Nano-Copper Supplementation Reduces Fecal Copper Excretion and Enhances Piglet Performance Under Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal, Diets, and Experimental Design
- (1)
- Basal diet without copper supplementation (Basal);
- (2)
- Basal diet + 50 mg/kg of Cu as copper sulfate (LC);
- (3)
- Basal diet + 150 mg/kg of Cu as copper sulfate (HC);
- (4)
- Basal diet + 50 mg/kg of Cu as nano-copper oxide (LNC);
- (5)
- Basal diet + 150 mg/kg of Cu as nano-copper oxide (HNC).
2.2. Animal Performances and Sampling
2.3. Measurement of Cu Concentration in Feces and Feed
2.4. Measurement of Cu Concentration in Serum
2.5. Measurement of Nutrient Digestibility
2.6. Cytokines Measurements
2.7. Measurements of Antioxidant-Related Enzymes and Metabolites
2.8. Analysis of Jejunal and Ileal Morphology
2.9. Quantitative Real-Time PCR (RT-qPCR) Analysis
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Nutrient Digestibility
3.3. Concentration in Serum, Feed, and Feces and Digestibility of Cu
3.4. Intestinal Health
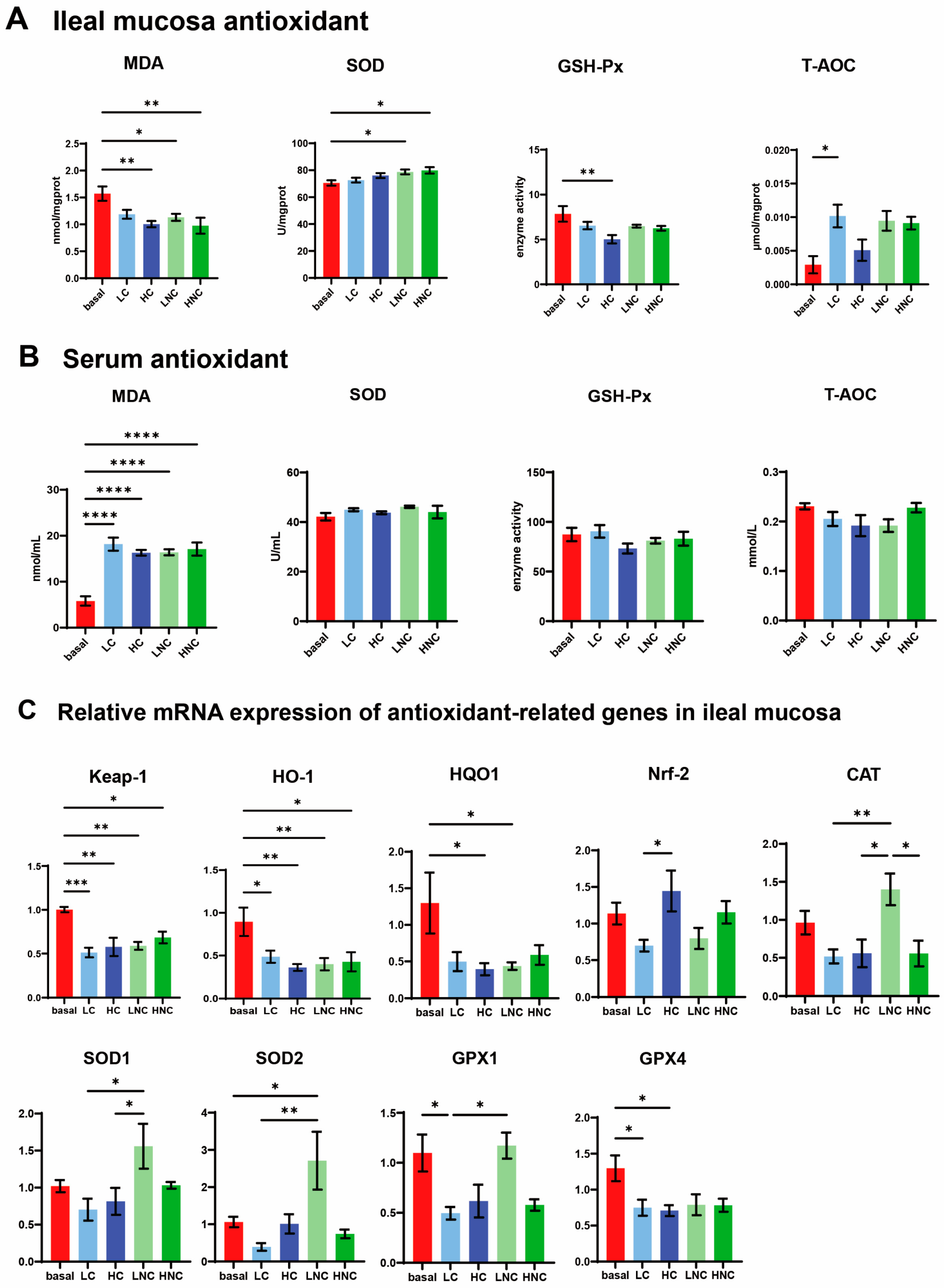
3.5. Anti-Oxidation
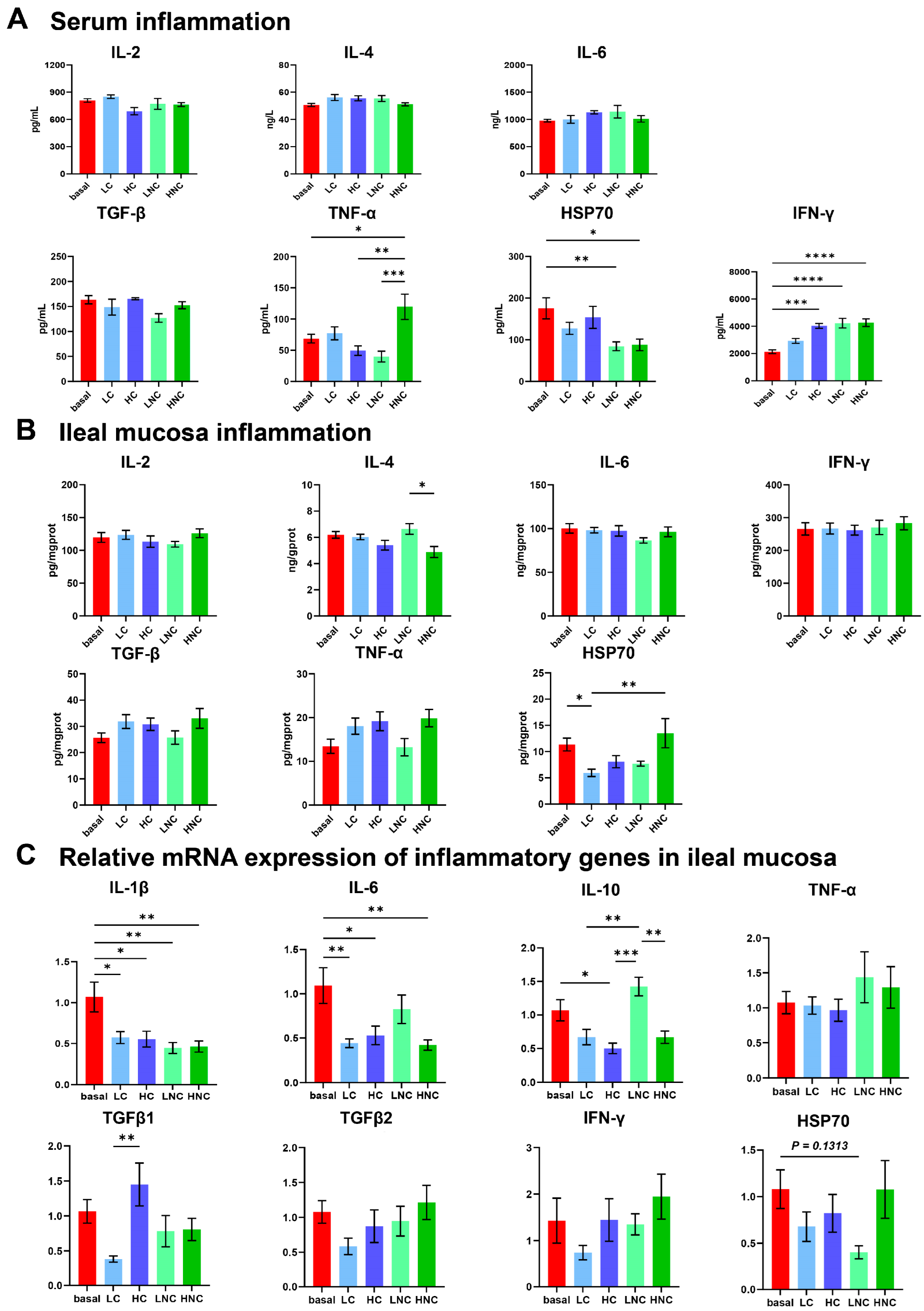
3.6. Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ATTD | Apparent total tract digestibility |
| BAD | Bcl-2-associated death promoter |
| Bax | B-cell lymphoma-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| BUN | Blood urea nitrogen |
| Ca | Calcium |
| Caspase-3 | Cysteine-aspartic acid protease-3 |
| Caspase-8 | Cysteine-aspartic acid protease-8 |
| Caspase-9 | Cysteine-aspartic acid protease-9 |
| CAT | Catalase |
| CP | Crude protein |
| DM | Dry matter |
| EE | Ether extract |
| F:G | Feed-to-gain ratio |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GPX1 | Glutathione peroxidase 1 |
| GPX4 | Glutathione peroxidase 4 |
| GSH-Px | Glutathione peroxidase |
| HC | High copper sulfate |
| HNC | High nano-copper |
| HO-1 | Heme oxygenase-1 |
| HSP70 | Heat shock protein 70 |
| IFN-γ | Interferon-γ |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1β |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| Keap-1 | Kelch-like ECH-associated protein 1 |
| LC | Low copper sulfate |
| LNC | Low nano-copper |
| MDA | Malondialdehyde |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| SOD1 | Superoxide dismutase 1 |
| SOD2 | Superoxide dismutase 2 |
| STTD | Standard total tract digestibility |
| T-AOC | Total antioxidant capacity |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| TP | Total phosphorus |
| T-SOD | Total superoxide dismutase |
| ZO-1 | Zonula occludens-1 |
References
- Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper Sources for Sod1 Activation. Antioxidants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chae, J.H.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, J.; Ju, J.S.; Choi, P.S.; Park, J.H. Theragnostic (64)Cu/(67)Cu Radioisotopes Production With RFT-30 Cyclotron. Front. Med. 2022, 9, 889640. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Gill, C.A.; Kurlak, L.O.; Seed, P.T.; Hesketh, J.E.; Méplan, C.; Schomburg, L.; Chappell, L.C.; Morgan, L.; Poston, L. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks’ gestation in nulliparous women who subsequently develop preeclampsia. Free. Radic. Biol. Med. 2015, 78, 147–155. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, J.Y.; Park, S.H.; Jung, H.; Kim, M. Effects of dietary copper sources and levels on growth performance, copper digestibility, fecal and serum mineral characteristics in growing pigs. J. Anim. Sci. Technol. 2022, 64, 885–896. [Google Scholar] [CrossRef]
- Forouzandeh, A.; Blavi, L.; Perez, J.F.; D’Angelo, M.; Gonzalez-Sole, F.; Monteiro, A.; Stein, H.H.; Sola-Oriol, D. How copper can impact pig growth: Comparing the effect of copper sulfate and monovalent copper oxide on oxidative status, inflammation, gene abundance, and microbial modulation as potential mechanisms of action. J. Anim. Sci. 2022, 100, skac224. [Google Scholar] [CrossRef]
- Xiong, X.; Yanxia, L.; Wei, L.; Chunye, L.; Wei, H.; Ming, Y. Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resour. Conserv. Recycl. 2010, 54, 985–990. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Stein, H.H. Digestibility and metabolism of copper in diets for pigs and influence of dietary copper on growth performance, intestinal health, and overall immune status: A review. J. Anim. Sci. Biotechnol. 2021, 12, 13. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.T.; Hu, H.W.; Ma, Y.B.; Zhang, L.M.; He, J.Z. Copper pollution decreases the resistance of soil microbial community to subsequent dry-rewetting disturbance. J. Environ. Sci. 2016, 39, 155–164. [Google Scholar] [CrossRef]
- Zhen, Y.; Ge, L.; Chen, Q.; Xu, J.; Duan, Z.; Loor, J.J.; Wang, M. Latent Benefits and Toxicity Risks Transmission Chain of High Dietary Copper along the Livestock-Environment-Plant-Human Health Axis and Mic robial Homeostasis: A Review. J. Agric. Food Chem. 2022, 70, 6943–6962. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Y.; Yang, Y.; Toor, G.S.; Zhang, X. Changes in heavy metal contents in animal feeds and manures in an intensive animal production region of China. J. Environ. Sci. 2013, 25, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Duan, G.; Han, M.; Gong, S.; Yang, Z.; Duan, Y.; Guo, Q.; Chen, Q.; Li, F. The Effect of Dietary Leucine Supplementation on Antioxidant Capacity and Meat Quality of Finishing Pigs under Heat Stress. Antioxidants 2022, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; Bouwmeester, H.; Gottardo, S.; Amenta, V.; Arena, M.; Brandhoff, P.; Marvin, H.J.P.; Mech, A.; Moniz, F.B.; Pesudo, L.Q.; et al. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci. Technol. 2016, 54, 155–164. [Google Scholar] [CrossRef]
- Tsao, C.W.; Ke, P.S.; Yang, H.Y.; Chang, T.C.; Liu, C.Y. Curcumin Remedies Testicular Function and Spermatogenesis in Male Mice with Low-Carbohydrate-Diet-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2022, 23, 10009. [Google Scholar] [CrossRef]
- Sharif, M.; Rahman, M.A.; Ahmed, B.; Abbas, R.Z.; Hassan, F.U. Copper Nanoparticles as Growth Promoter, Antioxidant and Anti-Bacterial Agents in Poultry Nutrition: Prospects and Future Implications. Biol. Trace Elem. Res. 2021, 199, 3825–3836. [Google Scholar] [CrossRef]
- FAO. State Food Agriculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Wen, Y.; Yang, L.; Wang, Z.; Liu, X.; Gao, M.; Zhang, Y.; Wang, J.; He, P. Blocked conversion of Lactobacillus johnsonii derived acetate to butyrate mediates copper-induced epithelial barrier damage in a pig model. Microbiome 2023, 11, 218. [Google Scholar] [CrossRef]
- Lee, J.; Hosseindoust, A.; Kim, M.; Kim, K.; Kim, T.; Moturi, J.; Chae, B. Effects of hot-melt extruded nano-copper on the Cu bioavailability and growth of broiler chickens. J. Anim. Sci. Technol. 2021, 63, 295–304. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Abdel-Rahman, G.; Elsisi, G.F.; Ayyat, M.S. Comparative effects of supplementary different copper forms on performance, protein efficiency, digestibility of nutrients, immune function and architecture of liver and kidney in growing rabbits. Anim. Biotechnol. 2023, 34, 2240–2250. [Google Scholar] [CrossRef]
- FAO. Climate-Smart Agriculture Sourcebook; FAO: Rome, Italy, 2013. [Google Scholar]
- Yue, Z.; Zhang, J.; Zhou, Z.; Ding, C.; Wan, L.; Liu, J.; Chen, L.; Wang, X. Pollution characteristics of livestock faeces and the key driver of the spread of antibiotic resistance genes. J. Hazard. Mater. 2021, 409, 124957. [Google Scholar] [CrossRef]
- Goel, A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1136–1145. [Google Scholar] [CrossRef]
- Medardus, J.J.; Molla, B.Z.; Nicol, M.; Morrow, W.M.; Rajala-Schultz, P.J.; Kazwala, R.; Gebreyes, W.A. In-feed use of heavy metal micronutrients in U.S. swine production sys tems and its role in persistence of multidrug-resistant salmonellae. Appl. Environ. Microbiol. 2014, 80, 2317–2325. [Google Scholar] [CrossRef]
- Wapnir, R.A. Copper absorption and bioavailability. Am. J. Clin. Nutr. 1998, 67, 1054S–1060S. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, R.; Piao, X.; Lin, G.; He, P. Different copper sources and levels affect growth performance, copper content, carcass characteristics, intestinal microorganism and metabolism of finishing pigs. Anim. Nutr. 2022, 8, 321–330. [Google Scholar] [CrossRef]
- Coble, K.F.; DeRouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Woodworth, J.C.; Usry, J.L. The effects of copper source and concentration on growth performance, carcass characteristics, and pen cleanliness in finishing pigs. J. Anim. Sci. 2017, 95, 4052–4059. [Google Scholar] [CrossRef]
- Mastrorilli, E.; Pietrucci, D.; Barco, L.; Ammendola, S.; Petrin, S.; Longo, A.; Mantovani, C.; Battistoni, A.; Ricci, A.; Desideri, A.; et al. A Comparative Genomic Analysis Provides Novel Insights Into the Ecological Success of the Monophasic Salmonella Serovar 4,[5],12:i. Front. Microbiol. 2018, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Sekizuka, T.; Tamamura, Y.; Kusumoto, M.; Hinenoya, A.; Yamasaki, S.; Iwata, T.; Watanabe-Yanai, A.; Kuroda, M.; Akiba, M. Salmonella Genomic Island 3 Is an Integrative and Conjugative Element and Contributes to Copper and Arsenic Tolerance of Salmonella enterica. Antimicrob. Agents Chemother. 2019, 63, 564534. [Google Scholar] [CrossRef]
- He, J.; Guo, H.; Zheng, W.; Xue, Y.; Zhao, R.; Yao, W. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Pardo, Z.; Fernández-Fígares, I.; Lachica, M.; Lara, L.; Nieto, R.; Seiquer, I. Impact of Heat Stress on Meat Quality and Antioxidant Markers in Iberian Pigs. Antioxidants 2021, 10, 1911. [Google Scholar] [CrossRef]
- Wang, T.; Xiang, P.; Ha, J.H.; Wang, X.; Doguer, C.; Flores, S.R.L.; Kang, Y.J.; Collins, J.F. Copper supplementation reverses dietary iron overload-induced pathologies in mice. J. Nutr. Biochem. 2018, 59, 56–63. [Google Scholar] [CrossRef]
- Pan, Z.; He, X.; Shao, Y.; Chen, W.; Fang, B. ROS/JNK-mediated lysosomal injury in rat intestinal epithelial-6 cells during heat stress. J. Therm. Biol. 2022, 109, 103326. [Google Scholar] [CrossRef]
- Yang, J.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; Mao, X.; He, J.; Luo, Y.; Luo, J.; Huang, Z.; et al. Dietary 25-Hydroxyvitamin D(3) Supplementation Alleviates Porcine Epidemic Diarrhea Virus Infection by Improving Intestinal Structure and Immune Response in Weaned Pigs. Animals 2019, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Cantet, J.M.; Yu, Z.; Ríus, A.G. Heat Stress-Mediated Activation of Immune-Inflammatory Pathways. Antibiotics 2021, 10, 1285. [Google Scholar] [CrossRef]
- Li, Y.; Liang, R.; Zhang, X.; Wang, J.; Shan, C.; Liu, S.; Li, L.; Zhang, S. Copper Chaperone for Superoxide Dismutase Promotes Breast Cancer Cell Proliferation and Migration via ROS-Mediated MAPK/ERK Signaling. Front. Pharmacol. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Lutsenko, S. An expanding range of functions for the copper chaperone/antioxidant protein Atox1. Antioxid. Redox Signal. 2013, 19, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Best, K.; McCoy, K.; Gemma, S.; Disilvestro, R.A. Copper enzyme activities in cystic fibrosis before and after copper supplementation plus or minus zinc. Metabolism 2024, 53, 37–41. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, L.; Feng, J.; Wang, X.; Wang, X.; Xu, X.; Chu, W. Aged polystyrene microplastics exacerbate alopecia associated with tight junction injuries and apoptosis via oxidative stress pathway in skin. Environ. Int. 2024, 186, 108638. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]


| Ingredients | % | Nutrient Level 1 | Content |
|---|---|---|---|
| Corn | 56.8 | Digestible energy (MJ/kg) | 14.55 |
| Soybean meal | 17.9 | Crude protein, % | 21.55 |
| Extruded soybean | 3 | Calcium, % | 0.83 |
| Fish meal | 6.6 | Total phosphorus, % | 0.70 |
| Whey powder | 9.1 | Available phosphorus, % | 0.48 |
| Soybean oil | 2.95 | Lysine, % | 1.39 |
| Sucrose | 1.2 | Methionine, % | 0.45 |
| CaCO3 (Ca ≥ 94.0%) | 0.55 | Cysteine + Methionine, % | 0.68 |
| CaHPO4 (P ≥ 16.5%) | 0.6 | Threonine, % | 0.81 |
| L-lysine-HCl (≥78.8%, dry basis) | 0.6 | Tryptophan, % | 0.17 |
| DL-Methionine (≥99.0%, dry basis) | 0.15 | Cu, (mg/kg) | 7.30 |
| L-Threonine (≥98.5%, dry basis) | 0.2 | ||
| NaCl (≥99.1%) | 0.05 | ||
| Vitamin-mineral premix 2 | 0.3 | ||
| Total | 100 |
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | Accession No. | Amplification Efficiency (%) |
|---|---|---|---|---|
| Occludin | Forward, CTACTCGTCCAACGGGAAAG | 158 | XM_005672525.3 | 97.2 |
| Reverse, ACGCCTCCAAGTTACCACTG | ||||
| Claudin-1 | Forward, TTTCCTCAATACAGGAGGGAAGC | 81 | NM_001244539.1 | 95.8 |
| Reverse, CCCTCTCCCCACATTCGAG | ||||
| ZO-1 | Forward, CAGCCCCCGTACATGGAGA | 114 | XM_005659811 | 96.4 |
| Reverse, GCGCAGACGGTGTTCATAGTT | ||||
| TGF-β1 | Forward, TGACCCGCAGAGAGGCTAT | 106 | NM_214015.2 | 94.3 |
| Reverse, CGGCCAGAATTGAACCCGT | ||||
| TGF-β2 | Forward, GGATCTTGGGTGGAAATGGA | 58 | XM_021064298.1 | 95.5 |
| Reverse, GGCACAGAAGTTGGCATTGT | ||||
| HO-1 | Forward, AGCTGTTTCTGAGCCTCCAA | 130 | NM_001004027.1 | 96.9 |
| Reverse, CAAGACGGAAACACGAGACA | ||||
| Keap-1 | Forward, GAGAGGTATGAACCCGAGCG | 147 | XM_005654811.3 | 97.5 |
| Reverse, ACACTCTGCTGAGTTGAGGC | ||||
| NQO1 | Forward, TGTAAAGCCGGGAAAGGTGT | 132 | NM_001159613.1 | 96.0 |
| Reverse, CCATTGAGGAGTTGGGTGCT | ||||
| NRF2 | Forward, GCCCCTGGAAGCGTTAAAC | 67 | XM_021075133.1 | 96.7 |
| Reverse, GGACTGTATCCCCAGAAGGTTGT | ||||
| GPX4 | Forward, TGTGTGAATGGGGACGATGC | 135 | NM_214407.1 | 97.0 |
| Reverse, CTTCACCACACAGCCGTTCT | ||||
| GPX1 | Forward, GGCGGCGGGTTCGA | 55 | NM_214201.1 | 97.2 |
| Reverse, CGCCATTCACCTCACACTTCT | ||||
| CAT | Forward, AGCTTTGCCCTTGCACAAAC | 119 | XM_021081498.1 | 95.8 |
| Reverse, ACATCCTGAACAAGAAGGGGC | ||||
| SOD1 | Forward, AGGGCACCATCTACTTCGAG | 81 | NM_001190422.1 | 96.4 |
| Reverse, GATCACCTTCAGCCAGTCCT | ||||
| SOD2 | Forward, CTGGACAAATCTGAGCCCTA | 156 | NM_214127.2 | 98.0 |
| Reverse, TTGAAACCGAGCCAACCC | ||||
| TNF-α | Forward, CGTCGCCCACGTTGTAGCCAAT | 128 | NM_214022.1 | 95.5 |
| Reverse, GCCCATCTGTCGGCACCACC | ||||
| IL-1β | Forward, CCAAAGAGGGACATGGAGAA | 123 | XM_021085847.1 | 96.9 |
| Reverse, GGGCTTTTGTTCTGCTTGAG | ||||
| IL-6 | Forward, TGGCTACTGCCTTCCCTACC | 153 | NM_214399.1 | 97.5 |
| Reverse, CACACATCTCCTTTCTCATTGC | ||||
| IL-10 | Forward, CGGCGCTGTCATCAATTTCTG | 89 | NM_214041.1 | 96.0 |
| Reverse, CCCCTCTCTTGGAGCTTGCTA | ||||
| IFN-γ | Forward, ACCAGGCCATTCAAAGGAGC | 90 | NM_213948.1 | 96.7 |
| Reverse, CGAAGTCATTCAGTTTCCCAGAG | ||||
| Bax | Forward, AAGCGCATTGGAGATGAACT | 121 | XM_013998624.2 | 97.0 |
| Reverse, TGCCGTCAGCAAACATTTC | ||||
| Bcl-2 | Forward, TGCCTTTGTGGAGCTGTATG | 144 | XM_021099593.1 | 97.2 |
| Reverse, GCCCGTGGACTTCACTTATG | ||||
| BAD | Forward, GAGTCGCCACAGCTCTTACC | 187 | XM_021082883.1 | 95.8 |
| Reverse, GCGAGGAAGTCCCTTCTTGA | ||||
| Caspase-3 | Forward, GTGGGACTGAAGATGACA | 190 | NM_214131.1 | 96.4 |
| Reverse, ACCCGAGTAAGAATGTG | ||||
| Caspase-8 | Forward, ATGTCGGACTGTCTGGGAGA | 84 | XM_021074714.1 | 98.4 |
| Reverse, GTATCCCCGAGGCTTGCTTT | ||||
| Caspase-9 | Forward, AATGCCGATTTGGCTTACGT | 195 | XM_003127618.4 | 95.5 |
| Reverse, CATTTGCTTGGCAGTCAGGTT | ||||
| HSP70 | Forward, GCCCTGAATCCGCAGAATA | 152 | NM_001123127.1 | 96.9 |
| Reverse, TCCCCACGGTAGGAAACG | ||||
| GAPDH | Forward, GGGCATGAACCATGAGAAGT | 133 | XM_021091114.1 | 99.5 |
| Reverse, TGTGGTCATGAGTCCTTCCA |
| Parameter | Treatment 1 | p Value 2 | ||||
|---|---|---|---|---|---|---|
| Basal | LC | HC | LNC | HNC | ||
| ADFI (g) | 200.97 ± 18.65 b | 274.3 ± 15.44 a | 284.5 ± 13.98 a | 242.58 ± 12.65 ab | 241.6 ± 15.23 ab | 0.005 |
| ADG (g) | 133.41 ± 18.71 b | 180.17 ± 13.88 a | 194.46 ± 5.93 a | 163.5 ± 13.4 ab | 174.11 ± 13.52 ab | 0.048 |
| F:G | 1.72 ± 0.35 | 1.54 ± 0.06 | 1.41 ± 0.03 | 1.52 ± 0.1 | 1.45 ± 0.05 | 0.793 |
| Diarrhea Index | 0.47 ± 0.05 a | 0.27 ± 0.07 b | 0.26 ± 0.06 b | 0.27 ± 0.05 b | 0.26 ± 0.02 b | 0.047 |
| Diarrhea Rate | 1.64 ± 0.08 a | 1.31 ± 0.12 b | 1.35 ± 0.1 b | 1.36 ± 0.09 b | 1.34 ± 0.04 b | 0.09 |
| Parameter | Treatment 1 | p Value 2 | ||||
|---|---|---|---|---|---|---|
| Basal | LC | HC | LNC | HNC | ||
| Liver index | 29.38 ± 1.59 ab | 29.02 ± 1.02 ab | 25.97 ± 0.95 b | 32.22 ± 1.27 a | 27.3 ± 0.86 b | 0.026 |
| Spleen index | 1.14 ± 0.05 | 1.29 ± 0.08 | 1.38 ± 0.11 | 1.28 ± 0.05 | 1.41 ± 0.11 | 0.163 |
| Parameter | Treatment 1 | p Value 2 | ||||
|---|---|---|---|---|---|---|
| Basal | LC | HC | LNC | HNC | ||
| DM (%) | 80.92 ± 0.42 c | 85.07 ± 0.56 ab | 84.13 ± 0.78 ab | 85.33 ± 0.41 a | 82.9 ± 1.62 bc | 0.04 |
| CP (%) | 71.16 ± 1.34 b | 78.25 ± 1.41 a | 76.98 ± 1.35 a | 79.25 ± 1.28 a | 76.4 ± 3.08 a | 0.021 |
| EE (%) | 61.15 ± 3.38 c | 78.76 ± 1.86 a | 71.14 ± 1.87 ab | 71.49 ± 1.2 ab | 68.99 ± 5.14 bc | 0.003 |
| Ca (%) | 57.46 ± 3.89 | 62.02 ± 2.05 | 65.23 ± 1.16 | 59.85 ± 7.46 | 54.9 ± 6.19 | 0.512 |
| TP (%) | 47.86 ± 4.31 a | 50.48 ± 1.69 a | 45.91 ± 0.91 ab | 40.56 ± 1.08 b | 40.66 ± 2.04 b | 0.006 |
| Parameter | Treatment 1 | p Value 2 | ||||
|---|---|---|---|---|---|---|
| Basal | LC | HC | LNC | HNC | ||
| Cu Serum (μmmol/L) | 13.94 ± 0.58 c | 14.92 ± 1.47 bc | 17.21 ± 0.8 ab | 15.41 ± 0.93 bc | 19.31 ± 1.13 a | 0.006 |
| Cu Feed (mg/kg) | 9.24 | 53.46 | 169.94 | 65.44 | 150.72 | |
| Cu Feces (ppm) | 65.99 ± 4.57 c | 410.4 ± 24.51 b | 890.13 ± 52.47 a | 325.44 ± 30.84 b | 841.5 ± 24.38 a | <0.001 |
| ATTD | −10.47 ± 4.78 c | −5.73 ± 7.47 c | 16.55 ± 4.05 ab | 30.29 ± 6.61 a | 12.87 ± 1.95 b | <0.001 |
| STTD | 0 ± 4.78 c | 4.75 ± 7.47 c | 27.02 ± 4.05 bc | 40.77 ± 6.61 a | 23.34 ± 1.95 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Xu, D.; Zhang, H.; Xing, Q.; Chen, D.; Mao, X.; Wang, Q.; Wang, H.; Yan, H. Nano-Copper Supplementation Reduces Fecal Copper Excretion and Enhances Piglet Performance Under Heat Stress. Agriculture 2025, 15, 1296. https://doi.org/10.3390/agriculture15121296
Xiao X, Xu D, Zhang H, Xing Q, Chen D, Mao X, Wang Q, Wang H, Yan H. Nano-Copper Supplementation Reduces Fecal Copper Excretion and Enhances Piglet Performance Under Heat Stress. Agriculture. 2025; 15(12):1296. https://doi.org/10.3390/agriculture15121296
Chicago/Turabian StyleXiao, Xiarui, Duo Xu, Haixin Zhang, Qian Xing, Daiwen Chen, Xiangbing Mao, Quyuan Wang, Huifen Wang, and Hui Yan. 2025. "Nano-Copper Supplementation Reduces Fecal Copper Excretion and Enhances Piglet Performance Under Heat Stress" Agriculture 15, no. 12: 1296. https://doi.org/10.3390/agriculture15121296
APA StyleXiao, X., Xu, D., Zhang, H., Xing, Q., Chen, D., Mao, X., Wang, Q., Wang, H., & Yan, H. (2025). Nano-Copper Supplementation Reduces Fecal Copper Excretion and Enhances Piglet Performance Under Heat Stress. Agriculture, 15(12), 1296. https://doi.org/10.3390/agriculture15121296







