Effects of Mepiquat Chloride and Chlormequat Chloride on the Growth and Fruit Quality of ‘Shine Muscat’ Grapevines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design and Treatments
2.3. Determination of Fruit Growth Parameters
2.4. Measurement of Fruit Quality
2.5. Statistical Analysis
3. Results
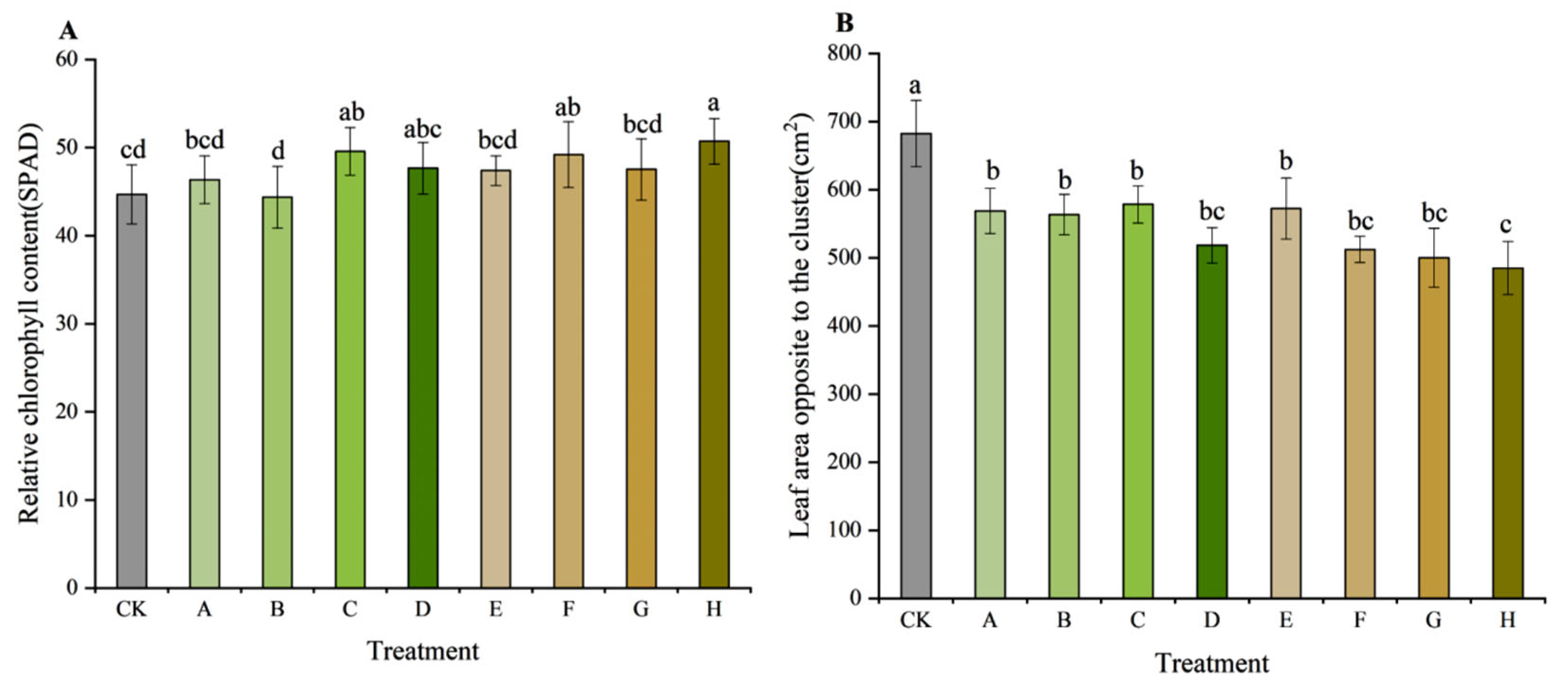
3.1. Growth of Shoots and Leaves
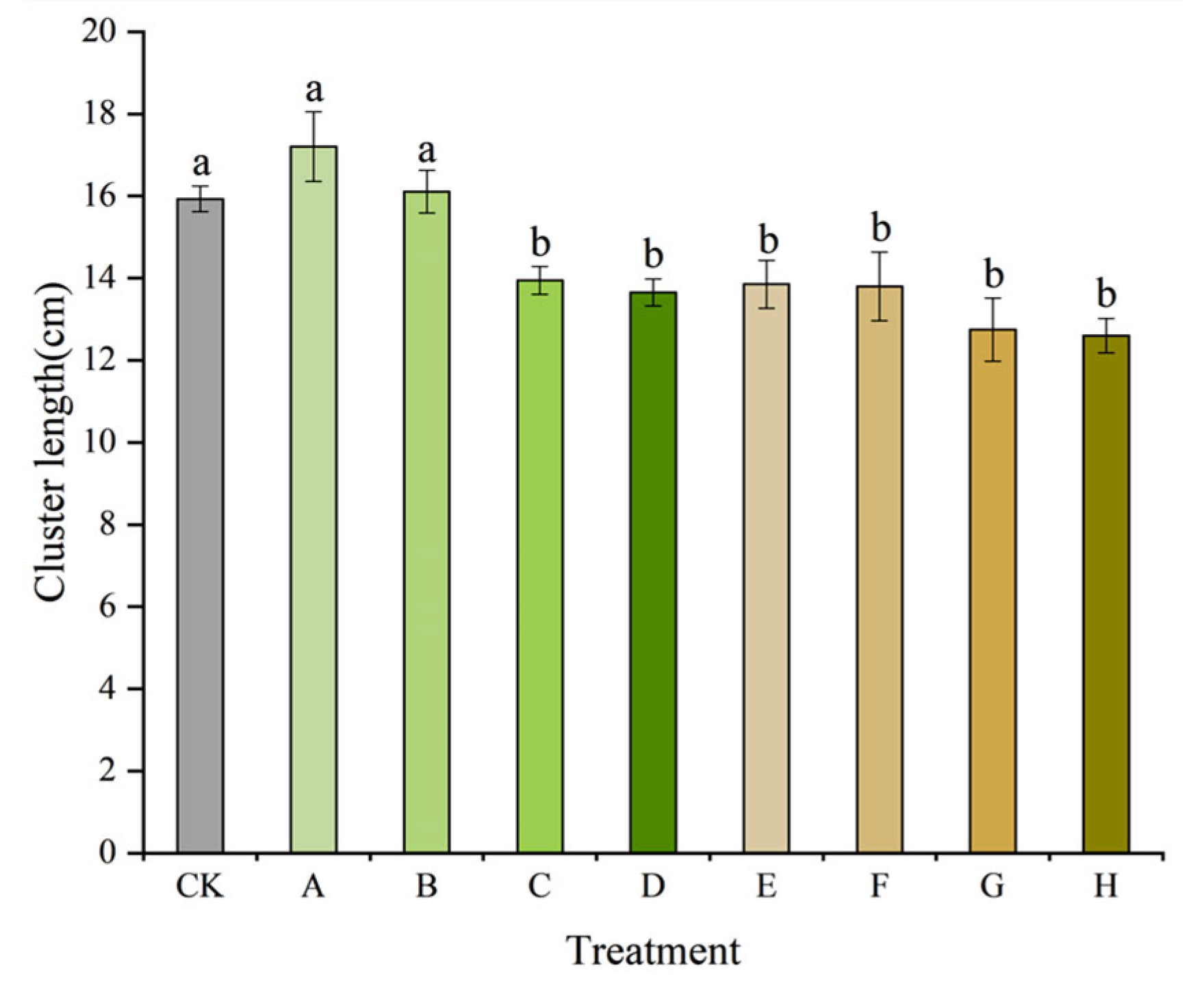
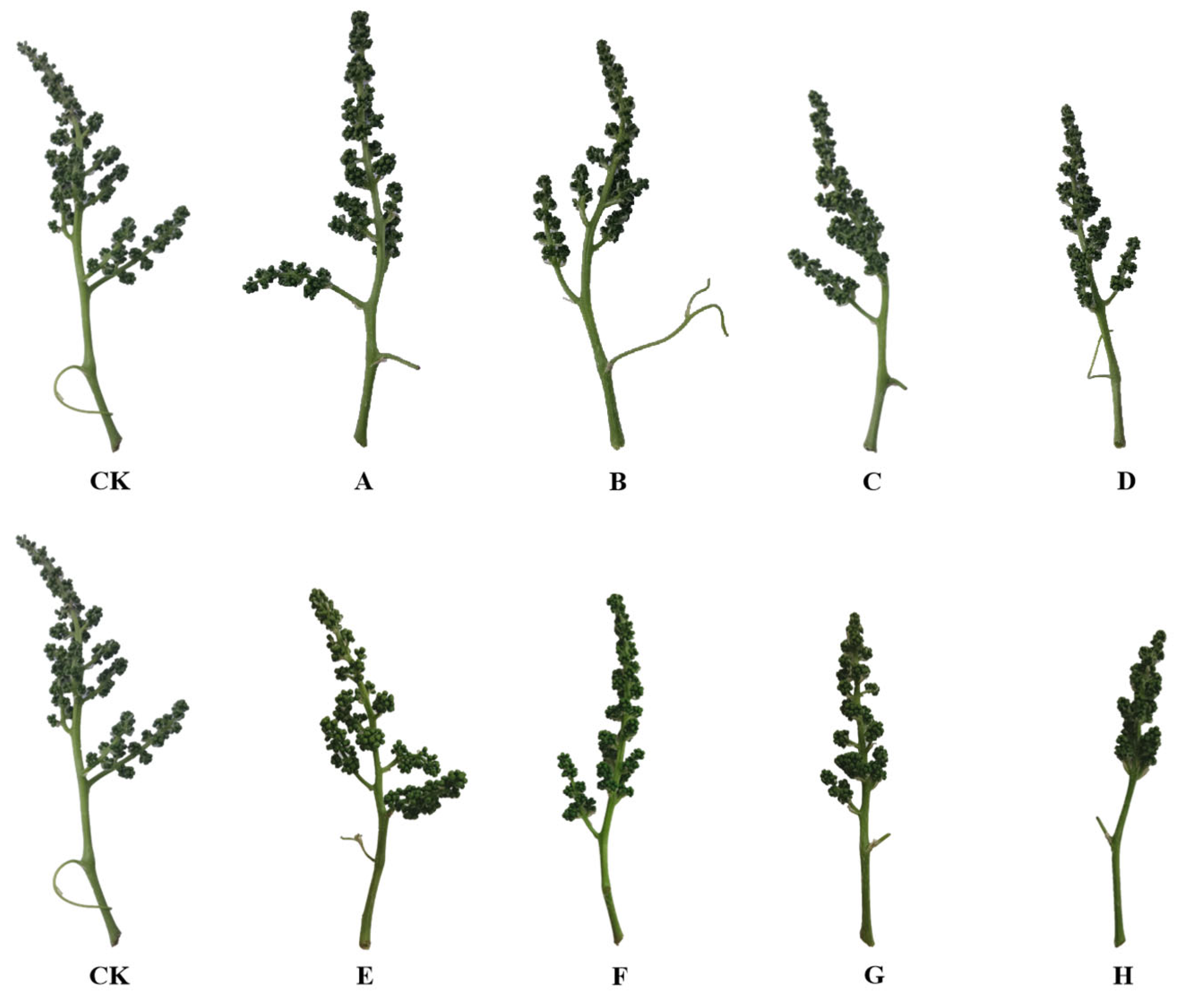
3.2. Growth of Clusters and Bunches
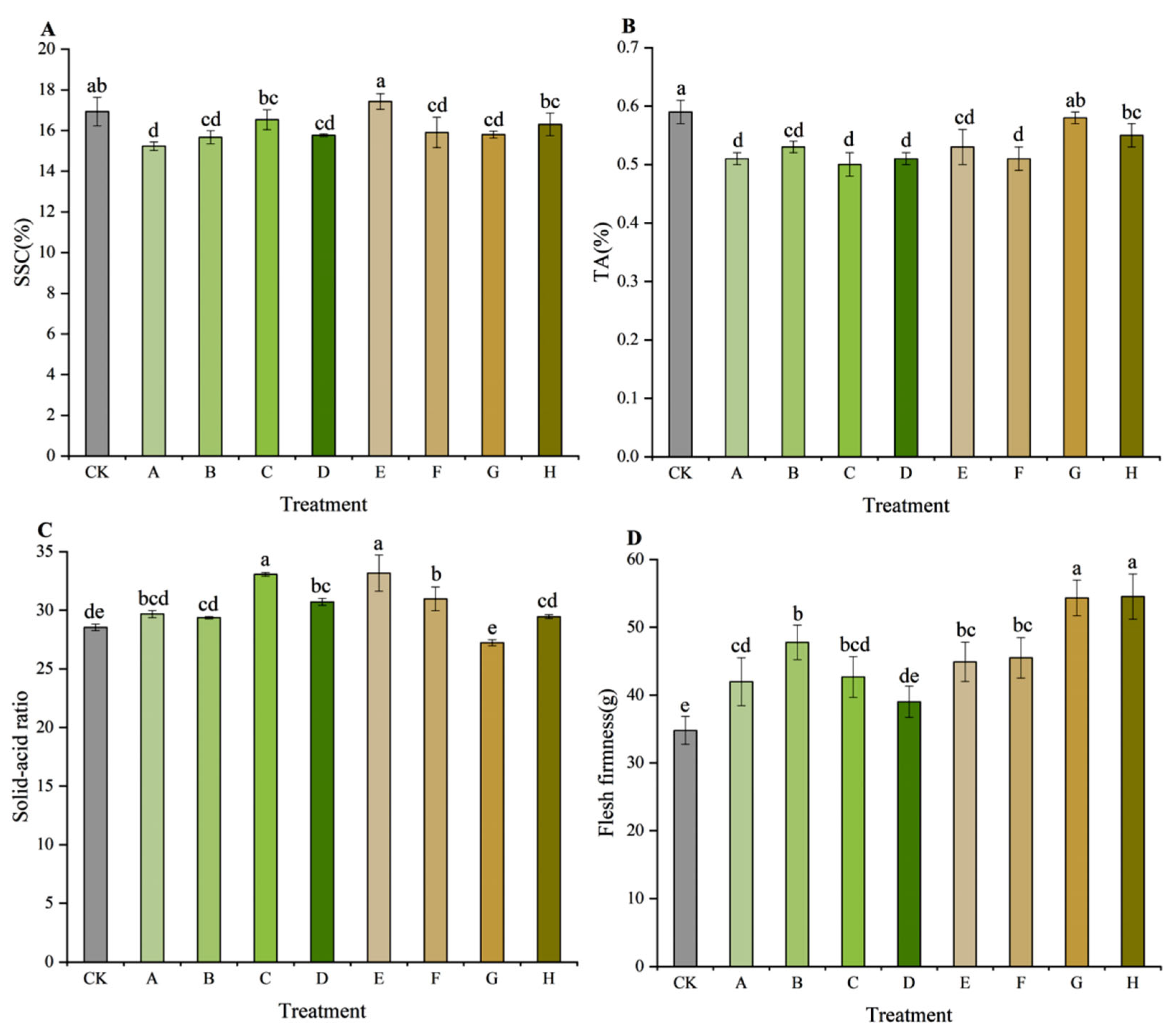
3.3. Fruit Quality Parameters
3.4. Comprehensive Evaluation of Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, R.N. Analysis of Influencing Factors of Soil Nutrients, Tree Nutrient Sand Yield in Vineyard. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2024. [Google Scholar] [CrossRef]
- Malhat, F.; Hegazy, A.; Barakat, D.A.; Ibrahim, E.D.; Hussien, M.; Saber, E.S.; Saber, A.N. Sulfoxaflor residues and exposure risk assessment in grape under egyptian field conditions. Environ. Sci. Pollut. Res. 2024, 31, 52038–52048. [Google Scholar] [CrossRef] [PubMed]
- Vanden, H.J.; Centinari, M. Under-Vine Vegetation Mitigates the Impacts of Excessive Precipitation in Vineyards. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Meintjes, J.J.; Jacobs, G.; Stassen, P.J.C.; Theron, K.I. Shoot growth control of pear trees (Pyrus communis L.) with prohexadione-calcium. Sci. Hortic. 2005, 106, 515–529. [Google Scholar] [CrossRef]
- Miller, S.S.; Tworkoski, T. Regulating Vegetative Growth in Deciduous Fruit Trees. PGRSA Q. 2003, 31, 8–46. [Google Scholar]
- Giudice, D.L.; Wolf, T.K.; Marini, R.P. Vegetative Response of Vitis vinifera to Prohexadione calcium. HortScience 2003, 38, 1435–1438. [Google Scholar] [CrossRef]
- Liu, L.; Gao, D.T.; Wei, Z.F.; Shi, C.Y.; Xu, Y.X. Effects of prohexadione calcium on growth and fruit quality of Fuji apple. J. Fruit Sci. 2021, 38, 1084–1091. [Google Scholar] [CrossRef]
- Mertoğlu, K.; Evrenosoğlu, Y. Effects of ethephon and mepiquat chloride on late blooming of ‘Angeleno’ plum. Mediterr. Agric. Sci. 2017, 30, 79–84. [Google Scholar]
- Mertoğlu, K.; Evrenosoğlu, Y.; Polat, M. Combined effects of ethephon and mepiquat chloride on late blooming, fruit set, and phytochemical characteristics of Black Diamond plum. Turk. J. Agric. For. 2019, 43, 544–553. [Google Scholar] [CrossRef]
- Meng, L.; Yu, K.K.; Wei, Z.X.; Li, K.X.; Dai, J.L.; Li, F.; Qi, H.K.; Sun, L.; Zhang, L.Z.; Dong, H.Z.; et al. High dosage of mepiquat chloride delays defoliation of harvest aids in cotton. Indl. Crop Prod. 2023, 202, 116998. [Google Scholar] [CrossRef]
- Chalise, D.P.; Snider, J.L.; Hand, L.C.; Roberts, P.; Vellidis, G.; Ermanis, A.; Collins, G.D.; Lacerda, L.N.; Cohen, Y.; Pokhrel, A.; et al. Cultivar, irrigation management, and mepiquat chloride strategy: Effects on cotton growth, maturity, yield, and fiber quality. Field Crop Res. 2022, 286, 108633. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.S.; Xing, J.P.; Yu, H.Y.; Zhang, R.; Chen, Y.Y.; Zhang, D.L.; Yin, P.; Tian, X.L.; Wang, Q.; et al. Introducing selective agrochemical manipulation of gibberellin metabolism into a cereal crop. Nat. Plants 2020, 6, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Prakash, R. Influence of mepiquat chloride on growth, yield and quality of cotton. Pestic. Res. J. 2000, 12, 261–262. [Google Scholar]
- Mao, L.L.; Zhang, L.Z.; Evers, J.B.; Werf, W.V.; Liu, S.D.; Zhang, S.P.; Wang, B.M.; Li, Z.H. Yield components and quality of intercropped cotton in response to mepiquat chloride and plant density. Field Crop. Res. 2015, 179, 63–71. [Google Scholar] [CrossRef]
- Lim, S.C.; Kim, Y.H.; Youn, C.K.; Yoon, T.; Kim, S.K. Vine growth and fruit quality of ‘Kyoho’ grapes as affected by mepiquat chloride and GA. Acta Hortic. 2004, 653, 145–149. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.A. Enhancing the bearing capacity and quality of Superior grapes via root pruning, ethephon and mepiquat chloride. Egypt. J. Hort. 2015, 42, 407–420. [Google Scholar]
- Hanaa, M.S.; Samia, A.A. Effect of some plant growth retardants on vegetative growth, spurs and fruiting of ‘leconte’ pear trees. Br. J. Appl. Sci. Technol. 2014, 4, 3785–3804. [Google Scholar] [CrossRef]
- El-Daen, Z. Effect of irrigation levels and spraying mepiquat chloride on growth and productivity of peach trees. Future J. Agric. 2019, 3, 9–19. [Google Scholar]
- Kaur, J.; Kaur, G.; Kaur, K.; Arora, N.K. Effect of Chlormequat Chloride Application on Vegetative Growth and Overall Quality of Grape Cultivars Under Protected Cultivation. Erwerbs-Obstbau 2023, 65, 1921–1929. [Google Scholar] [CrossRef]
- Li, F.F.; Huang, X.Y.; Li, Z.W.; Li, M.Y.; Wang, N.; Zhang, J.Q.; Wang, Y.F.; Sun, T.L.; Wang, H.L. Effects of chlormequat chloride treatment on the growth and physiological indices of wheat. Cereal Res. Commun. 2024, 53, 1–12. [Google Scholar] [CrossRef]
- Khan, H.; Parkash, O.; Mamrutha, H.M.; Bairwa, R.K.; Mishra, C.N.; Kumar, R.; Jasrotia, P.; Kumar, S.; Krishnappa, G.; Ahlawat, O.P.; et al. Foliar application of chlormequat chloride improves lodging resistance and grain yield in bread wheat. Plant Physiol. Rep. 2025, 30, 199–205. [Google Scholar] [CrossRef]
- Ajaykumar, R.; Krishnasamy, S.M. Impact of growth regulating compounds on leaf area index in transplanted rice under moisture stress condition. Int. J. Farm Sci. 2019, 9, 20–23. [Google Scholar] [CrossRef]
- Ma, Y.N.; Cao, Y.L.; Su, L.L.; Wang, H.; Xin, S.J.; Jiang, B.; Wang, F.; Zhu, W.Y. Effects of spraying chlormequat chloride on tomato plant and fruit quality. Shandong Agric. Sci. 2023, 55, 71–78. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, M.; Gill, P.P.S.; Singh, N.P. Effect of prohexadione calcium and chlormequat chloride on growth, yield and fruit quality of pear under high density planting. Indian J. Hortic. 2021, 78, 216–220. [Google Scholar] [CrossRef]
- Patra, S.; Nagar, S.; Swamy, K.M.R.; Pathak, A.; Kanth, N.; Singh, S.K.; Nanda, G.; Panda, A.K. Synergistic Effects of Pollarding and Chlormequat Chloride on Growth, Yield, Quality, and Pest-Disease Incidence in Papaya cv. F1-Red Lady Under Controlled Cultivation. J. Plant Growth Regul. 2025, 1–20. [Google Scholar] [CrossRef]
- Abitha, D.; Srinivasan, S.; Kavino, M.; Maheswari, P. Effect of chemicals, PGRs and pruning on flowering and yield in mango under high density planting. Int. J. Farm Sci. 2019, 9, 127–132. [Google Scholar] [CrossRef]
- Xiao, L.J.; Cao, Y.J.; Li, Y.R.; Feng, M.; Chen, G.; Huang, P.; Wang, H.Q. Effects of different concentrations of CCC on shoot growth and fruit quality of ‘Junzao’. Non-Wood Forest Res. 2021, 39, 150–155. [Google Scholar] [CrossRef]
- Qin, H.H.; Xu, J.; Ma, X.J.; Wei, R.C.; Luo, Z.L. Regulatory Effects of Chlormequat Chloride on the Yield and Chemical Composition of Angelica sinensis Radix. Molecules 2024, 29, 4725. [Google Scholar] [CrossRef]
- Yang, H.; Ding, W.B.; Chen, C.Y.; Dai, M.T.; Chen, X.L.; Liang, J.; Xu, Y.H. Effect of chlormequat chloride on the growth and development of panax ginseng seedlings. HortTechnology 2024, 34, 801–811. [Google Scholar] [CrossRef]
- Lal, M.; Mir, M.M.; Iqbal, U.; Kumar, A. Response of prohexadione calcium and paclobutrazol on growth and physio-chemical characteristics of pear cv. Clapp’s Favorite. Indian J. Hortic. 2018, 75, 191–196. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Qi, X.T.; Yang, D.J.; Li, T.T. Investigation on application of several plant growth regulators on grape. Sino-Overseas Grapevine Wine 2018, 5, 34–39. [Google Scholar] [CrossRef]
- Zheng, R.R.; Wu, Y.; Xia, Y.P. Chlorocholine chloride and paclobutrazol treatments promote carbohydrate accumulation in bulbs of Lilium Oriental hybrids ‘Sorbonne’. J. Zhejiang Univ. Sci. B 2012, 13, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Rogach, V.; Reshetnyk, K.; Kuryata, V.; Rogach, T. Influence of gibberellin inhibitors on the accumulation and redistribution of various forms of carbohydrates and nitrogen-containing compounds in plants of Solanum melongena L. Biologija 2020, 66, 35–46. [Google Scholar] [CrossRef]
- Suliman, A.A.; Saleh, S.A. Effect of chloromequate chloride and indole-3-butric acid as chemical growth regulators on tomato productivity and its chemical composition. Egypt. J. Chem. 2022, 65, 617–623. [Google Scholar] [CrossRef]
- Kashid, D.A.; Doddamani, M.B.; Chetti, M.B.; Hiremath, S.M.; Arvindkumar, B.N. Effect of growth retardants on morpho-physiological traits and yield in sunflower. Karnataka J. Agric. Sci. 2010, 23, 347–349. [Google Scholar]
- Ahmad, I.; Zhu, G.L.; Zhou, G.S.; Younas, M.U.; Yan, X.B. Melatonin and mepiquat chloride impact on cotton growth, physiological and yield at different growth stages. Ind. Crops Prod. 2025, 226, 120702. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, Y.; Zhang, J.Y.; Li, X.M.; Jiang, Z.; Dong, S.K. Effects of DA-6 and MC on the growth, physiology, and yield characteristics of soybean. BMC Plant Biol. 2025, 25, 304. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.G.; Cuadros, L. Study on the effect of girdling and mepiquat chloride application on the yield and quality of avocado (Persea americana Mill.) cv. Fuerte fruits. Proc. Interamer. Soc. Trop. Hort. 2004, 47, 252–254. [Google Scholar]
- Pant, N.; Kumar, R. Effect of paclobutrazol and chlormequat on growth, flowering, yield and quality of ‘Red delicious apple’. Progress. Hort. 2004, 36, 167–170. [Google Scholar]
- Chit-Aree, L.; Suwannawong, P.; Somboonchai, P.; Matta, F.B.; Prathumyot, W. Effect of potassium chlorate combined with paclobutrazol, monopotassium phosphate and mepiquat chloride on fruit quality of longan (Dimocarpus longan). Int. J. Agric. Technol. 2019, 15, 241–248. [Google Scholar]

| Treatments | Reagent | Concentration |
|---|---|---|
| CK (control) | Water | - |
| A | MC | 100 mg/L |
| B | MC | 300 mg/L |
| C | MC | 500 mg/L |
| D | MC | 700 mg/L |
| E | CCC | 100 mg/L |
| F | CCC | 300 mg/L |
| G | CCC | 500 mg/L |
| H | CCC | 700 mg/L |
| Treatments | 17 April | 26 April | 6 May | ||||
|---|---|---|---|---|---|---|---|
| Shoot Length (cm) | Shoot Length (cm) | Growth Rate (%) | Compared with CK Increase or Decrease (%) | Shoot Length (cm) | Growth Rate (%) | Compared with CK Increase or Decrease (%) | |
| CK | 34.30 ± 7.12 bc | 94.85 ± 8.03 a | 176.53 | 0.00 | 148.10 ± 10.40 a | 56.14 | 0.00 |
| A | 37.33 ± 6.32 abc | 84.10 ± 13.10 ab | 125.15 | −51.38 | 137.00 ± 20.80 a | 63.00 | 6.86 |
| B | 39.38 ± 7.09 ab | 81.40 ± 7.00 b | 106.58 | −69.95 | 122.80 ± 14.90 b | 50.95 | −5.19 |
| C | 41.00 ± 4.75 a | 82.80 ± 8.50 b | 101.90 | −74.63 | 124.50 ± 12.50 b | 50.38 | −5.77 |
| D | 32.70 ± 4.39 c | 67.00 ± 8.00 de | 104.89 | −71.64 | 98.70 ± 16.10 cd | 47.31 | −8.83 |
| E | 34.80 ± 4.61 bc | 78.20 ± 6.00 bc | 124.71 | −51.82 | 114.30 ± 15.00 bc | 46.14 | −10.00 |
| F | 37.20 ± 5.94 abc | 73.00 ± 6.30 cd | 96.10 | −80.43 | 105.70 ± 12.80 cd | 44.89 | −11.25 |
| G | 42.32 ± 5.24 a | 70.20 ± 6.70 de | 65.77 | −110.76 | 98.90 ± 7.80 d | 40.91 | −15.23 |
| H | 40.05 ± 3.52 ab | 65.10 ± 3.10 e | 62.55 | −113.98 | 91.90 ± 9.90 d | 41.17 | −14.97 |
| Treatments | Fruit Axis Length (cm) | Branch Number (No.) | Branch Density (No./cm) |
|---|---|---|---|
| CK | 13.60 ± 1.61 ab | 21.10 ± 2.69 cd | 1.56 ± 0.19 b |
| A | 13.25 ± 1.23 ab | 20.80 ± 2.30 d | 1.58 ± 0.20 b |
| B | 12.86 ± 1.10 ab | 22.27 ± 2.15 cd | 1.73 ± 0.10 ab |
| C | 13.10 ± 1.51 ab | 22.30 ± 2.45 cd | 1.72 ± 0.25 ab |
| D | 12.27 ± 0.82 b | 21.73 ± 2.94 cd | 1.77 ± 0.22 a |
| E | 14.00 ± 2.37 a | 25.18 ± 2.48 a | 1.72 ± 0.14 ab |
| F | 13.30 ± 1.46 ab | 22.70 ± 1.70 bcd | 1.83 ± 0.27 a |
| G | 13.23 ± 1.15 ab | 24.82 ± 2.5 2 ab | 1.88 ± 0.12 a |
| H | 12.65 ± 1.68 ab | 23.50 ± 2.95 abc | 1.87 ± 0.17 a |
| Treatments | Single Berry Weight (g) | Longitudinal Diameters (cm) | Transverse Diameters (cm) | Fruit Shape Index |
|---|---|---|---|---|
| CK | 12.18 ± 0.82 bc | 2.84 ± 0.03 bc | 2.66 ± 0.01 cd | 1.07 ± 0.01 de |
| A | 14.08 ± 1.14 a | 2.98 ± 0.02 a | 2.81 ± 0.02 a | 1.06 ± 0.00 e |
| B | 13.29 ± 1.20 ab | 3.00 ± 0.03 a | 2.79 ± 0.03 a | 1.07 ± 0.02 de |
| C | 13.38 ± 1.31 ab | 3.01 ± 0.03 a | 2.77 ± 0.03 a | 1.09 ± 0.02 cd |
| D | 12.49 ± 1.93 bc | 2.99 ± 0.05 a | 2.62 ± 0.06 d | 1.14 ± 0.01 a |
| E | 13.29 ± 1.34 ab | 3.06 ± 0.04 a | 2.76 ± 0.05 ab | 1.11 ± 0.02 bc |
| F | 12.24 ± 1.29 bc | 2.91 ± 0.07 b | 2.69 ± 0.07 bc | 1.08 ± 0.01 de |
| G | 12.85 ± 0.84 ab | 2.99 ± 0.04 a | 2.67 ± 0.02 cd | 1.12 ± 0.02 ab |
| H | 11.41 ± 1.20 c | 2.82 ± 0.05 c | 2.54 ± 0.03 e | 1.11 ± 0.01 bc |
| Principal Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigen value | 2.397 | 2.132 | 1.416 |
| Variance contribution rate (%) | 34.245 | 30.453 | 20.226 |
| Cumulative variance contribution rate (%) | 34.245 | 64.698 | 84.924 |
| Branch density | 0.843 * | −0.271 | 0.116 |
| Relative chlorophyll content | −0.660 * | 0.597 | 0.004 |
| Single berry weight | 0.641 * | 0.230 | −0.476 |
| SSC | −0.057 | 0.236 | 0.906 * |
| TA | 0.304 | 0.843 * | −0.379 |
| Solid–acid ratio | 0.211 | 0.938 * | 0.244 |
| Flesh firmness | −0.836 * | −0.041 | −0.390 |
| Treatments | CK | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|---|
| Z value | 0.315 | 0.357 | −0.294 | 1.038 | 0.252 | 0.92 | 0.069 | −1.571 | −1.086 |
| Rank | 4 | 3 | 7 | 1 | 5 | 2 | 6 | 9 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; He, S.; Li, L.; Tong, X.; Gu, H.; Sun, X.; Li, M.; Chen, J. Effects of Mepiquat Chloride and Chlormequat Chloride on the Growth and Fruit Quality of ‘Shine Muscat’ Grapevines. Agriculture 2025, 15, 1267. https://doi.org/10.3390/agriculture15121267
Cheng D, He S, Li L, Tong X, Gu H, Sun X, Li M, Chen J. Effects of Mepiquat Chloride and Chlormequat Chloride on the Growth and Fruit Quality of ‘Shine Muscat’ Grapevines. Agriculture. 2025; 15(12):1267. https://doi.org/10.3390/agriculture15121267
Chicago/Turabian StyleCheng, Dawei, Shasha He, Lan Li, Xiangyang Tong, Hong Gu, Xiaoxu Sun, Ming Li, and Jinyong Chen. 2025. "Effects of Mepiquat Chloride and Chlormequat Chloride on the Growth and Fruit Quality of ‘Shine Muscat’ Grapevines" Agriculture 15, no. 12: 1267. https://doi.org/10.3390/agriculture15121267
APA StyleCheng, D., He, S., Li, L., Tong, X., Gu, H., Sun, X., Li, M., & Chen, J. (2025). Effects of Mepiquat Chloride and Chlormequat Chloride on the Growth and Fruit Quality of ‘Shine Muscat’ Grapevines. Agriculture, 15(12), 1267. https://doi.org/10.3390/agriculture15121267






