandha, G.G.; Poling, S.M.; Maragos, C.M. Fusarium species from Nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl. Environ. Microbiol. 2000, 66, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Ma, Z. Simultaneous detection of Fusarium asiaticum and Fusarium graminearum in wheat seeds using a real-time PCR method. Soc. Appl. Microbiol. 2009, 48, 680–686. [Google Scholar]
- Xu, M.; Ye, W.; Zeng, D.; Wang, Y.; Zheng, X. Rapid diagnosis of wheat head blight caused by Fusarium asiaticum using a loop-mediated isothermal amplification assay. Australas. J. Plant Pathol. 2017, 46, 261–266. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, J.L.; Hao, X.A.; Tian, X.M.; Wu, Y.F. Rapid detection of tobacco viruses by reverse transcription loop-mediated isothermal amplification. Arch. Virol. 2012, 157, 2291–2298. [Google Scholar] [CrossRef]
- Ortega, F.S.; Tomlinson, J.; Gilardi, G.; Spadaro, D.; Gullino, M.L.; Garibaldi, A.; Boonham, N. Rapid detection of Fusarium oxysporum f. sp. lactucae on soil, lettuce seeds and plants using loop-mediated isothermal amplification. J. Plant Pathol. 2018, 67, 1462–1473. [Google Scholar] [CrossRef]
- Lai, D.W.; Zhang, Y.L.; Huang, Q.X.; Yin, G.H.; Pennerman, K.K.; Liu, Z.X.; Guo, A.P. Reverse transcription loop-mediated isothermal amplification to rapidly detect Rice ragged stunt virus. Saudi J. Biol. Sci. 2018, 25, 1577–1584. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxynaphthol blue. BioTechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.; Spadaro, D.; Gullino, M.L.; Mezzalama, M. Optimization of a loop-mediated isothermal amplification assay for on-site detection of Fusarium fujikuroi in rice seed. J. Agron. 2021, 11, 1580. [Google Scholar] [CrossRef]
- Quazi, S.A.; Meon, S.; Abidin, B. Characterization of Fusarium proliferatum through species specific primers and its virulence on rice seeds. Int. J. Agric. Biol. 2013, 15, 649–656. [Google Scholar]
- Rahjoo, V.; Zad, J.; Javan-Nikkhah, M.; Gohari, M.A. Morphological and molecular identification of Fusarium isolated from maize ears in Iran. J. Plant Pathol. 2008, 90, 463–468. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Zhang, S.Y.; Dai, D.J.; Wang, H.D.; Zhang, C.Q. One-step loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Fusarium fujikuroi in bakanae disease through NRPS31, an important gene in the gibberellic acid bio-synthesis. Sci. Rep. 2019, 9, 3726. [Google Scholar] [CrossRef]
- Gerlach, W.; Nirenberg, H. The Genus Fusarium: A pictorial Atlas. Mitteilungen Biol. Bundesanst. Land-Und Forstwirtsch. 1983, 209, 1–406. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Rheeder, J.P.; Lamprecht, S.C.; Zeller, K.A.; Leslie, J.F. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia 2001, 93, 1203–1210. [Google Scholar] [CrossRef]
- Zhang, X.; Harrington, T.C.; Batzer, J.C.; Kubota, R.; Peres, N.A.; Gleason, M.L. Detection of Colletotrichum acutatum sensu lato on strawberry by loop-mediated isothermal amplification. Plant Dis. 2016, 100, 1804–1812. [Google Scholar] [CrossRef]
- Denschlag, C.; Vogel, R.F.; Niessen, L. Hyd5 gene-based detection of the major gushing-inducing Fusarium spp. in a loop-mediated isothermal amplification (LAMP) assay. Int. J. Food Microbiol. 2012, 156, 189–196. [Google Scholar] [CrossRef]
- Berruezo, L.A.; Mercado Crdenas, G.E.; Harries, E.M.; Stenglein, S.A.; Curti, R.N.; Rodriguero, M.S.; Galvan, M.Z. Characterization of Fusarium species associated with to bacco diseases in Northwestern Argentina. Eur. J. Plant Pathol. 2018, 151, 1065–1079. [Google Scholar] [CrossRef]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Liu, Y.L.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Mule, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Jurado, M.; Vázquez, C.; Marin, S.; Sanchis, V.; González-Jaén, M.T. PCR-based strategy to detect contamination with mycotoxi-genic Fusarium species in maize. Syst. Appl. Microbiol. 2006, 29, 681–689. [Google Scholar] [CrossRef]
- Amoah, B.K.; Macdonald, M.V.; Rezanoor, N.; Nicholson, P. Theuse of the random amplified polymorphic DNA technique to identify matinggroups in the Fusarium section Liseola. J. Plant Pathol. 1996, 45, 115–125. [Google Scholar] [CrossRef]
- Almasi, M.A.; Dehabadi, S.M.H.; Moradi, A.; Eftekhari, Z.; Ojaghkandi, M.A.; Aghaei, S. Development and application of loop-mediated isothermal amplification assay for rapid detection of Fusarium oxysporum f. sp. lycopersici. J. Plant Pathol. Microbiol. 2013, 4, 177–183. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.; Nah, J.Y.; Kim, H.K.; Paek, J.S.; Lee, S.; Ham, H.; Hong, S.K.; Yun, S.H.; Lee, T. Species composition of and Fumonisin production by the Fusarium fujikuroi species complex isolated from Korean cereals. Int. J. Food Microbiol. 2018, 267, 62–69. [Google Scholar] [CrossRef]
- Amoah, B.K.; Rezanoor, H.N.; Nicholson, P.; Macdonald, M.V.P. Variation in the Fusarium section Liseola: Pathogenicity and genetic studies of Fusarium moniliforme sheldon from different hosts in Ghana. J. Plant Pathol. 1995, 44, 563–572. [Google Scholar] [CrossRef]

| Assay | Primer Type | Sequence (5′–3′) | Length |
|---|---|---|---|
| Ff-NRPS31-LAMP | F3 | TTTTTTAAGTCCGTATTG | 18 |
| B3 | GTGCTCATCGCTTCCTCAAC | 20 | |
| FIP | GGGTGGTGGCAGCTC-TTGCACGCGTTGAGTTTAC | 34 | |
| BIP | CATTGCACGATAGCTAGC-CTAATCAGATATTTTCTCTA | 38 | |
| LF | GCCCCTGATTCTACC | 15 | |
| LB | ACCTACCTACTCTCAAG | 17 | |
| Fa-TEF-LAMP | F3 | ACCAGTCACTAACCACCTGT | 20 |
| B3 | GAGCGTCTGATAGCCATGTT | 20 | |
| FIP | TCACGCTCGGCTTTGAGCTTG-GAGCTCGGTAAGGGTTCCT | 40 | |
| BIP | ATCGCCCTCTGGAAGTTCGAGA-GACAGCAGTGGTGACAACAT | 42 | |
| LF | AGAACCCAGGCGTACTTGA | 19 | |
| LB | CTCCTCGCTACTATGTCACCGT | 22 | |
| Fp-TEF-LAMP | F3 | CATTTACCCCGCCACTCG | 18 |
| B3 | AGGAGTCTCGAACTTCCAGA | 20 | |
| FIP | CGGACGGTTAGTGACTGCTTGA-CGTTTGCCCTCTCCACAA | 40 | |
| BIP | CGCTGAGCTCGGTAAGGGTTC-GGTGATACCACGCTCACG | 39 | |
| LF | GACGATGCGCTCATTGAGG | 19 | |
| LB | CTTCAAGTACGCCTGGGTTC | 20 | |
| Fan-TEF-LAMP | F3 | GCATTTACCCCGCCACTC | 18 |
| B3 | AGCGAGGAGTCTCGAACTTC | 20 | |
| FIP | CTCAGCGGCTTCCTATTGTCGA-CCTCTCCCATTCCACAACC | 41 | |
| BIP | AGGGTTCCTTCAAGTACGCCTG-GATGGTGATACCACGCTCAC | 42 | |
| LF | CGTGACAATGCGCTCAGTGA | 20 | |
| LB | GGTTCTTGACAAGCTCAAGGC | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Y.; Zhang, Y.; Xia, J.; Liu, C.; Song, Y.; Han, T.; Wei, S.; Zheng, W. Establishment and Application of Loop-Mediated Isothermal Amplification Assays for Pathogens of Rice Bakanae Disease. Agriculture 2025, 15, 1319. https://doi.org/10.3390/agriculture15121319
Liu X, Wang Y, Zhang Y, Xia J, Liu C, Song Y, Han T, Wei S, Zheng W. Establishment and Application of Loop-Mediated Isothermal Amplification Assays for Pathogens of Rice Bakanae Disease. Agriculture. 2025; 15(12):1319. https://doi.org/10.3390/agriculture15121319
Chicago/Turabian StyleLiu, Xinchun, Yan Wang, Yating Zhang, Jingzhao Xia, Chenxi Liu, Yu Song, Tao Han, Songhong Wei, and Wenjing Zheng. 2025. "Establishment and Application of Loop-Mediated Isothermal Amplification Assays for Pathogens of Rice Bakanae Disease" Agriculture 15, no. 12: 1319. https://doi.org/10.3390/agriculture15121319
APA StyleLiu, X., Wang, Y., Zhang, Y., Xia, J., Liu, C., Song, Y., Han, T., Wei, S., & Zheng, W. (2025). Establishment and Application of Loop-Mediated Isothermal Amplification Assays for Pathogens of Rice Bakanae Disease. Agriculture, 15(12), 1319. https://doi.org/10.3390/agriculture15121319





