Effects of Dietary Rhodotorula Yeast Culture Supplementation on Physicochemical Properties, Antioxidant Capacity, Shelf Life, and Flavor Substance of the Longissimus dorsi Muscle in Fattening Lambs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Diet Composition
2.3. Physicochemical Properties
2.4. Antioxidant Capacity
2.5. Shelf Life for Sale
2.6. Meat Flavor Substances
2.7. Data Analysis
3. Results
3.1. Carcass Traits
3.2. Physicochemical Properties
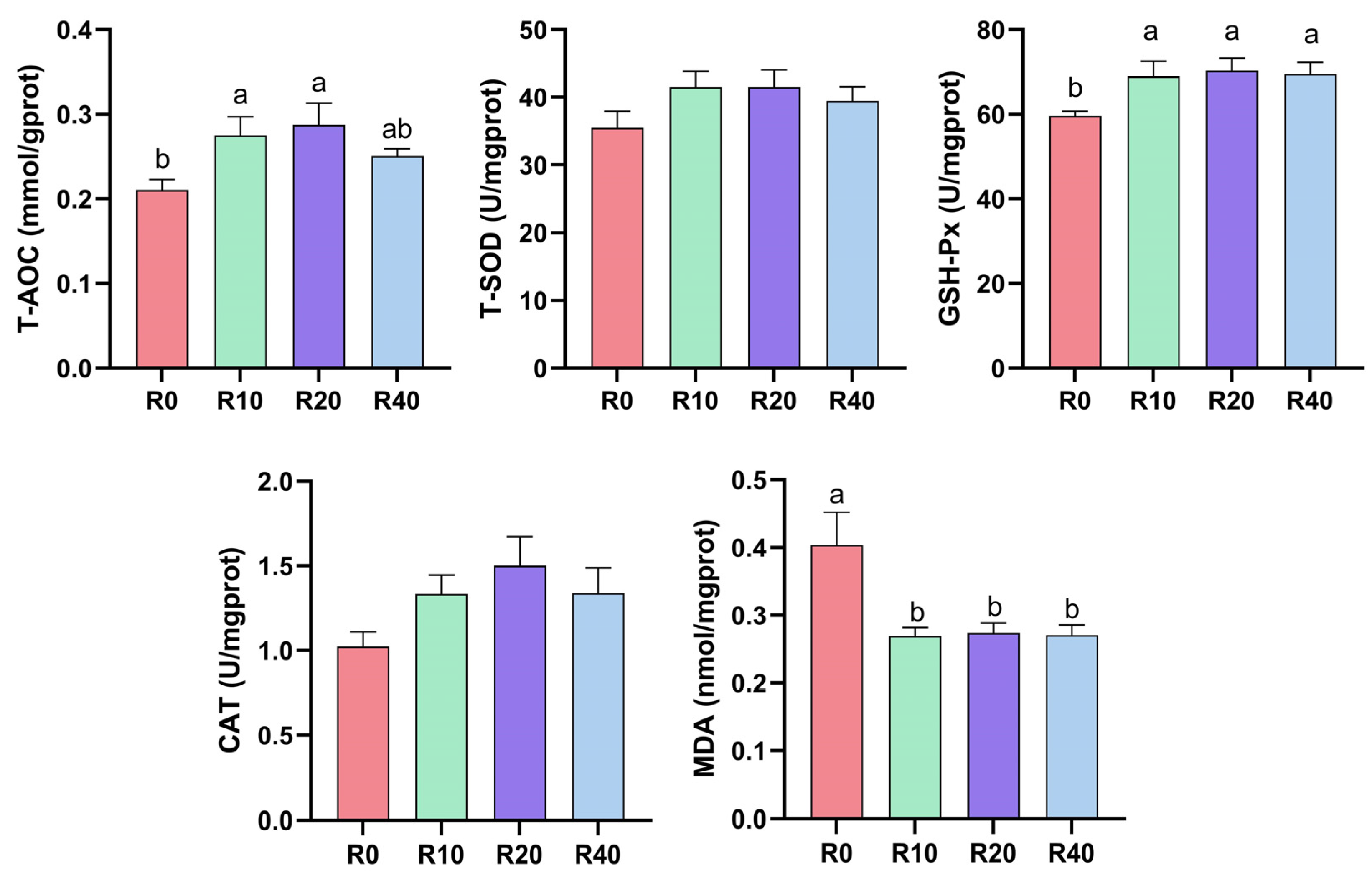
3.3. Antioxidant Capacity
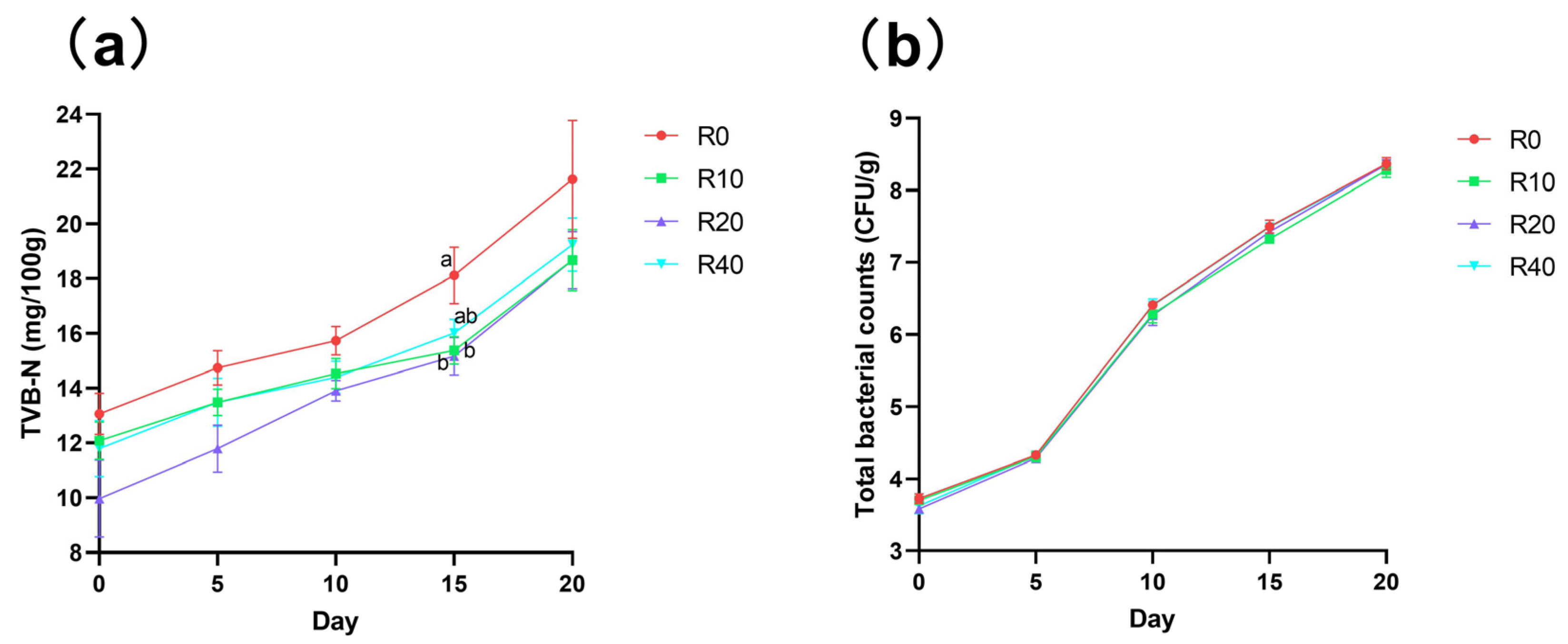
3.4. Shelf Life for Sale
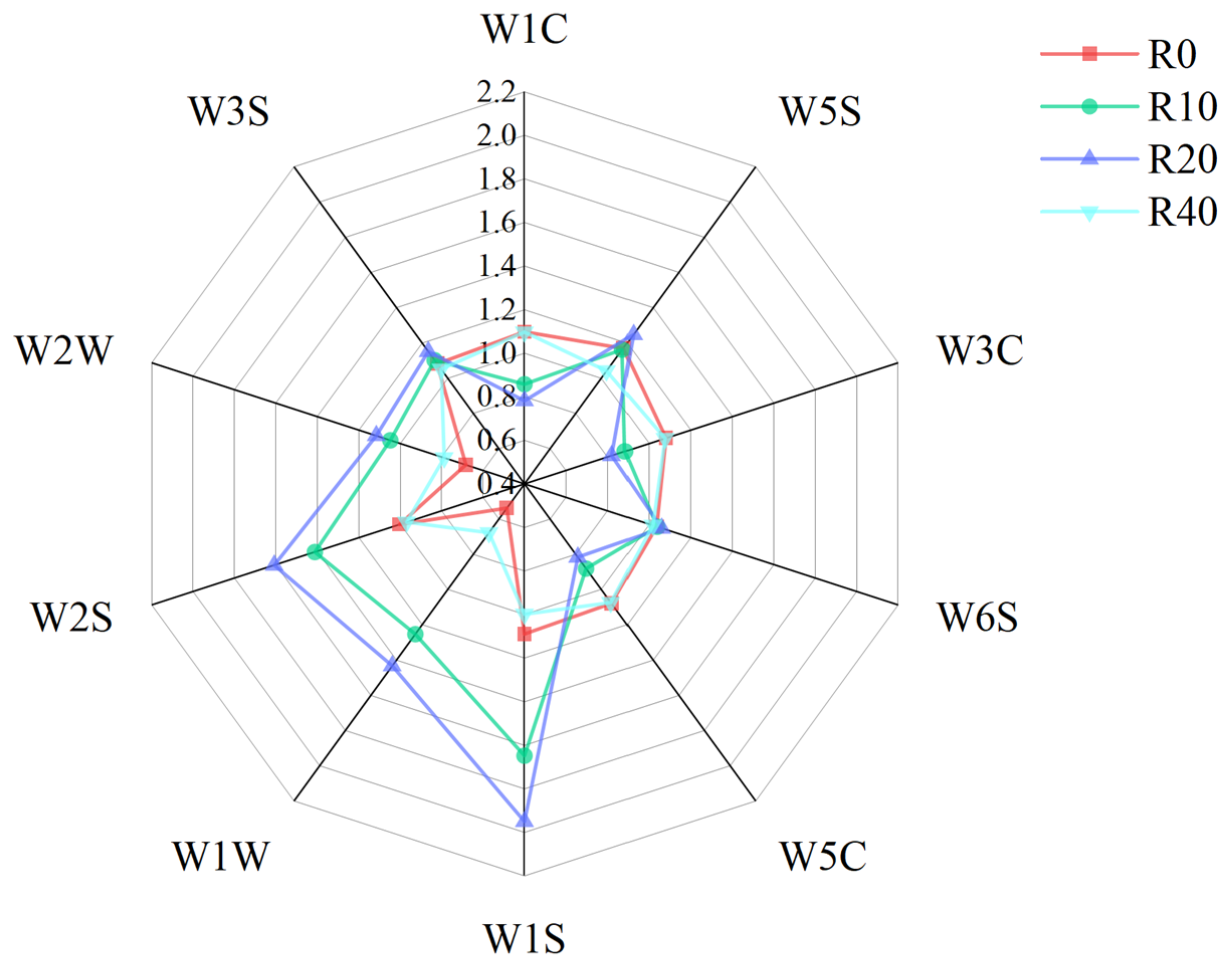
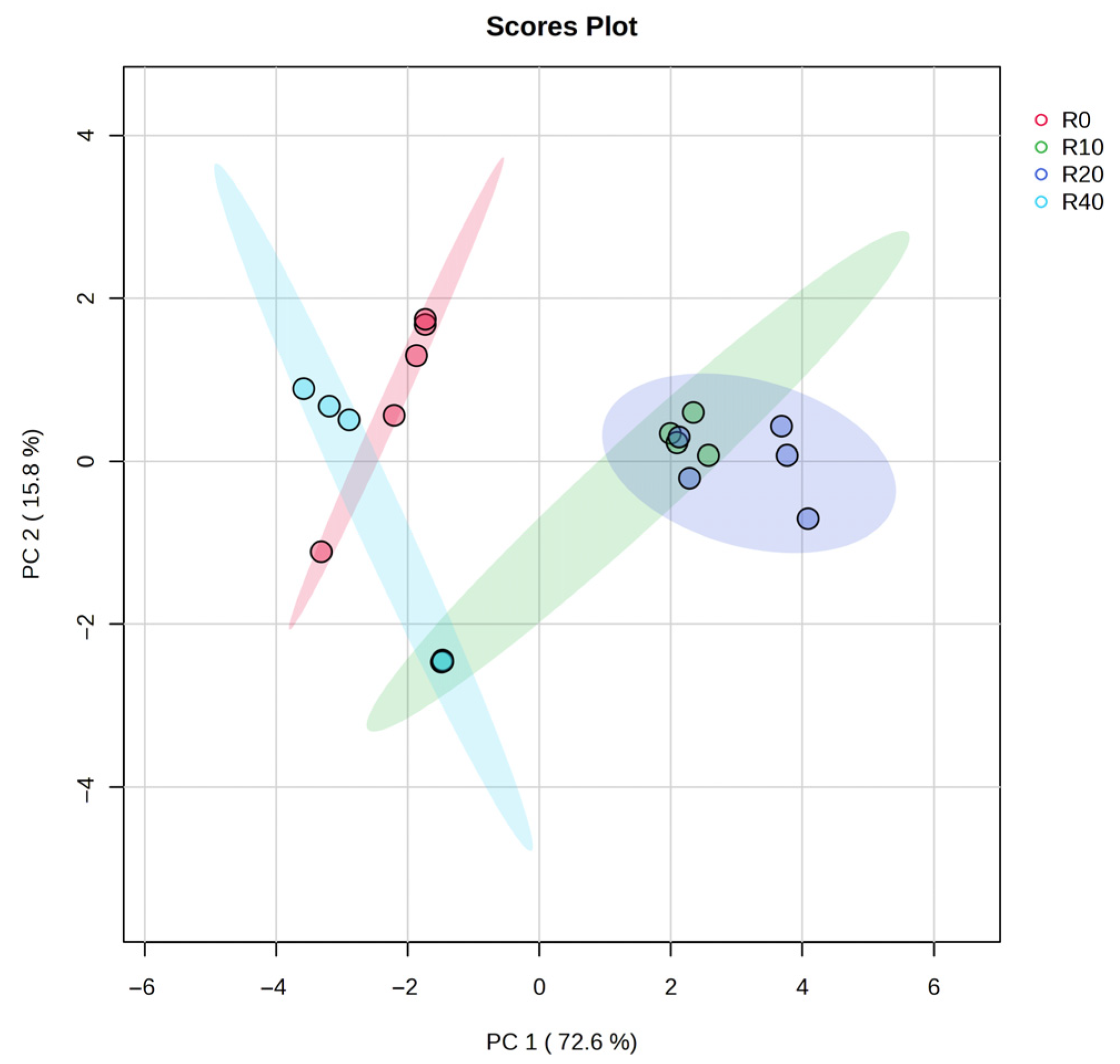
3.5. Meat Flavor Substances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, M.; Li, X.; Huo, R.; Chang, G.; Shen, X. Effects of dietary disodium fumarate supplementation on muscle quality, chemical composition, oxidative stress and lipid metabolism of Hu sheep induced by high concentrate diet. Meat Sci. 2023, 201, 109176. [Google Scholar] [CrossRef]
- Lin, L.; Cao, Q.; Zhang, C.; Xu, T.; Yue, K.; Li, Q.; Liu, F.; Wang, X.; Dong, H.; Huang, S.; et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol. Environ. Saf. 2022, 232, 113225. [Google Scholar] [CrossRef]
- Van, W.; Kind, K.; Gatford, K.; Swinbourne, A.; Leu, S.; Hayman, P.; Kelly, J.; Weaver, A.; Kleemann, D.; Walker, S. Review of the impact of heat stress on reproductive performance of sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar]
- Cui, Y.; Li, X.; Yu, C.; Li, D.; Gao, M.; Hu, H.; Liu, D. Effects of compound bacteria culture on growth performance, immunity and antioxidant function of meat sheep. Chin. J. Anim. Nutr. 2019, 31, 5065–5073. [Google Scholar]
- Liu, G.; Liu, D.; Gao, M.; Hu, H.; Lu, D. Research and development of nutritive active matter omics and its application technology of compound yeast culture. Chin. J. Anim. Husb. 2014, 50, 84–88. [Google Scholar]
- Lim, D.; Han, M.; Ki, K.; Kim, T.; Park, S.; Kim, D.; Kim, Y. Changes in milk production and blood metabolism of lactating dairy cows fed Saccharomyces cerevisiae culture fluid under heat stress. J. Anim. Sci. Technol. 2021, 63, 1433–1442. [Google Scholar] [CrossRef]
- Ramani, K.; Desai, M.; Changela, D.; Dangar, K. Active Role of Yeast in Environmental Sustainability. In Microbial BioTechnology for Sustainable Agriculture Volume 1; Springer: Singapore, 2022; Volume 33, pp. 429–477. [Google Scholar]
- Pang, Y.; Zhang, H.; Wen, H.; Wan, H.; Wu, H.; Chen, Y.; Li, S.; Zhang, L.; Sun, X.; Li, B.; et al. Yeast Probiotic and Yeast Products in Enhancing Livestock Feeds Utilization and Performance: An Overview. J. Fungi 2022, 8, 1191. [Google Scholar] [CrossRef] [PubMed]
- Denev, S.; Popova, T.; Radulova, P.; Stancheva, P.; Staykova, G.; Beev, G.; Todorova, P.; Tchobanova, S. Yeast cultures in ruminant nutrition. Bulg. J. Agric. Sci. 2007, 13, 357–374. [Google Scholar]
- Liu, Y.; Lang, M.; Zhen, Y.; Chen, X.; Sun, Z.; Zhao, W.; Zhang, X.; Wang, T.; Qin, G. Effects of yeast culture supplementation and the ratio of non-structural carbohydrate to fat on growth performance, carcass traits and the fatty acid profile of the longissimus dorsi muscle in lambs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1274–1282. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xu, J.; Yang, Q.; Sha, Y.; Jiao, T.; Zhao, S. Effects of yeast cultures on meat quality, flavor composition and rumen microbiota in lambs. Curr. Res. Food Sci. 2024, 9, 100845. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, C.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Zhang, J.; et al. Effects of Yeast Culture Supplementation in Wheat-Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing-Finishing Pigs. Animals 2022, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Geng, C.; Zhang, M. Effects of selenium-enriched and germanium-enriched yeast cultures on growth performance, muscle fatty acid and amino acid contents of Yanbian yellow cattle. Acta Anim. Nutr. Sin. 2018, 30, 2850–2856. [Google Scholar]
- Lu, X.; Ma, H.; Liu, Y.; Chen, M.; Dang, J.; Su, X.; Zhao, Y.; Wang, K.; Yang, G.; Zhang, G.; et al. Rhodotorula Yeast Culture Improved the Antioxidant Capacity, Lipid Metabolism, and Immunity of Sheep Livers. Vet. Sci. 2025, 12, 314. [Google Scholar] [CrossRef]
- Li, X.; An, N.; Chen, H.; Liu, D. Effects of yeast culture on growth performance, antioxidant capacity, immune function, and intestinal microbiota structure in Simmental beef cattle. Front. Vet. Sci. 2025, 11, 1533081. [Google Scholar] [CrossRef]
- Mussagy, C.; Ribeiro, H.; Santos-Ebinuma, V.; Schuur, B.; Pereira, J. Rhodotorula sp.-based biorefinery: A source of valuable biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar] [CrossRef]
- Kang, K.; Deng, X.; Xie, W.; Chen, J.; Lin, H.; Chen, Z. Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice. Animals 2023, 13, 3376. [Google Scholar] [CrossRef]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.; Shene, C. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Jiang, Y.; Chu, W. Prebiotic, Antioxidant, and Immunomodulatory Properties of Acidic Exopolysaccharide From Marine Rhodotorula RY1801. Front. Nutr. 2021, 8, 710668. [Google Scholar] [CrossRef]
- Hu, P.; Mao, J.; Zeng, Y.; Sun, Z.; Deng, H.; Chen, C.; Sun, W.; Tang, Z. Isolation, Identification, and Function of Rhodotorula mucilaginosa TZR2014 and Its Effects on the Growth and Health of Weaned Piglets. Front. Microbiol. 2022, 13, 922136. [Google Scholar] [CrossRef]
- Coutinho, J.; Peixoto, T.; de Menezes, G.; Carvalho, C.; Ogaki, M.; Gomes, E.; Rosa, C.; Rosa, L.; Arantes, R.; Nicoli, J.; et al. In Vitro and In Vivo Evaluation of the Probiotic Potential of Antarctic Yeasts. Probiotics Antimicrob. Proteins 2021, 13, 1338–1354. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Galano, A.; Vargas, R.; Martínez, A. Carotenoids can act as antioxidants by oxidizing the superoxide radical anion. Phys. Chem. Chem. Phys. 2010, 12, 193–200. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef]
- Wu, J.; Hu, Y.; Zhao, N.; Yang, W.; Chen, Z. The active roles of Rhodotorula mucilaginosa ZTHY2 in regulating antioxidant capacity and immune function of Leizhou black ducks. Front. Vet. Sci. 2025, 12, 1494892. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Tang, Z.; Zhang, X.; Chen, J.; Sun, Z. Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. J. Appl. Microbiol. 2020, 128, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Zhou, D.; Qin, J.; Xu, Y.; Lu, Q.; Tian, X. Effects of Rosa roxburghii Tratt seed on the growth performance, meat quality, and sensory evaluation characteristics in growing rabbits. Meat Sci. 2024, 208, 109394. [Google Scholar] [CrossRef] [PubMed]
- Kannan, G.; Mahapatra, A.; Degala, H. Preharvest Management and Postharvest Intervention Strategies to Reduce Escherichia coli Contamination in Goat Meat: A Review. Animals 2021, 11, 2943. [Google Scholar] [CrossRef]
- Mazhangara, I.; Chivandi, E.; Mupangwa, J.; Muchenje, V. The Potential of Goat Meat in the Red Meat Industry. Sustainability 2019, 11, 3671. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O. Nutritive value and quality characteristics of goat meat. In Goat Meat Production and Quality; Mahgoub, O., Kadim, I.T., Webb, E.C., Eds.; CAB international: Cambridge, MA, USA, 2011; pp. 305–306. [Google Scholar]
- Ding, W.; Lu, Y.; Xu, B.; Chen, P.; Li, A.; Jian, F.; Yu, G.; Huang, S. Meat of Sheep: Insights into Mutton Evaluation, Nutritive Value, Influential Factors, and Interventions. Agriculture 2024, 14, 1060. [Google Scholar] [CrossRef]
- Tshabalala, P.; Strydom, P.; Webb, E.; Kock, H. Meat quality of designated South African indigenous goat and sheep breeds. Meat Sci. 2003, 65, 563–570. [Google Scholar] [CrossRef] [PubMed]
- GB/T 43562-2023; Sheep Slaughter and Inspection Operation Specification of China. China Standards Press: Beijing, China, 2023.
- Bao, G.; Liu, X.; Wang, J.; Hu, J.; Shi, B.; Li, S.; Luo, Y. Effects of Slaughter Age on Myosin Heavy Chain Isoforms, Muscle Fibers, Fatty Acids, and Meat Quality in Longissimus Thoracis Muscle of Tibetan Sheep. Front. Vet. Sci. 2021, 8, 689589. [Google Scholar] [CrossRef]
- Silva, J.; Rodriguez, F.; Trettel, M.; Abal, R.; Lima, C.; Yoshikawa, C.; Zanetti, M. Performance, carcass characteristics and meat quality of Nellore cattle supplemented with supranutritional doses of sodium selenite or selenium-enriched yeast. Animal 2020, 14, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.P. Ash Content Determination. In Food Analysis Laboratory Manual; Food Science Text Series; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- GB 5009.228-2016; Determination of Volatile Base Nitrogen in Food of China. China Standards Press: Beijing, China, 2016.
- GB 4789.2-2022; Food Microbiological Examination of China. China Standards Press: Beijing, China, 2022.
- GB/T 18246-2019; Determination of Amino Acids in Feed of China. China Standards Press: Beijing, China, 2019.
- GB/T 9961-2008; Fresh and Frozen Carcass Mutton of China. China Standards Press: Beijing, China, 2008.
- Li, Q.; Xu, G.; Yang, D.; Tu, Y.; Zhang, J.; Ma, T.; Diao, Q. Effects of Feed Ingredients with Different Protein-to-Fat Ratios on Growth, Slaughter Performance and Fat Deposition of Small-Tail Han Lambs. Animals 2024, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Sun, X.; Zhao, S.; Hu, M.; Li, D.; Qi, S.; Jiao, X.; Sun, Y.; Wang, C.; Zhu, X.; et al. Dietary alfalfa powder supplementation improves growth and development, body health, and meat quality of Tibetan sheep. Food Chem. 2022, 396, 133709. [Google Scholar] [CrossRef]
- Eun, J.; Jie, C. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 2004, 53, 2748–2756. [Google Scholar]
- Blanchard, P.-G.; Festuccia, W.T.; Houde, V.P.; St-Pierre, P.; Brûlé, S.; Turcotte, V.; Côté, M.; Bellmann, K.; Marette, A.; Deshaies, Y. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion. J. Lipid Res. 2012, 53, 1117–1125. [Google Scholar] [CrossRef]
- Andrade, M.; Gilio, G.; Perandini, L.; Peixoto, A.; Moreno, M.; Castro, É.; Oliveira, T.; Vieira, T.; Ortiz, S.; Thomazelli, C.; et al. PPAR γ-induced upregulation of subcutaneous fat adiponectin secretion, glyceroneogenesis and BCAA oxidation requires mTORC1 activity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158967. [Google Scholar]
- Laplante, M.; Sabatini, M. An Emerging Role of mTOR in Lipid Biosynthesis. Curr. Biol. 2009, 19, 1046–1052. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Xu, H.; Yao, D.; Tian, H.; Luo, J.; Loor, J. Sterol regulatory element binding protein-1 (SREBP-1) promoter: Characterization and transcriptional regulation by mature SREBP-1 and liver X receptor α in goat mammary epithelial cells. J. Dairy Sci. 2016, 99, 1595–1604. [Google Scholar]
- Ren, F.; Yang, F.; Guo, C.; Zong, S.; Maimaitijiang, A.; Chen, K. Effects of yeast culture supplementation on production performance, slaughter performance and organ index of fattening sheep. Anim. Husb. Feed Sci. 2023, 44, 39–43+54. [Google Scholar]
- Hao, Z.; Su, M.; Qin, Z.; Li, T.; Shi, H.; An, X.; Wang, H.; Duan, X.; Ma, Y. Effects of dietary yeast culture on growth performance, nutrient digestion, slaughter performance and organ index of male Hu lambs. J. Gansu Agric. Univ. 2023, 58, 30–37. [Google Scholar]
- Wang, L.; Liu, Y.; Zhao, J.; Wang, B.; Te, R.; He, X.; Fu, S.; Jiang, L.; Tang, Y.; He, J. Effects of dietary yeast culture supplementation on slaughter performance, intramuscular amino acids and functional components of Small Tail Han sheep. Anim. Husb. Feed Sci. 2023, 44, 18–26. [Google Scholar]
- Zhang, J.; Zhang, R.; Zhang, Y. Application of High-Carotenoid-Producing Rhodotorula Yeast in Antibiotic-Free Broiler Production and Its Improvement on Meat Quality. In Proceedings of the 3rd Symposium and 8th National Academic Conference with the Forum on Development Strategy of Animal Microecological Enterprises, Guangzhou, China, 1 November 2006; pp. 240–245. [Google Scholar]
- Paryad, A.; Mahmoudi, M. Effect of different levels of supplemental yeast (Saccharomyces cerevisiae) on performance, blood constituents and carcass characteristics of broiler chicks. Afr. J. Agric. Res. 2008, 3, 835–842. [Google Scholar]
- Zhou, J.; Fu, Y.; Qi, G.; Dai, J.; Zhang, H.; Wang, J.; Wu, S. Yeast cell-wall polysaccharides improve immunity and attenuate inflammatory response via modulating gut microbiota in LPS-challenged laying hens. Int. J. Biol. Macromol. 2023, 224, 407–421. [Google Scholar] [CrossRef]
- Jach, M.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Seidemann, S.; Schollmeyer, J.; Dutson, T.; Crouse, J. Effect of post-mortem storage on Ca(++)-dependent proteases, their inhibitor and myofibril fragmentation. Meat Sci. 1987, 19, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Kent, M.; Shackelford, S.; Veiseth, E.; Wheeler, T. Meat tenderness and muscle growth: Is there any relationship? Meat Sci. 2002, 62, 345–352. [Google Scholar] [CrossRef]
- Lepetit, J. A theoretical approach of the relationships between collagen content, collagen cross-links and meat tenderness. Meat Sci. 2007, 76, 147–159. [Google Scholar] [CrossRef]
- Burke, R.; Monahan, F. The tenderisation of shin beef using a citrus juice marinade. Meat Sci. 2003, 63, 161–168. [Google Scholar] [CrossRef]
- Xu, X.-L.; Li, C.-B.; Chang, H.-J.; Zhou, G.-H.; Huang, M. Effects of characteristics changes of collagen on meat physicochemical properties of beef Semitendinosus muscle during ultrasonic processing. Food Bioprocess Technol. 2012, 5, 285–297. [Google Scholar]
- Lu, G.; Wang, F.; Zhu, Y.; Wang, K.; Peng, Z. The relationship between shear force and pyridine cross-linking and thermal solubility of collagen in Qinchuan cattle patterned meat. China Agric. Sci. 2013, 46, 130–135. [Google Scholar]
- Felipe, A.; Mauricio, R. Yeast carotenoids: Production and activity as antimicrobial bio-molecule. Arch. Microbiol. 2020, 203, 873–888. [Google Scholar]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef]
- Lavigne, L.; Albina, J.; Reichner, J. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J. Immunol. 2006, 177, 8667–8675. [Google Scholar] [CrossRef]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz, C. Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods 2018, 7, 132. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Li, M.; Yang, H.; Li, S.; Li, J.; Xu, Q.; Yang, W.; Jiang, S. Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs. Animals 2020, 10, 615. [Google Scholar] [CrossRef]
- Georgescu, A.; Corbu, V.; Csutak, O. Molecular Basis of Yeasts Antimicrobial Activity-Developing Innovative Strategies for Biomedicine and Biocontrol. Curr. Issues Mol. Biol. 2024, 46, 4721–4750. [Google Scholar] [CrossRef]
- Sameera, S.; Sitha, C.; Nic, R.; Maso, S.D.; Gras, S.L.; Martin, G.J.O. Influence of yeast growth conditions and proteolytic enzymes on the amino acid profiles of yeast hydrolysates: Implications for taste and nutrition. Food Chem. 2024, 437, 137906. [Google Scholar]
- Dung, T.; Oh, Y.; Choi, S.; Kim, I.; Oh, M.; Kim, M. Applications and Advances in Bioelectronic Noses for Odour Sensing. Sensors 2018, 18, 103. [Google Scholar] [CrossRef]
- Wang, R.; Yang, C.; Song, H. Key meat flavor compounds formation mechanism in a glutathione–xylose Maillard reaction. Food Chem. 2012, 131, 280–285. [Google Scholar] [CrossRef]
- Park, K.; Choi, S. Effective Strategies for Understanding Meat Flavor: A Review. Food Sci. Anim. Resour. 2025, 45, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.; Roullier, G.; Morge, C.; Sparrow, C.; Gobert, A.; Vichi, S.; Alexandre, H. Thiamine and Biotin: Relevance in the Production of Volatile and Non-Volatile Compounds during Saccharomyces cerevisiae Alcoholic Fermentation in Synthetic Grape Must. Foods 2023, 12, 972. [Google Scholar] [CrossRef]
- Guo, Q.; Kong, X.; Hu, C.; Zhou, B.; Wang, C.; Shen, Q. Fatty Acid Content, Flavor Compounds, and Sensory Quality of Pork Loin as Affected by Dietary Supplementation with l-arginine and Glutamic Acid. J. Food Sci. 2019, 84, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Sohaib, M.; Ahmad, R.; Nadeem, M.; Imran, A.; Arshad, M.; Kwon, J.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef]
| Items | Content (%) |
|---|---|
| Ingredients | |
| Leymus chinensis | 10.76 |
| Corn stalks | 30.79 |
| Concentrate supplements 1 | 52.33 |
| Corn silage | 6.12 |
| Total | 100.00 |
| Nutrient levels | |
| Metabolic energy 2 (MJ/kg) | 8.91 |
| Crude protein | 14.31 |
| Crude fat | 2.22 |
| Neutral detergent fibers | 50.88 |
| Acid detergent fibers | 19.85 |
| Calcium | 0.96 |
| Phosphorus | 0.35 |
| Sensor | Sensitive Materials |
|---|---|
| W1C | Aromatic hydrocarbon |
| W5S | Nitoxides |
| W3C | Ammonia |
| W6S | Hydride |
| W5C | Olefins, aromatics, polar molecules |
| W1S | Ethers |
| W1W | Sulfide |
| W2S | Alcohols, some aromatic compounds |
| W2W | Aromatic components, organic sulfides |
| W3S | Ethers, aliphatic |
| Items | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| R0 | R10 | R20 | R40 | |||
| Carcass weight (kg) | 27.13 | 28.31 | 28.45 | 27.81 | 0.85 | 0.70 |
| Pre-slaughter live weight (kg) | 53.67 | 55.82 | 54.87 | 54.79 | 1.39 | 0.76 |
| Slaughter rate (%) | 50.56 | 50.73 | 51.78 | 50.80 | 0.83 | 0.73 |
| GR value (cm) | 0.86 b | 1.02 ab | 0.90 b | 1.10 a | 0.06 | 0.04 |
| Backfat thickness (cm) | 2.52 b | 2.56 b | 2.70 a | 2.62 ab | 0.04 | 0.05 |
| Eye muscle area (cm2) | 17.80 | 18.01 | 19.19 | 17.81 | 1.60 | 0.91 |
| Items | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| R0 | R10 | R20 | R40 | |||
| Shear force (N) | 37.02 a | 34.24 b | 33.77 b | 32.76 b | 0.76 | <0.01 |
| Cooking loss (%) | 34.40 | 33.94 | 32.87 | 33.35 | 0.95 | 0.69 |
| Water loss rate (%) | 15.92 | 15.85 | 15.31 | 15.78 | 0.62 | 0.90 |
| Drip loss (%) | 3.87 | 3.56 | 3.22 | 3.35 | 0.19 | 0.13 |
| 45 min pH | 6.50 | 6.77 | 6.63 | 6.54 | 0.11 | 0.32 |
| 24 h pH | 5.45 | 5.46 | 5.53 | 5.46 | 0.15 | 0.98 |
| Lightness (L*) | 37.14 | 36.48 | 36.45 | 35.65 | 0.66 | 0.48 |
| Redness (a*) | 13.18 | 14.16 | 13.76 | 14.15 | 0.57 | 0.59 |
| Yellowness (b*) | 8.95 | 8.84 | 8.61 | 8.42 | 0.49 | 0.88 |
| Items | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| R0 | R10 | R20 | R40 | |||
| Moisture (%) | 74.13 | 75.09 | 74.75 | 74.22 | 0.61 | 0.65 |
| Crude protein (%) | 16.37 c | 16.83 b | 17.22 a | 16.96 ab | 0.11 | <0.01 |
| Crude fat (%) | 1.73 | 1.74 | 1.75 | 1.73 | 0.03 | 0.95 |
| Crude ash (%) | 5.92 | 5.95 | 6.07 | 5.93 | 0.31 | 0.98 |
| Items | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| R0 | R10 | R20 | R40 | |||
| Asp (%) * | 8.82 b | 9.78 ab | 10.25 a | 10.12 a | 0.35 | 0.04 |
| Thr (%) # | 4.23 | 4.68 | 4.82 | 4.65 | 0.16 | 0.09 |
| Ser (%) | 3.24 | 3.51 | 3.65 | 3.53 | 0.10 | 0.07 |
| Glu (%) * | 13.76 b | 13.91 b | 14.95 a | 14.40 ab | 0.24 | <0.01 |
| Gly (%) | 5.07 | 4.73 | 4.81 | 5.08 | 0.23 | 0.62 |
| Ala (%) | 5.56 | 5.97 | 5.90 | 5.87 | 0.13 | 0.19 |
| Cys (%) | 2.09 | 2.12 | 1.62 | 1.53 | 0.25 | 0.24 |
| Val (%) # | 4.16 | 4.43 | 4.47 | 4.35 | 0.09 | 0.11 |
| Met (%) # | 3.21 | 3.12 | 2.87 | 2.78 | 0.24 | 0.57 |
| Ile (%) # | 4.34 b | 4.60 a | 4.76 a | 4.62 a | 0.08 | 0.01 |
| Leu (%) # | 7.35 b | 8.37 a | 8.44 a | 8.12 a | 0.24 | 0.02 |
| Tyr (%) | 3.62 | 4.01 | 3.61 | 3.43 | 0.24 | 0.43 |
| Phe (%) # | 4.43 | 5.12 | 5.00 | 4.91 | 0.21 | 0.13 |
| Lys (%) # | 7.90 b | 9.02 a | 9.39 a | 9.01 a | 0.30 | 0.02 |
| His (%) # | 3.66 | 3.60 | 3.72 | 3.71 | 0.23 | 0.98 |
| Arg (%) | 6.56 | 7.12 | 6.40 | 6.99 | 0.64 | 0.83 |
| Pro (%) | 10.49 | 4.16 | 3.72 | 4.94 | 2.04 | 0.11 |
| EAA (%) | 39.27 | 42.95 | 43.47 | 42.15 | 1.04 | 0.05 |
| DAA (%) | 22.58 b | 23.68 ab | 25.21 a | 24.52 a | 0.49 | <0.01 |
| TAA (%) | 13.80 | 9.73 | 14.51 | 12.31 | 2.62 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Chen, M.; Lu, X.; Zhang, G.; Wang, K.; Su, X.; Gao, A. Effects of Dietary Rhodotorula Yeast Culture Supplementation on Physicochemical Properties, Antioxidant Capacity, Shelf Life, and Flavor Substance of the Longissimus dorsi Muscle in Fattening Lambs. Agriculture 2025, 15, 1265. https://doi.org/10.3390/agriculture15121265
Yang G, Chen M, Lu X, Zhang G, Wang K, Su X, Gao A. Effects of Dietary Rhodotorula Yeast Culture Supplementation on Physicochemical Properties, Antioxidant Capacity, Shelf Life, and Flavor Substance of the Longissimus dorsi Muscle in Fattening Lambs. Agriculture. 2025; 15(12):1265. https://doi.org/10.3390/agriculture15121265
Chicago/Turabian StyleYang, Guang, Meiru Chen, Xinyu Lu, Gaowei Zhang, Ke Wang, Xiangtan Su, and Aiqin Gao. 2025. "Effects of Dietary Rhodotorula Yeast Culture Supplementation on Physicochemical Properties, Antioxidant Capacity, Shelf Life, and Flavor Substance of the Longissimus dorsi Muscle in Fattening Lambs" Agriculture 15, no. 12: 1265. https://doi.org/10.3390/agriculture15121265
APA StyleYang, G., Chen, M., Lu, X., Zhang, G., Wang, K., Su, X., & Gao, A. (2025). Effects of Dietary Rhodotorula Yeast Culture Supplementation on Physicochemical Properties, Antioxidant Capacity, Shelf Life, and Flavor Substance of the Longissimus dorsi Muscle in Fattening Lambs. Agriculture, 15(12), 1265. https://doi.org/10.3390/agriculture15121265




