Factors Influencing the Formation, Development of Buds, and Flowering of Temperate Fruit Trees
Abstract
1. Genetic Diversity Among Temperate Fruit Plants
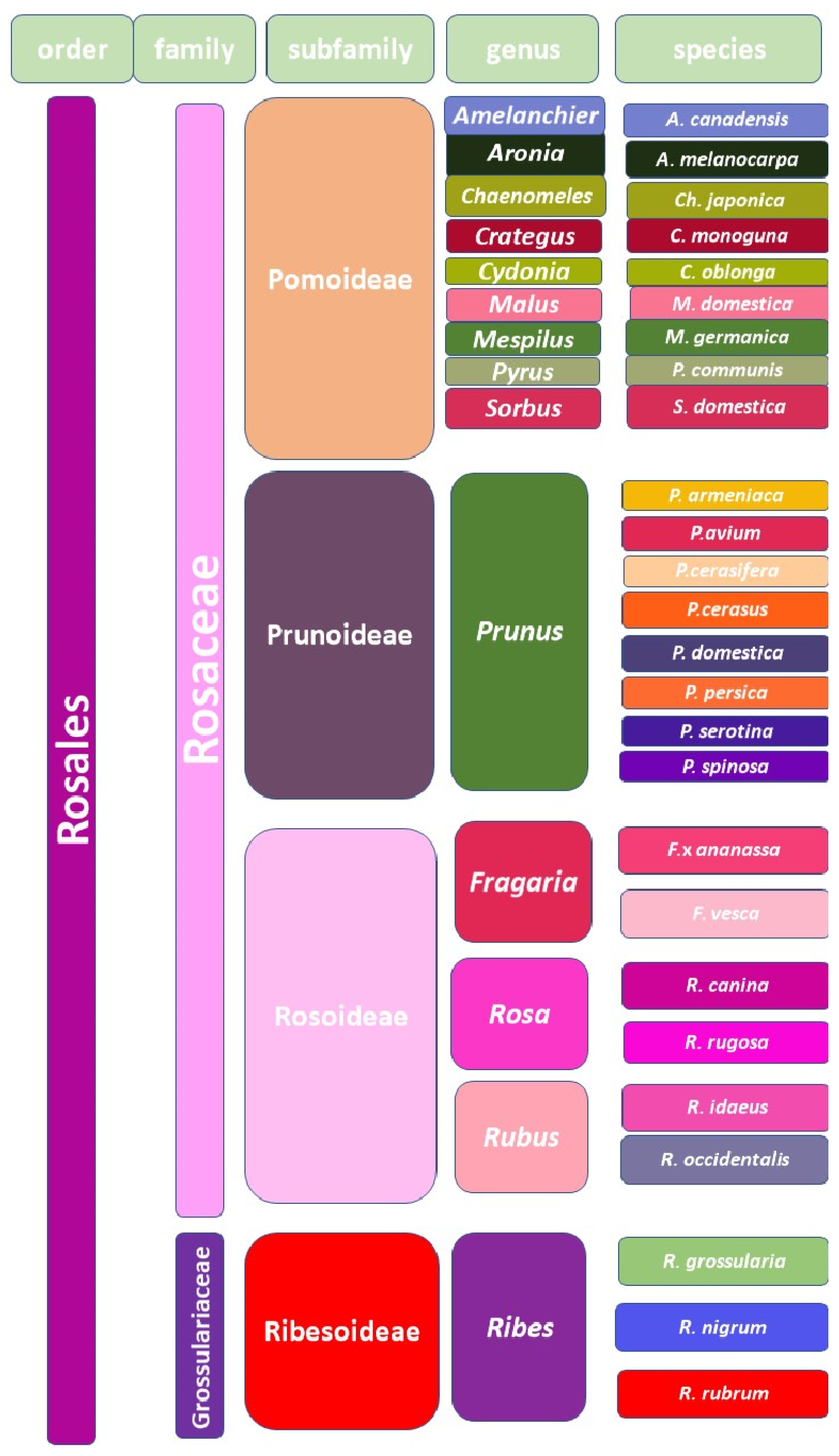
2. Systematics of Fruit Plants
3. Types of Flowers and Fruits
Place of Flower Bud Formation
4. The Juvenile Phase
4.1. Effects of Light
4.2. Method of Reproduction
5. Mature Phase
5.1. Structure of the Flower Bud
5.1.1. Induction and Initiation of Flower Buds
5.1.2. Differentiation and Maturation of Flower Buds
6. Flowering
| Fruit Plant | GDD to Full Bloom (°C) | Base Temperature (°C) | References |
|---|---|---|---|
| Apple | 598–655 | 4 or 0 | [140] |
| Apricot | 332–425 | −1 | [141] |
| Blueberry | 376–409 | 0 | [142] |
| Grapes | 222–414 | 10 | [143] |
| Hazelnut | Catkins: 217–387, Female flowers: 128–276 | 0 | [102] |
| Peach | 217–333 | 4.4 | [144] |
| Pear | 202–260 | 4.4; 8.2 | [145] |
| Plum (European) | 462–548 | 0 | [142] |
| Plum (Japanese) | 381–428 | 0 | [142] |
| Sour cherry | 123 | 4 | [146] |
| Sweet cherry | 109–130 | 4 | [147] |
| Strawberry | 1892–2039 | 3 | [148] |
| Walnut | 721–827 | 0 | [149] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Park, Y.; Muthuramalingam, P.; Jeong, J.H.; Kim, S.H.; Shin, H. Physiological and Metabolic Analyses Reveal the Proline-Mediated Flowering Delay Mechanism in Prunus persica. Front. Plant Sci. 2024, 15, 1302975. [Google Scholar] [CrossRef] [PubMed]
- Ohl, M. Principles of Taxonomy and Classification: Current Procedures for Naming and Classifying Organisms. In Handbook of Paleoanthropology; Henke, W., Tattersall, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–20. ISBN 978-3-642-27800-6. [Google Scholar]
- Senters, A.E.; Soltis, D.E. Phylogenetic Relationships in Ribes (Grossulariaceae) Inferred from ITS Sequence Data. Taxon 2003, 52, 51–66. [Google Scholar] [CrossRef]
- Pikunova, A.V.; Martirosian, E.V.; Kniazev, S.D.; Ryzhova, N.N. Application of the RAPD-Analysis for the Study of Genetic Polymorphism and Phylogenetic Relationships in the Ribes L. Genus. Russ. J. Genet. Appl. Res. 2012, 2, 141–151. [Google Scholar] [CrossRef]
- Woziwoda, B. Leśne Rośliny o Jadalnych Owocach—Przegląd Botaniczny. Stud. I Mater. CEPL W Rogowie 2014, 38, 105–118. [Google Scholar]
- Szot, I.; Łysiak, G.P. Effect of the Climatic Conditions in Central Europe on the Growth and Yield of Cornelian Cherry Cultivars. Agriculture 2022, 12, 1295. [Google Scholar] [CrossRef]
- Pescie, M.; Lovisolo, M.; De Magistris, A.; Strik, B.; López, C. Flower Bud Initiation in Southern Highbush Blueberry Cv. O’Neal Occurs Twice per Year in Temperate to Warm-Temperate Conditions. J. Appl. Hortic. 2011, 13, 8–12. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Wang, Z.; Zong, Y.; Yu, L.; Li, Y.; Liao, F.; Chen, M.; Cai, K.; Guo, W. Comparative Anatomical and Transcriptomic Insights into Vaccinium corymbosum Flower Bud and Fruit throughout Development. BMC Plant Biol. 2021, 21, 289. [Google Scholar] [CrossRef]
- Westwood, M.N. Temperature Zone, Pomology, Physiology and Culture; Timber Press: Portland, OR, USA, 1993. [Google Scholar]
- Taghavi, T.; Dale, A.; Saxena, P.; Galic, D.; Rahemi, A.; Kelly, J.; Suarez, E. Flowering of Hazelnut Cultivars and How It Relates to Temperature in Southern Ontario. Acta Hortic. 2018, 1226, 131–136. [Google Scholar] [CrossRef]
- Hassankhah, A.; Rahemi, M.; Mozafari, M.R.; Vahdati, K. Flower Development in Walnut: Altering the Flowering Pattern by Gibberellic Acid Application. Not. Bot. Horti Agrobo 2018, 46, 700–706. [Google Scholar] [CrossRef]
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and Classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Lespinass, J.M. La Conduite Du Pommier—Types de Fructication, Incidence Sur La Conduite de L’arbe; INR-INVUFLEC: Paris, France, 1977. [Google Scholar]
- Pan, T.; Fan, X.; Sun, H. Juvenile Phase: An Important Phase of the Life Cycle in Plants. Ornam. Plant Res. 2023, 3, 18. [Google Scholar] [CrossRef]
- Tsuruyama, J.; Shibuya, T. Effects of Far-Red Light and Photoperiod during Early Growth Stages on Flower Bud Development of Seed-Propagated Strawberry Seedlings. Sci. Hortic. 2023, 317, 112051. [Google Scholar] [CrossRef]
- Ohishi-Yamazaki, M.; Watanabe, M.; Nakanishi, A.; Che, J.; Horiuchi, N.; Ogiwara, I. Shortening of the Juvenile Phase of the Southern Highbush Blueberry (Vaccinium corymbosum L. Interspecific Hybrid) Grown in Controlled Rooms under Artificial Light. Hortic. J. 2018, 87, 329–339. [Google Scholar] [CrossRef]
- Campa, C.; Urban, L.; Mondolot, L.; Fabre, D.; Roques, S.; Lizzi, Y.; Aarrouf, J.; Doulbeau, S.; Breitler, J.-C.; Letrez, C.; et al. Juvenile Coffee Leaves Acclimated to Low Light Are Unable to Cope with a Moderate Light Increase. Front. Plant Sci. 2017, 8, 1126. [Google Scholar] [CrossRef]
- Aldwinckle, H.S. Flowering of Apple Seedlings 16–20 Months after Germination. HortScience 1975, 10, 124–126. [Google Scholar] [CrossRef]
- Way, R.D. Hastening the Fruiting of Apple Seedlings. J. Am. Soc. Hortic. Sci. 1971, 96, 389–391. [Google Scholar]
- Mehlenbacher, S.A.; Smith, D.C. Effect of Spacing and Sucker Removal on Precocity of Hazelnut Seedlings. J. Am. Soc. Hortic. Sci. 1992, 117, 523–526. [Google Scholar] [CrossRef]
- Szot, I.; Lipa, T.; Sosnowska, B. Evaluation of Yield and Fruit Quality of Several Ecotypes of Cornelian Cherry (Cornus mas L.) In Polish Condiotions. Acta Sci. Pol. Hortorum Cultus 2019, 18, 139–148. [Google Scholar] [CrossRef]
- Szot, I.; Lipa, T.; Yareshchenko, A. Comparison of Growth of Maiden Trees of Cultivars and Genotypes of Cornelian Cherry (Cornus mas L.) in a Nursery. Agron. Res. 2020, 18, 1526–1536. [Google Scholar] [CrossRef]
- Hamid, M.; Hassan, G.I.; Wani, A.W.; Mumtaz, S.; Ashraf, S.; Ahad, S.; Wani, M.Y.; Kumar, A. Physiology of Flowering in Apple and Almond: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1912–1929. [Google Scholar] [CrossRef]
- Awasthi, P.; Adhikari, S.P.; Paudel, B.; Bogati, S.; Joshi, D. Flower Bud Formation in Fruit Crops. Asian J. Res. Biosci. 2024, 6, 185–191. [Google Scholar]
- Katoda, N.; Wada, M.; Masuda, T.; Soejima, J. The Break-through in the Reduction of Juvenile Phase in Apple Using Transgenic Approaches. Acta Hortic. 2003, 625, 337–343. [Google Scholar] [CrossRef]
- Song, G.; Walworth, A.; Zhao, D.; Jiang, N.; Hancock, J.F. The Vaccinium Corymbosum FLOWERING LOCUS T-like Gene (VcFT): A Flowering Activator Reverses Photoperiodic and Chilling Requirements in Blueberry. Plant Cell Rep. 2013, 32, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Brukhin, V.; Morozova, N. Plant Growth and Development—Basic Knowledge and Current Views. Math. Model. Nat. Phenom. 2011, 6, 1–53. [Google Scholar] [CrossRef]
- Zimmerman, R.H. Session III—Juvenility—Juvenility and Flowering of Fruit Trees. Acta Hortic. 1973, 34, 139–142. [Google Scholar] [CrossRef]
- Bijhouwer, J. De Periodiciteit van de Knopontwikkeling Bij Den Appel (Periodicity of the Bud Development of Apple). Meded. Landb. Hoogesch. 1924, 27, 1–64. [Google Scholar]
- Buban, T.; Faust, M. Flower Bud Induction in Apple Trees: Internal Control and Differentiation. In Horticultural Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 1982; pp. 174–203. ISBN 978-1-118-06430-6. [Google Scholar]
- Schaffer, B.; Andersen, P.C. Handbook of Environmental Physiology of Fruit Crops: Volume I: Temperate Crops, 1st ed.; Schaffer, B., Andersen, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-0-203-71929-9. [Google Scholar]
- McLaughlin, J.M.; Greene, D.W. Fruit and Hormones Influence Flowering of Apple. II. Effects of Hormones. J. Am. Soc. Hortic. Sci. 1991, 16, 450–453. [Google Scholar] [CrossRef]
- Abbott, D.L. Fruit Bud Formation in Cox’s Orange Pippin. Rep. Long Asht Res. Stn. 1977, 167–176. [Google Scholar]
- Hirst, P.M.; Ferree, D. Rootstock Effects on the Flowering of ‘Delicious’ Apple. I. Bud Development. J. Am. Soc. Hortic. Sci. 1995, 120, 1010–1017. [Google Scholar] [CrossRef]
- Luckwill, L.C.; Silva, J.M. The Effects of Daminozide and Gibberellic Acid on Flower Initiation, Growth and Fruiting of Apple Cv Golden Delicious. J. Hortic. Sci. 1979, 54, 217–223. [Google Scholar] [CrossRef]
- Tromp, J. Flower—Bud Formation. In Fundamentals of Temperate Zone Tree Fruit Production; Backhuys Publishers: Leiden, The Netherlands, 2005; pp. 204–215. [Google Scholar]
- Luckwill, L.C. A New Look at the Process of Fruit Bud Formation in Apple. In Proceedings of the XIX International Horticultural Congress, Warsaw, Poland, 10 September 1974; pp. 237–245. [Google Scholar]
- Guimond, M.; Andrews, P.; Lang, G. Scanning Electron Microscopy of Floral Initiation in Sweet Cherry. J. Am. Soc. Hortic. Sci. 1998, 123, 509–512. [Google Scholar] [CrossRef]
- Csihon, Á.; Gonda, I.; Szabó, S.; Holb, I.J. Tree Vegetative and Generative Properties and Their Inter-Correlations for Prospective Apple Cultivars under Two Training Systems for Young Trees. Hortic. Environ. Biotechnol. 2022, 63, 325–339. [Google Scholar] [CrossRef]
- Manchanda, P.; Kaur, M.; Sharma, S.; Sidhu, G.S. Biotechnological Interventions for Reducing the Juvenility in Perennials. Horticulturae 2022, 9, 33. [Google Scholar] [CrossRef]
- Milyaev, A.; Kofler, J.; Moya, Y.A.T.; Lempe, J.; Stefanelli, D.; Hanke, M.-V.; Flachowsky, H.; Von Wirén, N.; Wünsche, J.-N. Profiling of Phytohormones in Apple Fruit and Buds Regarding Their Role as Potential Regulators of Flower Bud Formation. Tree Physiol. 2022, 42, 2319–2335. [Google Scholar] [CrossRef]
- Ghosh, A.; Dey, K.; Das, S.; Dutta, P. Effect of Light on Flowering of Fruit Crops. Adv. Life Sci. 2016, 5, 2597–2603. [Google Scholar]
- Aucher, E.C.; Lagasse, F.S.; Aldric, W.W. The Effect of Shade on the Growth, Fruit Bud Formation and Chemical Composition of Apple Trees. Hortic. Sci. 1926, 23, 368–372. [Google Scholar]
- Tromp, J. Flower-Bud Formation in Apple as Affected by Air and Root Temperature, Air Humidity, Light Intensity, and Day Length. Acta Hortic. 1984, 149, 39–48. [Google Scholar] [CrossRef]
- Jackson, B.I. Effects of Water, Light and Nutrition on Flower Bud Initiation in Apricots. Aust. J. Biol. Sci. 1969, 22, 69–75. [Google Scholar] [CrossRef]
- Sidhu, V.; Bernier-English, V.; Lamontagne-Drolet, M.; Gravel, V. Effect of Light Quality and Extended Photoperiod on Flower Bud Induction during Transplant Production of Day-Neutral Strawberry Cultivars. Can. J. Plant Sci. 2022, 102, 356–367. [Google Scholar] [CrossRef]
- Heide, O.M.; Rivero, R.; Sønsteby, A. Temperature Control of Shoot Growth and Floral Initiation in Apple (Malus × Domestica Borkh.). CABI Agric. Biosci. 2020, 1, 8. [Google Scholar] [CrossRef]
- Hendrickson, J.R.; Hanson, J.D.; Tanaka, D.L.; Sassenrath, G. Principles of Integrated Agricultural Systems: Introduction to Processes and Definition. Renew. Agric. Food Syst. 2008, 23, 265–271. [Google Scholar] [CrossRef]
- Wilkie, J.D.; Sedgley, M.; Olesen, T. Regulation of Floral Initiation in Horticultural Trees. J. Exp. Bot. 2008, 59, 3215–3228. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.B.M.; Ben Laya, M.; Baazaoui, N.; Sghaier-Hammami, B. Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree. Sustainability 2022, 14, 15614. [Google Scholar] [CrossRef]
- Beyá-Marshall, V.; Herrera, J.; Fichet, T.; Trentacoste, E.R.; Kremer, C. The Effect of Water Status on Productive and Flowering Variables in Young ‘Arbequina’ Olive Trees under Limited Irrigation Water Availability in a Semiarid Region of Chile. Hortic. Environ. Biotechnol. 2018, 59, 815–826. [Google Scholar] [CrossRef]
- Bally, I.S.E.; Harris, M.; Whiley, A.W. Effect of Water Stress on Flowering and Yield of ‘Kensington Pride’ Mango (Mangifera indica L.). Acta Hortic. 2000, 509, 277–282. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Rodríguez Pleguezuelo, C.R.; Gálvez Ruiz, B.; Gutiérrez Gordillo, S.; García-Tejero, I.F. Water Use and Fruit Yield of Mango (Mangifera indica L.) Grown in a Subtropical Mediterranean Climate. Int. J. Fruit Sci. 2019, 19, 136–150. [Google Scholar] [CrossRef]
- Borchert, R. Phenology and Control of Flowering in Tropical Trees. Biotropica 1983, 15, 81–89. [Google Scholar] [CrossRef]
- Kumar, A.; Bhuj, B.D.; Singh, C.P. Alternate Bearing in Fruits Trees: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1218–1235. [Google Scholar] [CrossRef]
- Zhou, J.N.; Shan, L.U.; Juntao, J.; Shouren, L.; Nam, Z.; Xaoyu, J.; Fan, W. Research Progress on Influencing Factors and Mechanisms of Flower Bud Differentiation in Horticultural Plants. Acta Hortic. Sin. 2023, 50, 1151–1164. [Google Scholar]
- Sosna, I. Effect of Hand and Chemical Thinning on Yielding and Fruit Quality of Two Late-Ripening Plum Cultivars. Acta Sci. Pol. Hortorum Cultus 2012, 11, 41–51. [Google Scholar]
- Rutkowski, K.; Łysiak, G.P. Thinning Methods to Regulate Sweet Cherry Crops—A Review. Appl. Sci. 2022, 12, 1280. [Google Scholar] [CrossRef]
- Khan, I.A.; Hassan, S.; Mir, M.A.; Nisar, S.; Khalil, A.; Amin, Z. Impact of Crop Load on Growth, Flowering and Fruiting in Apple Cv. Gala Redlum. Int. J. Environ. Clim. Change 2023, 13, 4388–4395. [Google Scholar] [CrossRef]
- Szot, I.; Lipa, T. Apple Trees Yielding and Fruit Quality Depending on the Crop Load, Branch Type and Position in the Crown. Acta Sci. Pol. Hortorum Cultus 2019, 18, 205–215. [Google Scholar] [CrossRef]
- Bound, S.A. Managing Crop Load in European Pear (Pyrus communis L.)—A Review. Agriculture 2021, 11, 637. [Google Scholar] [CrossRef]
- Bound, S.A. Crop Load Management in Nashi Pear—A Review. Horticulturae 2022, 8, 923. [Google Scholar] [CrossRef]
- Parveze, M.U.; Mir, M.M.; Rehman, M.U.; Iqbal, U.; Khan, S.Q.; Khan, F.A.; Khan, I.; Qayoom, S.; Mushtaq, I.; Shah, H.K.; et al. Regulation of Crop Load and Quality in Sweet Cherry Cv. ‘Sweet Heart’ Using Blossom Thinning. Folia Hortic. 2024, 36, 311–321. [Google Scholar] [CrossRef]
- Meland, M.; Vangdal, E.; Kaiser, C. Effects of Different Flowering Intensities and Crop Load of ‘Opal’ European Plum on Yield, Fruit Quality and Return Bloom. Acta Hortic. 2016, 1130, 235–240. [Google Scholar] [CrossRef]
- Johnson, H.A.; Weinbaum, S.A.; DeJong, T.M. Crop Load Effects on Subsequent Peach Floral Development, Pistil Size at Anthesis and Fruit Size at Maturity. HortScience 2004, 39, 851. [Google Scholar] [CrossRef]
- Alburquerque, N.; Burgos, L.; Egea, J. Influence of Flower Bud Density, Flower Bud Drop and Fruit Set on Apricot Productivity. Sci. Hortic. 2004, 102, 397–406. [Google Scholar] [CrossRef]
- Reddy, P.; Plozza, T.; Ezernieks, V.; Stefanelli, D.; Scalisi, A.; Goodwin, I.; Rochfort, S. Metabolic Pathways for Observed Impacts of Crop Load on Floral Induction in Apple. Int. J. Mol. Sci. 2022, 23, 6019. [Google Scholar] [CrossRef]
- Micklem, T. Studies on Fruit Bud Formation in Deciduous Fruit Trees in South. Afr. J. Pomol. Hortic. Sci. 1938, 16, 201–209. [Google Scholar] [CrossRef]
- Georgiev, V. Phenophase of Flower Bud Formation. In Cheresha (Sweet Cherry) [Bul]; Zemizdat: Sofia, Bulgaria, 2001; pp. 64–70. [Google Scholar]
- Faust, M. Fruiting. In Physiology of Temperate Fruit Trees; John Wiley: Hoboken, NJ, USA, 1989; pp. 169–234. [Google Scholar]
- Szpadzik, E.; Jadczuk-Tobjasz, E.; Łotocka, B. Pollination, Fertilization, Autogamy Degree and Fruit Set of Some Sour Cherry Cultivars in Growing Conditions of Poland. Acta Hortic. 2014, 1020, 257–263. [Google Scholar] [CrossRef]
- Zhu, L.H.; Borsboom, O.; Tromp, J. The Effect of Temperature on Flower-Bud Formation in Apple Including Some Morphological Aspects. Sci. Hortic. 1997, 70, 1–8. [Google Scholar] [CrossRef]
- Beppu, K.; Ikeda, T.; Kataoka, I. Effect of High Temperature Exposure Time during Flower Bud Formation on the Occurrence of Double Pistils in ‘Satohnishiki’ Sweet Cherry. Sci. Hortic. 2001, 87, 77–84. [Google Scholar] [CrossRef]
- Bartolini, S.; Lo Piccolo, E.; Remorini, D. Different Summer and Autumn Water Deficit Affect the Floral Differentiation and Flower Bud Growth in Apricot (Prunus armeniaca L.). Agronomy 2020, 10, 914. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.P.; Gu, C.; Tao, S.T.; Wang, D.S.; Guo, X.P.; Qi, K.J.; Zhang, S.L. Transcriptome Profiling Reveals Differentially Expressed Genes Associated with Wizened Flower Bud Formation in Chinese Pear (Pyrus bretschneideri Rehd.). J. Hortic. Sci. Biotechnol. 2016, 91, 227–235. [Google Scholar] [CrossRef]
- Papachnatzis, A.; Lichev, V.; Botu, M. Influence of the Rootstock on the Autumn-Winter Development of the Differentiating Flower Buds in Two Sweet Cherry Cultivars. Univ. Craiova Univ. Craiova 2008, 13, 149–154. [Google Scholar]
- Soysal, D.; Doğan, D.E.; Lizalo, A.; Demirsoy, L.; Demirsoy, H. Rootstock Affects the Number of Flowers in the Floral Bud in Sweet Cherries. Erwerbs-Obstbau 2023, 65, 2281–2288. [Google Scholar] [CrossRef]
- Jacyna, T.; Lipa, T.; Seliga, Ł. Basic Shoot Characteristics Predict the Cropping Potential of Young Sweet Cherry (Prunus avium L.) Trees. N. Z. J. Crop Hortic. Sci. 2021, 49, 63–73. [Google Scholar] [CrossRef]
- Dziedzic, E.; Bieniasz, M.; Kowalczyk, B. Morphological and Physiological Features of Sweet Cherry Floral Organ Affecting the Potential Fruit Crop in Relation to the Rootstock. Sci. Hortic. 2019, 251, 127–135. [Google Scholar] [CrossRef]
- Bartolini, S.; Iacona, C.; Remorini, D.; Viti, R. Influence of Weather Conditions and Rootstock Genotypes on Flower Bud Biology and Xylem Vessel Differentiation in Apricot. J. Appl. Hortic. 2016, 18, 171–177. [Google Scholar] [CrossRef]
- Selamovska, A.; Miskoska-Milevska, E. Differentiation of Mixed Flower Buds in Some Traditional Pear Varieties in the Region of Skopje. Acta Agric. Serbica 2018, 23, 277–284. [Google Scholar] [CrossRef]
- Bieniek, A.; Markuszewski, B.; Kopytowski, J.; Pluta, S.; Markowski, J. Yielding and Fruit Quality of Several Cultivars and Breeding Clones of Amelanchier Alnifolia Grown in North-Eastern Poland. Zemdirb.-Agric. 2019, 106, 351–358. [Google Scholar] [CrossRef]
- Steeves, M.W.; Steeves, T.A. Inflorescence Development in Amelanchier Alnifolia. Can. J. Bot. 1990, 68, 1680–1688. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in Temperate Fruit Trees in a Global Warming Context: A Review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Cattani, A.M.; Sartor, T.; Da Silveira Falavigna, V.; Porto, D.D.; Silveira, C.P.; Dias De Oliveira, P.R.; Revers, L.F. The Control of Bud Break and Flowering Time in Plants. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 88, pp. 277–325. ISBN 978-0-12-815403-8. [Google Scholar]
- Pérez, F.J.; Ormeño, N.J.; Reynaert, B.; Rubio, S. Use of the Dynamic Model for the Assessment of Winter Chilling in a Temperate and a Subtropical Climatic Zone of Chile. Chil. J. Agric. Res. 2008, 68, 198–206. [Google Scholar] [CrossRef]
- Benmoussa, H.; Ghrab, M.; Ben Mimoun, M.; Luedeling, E. Chilling and Heat Requirements for Local and Foreign Almond (Prunus dulcis Mill.) Cultivars in a Warm Mediterranean Location Based on 30 Years of Phenology Records. Agric. For. Meteorol. 2017, 239, 34–46. [Google Scholar] [CrossRef]
- Tabuenca, M.C.; Mut, M.; Herrero, J. The Effect of Temperature on Flowering Date in Almond Varieties. An. Estac. Exp. Aula Dei 1972, 11, 378–395. [Google Scholar]
- Kumar, A.; Mir, Y. Assessment of Chilling Requirement of Different Varietiess of Apple in South Kashmir. Progress. Hortic. 2012, 44, 84–88. [Google Scholar]
- Butcaru, A.C.; Gogoț, R.; Mihai, C.A.; Asănică, A.; Stănică, F. Methods of Evaluating the Chilling and Heat Requirements of Apple and Pear Trees. Sci. Pap. Ser. B Hortic. 2023, 67, 33–38. [Google Scholar]
- Labuschagné, I.F.; Louw, J.H.; Schmidt, K.; Sadie, A. Genetic Variation in Chilling Requirement in Apple Progeny. J. Am. Soc. Hortic. Sci. 2002, 127, 663–672. [Google Scholar] [CrossRef]
- Valentini, N.; Me, G.; Spanna, F.; Lovisetto, M. Chilling and Heat Requirement in Apricot and Peach Varieties. Acta Hortic. 2004, 636, 199–203. [Google Scholar] [CrossRef]
- Gao, Z.; Wong, S.S.; House, L.A.; Spreen, T.H. French Consumer Perception, Preference of, and Willingness to Pay for Fresh Fruit Based on Country of Origin. Br. Food J. 2014, 116, 805–820. [Google Scholar] [CrossRef]
- Warmund, M.R.; Krumme, J. A Chilling Model to Estimate Rest Completion of Erect Blackberries. HortScience 2005, 40, 1259–1262. [Google Scholar] [CrossRef]
- Spiers, J.M.; Marshall, D.A.; Smith, B.J.; Braswell, J.H. Method to Determine Chilling Requirement in Blueberries. Acta Hortic. 2006, 715, 105–110. [Google Scholar] [CrossRef]
- Jones, H.G.; Gordon, S.L.; Brennan, R.M. Chilling Requirement of Ribes Cultivars. Front. Plant Sci. 2015, 5, 767. [Google Scholar] [CrossRef]
- Kampuss, K.; Pedersen, H.L. A Review of Red and White Currant (Ribes rubrum L.): Research and Literature. Small Fruits Rev. 2003, 2, 23–46. [Google Scholar] [CrossRef]
- Sønsteby, A.; Sadojevic, M.; Heide, O.M. Chilling Requirements for Dormancy Release in Red Currants and Gooseberries. Acta Hortic. 2024, 1388, 177–182. [Google Scholar] [CrossRef]
- Rahemi, A.; Fisher, H.; Dale, A.; Taghavi, T.; Kelly, J. Bud Dormancy Pattern, Chilling Requirement, and Cold Hardiness in Vitis vinifera L. ‘Chardonnay’ and ‘Riesling’. Can. J. Plant Sci. 2021, 101, 871–885. [Google Scholar] [CrossRef]
- Andersen, P.C.; Sarkhosh, A.; Huff, D.; Breman, J. The Muscadine Grape (Vitis rotundifolia Michx). EDIS 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Londo, J.P.; Johnson, L.M. Variation in the Chilling Requirement and Budburst Rate of Wild Vitis Species. Environ. Exp. Bot. 2014, 106, 138–147. [Google Scholar] [CrossRef]
- Hlubik, D.; Capik, J.M.; Molnar, T.J. Chilling and Growing Degree Day Requirements of New Hazelnut Cultivars in New Jersey. Acta Hortic. 2023, 1379, 179–186. [Google Scholar] [CrossRef]
- Makhoul, G.; Choumane, W.; Boroudi, H. Effect of Chilling Hour Requirements in Breaking Buds Dormancy in Some Al- Shami Mulberry (Morus nigra L.) Genotypes. Tishreen Univ. J. Res. Sci. Stud.-Biol. Sci. Ser. 2021, 43, 211–227. [Google Scholar]
- Navarro, A.C.; Gazquez, A.G.; Montiel, F.G. Estimación de las necesidades de frío de variedades de melocotón de forma plana (paraguayos). In Proceedings of the XIV Congreso Nacional De Ciencias Hortícolas, Orihuela, Spain, 21–22 May 2015. [Google Scholar]
- Maulión, E.; Valentini, G.H.; Kovalevski, L.; Prunello, M.; Monti, L.L.; Daorden, M.E.; Quaglino, M.; Cervigni, G.D.L. Comparison of Methods for Estimation of Chilling and Heat Requirements of Nectarine and Peach Genotypes for Flowering. Sci. Hortic. 2014, 177, 112–117. [Google Scholar] [CrossRef]
- Fadón, E.; Herrera, S.; Guerrero, B.; Guerra, M.; Rodrigo, J. Chilling and Heat Requirements of Temperate Stone Fruit Trees (Prunus sp.). Agronomy 2020, 10, 409. [Google Scholar] [CrossRef]
- Ravazi, F.; Hajilou, J.; Tabatabaci, J.; Dadpour, M.R. Comparison of Chilling and Heat Requirement in Some Peach and Apricot Cultivars. Res. Plant Biol. 2011, 1, 40–47. [Google Scholar]
- Ferlito, F.; Di Guardo, M.; Allegra, M.; Nicolosi, E.; Continella, A.; La Malfa, S.; Gentile, A.; Distefano, G. Assessment of Chilling Requirement and Threshold Temperature of a Low Chill Pear (Pyrus communis L.) Germplasm in the Mediterranean Area. Horticulturae 2021, 7, 45. [Google Scholar] [CrossRef]
- Kretzschmar, A.A.; Brighenti, L.M.; Rufato, L.; Pelizza, T.R.; Silveira, F.N.; Miquelutti, D.J.; Faoro, I.D. Chilling Requirement for Dormancy Bud Break in European Pear. Acta Hortic. 2011, 909, 85–88. [Google Scholar] [CrossRef]
- Marafon, A.C.; Citadin, I.; Amarante, L.D.; Herter, F.G.; Hawerroth, F.J. Chilling Privation during Dormancy Period and Carbohydrate Mobilization in Japanese Pear Trees. Sci. Agric. 2011, 68, 462–468. [Google Scholar] [CrossRef]
- Sawant, S.S.; Choi, E.D.; Song, J.; Seo, H.J. Pear Production Trends and Characteristics of Important Pests in India. J. Korean Soc. Int. Agric. 2021, 33, 265–269. [Google Scholar] [CrossRef]
- Tabuenca, A. Winter Chilling Requirernents of European Plum Varieties (Prunus domestica L.). An. Estac. Exp. Aula Dei 1967, 8, 311–383. [Google Scholar]
- Ruiz, D.; Egea, J.; Salazar, J.A.; Campoy, J.A. Chilling and Heat Requirements of Japanese Plum Cultivars for Flowering. Sci. Hortic. 2018, 242, 164–169. [Google Scholar] [CrossRef]
- Martínez-Valero, R.; Melgarejo, P.; Salazar, D.M.; Martínez, R.; Martínez, J.J.; Hernández, F. Phenological Stages of the Quince Tree (Cydonia oblonga). Ann. Appl. Biol. 2001, 139, 189–192. [Google Scholar] [CrossRef]
- Felker, F.C.; Robitaille, H.A. Chilling Accumulation and Rest of Sour Cherry Flower Buds. J. Am. Soc. Hortic. Sci. 1985, 110, 227–232. [Google Scholar] [CrossRef]
- Alburquerque, N.; García-Montiel, F.; Carrillo, A.; Burgos, L. Chilling and Heat Requirements of Sweet Cherry Cultivars and the Relationship between Altitude and the Probability of Satisfying the Chill Requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Wójcik, K.; Klamkowski, K.; Treder, W.; Masny, A.; Tryngiel-Gać, A. The Influence of Chilling Hours on Root Starch Content, Growth and Yield of Strawberry Tray Plants. Acta Sci. Pol. Hortorum Cultus 2023, 22, 41–51. [Google Scholar] [CrossRef]
- Aslamarz, A.A.; Vahdati, K.; Rahemi, M.; Hassani, D. Estimation of Chilling and Heat Requirements of Some Persian Walnut Cultivars and Genotypes. HortScience 2009, 44, 697–701. [Google Scholar] [CrossRef]
- Gaurha, A.; Dewangan, R.K.; Minz, V.; Shukla, S.; Shrivastava, P.; Yamini, Y. A Comprehensive Review on Fast Track Breeding of Fruit Crops: A New Approach. Plant Arch. 2024, 24, 923–932. [Google Scholar] [CrossRef]
- Naor, A.; Flaishman, M.; Stern, R.; Moshe, A.; Erez, A. Temperature Effects on Dormancy Completion of Vegetative Buds in Apple. J. Am. Soc. Hortic. Sci. 2003, 128, 636–641. [Google Scholar] [CrossRef]
- Chandler, W.H.; Brown, D.S.; Kimball, M.H.; Philip, G.L.; Tufts, W.P.; Weldon, G.P. Chilling Requirements for Opening of Buds on Deciduous Orchard Trees and Some Other Plants in California. Calif. Agric. Exp. Stat. Bull. 1937, 611, 63. [Google Scholar]
- Shaltout, A.D.; Unrath, C.R. Rest Completion Prediction Model for ‘Starkrimson Delicious’ Apples. J. Am. Soc. Hortic. Sci. 1983, 108, 957–961. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Szot, I. The Use of Temperature Based Indices for Estimation of Fruit Production Conditions and Risks in Temperate Climates. Agriculture 2023, 13, 960. [Google Scholar] [CrossRef]
- Weinberger, J.H. Chilling Requirements of Peach Varieties. Proc. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Bennet, J.P. Temperature and Bud Rest Period. Calif. Agric. 1949, 3, 9–12. [Google Scholar]
- Richardson, E.; Seeley, S.D.; Walker, D.R. A Model for Estimating the Completion of Rest for ‘Redhaven’ and ‘Elberta’ Peach Trees1. HortScience 1974, 9, 331–332. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The Temperature Dependence of Dormancy Breaking in Plants: Computer Simulation of Processes Studied under Controlled Temperatures. J. Theor. Biol. 1987, 126, 309–321. [Google Scholar] [CrossRef]
- Ruiz, D.; Campoy, J.; Egea, J. Chilling and Heat Requirements of Apricot Cultivars for Flowering. Environ. Exp. Bot. 2007, 61, 254–263. [Google Scholar] [CrossRef]
- Erez, A.; Fishman, S.; Linsley-Noakes, G.C.; Allan, P. The Dynamic Model For Rest Completion in Peach Buds. Acta Hortic. 1990, 276, 165–174. [Google Scholar] [CrossRef]
- Finetto, G. Are the Chilling Temperatures the Only Climatic Factor to Overcome Endo-Dormancy in the Temperate Fruit Trees? Which Other Climatic Factors Can Affect the Endo-Dormancy and the Effect of the Chilling Temperatures? Acta Hortic. 2020, 1281, 421–436. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P.; Zydlik, Z. Effect of Nitrogen Fertilization in the Sour Cherry Orchard on Soil Enzymatic Activities, Microbial Population, and Fruit Quality. Agriculture 2022, 12, 2069. [Google Scholar] [CrossRef]
- Hassan, G.I.; Wani, A.W.; Dar, S.Q.; Wani, M.Y.; Sofi, J.A.; Baba, T.R.; Parray, E.; Rasool, A. Physiology of Fruit Set and Development in Apple under Temperate Conditions: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 618–638. [Google Scholar] [CrossRef]
- Badr, G.; Hoogenboom, G.; Abouali, M.; Moyer, M.; Keller, M. Analysis of Several Bioclimatic Indices for Viticultural Zoning in the Pacific Northwest. Clim. Res. 2018, 76, 203–223. [Google Scholar] [CrossRef]
- Kryza, M.; Szymanowski, M.; Błaś, M.; Migała, K.; Werner, M.; Sobik, M. Observed Changes in SAT and GDD and the Climatological Suitability of the Poland-Germany-Czech Republic Transboundary Region for Wine Grapes Cultivation. Theor. Appl. Climatol. 2015, 122, 207–218. [Google Scholar] [CrossRef]
- Ruml, M.; Milatović, D.; Vuković, A. Predicting Flowering of Apricot Cultivars Using Growing Degree Days. Acta Hortic. 2012, 966, 87–91. [Google Scholar] [CrossRef]
- Lanzes, T.; Khenrab, S.; Sharma, P. Low Chilling Apples: Sustainable Orchards Amidst Climate Change. Agric. Food E-Newsl. 2024, 6, 357–360. [Google Scholar]
- Guo, L.; Dai, J.; Ranjitkar, S.; Yu, H.; Xu, J.; Luedeling, E. Chilling and Heat Requirements for Flowering Temperate Fruit Trees. Int. J. Biometeorol. 2014, 58, 1195–1206. [Google Scholar] [CrossRef]
- Sarisu, H. Change of Flowering and Harvest Dates of CherryVarieties with Air Temperature. Pol. J. Environ. Stud. 2020, 30, 351–359. [Google Scholar] [CrossRef]
- Sun, L.; Nie, T.; Chen, Y.; Yin, Z. From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants. Int. J. Mol. Sci. 2022, 23, 10959. [Google Scholar] [CrossRef]
- Łysiak, G. The Sum of Active Temperatures as a Method of Determining the Optimum Harvest Date of “Šampion” and “Ligol” Apple Cultivars. Acta Sci. Pol.—Hortorum Cultus 2012, 11, 3–13. [Google Scholar]
- Ruml, M.; Milatović, D.; Đurđević, M.; Boškov, Đ. Heat Requirements for Flowering of European and Japanese Plum Cultivars in the Belgrade Region. Acta Hortic. 2021, 1322, 215–220. [Google Scholar] [CrossRef]
- White, S.N.; Boyd, N.S.; Van Acker, R.C. Growing Degree-Day Models for Predicting Lowbush Blueberry (Vaccinium angustifolium Ait.) Ramet Emergence, Tip Dieback, and Flowering in Nova Scotia, Canada. HortScience 2012, 47, 1014–1021. [Google Scholar] [CrossRef]
- Verdugo-Vásquez, N.; Fuente, C.P.-D.L.; Ortega-Farías, S. Model Development to Predict Phenological Scale of Table Grapes (Cvs. Thompson, Crimson and Superior Seedless and Red Globe) Using Growing Degree Days. OENO One 2017, 51, 277–288. [Google Scholar] [CrossRef]
- El-Baz, E.; Arafa, A.; Awad, W. Studies on Growing Degree Days Required for Growth and Fruiting of Some Peach Trees. J. Plant Prod. 2007, 32, 6575–6582. [Google Scholar] [CrossRef]
- Rafael, A.; Biasi, L.A. Base Temperature as a Function of Genotype: A Foundation for Modeling Phenology of Temperate Fruit Species. Semin. Ciências Agrárias 2016, 37, 1811. [Google Scholar] [CrossRef]
- Zavalloni, C.; Andresen, J.A.; Flore, J.A. Phenological Models of Flower Bud Stages and Fruit Growth of `Montmorency’ Sour Cherry Based on Growing Degree-Day Accumulation. J. Am. Soc. Hortic. Sci. 2006, 131, 601–607. [Google Scholar] [CrossRef]
- Hochmaier, V. Chilling Unit Accumulation and Degree-day Requirements of Four Sweet Cherry (Prunus avium L.) Cultivars. Acta Hortic. 2014, 1020, 203–207. [Google Scholar] [CrossRef]
- Alvarado-Raya, H.E.; Vázquez-Rodríguez, J.C.; Ramírez-Arias, A.; Calderón-Zavala, G.; Rivera-del Río, R. Phenology and Growing Degree Days of Festival Strawberry Grown on Red Volcanic Rock at Two Plant Densities. Rev. Fitotec. Mex. 2021, 44, 349. [Google Scholar] [CrossRef]
- Soleimani, A.; Dastjerdi, R.; Nankali, A.; Rahemi, A.; Hassani, D. Growing-Degree-Days (GDDs) in a Uniform Phenology Scale (BBCH) for Two Walnut (Juglans regia L.) Cultivars. J. Hortic. Sci. Biotechnol. 2024, 100, 371–384. [Google Scholar] [CrossRef]
- Gambardella, M.; Contreras, E.; Alcalde, J.; Neri, D. Phenotyping Primocane Fruiting Trait in Raspberry (Rubus idaeus). Acta Hortic. 2016, 1133, 67–74. [Google Scholar] [CrossRef]

| Species | Place of Flower Bud Formation | Description of Buds | Description of Flowers | Description of Fruit |
|---|---|---|---|---|
| Monoecious species | ||||
| Corylus avellana. | Male buds are single in the axils of the scaly leaflets. Female buds on the flanks or at the end of last year’s shoot. | Male buds in the form of long, roller-shaped catkins. Female buds are ovoid and flattened. | Male in inflorescences (♂K0C0A4G0). Female as small, red-centred clusters (♀K0C0A0G2). | Globular-ovoid achene composed of a woody pericarp containing the kernel, an edible nut [5]. |
| Juglans regia | Male flowers at the end of the shoot and on the axils of the leaves. Female flowers at the top of the shoots from this year. | Male flower buds, single, less often several, in the form of shortened catkins. Female flower buds are gathered in spikes of 2–24, depending on the cultivar. | Male inflorescences, 5–10 cm long with many stamens (♂K0C0A~G0). The female flower has a pistil with two stigmas (♀K0C0A0G2). | A pseudo-drupe, it is formed from a lower pistil, covered with the remains of the perianth. The soft covering is the exocarp, whereas the hard shell is the endocarp. |
| Hermaphoriditic species | ||||
| Cornus mas | The flowers are on short stems, opposite each other. | The flower buds are round and larger. The leaf buds are narrowly conical and pointed [6]. | Small yellow flowers are clustered in umbels (* K4C4A4G1). | A juicy drupe weighing about 2–8 g. |
| Vaccinium corymbosum | The flowers are at the ends of one-year-old shoots or on short shoots [7]. | Flower buds (inflorescence) are clearly different from leaf buds. | The petals of the corolla of the flowers (4–5) and the sepals of the calyx are fused, white or pinkish, and bell-shaped. The calyx is fused to the ovary. | A pseudoberry that develops not only from the lower ovary but also from the sepals and the floral receptacle [8]. |
| Vitis vinifera | Flowers are formed on fruiting shoots, growing on 3–4 nodes of the stem. | Buds are formed on a one-year-old cane. | The flowers are inconspicuous, with yellow-green petals of the corolla, gathered in panicle-type inflorescences. | Berry. |
| Amelanchier canadensis | The flowers are on one-year-old, well-lit shoots. | Flower buds are thicker and rounder than vegetative ones. | White, gathered in several or a dozen or so clusters of inflorescences, 12 cm long | False fruits, developed from the ovary and the expanded floral receptacle, are spherical, and have a mass of 0.5–0.7 g. |
| Aronia melanocarpa | Flowers at the ends of the branches [9]. | Flower and leaf buds are similar. Leaf buds are smaller, pointed and attached to the shoot. | Flowers in corymbs, white or pale pink, with stamens with purple anthers. | False fruits, about 1 g, dark blue, almost black, with a waxy coating. |
| Chaenomeles japonica | The flowers are grouped on short shoots. | Flower heads are spherical, arranged in several. Leaves in threes, central conical, lateral ones smaller and almost spherical. | Brick red, sitting on branches in bunches of 2–6, up to 5 cm in diameter. | Pseudo-fruit, yellow, apple-shaped, sometimes with a pink-red blush. The flesh is hard, sour, and very aromatic. |
| Crataegus monogyna, C. laevigata | Flowers set on short shoots. | The terminal buds are wide and conical; the lateral buds are ovate or almost spherical, protruding. The flower buds are slightly larger. | White or pink, five-petalled, forming terminal corymbs. C. monogyna has one pistil (* K5C5A~G-1) and C. laevigata has two (* K5C5A~G-2). | Pseudo-fruit, apple-like, spherical, brownish-red. The fruit of C. monogyna has one seed with a brittle hull, and C. laevigata has two or three seeds without a hull. |
| Cydonia oblonga | The flowers are formed on short shoots from the previous year and on short shoots. | The buds are conical, rounded at the top, adhering to the shoot. | White-pink, five petalled (K5C5A~G-(5)). The flowers are on short, hairy pedicels. | A pseudofruit. Shape variable, depending on cultivar. It has large, often green calyx sepals. Lemon yellow, covered with hairs. |
| Malus domestica | The location of flower buds, depending on the cultivar, on short shoots or long shoots. | The flower buds are always larger, rounded and more hairy. The leaf buds are pointed. | Inflorescences with 5–7 flowers. Flowers on 1–2 cm pedicels, white or pink, five-petalled, 5 sepals, 15–50 stamens, and 1 lower pistil (K5C5A~G-(5)). | Pseudofruit, formed from the ovary, the floral receptacle, the sepals, the petals of the corolla and the stamens. Fruit weight from 25 to 300 g. |
| Mespilus germanica | The flowers develop at the ends of short branches. | The winter buds are pointed, ovoid and up to 5 mm. | The flowers are single, five-petalled, white (K5C5AG-(5)). | Pseudofruit, spherical or pear-shaped, with 5 calyx sepals at the top. Weight from 3 to 35 g. |
| Pyrus communis | Flowers on the tops of shoots, 2–3-year-old wood and on short shoots. | Mixed buds are larger and rounder than leaf buds. | Flowers are 5–9 in number, collected in umbels of shaped inflorescences (K5C5A~G-(5)). | Pseudofruit. It has stone cells that form around the sieve-vascular bundles. Fruit weight from 25 to over 300 g. |
| Sorbus aucuparia | The flowers are formed at the ends of the shoots and on short shoots. | Mixed buds: the apical ones are large, slightly bent at the top and indistinctly three-scaled; the lateral ones are apparently single-scaled. | Fivefold (* K5C5A~G-(3–5)), numerous, white-yellow, small, gathered in terminal corymbs. | A pseudo-fruit in the shape of a berry. Initially orange, ripe scarlet red. |
| Prunus armeniaca | Flowers are formed on one-year-old shoots and spurs. | The leaves are smaller and broadly conical; the flowers are larger and spherical. | The flower is almost sedentary. The petals are white or slightly pink. | A drupe weighing from 9 to 90 g [9]. |
| Prunus avium | The flowers are most often found at the base of long shoots and on short shoots. | The buds are not very differentiated, the flower buds are larger, wider and rounder. | Flowers (* K5C5A10G1) are gathered in umbels, usually 2–3, white. | Drupe. |
| Prunus cerasifera | The flowers are on spurs of perennial shoots. | Single or triple leaf buds, central leaf bud conical, lateral flower buds ovate. | Flowers (* K5C5A10G1) in buds are usually single, diameter 2–2.5 cm. | A spherical drupe weighing about 20–30 g, yellow or red in colour. |
| Prunus cerasus | The flowers are on one-year-old shoots or at the base of two-year-old shoots. | The buds are not very differentiated. Ovoid, pointed at the top, deviating from the shoot. | Flowers (* K5C5A10G1) are gathered in several in the inflorescence, white. | A spherical, single-seeded drupe of red colour. |
| Prunus domestica | Flower buds are formed on long and short shoots, often several in number. | The leaf bud is pointed; the flower bud is smaller and more rounded. | Flowers (* K5C5A10G1), gathered in groups of 2–3 on short pedicels, 1.5–3 cm in diameter. | A drupe weighing 10–50 g. |
| Prunus persica | Flower buds are formed on one-year-old shoots. | Flower buds broadly ovate, leaf buds smaller, narrowly conical, often in threes: central leaf bud and lateral flower buds. | Two types of flowers, depending on the cultivar. With wide petals—rosaceous—and with narrow petals—bell-shaped (* K5C5A10G1). | Spherical drupe weighing 60 to 300 g. |
| Prunus serotina | The flowers are formed on the tops of the shoots and young shoots. | Buds narrowly egg-shaped, pointed, adhering to or slightly protruding from the shoot. | White flowers (* K5C5A10G1) are gathered in clusters. | A single-seeded drupe, spherical, black, and shiny. |
| Prunus spinosa | The flowers are formed mainly at the end of young shoots. | The buds are small, single or in pairs or threes. Leaf buds are wider and pointed; flower buds are spherical. | Flowers singly (* K5C5A10G1) or in clusters of 2 (−5), up to 20 mm in diameter. | A spherical drupe, navy blue in colour, covered with a waxy coating. |
| Fragaria ananassa, F. vesca | The flowers arise from leaf buds on the shoot crown. | Flower buds of 5–25 flowers in the inflorescence at the top in the rosette. | White corolla, occurring singly or in inflorescence (* K5C5A~G~). | Aggregate fruits, developed from multiple ovaries with the fleshy part being the swollen receptacle and the “seeds” being the actual fruits (achenes). |
| Rosa canina | Flowers are formed on woody shoots that are two years old or older. | Apical buds are wide, rounded, lateral protruding, and variable in shape. | The flowers are pink or whitish, growing singly or in groups of several on long pedicels. | Rose hip—an actual fruits—achenes are embedded inside a fleshy and colourful flower receptacle, the so-called hypanthium. |
| Rubus idaeus | The flowers grow at nodes on annual shoots or at the tips of shoots and in the axils of the upper leaves. | The terminal buds are slightly larger than the axillary buds. The lateral buds are conical and andhered to the shoot. | Large, fivefold (* K5C5A~G~) flowers with a white corolla are gathered in clusters. | Aggregate fruit, composed of numerous small fruits, called drupelets, that are fused together on a single receptacle. |
| Ribes glossularia | Flower buds on short shoots, 3–4-year-old branches. | Narrowly conical buds, slightly deviated from the shoot. | Flowers (* K5C5A5G-(2)) small, bell-shaped, pendulous, single or 2–3 in short clusters, largely self-pollinating. | A spherical or oval berry, yellow, green or red, covered with glandular hairs. |
| Ribes nigrum | Flowers are most numerous on one- or two-year-old shoots. | Buds are ovoid, set on short stems, deviating from the shoot. | Small, cup-shaped flowers (* K5C5A5G-(2)), with reddish petals, gathered in hanging clusters of 4 to 40 flowers. | A black, spherical berry with a dried perianth at the top. |
| Genus (in English) | May | June | July | August | September | October | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | I | II | III | I | II | III | I | II | III | |
| Raspbery primocane | ||||||||||||||||||
| Walnut | ♂ | ♀ | ||||||||||||||||
| Hazelnut | ♂ | ♀ | ||||||||||||||||
| Sour cherry Sweet cherry | ||||||||||||||||||
| Red curant | ||||||||||||||||||
| Vine | ||||||||||||||||||
| Pear | ||||||||||||||||||
| Apricot | ||||||||||||||||||
| Apple Plum Rapsberry floricane Blackberry Gooseberry | ||||||||||||||||||
| Black currant | ||||||||||||||||||
| Peach Highbush blueberry | ||||||||||||||||||
| Strawberry | ||||||||||||||||||
 —Initiation period.
—Initiation period.| Genus (in English) | Species (in Latin) | Chilling Hours | References |
|---|---|---|---|
| Almond | Prunus dulcis | 8–713 | [87,88] |
| Apple | Malus domestica | 200–2900 | [89,90,91] |
| Apricot (European) | Prunus armeniaca | 171–1812 | [84,92] |
| Apricot (Japanese) | Prunus mume | 239–1148 | [93] |
| Blackberry | Rubus sp. | 200–800 | [94] |
| Blueberry | Vaccinium sp | 150–1100 | [95] |
| Currant | Ribes sp. | 800–1600 | [96,97] |
| Goosberry | Ribes grossularia | [98] | |
| Grapes | Vitis vinifera | 100–500 | [99] |
| Vitis rotundifolia | 500–1000 | [100] | |
| Vitis labrusta | 1000–1400 | [101] | |
| Hazelnut | Corylus avellana | 800–1600 | [102] |
| Mulbery | Morus nigra | 400–1400 | [103] |
| Peach | Flat peach (Prunus persica) | 239–536 | [104] |
| Nectarine (Prunus persica) | 93–426 | [105] | |
| Peach (Prunus persica) | 79–1390 | [106,107] | |
| Pear | Pyrus communis | 400–1050 | [108,109] |
| Pyrus pyrifolia | 150–720 | [110,111] | |
| Plum (European) | Prunus domestica | 579–1323 | [112] |
| Plum (Japanese) | Prunus saliciana | 118–685 | [112,113] |
| Quince | Cydonia oblonga | 100–400 | [114] |
| Sour cherry | Prunus cerasus | 700–1200 | [115] |
| Sweet cherry | Prunus avium | 176–1100 | [112,116] |
| Strawberry | Fragaria x ananassa | 200–800 | [117] |
| Walnut | Juglans sp. | 400–1500 | [118] |
| Model | Description | References |
|---|---|---|
| Chilling Hours (CH) | Temperature 0–7.2 °C | [121,124,125] |
| Chilling Units (CU), syn. Utah model | 1 h below 1.4 °C—0.0 chill units 1 h between 1.5 and 2.4 °C = 0.5 chill units 1 h between 2.5 and 9.1 °C = 1.0 chill units 1 h between 9.2 and 12.4 °C—0.5 chill units 1 h between 12.5 and 15.9 °C = 0.0 chill units 1 h between 19 and 18 °C = −0.5 chill units 1 h above 18.1 °C = −1.0 chill units | [70,126] |
| Chilling Portions (CP), syn. dynamic model | The dynamic model assumes that winter cold accumulates in a two-step process. Initially, low temperatures lead to the formation of an intermediate product. Once a certain amount of this intermediate product has been accumulated, it can be converted into what is known as a chill portion in a process requiring relatively high temperatures. | [127] |
| Species (in Latin) | February | March | April | May | June | July | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | I | II | III | I | II | III | I | II | III | |
| Corylus avellana | ♂ | ♂ | ♀ | ♀ | ||||||||||||||
| Cornus mas | ||||||||||||||||||
| Prunus cersifera | ||||||||||||||||||
| Prunus armeniaca | ||||||||||||||||||
| Ribes grossularia | ||||||||||||||||||
| Ribes nigrum | ||||||||||||||||||
| Chaenomeles japonica | ||||||||||||||||||
| Prunus persica | ||||||||||||||||||
| Prunus cerasus | ||||||||||||||||||
| Pyrus communis | ||||||||||||||||||
| Prunus domestica | ||||||||||||||||||
| Malus domestica | ||||||||||||||||||
| Amelanchier alnifolia | ||||||||||||||||||
| Fragaria x ananassa | ||||||||||||||||||
| Juglans regia | ♂ | ♂ | ♂ | ♀ | ♀ | |||||||||||||
| Rubus idaeus | flII prI | flII, prI | flII, prI | flI, prI | prI | prI | prI | prI | prI | |||||||||
| Aronia melanocarpa | ||||||||||||||||||
| Vaccinium corymbosum | ||||||||||||||||||
| Crataegus monogyna, C. laevigata | ||||||||||||||||||
| Cydonia oblonga | ||||||||||||||||||
| Mespilus germanica | ||||||||||||||||||
| Sorbus aucuparia | ||||||||||||||||||
| Rosa canina | ||||||||||||||||||
| Vitis vinifera | ||||||||||||||||||
 —flowering period; fl—floricane raspberries, pr—primocane raspberries.
—flowering period; fl—floricane raspberries, pr—primocane raspberries.| Species | Flowering | How Flowers Are Pollinated |
|---|---|---|
| Corylus avellana | There is a phenomenon of nonsimultaneous development of male and female flowers. Male flowers bloom even before the leaves develop. | Anemophilous, bees collect only pollen from hazel trees (there are no honey and nectaries) and do not participate in the pollination of flowers. Almost all hazel are open-pollinated. |
| Cornus mas | For about 30 days, before leaf development. | Open-pollinated, insect-pollinated. |
| Chaenomeles japonica | Mostly before the leaves appear. | The flowers are eagerly visited by insects, especially bumblebees. |
| Prunus cersifera | With leaf development. | Most cultivars are self-pollinating. |
| Prunus armeniaca | before leaf development for about 8 days. | Most cultivars are self-fertile. |
| Ribes grossularia | For about 2 weeks. | The flowers secrete nectar abundantly on the entire surface of the flower base and are eagerly flown to by insects. |
| Ribes nigrum | For about 2 weeks. | It provides bees with nectar and pollen, and if there are no other flowering plants in the area, it is quite eagerly visited by them. |
| Prunus avium | For 10–12 days. | All are open-pollinated; intersteriality occurs between some pairs of cultivars. |
| Prunus persica | Before leaves appear. | Most cultivars are self-pollinating. |
| Prunus cerasus | The flowers develop almost simultaneously with the leaves. | Most cultivars are self-pollinating. |
| Pyrus communis | With leaf development. | It requires cross-pollination. Not very eagerly visited by bees. They are prone to parthenocarpy. |
| Prunus domestica | With the development of leaves or shortly before them. | Self- and open-pollinated, generally pollinated by insects |
| Malus domestica | With leaf development. | Most cultivars are open-pollinated by insects. |
| Amelanchier alnifolia | Slightly earlier than leaves develop. | Self-pollinating, pollinated by insects or wind. |
| Fragaria x ananassa | The flowers develop consecutively, depending on their position at the apex, for 3–4 weeks. They are the first to bloom in the middle of the inflorescence. | The vast majority of cultivars are self-pollinating; only dioecious cultivars need a pollinator. |
| Juglans regia | The male flowers begin right after the development of leaves and can last for 30 days. Female later, when the leaves are already developed. | Anemophilous, pollen is carried by the wind only up to 100 m. In exceptional conditions, the fruits can develop without fertilisation. Rain makes dust very difficult. |
| Rubus idaeus | About 3 weeks. | Most cultivars are self-pollinating or insect-pollinated. |
| Aronia melanocarpa | About 2 weeks. | It is insect-pollinated, but in unfavourable conditions it can self-pollinate. Pollination with foreign pollen increases fruit set. |
| Vaccinium corymbosum | About 3 weeks. | Most cultivars are self-pollinating, but cross-pollination guarantees a higher yield. |
| Crataegus monogyna, C. laevigata | The lower flowers open first, and the axis of a corymb continues to produce flowers (indeterminate growth). | Open-pollinated, insect-pollinated. |
| Cydonia oblonga | The flowers develop later than the leaves. | Most cultivars need cross-pollination. |
| Mespilus germanica | Flowering 6—11 days. | Most varieties are self-pollinating. |
| Sorbus aucuparia | After leaf development. | Most cultivars are self-pollinating. |
| Rosa canina | Flowering for about 30 days. | Flowers do not give nectar, only pollen. |
| Vitis vinifera | Flowering for 10–14 days. | Mostly self-pollinating. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szot, I.; Łysiak, G.P. Factors Influencing the Formation, Development of Buds, and Flowering of Temperate Fruit Trees. Agriculture 2025, 15, 1304. https://doi.org/10.3390/agriculture15121304
Szot I, Łysiak GP. Factors Influencing the Formation, Development of Buds, and Flowering of Temperate Fruit Trees. Agriculture. 2025; 15(12):1304. https://doi.org/10.3390/agriculture15121304
Chicago/Turabian StyleSzot, Iwona, and Grzegorz P. Łysiak. 2025. "Factors Influencing the Formation, Development of Buds, and Flowering of Temperate Fruit Trees" Agriculture 15, no. 12: 1304. https://doi.org/10.3390/agriculture15121304
APA StyleSzot, I., & Łysiak, G. P. (2025). Factors Influencing the Formation, Development of Buds, and Flowering of Temperate Fruit Trees. Agriculture, 15(12), 1304. https://doi.org/10.3390/agriculture15121304






