1. Introduction
Microbial feed fermentation technology has gained wide-spread acceptance in livestock and poultry feeding during breeding. The use of unconventional raw materials is crucial in reducing reliance on corn and soybean meal, and research on the fermentation of these alternative ingredients for duck feed has attracted increasing attention. Fermentation technology has been proven to substantially boost the nutritional value and digestibility of feed, thereby improving poultry growth and production performance [
1,
2]. For example, a study revealed that fermented feed enhances digestibility, improving growth performance in poultry [
3]. Fermented feed made from soybean hulls and rapeseed cakes enhances immunity, increases antioxidant levels, and improves gut health, resulting in improved chicken growth [
4]. Furthermore, food waste processed through fermentation technology has been demonstrated to be a viable source of chicken feed and liquid fertilizers, contributing to both resource recycling while lowering feed costs [
5]. Moreover, the application of microbial fermentation technology to soybean meal and other plant-based protein sources has been proven to be both cost-effective and nutritionally viable [
6,
7]. Recently, a study reported that incorporating fermented feed into duck diets fosters intestinal health and reduces pathogen proliferation [
8]. All these findings reported above indicate that exploring fermentation techniques for non-traditional raw materials may not only reduce feed costs but also enhance livestock and poultry production performance and overall health levels. In other words, microbial fermentation technology has a wide range of applications in livestock and poultry feed, providing sustainable alternatives to conventional feed ingredients while promoting efficient and environmentally friendly breeding practices.
Unconventional protein feeds such as sesame meal, sunflower seed meal, and corn starch residue are widely available in China and its surrounding production regions. However, their incorporation into livestock and poultry farming is frequently hindered by challenges such as unappealing taste, inadequate nutritional content, unbalanced nutrient profiles, and the existence of several anti-nutritional elements [
9]. Fermentation technology, characterized by its relatively mild and efficient processes, is the preferred method for developing non-traditional protein feed resources. This approach offers an effective solution to the aforementioned challenges. However, its application still encounters various technical limitations and potential drawbacks. For instance, controlling the fermentation process is complex, and dynamic regulation poses challenges. The microbial fermentation process is influenced by multiple factors, including time, temperature, pH levels, and substrate concentration [
10]. Specifically, during prolonged fermentation, microbial metabolic pathways may adjust in response to changes in growth phases, complicating the widespread promotion and adoption of related technologies in production. Therefore, investigating relatively straightforward fermentation technologies is particularly crucial for poultry production. The Longyan Shan-ma duck, a local breed from southern China, represents one of the country’s most popular laying duck, known for its high egg production performance, with over 300 million raised there [
11,
12]. Nevertheless, there is limited information regarding the effects of microbially fermented feed on the production and intestinal health of Longyan Shan-ma ducks. Herein, we investigated the effects of dietary supplementation with fermented mixed feed (FMF, composed of several unconventional protein feeds, including brown rice, rice bran, sunflower meal, cottonseed meal, and corn starch residue) on the egg performance and quality, serum biochemical indices, and gut health of Fujian Longyan Shan-ma laying ducks.
2. Materials and Methods
2.1. FMF Preparation
The feed, consisting of brown rice, wheat, rice bran, wheat bran, soybean meal, sunflower meal, cottonseed meal, and corn starch residue, was provided by Shanghai Nonghao Feed Co., Ltd. (Shanghai, China). The fermentation process was carried out using the fermentation bacterial liquid supplied by Henan Nong-fukang Biotechnology Co., Ltd., (Nanyang, China). The fermentation bacterial liquid consists of a mixed liquid culture containing about 2 × 1011 CFU/ml of Bacillus licheniformis, Lactobacillus plantarum, and Bifidobacterium plantarum in approximately equal proportions. Briefly, before the fermentation process, 1000 mL of bacterial fermentation liquid was combined with 1 kg of brown sugar in 100 kg of water to prepare the fermentation broth. Subsequently, the dry basal feed consisting of 29.86% brown rice, 27.40% wheat, 13.70% rice bran, 6.85% wheat bran, 7.81% soybean meal, 8.22% sunflower meal, 2.05% cottonseed meal, and 4.11% corn starch residue was thoroughly homogenized and then integrated with the fermentation broth to ensure even mixing. The feed-to-liquid ratio was maintained at a ratio of 3:1 (m:m). Finally, the mixture was uniformly combined, compacted, and sealed in plastic bags (25 kg per bag) for fermentation at a temperature of 37 °C for a period of 14 days. Upon completion of the fermentation process, the moist fermented feed was stored in a refrigeration facility at a temperature of 4 °C.
Using the Kjeldahl method, the crude protein (CP) content in FMF was analyzed, and the crude fat was evaluated through the Soxhlet extraction method (AOAC, 2000) [
13]. Crude fiber content was analyzed through filter bag technology [
14]. Lactic acid content was quantified using a detection kit from Suzhou Mengxi Biomedical Technology Co., Ltd., (Suzhou, China). The amino acids were detected by high-pressure liquid chromatography (HPLC), which was performed on Agilent 1100 HPLC (Agilent, Folsom, CA, USA), as previous described by Wassie et al. [
15], with a UV detector set at 254 nm.
2.2. Animals, Experimental Design, and Diets
A total of 360 Fujian Longyan Shan-ma laying ducks, each aged 140 days, were randomly divided into a control group receiving a basal diet and an FMF-treated group where 4% of the basal diet was substituted with FMF. Each treatment had six replicates with 30 ducks per replicate. The ducks were raised using the ground flat-feeding method, maintaining a water-to-land area ratio of 1:3 (each replicate was housed in indoor 4 × 5 m, outdoor 5 × 5 m, and pool 3 × 5 m areas). The feed formulation for both groups adhered to the recommended nutritional standards for ducks, featuring a metabolic energy level of 10.45 MJ/kg and a CP content of 15.3%. Both diets contained similar levels of protein and metabolizable energy (shown in
Table 1). Ducks had unlimited access to water and food. They underwent a 7-day adaptation phase before a 98-day experimental period. During the experiment, the ducks were not vaccinated and were exposed to both natural and artificial lighting conditions (16 h light and 8 h dark).
2.3. Sample Collection
Two ducks per replicate, making up 12 ducks per group, were randomly selected and slaughtered at an age of 245 days for the analysis of intestinal tissues and contents. After euthanizing the ducks with sodium pentobarbital, the ileum samples were separated from the mesentery, rinsed with cold PBS, and fixed in 4% paraformaldehyde. The ileal contents were collected, frozen in liquid nitrogen, and stored at −80 °C.
2.4. Performance and Egg Quality Measurements
Throughout the experiment, daily records of egg production, egg weight, and feed consumption were kept for each replicate to determine the average daily feed intake (ADFI), feed conversion rate (FCR), and average egg weight. To minimize the risk of outcome variations caused by differences in duck feed consumption, each duck in every group was provided with 140 g of feed per day. At 245 days of age, ten eggs from each replicate (n = 60 per group) were randomly selected and assessed for egg quality. The egg shape index, eggshell strength, Haugh unit, albumen height, and yolk color were analyzed using an automatic egg analyzer (DET6500, Nabel, Kyoto, Japan).
2.5. Serum Analysis
At 245 days old, three ducks were randomly chosen for blood collection from the wing vein. The serum concentrations of blood urea nitrogen (BUN) and uric acid (UA) were determined using a Beckman AU5821 automatic analyzer (Beckman Coulter Inc., Brea, CA, USA). Interleukin-1β (IL-1β), interleukin-4 (IL-4), immunoglobulin A (IgA), endotoxin (ET), and tumor necrosis factor-α (TNF-α) levels were detected by ELISA kits according to the manufacture’s instruction (Jiangsu Jingmei Bio-technology Co., Ltd., Yancheng, China). Further, total antioxidant capacity (T-AOC) and superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) contents were assessed with kits from the Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China) according to the manufacturer’s instructions.
2.6. Histological Analysis
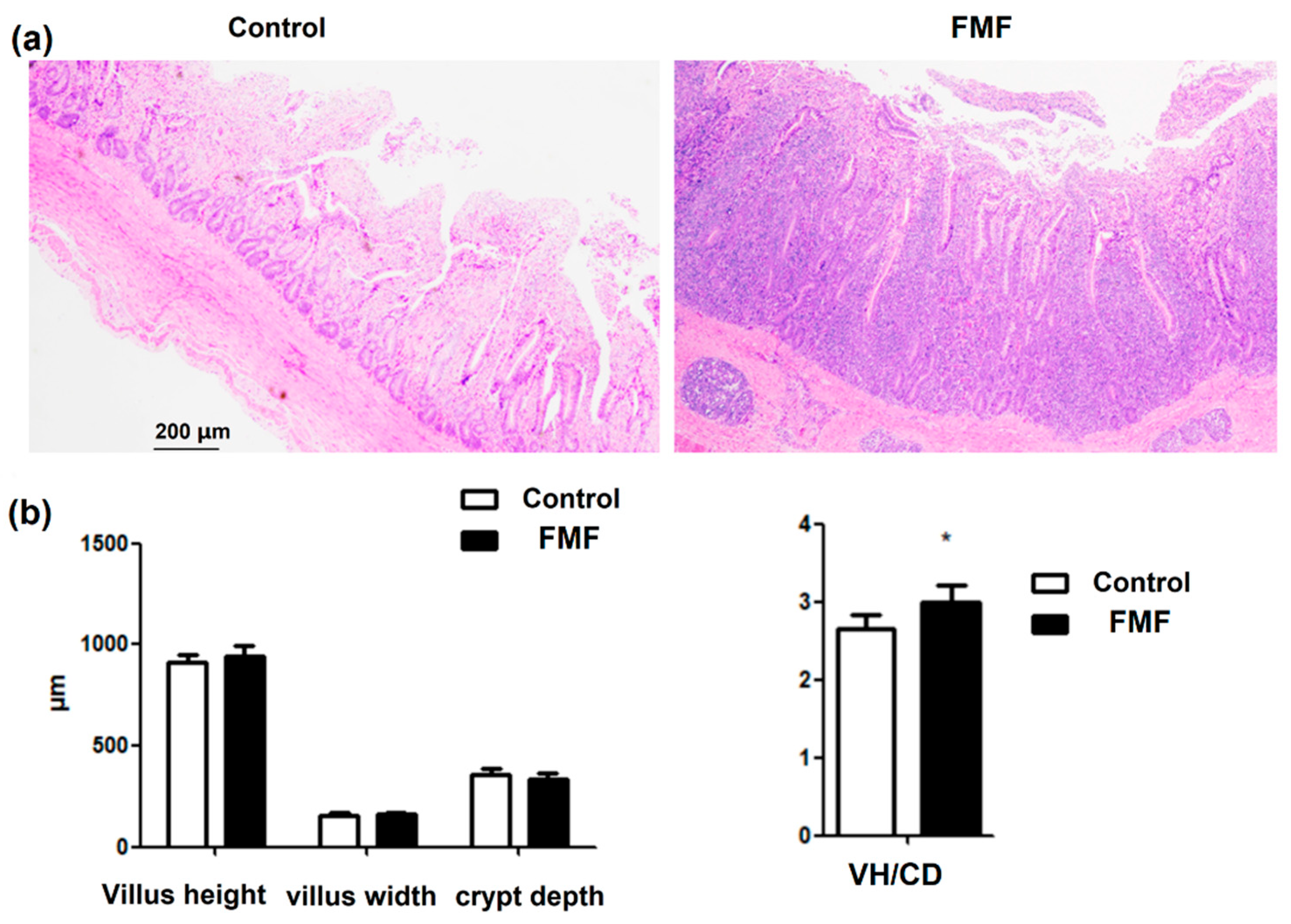
Ileal samples, with 12 in each group, were sectioned and fixed in 4% formaldehyde for histological examination. They were stained with hematoxylin and eosin and observed using an Olympus light microscope (Olympus, Center Valley, PA, USA). Images of the proximal small intestine were selected for analysis, particularly those showing regions where the crypts in the cross-section merged with the villi. To obtain a statistical value for each animal, five distinct sections were analyzed and averaged. The measurements focused on the height and width of the villi and the depth of the crypts, using six well-aligned villi per section.
2.7. Gut Microbiome and SCFA Analyses
The TIANGEN DNA stool mini kit (TIANGEN, Beijing, China) was used to extract microbial genomic DNA from ileum content samples, following the manufacturer’s guidelines. The V3–V4 region of the 16S rRNA gene was subsequently amplified using specific primers as previously outlined [
16]. PCR-amplified 16S rRNA gene sequences from both the control group (
n = 12) and the FMF (
n = 12) group were sequenced using the Illumina MiSeq PE300 platform (San Diego, CA, USA). The Trimmomatic software (v0.39,
http://www.usadellab.org/cms/?page=trimmomatic, accessed on 15 March 2024) was employed to filter raw FASTQ sequences by eliminating adapter contamination, trimming low-quality bases, and discarding reads shorter than 50 bp or with a quality score below 20. Subsequently, we utilized DADA2 (
https://github.com/benjjneb/dada2, accessed on 15 March 2024) to infer precise amplicon sequence variants from the filtered data. Taxonomic assignments, as well as analyses of alpha diversity, beta diversity, and species differences, were conducted with QIIME2. Functional metagenomic predictions based on 16S rRNA data were performed by utilizing Phylogenetic Investigation of Communities by Reconstruction of Unobserved States.
For short-chain fatty acid (SCFA) analyses, gas chromatography was used as previously described [
17]. Briefly, ileal contents (50 mg) were homogenized in 800 μL of 0.5% phosphoric acid water (containing an internal standard of 2-ethylbutyric acid at a concentration of 10 μg/mL) and subsequently centrifuged at 13,000×
g for 15 min. The supernatants were analyzed using an HP FFAP column (30 m × 0.25 mm × 0.25 μm, Agilent J&W Scientific, Folsom, CA, USA) under the following conditions: an injector volume of 1 μL, a split ratio of 10:1, an injector temperature of 180 °C, helium as the carrier gas, and an oven temperature that started at 80 °C for 10 min, increased at a rate of 20 °C/min to 120 °C, and subsequently programmed to 160 °C at a rate of 5 °C/min. Then, it was maintained at 220 °C for 3 min. Finally, a detector temperature of 230 °C was employed.
2.8. Statistical Analysis
Values are presented as the Mean ± SEM. Data analysis was conducted using GraphPad Prism version 8.0 (San Diego, CA, USA). Student’s t-test was utilized to compare the biological parameters, gut morphology, and microbiota between the groups. For diversity indices and differential microbiota analyses, the Wilcoxon rank-sum test was employed. Statistical significance was determined at a threshold of p < 0.05, with adjustments made for the false discovery rate. Additionally, a Spearman correlation analysis was performed to investigate the relationships between significantly altered parameters and the microbiota, considering a p-value of less than 0.05 as indicative of statistical significance.
4. Discussion
The diets of laying ducks are predominantly composed of corn and soybean meal. However, the increasing costs associated with these ingredients necessitate the exploration of alternative protein sources for egg production. Nutrient-rich feedstuffs, such as rough rice, wheat, rice bran, rice bran meal, soybean meal, sunflower meal, cottonseed meal, and corn starch residue, have been investigated for poultry production. Nevertheless, these alternatives in Chinese duck egg production are constrained due to the presence of anti-nutritional factors, which may negatively affect the health and performance of poultry.
Microbial fermentation has been shown to effectively degrade anti-nutritional factors in feed ingredients, enhance the release of small peptides and amino acids, and generate functional compounds, such as probiotics, antimicrobial agents, and organic acids [
10]. These changes can significantly improve intestinal health, enhance immune function, and increase feed palatability [
18,
19]. Previous studies have demonstrated that fermentation significantly reduces free gossypol content in cottonseed meal while increasing CP and lactic acid bacteria content [
19], a finding consistent with our research results. Herein, we found that fermentation significantly reduced the phytic acid and crude fiber content in FMF while markedly increasing the CP and lactic acid content. In addition, fermentation significantly enhanced the concentrations of several essential amino acids in duck feed, suggesting its efficacy in enhancing the nutritional value of unconventional feed and facilitating the release of digestible amino acids, thereby enhancing poultry production performance.
Egg quality is evaluated using key indicators such as eggshell strength, the Haugh unit, eggshell thickness, and yolk color. Previous studies have shown that incorporating fermented feed into chicken diets can improve egg weight, eggshell weight, and eggshell hardness [
20]. Moreover, FMF supplementation has been reported to enhance egg production, albumen height, and the Haugh unit in laying hens [
21]. Eggshell strength and thickness are critical factors in determining the overall egg quality. These characteristics not only ensure the protection of the egg’s contents but also contribute to the egg’s marketability and consumer acceptance. Fermented feed can improve nutrient absorption and gut health, leading to better calcium utilization and deposition in the eggshell [
22]. This is supported by research indicating that dietary interventions, such as the inclusion of probiotics and other additives, can significantly enhance eggshell quality by improving the laying performance and immune function of hens [
22]. In the present study, align with these findings in laying hens, we demonstrate that the inclusion of FMF is advantageous for the egg performance and quality in ducks, along with enhancing the yolk ratio, Haugh unit, eggshell strength, and eggshell thickness.
Serum biochemical indicators provide a comprehensive assessment of nutritional metabolism, stress, and overall health. The inclusion of fermented feed in the diet of laying hens enhances the function of antioxidant enzymes, including T-AOC, SOD, and GSH-Px, and decreases MDA levels [
22,
23]. Additionally, IL-1β is a critical pro-inflammatory cytokine implicated in immune responses and tumor progression [
24]. BUN is a critical biomarker for assessing kidney function and has been linked to the development and prognosis of various diseases [
25]. Herein, it was observed that FMF supplementation led to a significant enhancement in T-AOC and GSH-Px concentrations while concurrently reducing blood BUN and IL-1β levels. These findings suggest that FMF supplementation may enhance the antioxidant capacity and modulate the immune function in egg-laying ducks.
The small intestine is the primary site for nutrient digestion and absorption in poultry, with the intestinal villi playing a critical role in nutrient uptake. Maintaining an intact intestinal structure is essential for optimal nutrient utilization and poultry growth. Key indicators of intestinal development include villus height, crypt depth, and the VH/CD ratio. An increase in villus height enhances the digestive surface area, improving nutrient absorption efficiency, while a reduction in crypt depth promotes intestinal epithelial regeneration. The VH/CD ratio is an important indicator of intestinal absorptive function, with higher values correlating with improved nutrient uptake [
26]. Our findings showed that FMF supplementation significantly increased the VH/CD ratio and enhanced intestinal villi density, suggesting that FMF improves the intestinal morphology and gut health in laying ducks. These findings are consistent with previous reports by [
10,
22].
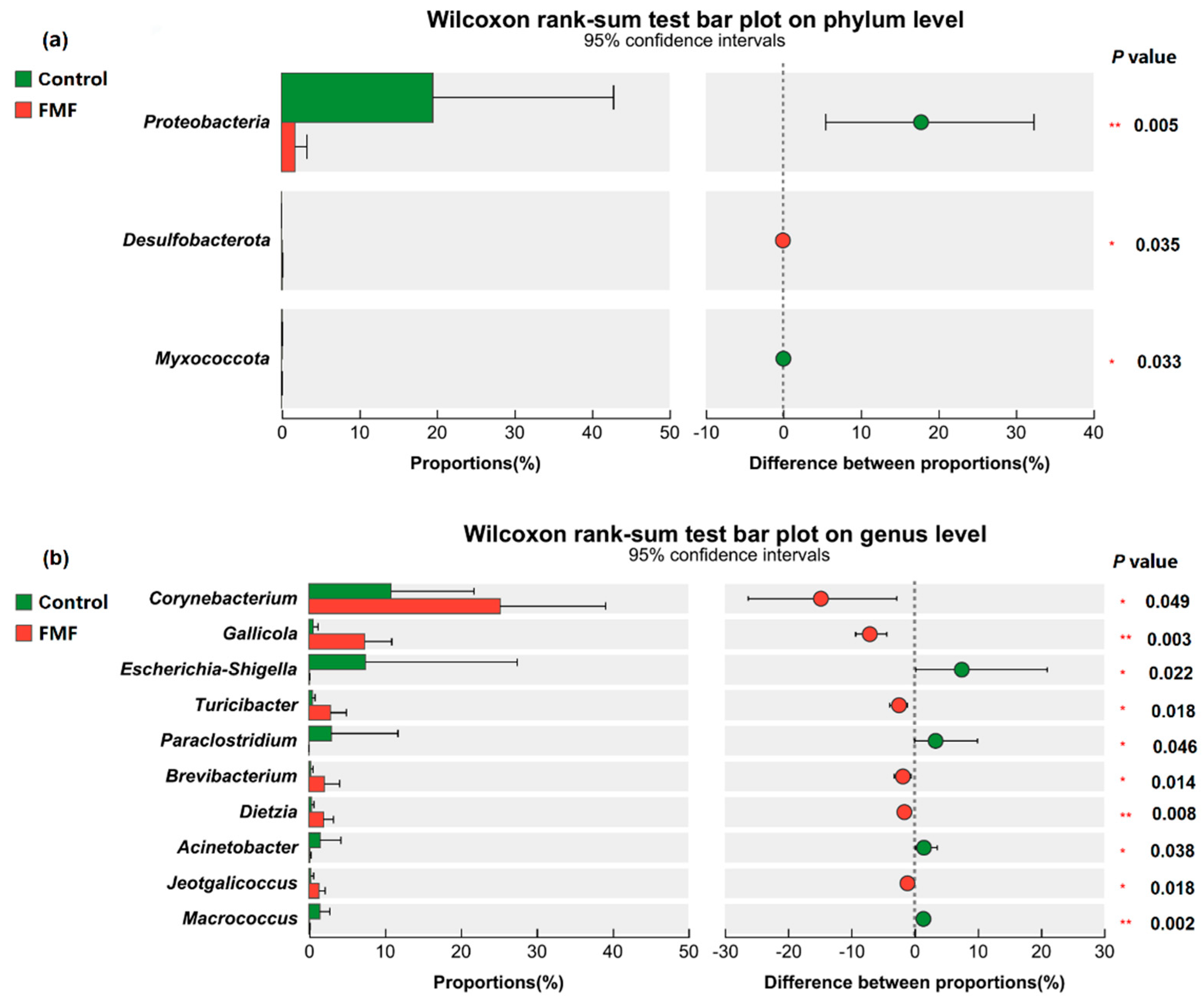
Probiotics are essential for adjusting gut microbiota by boosting the presence and function of beneficial bacteria while suppressing harmful bacteria, thus enhancing the intestinal biochemical barrier [
27]. Incorporating FMF into broiler diets has been shown to boost beneficial gut bacteria and decrease
E. coli and
Morganella morganii [
28]. Probiotic-fermented feed reshapes the intestinal microbiota of ducks by reducing the abundance of
Ruminococcaceae,
Lachnospiraceae, and
Prevotaceae [
29]. Recent data have demonstrated that
Firmicutes harbor numerous genes responsible for dietary fiber fermentation and may interact with the intestinal mucosa, contributing to gut homeostasis [
30].
Lactobacillus also plays a significant role in maintaining intestinal function in both humans and animals [
31].
Lactobacillus can eliminate pathogenic bacteria, enhance intestinal health, and modulate immune and metabolic responses [
32,
33]. In contrast,
Helicobacter is a pathogenic bacterial genus associated with gastrointestinal diseases [
34].
Kocuria overabundance has been linked to peritonitis [
35], while
Rothia is associated with respiratory diseases in animals [
36].
Escherichia-Shigella is another prevalent pathogen that contributes to intestinal diseases in animals [
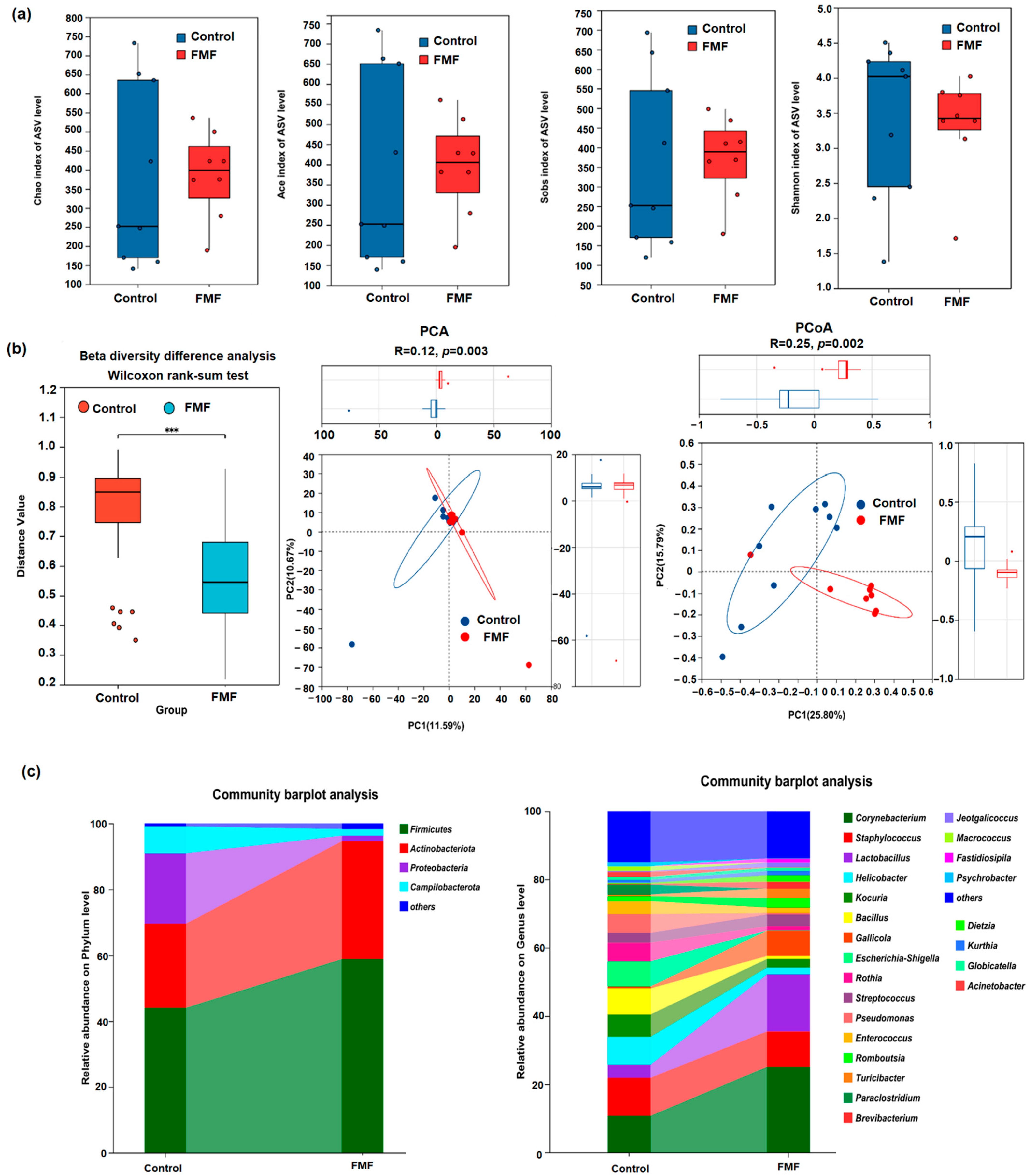
37]. In the present study, FMF supplementation influenced β-diversity in the intestinal microbiota in laying ducks, suggesting that FMF alters both microbial composition and abundance. Notably, the abundance of
Lactobacillus increased while that of
Kocuria,
Helicobacter, and
Escherichia-Shigella decreased in the FMF group. These findings supported the hypothesis that FMF supplementation promoted the proliferation of beneficial gut bacteria while inhibiting harmful microbes. In addition, we found that
Escherichia-Shigella,
Enterococcus, and
Rothia were positively correlated with IL-1β, whereas
Gallicola exhibited a negative correlation. In addition,
Rothia abundance was negatively correlated with T-AOC. These findings confirmed the beneficial effects of FMF on the intestinal health of laying ducks.
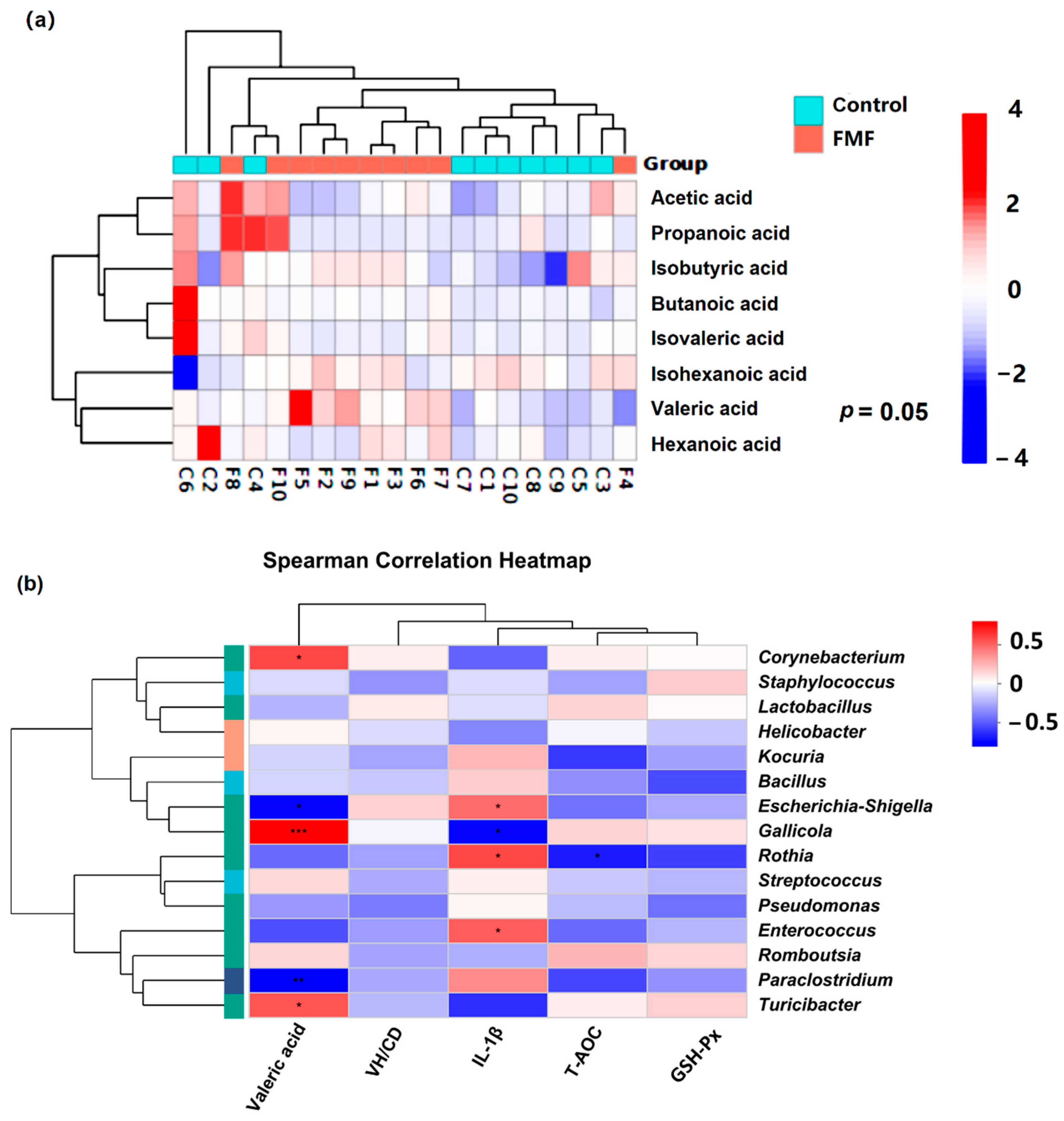
SCFAs, produced through the fermentation of dietary fiber by intestinal microbes, are key energy providers for intestinal epithelial cells and aid in systemic equilibrium [
38,
39]. Valeric acid, an important SCFA generated by gut microbiota, is crucial for sustaining a balanced intestinal environment and supporting the growth of beneficial bacteria in the colon [
40]. It reportedly enhances gastrointestinal function and preserves intestinal epithelial integrity in mice exposed to radiation [
41]. Furthermore, valeric acid could suppress the production of the RELA protein, a pro-inflammatory factor, while upregulating the mRNA expression of the anti-inflammatory factor IL-10, thereby inhibiting osteoclast-like cell maturation [
42]. Herein, FMF supplementation was found to increase valeric acid concentrations in the intestines of laying ducks. Furthermore, valeric acid levels showed a positive correlation with the abundance of
Turcibacter,
Gallicola, and
Corynebacterium and a negative correlation with that of
Paraclostridium and
Escherichia-Shigella. These results confirmed a strong association between SCFA production and gut microbial composition in FMF-fed ducks.