Milk Lactose and Inflammatory Marker Changes: Early Indicators of Metabolic and Inflammatory Stress in Early Lactation Dairy Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animal Selection
2.2. Experimental Group Classification
2.3. Housing and Feeding Management
2.4. Data Acquisition and Monitoring Systems
2.5. Blood Sampling and Biochemical Analysis
2.6. Milk Composition
2.7. Cow Behavioural Monitoring and Activity Tracking
2.8. Data Processing and Statistical Evaluation
3. Results
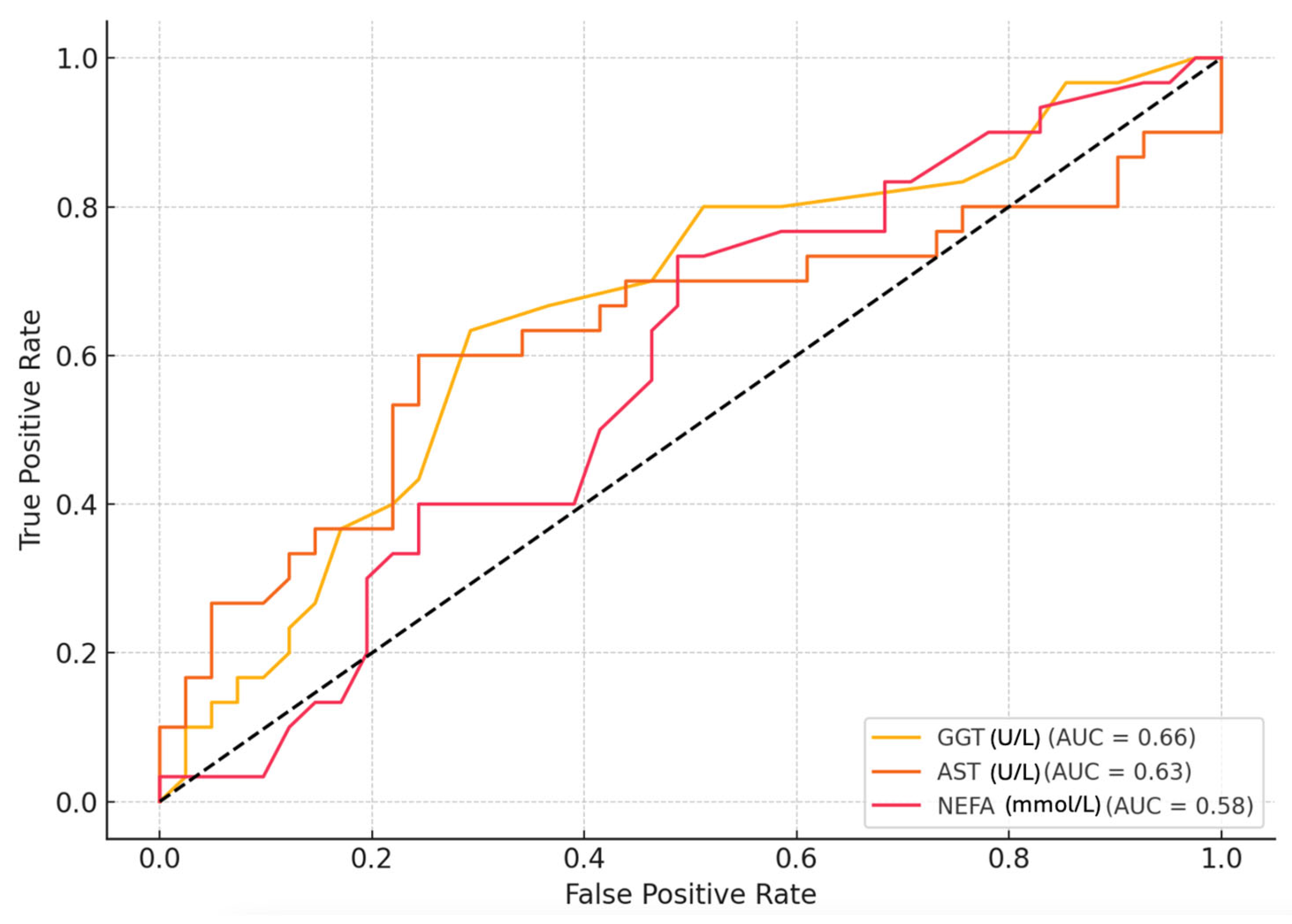
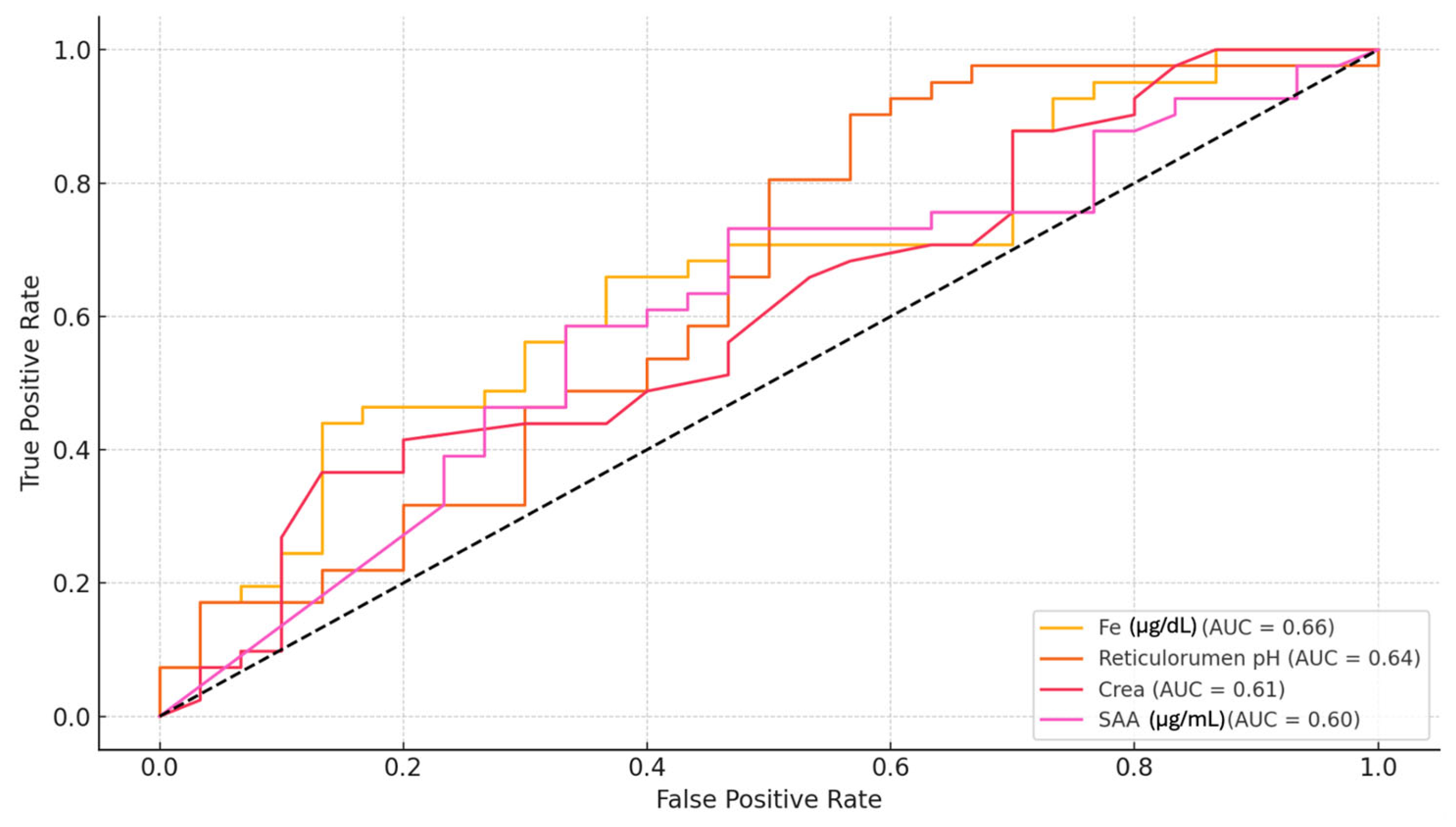
3.1. Biomarker Description of ROC Analysis Results for Milk Lactose
3.2. Behaviour and Blood Parameters Differences in Dairy Cows with Low and High Milk Lactose Levels
3.3. Correlation Analysis with CRP
4. Discussion
4.1. Interpretation of Biomarker Associations and Physiological Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| CRP | C-reactive protein |
| AST | Aspartate transaminase |
| Fe | Iron |
| NEFA | Non-esterified fatty acid |
| APP | Acute phase protein |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| TNFα | Tumour necrosis factor α |
| GGT | Gamma-glutamyl transferase |
| SAA | Serum amyloid A |
| BSC | Body condition score |
| CMT | California mastitis test |
| TMR | Total mixed ration |
| CREA | Creatinine |
| A/D | Analog-to-digital |
| AUC | Area Under the Curve |
| ROC | Receiver operating characteristic |
| RMSEP | Root mean square error of prediction |
| SARA | Sub-acute ruminal acidosis |
| BHB | β-hydroxybutyrate |
| VFA | Volatile fatty acid |
| SCC | Somatic cell count |
| NEB | Negative energy balance |
| Hp | Haptoglobin |
| DMI | Dry matter intake |
| BHBA | β-hydroxybutyrate |
| MDA | Malondialdehyde |
| TAS | Total antioxidant status |
References
- Costa, A.; Lopez-Villalobos, N.; Sneddon, N.W.; Shalloo, L.; Franzoi, M.; De Marchi, M.; Penasa, M. Invited Review: Milk Lactose-Current Status and Future Challenges in Dairy Cattle. J. Dairy Sci. 2019, 102, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Lactose and Its Relationship with Other Milk Constituents, Somatic Cell Count, and Total Bacterial Count. Livest. Sci. 2021, 252, 104678. [CrossRef]
- Gross, J.J.; Bruckmaier, R.M. Review: Metabolic challenges in lactating dairy cows and their assessment via established and novel indicators in milk. Animal 2019, 13, s75–s81. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, A.; Lv, X.; Zhou, C.; Tan, Z. Metabolic Changes in Serum and Milk of Holstein Cows in Their First to Fourth Parity Revealed by Biochemical Analysis and Untargeted Metabolomics. Animals 2024, 14, 407. [Google Scholar] [CrossRef]
- Tian, H.; Zheng, N.; Wang, W.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Integrated Metabolomics Study of the Milk of Heat-Stressed Lactating Dairy Cows. Sci. Rep. 2016, 6, 24208. [Google Scholar] [CrossRef]
- Antanaitis, R.; Džermeikaitė, K.; Krištolaitytė, J.; Girdauskaitė, A.; Arlauskaitė, S.; Tolkačiovaitė, K.; Baumgartner, W. The Relation between Milk Lactose Concentration and the Rumination, Feeding, and Locomotion Behavior of Early-Lactation Dairy Cows. Animals 2024, 14, 836. [Google Scholar] [CrossRef]
- Kovacikova, E.; Kovacik, A.; Harangozo, L.; Tokarova, K.; Knazicka, Z.; Tvrda, E.; Jambor, T.; Tomka, M.; Massanyi, P.; Lukac, N. Canonical Correlation of Milk Composition Parameters and Blood Biomarkers in High-Producing Dairy Cows During Different Lactation Stages. Animals 2024, 14, 3294. [Google Scholar] [CrossRef]
- Portnoy, M.; Barbano, D.M. Lactose: Use, Measurement, and Expression of Results. J. Dairy Sci. 2021, 104, 8314–8325. [Google Scholar] [CrossRef]
- Horst, E.A.; Kvidera, S.K.; Baumgard, L.H. Invited Review: The Influence of Immune Activation on Transition Cow Health and Performance—A Critical Evaluation of Traditional Dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef]
- Shahbazkia, H.R.; Aminlari, M.; Tavasoli, A.; Mohamadnia, A.R.; Cravador, A. Associations among Milk Production Traits and Glycosylated Haemoglobin in Dairy Cattle; Importance of Lactose Synthesis Potential. Vet. Res. Commun. 2010, 34, 1–9. [Google Scholar] [CrossRef]
- Costa, A.; Bovenhuis, H.; Egger-Danner, C.; Fuerst-Waltl, B.; Boutinaud, M.; Guinard-Flament, J.; Obritzhauser, W.; Visentin, G.; Penasa, M. Mastitis Has a Cumulative and Lasting Effect on Milk Yield and Lactose Content in Dairy Cows. J. Dairy Sci. 2025, 108, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Qi, J.; An, X.; Wang, Y.; Wang, B.; Li, X.; Zhang, Z.; Huo, X. Changes in Feeding Behavior, Milk Yield, Serum Indexes, and Metabolites of Dairy Cows in Three Weeks Postpartum. Sci. Rep. 2025, 15, 7925. [Google Scholar] [CrossRef] [PubMed]
- Haile-Mariam, M.; Pryce, J.E. Genetic Parameters for Lactose and Its Correlation with Other Milk Production Traits and Fitness Traits in Pasture-Based Production Systems. J. Dairy Sci. 2017, 100, 3754–3766. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.A.A.; Larsen, T.; Leroux, C. Milk Metabolites and Fatty Acids as Noninvasive Biomarkers of Metabolic Status and Energy Balance in Early-Lactation Cows. J. Dairy Sci. 2022, 105, 201–220. [Google Scholar] [CrossRef]
- Bagga, A.; Randhawa, S.S.; Sharma, S.; Bansal, B.K. Acute Phase Response in Lame Crossbred Dairy Cattle. Vet. World 2016, 9, 1204–1208. [Google Scholar] [CrossRef]
- Prediction of metabolic status of dairy cows in early lactation with on-farm cow data and machine learning algorithms. J. Dairy Sci. 2019, 102, 10186–10201. [CrossRef]
- Kuczyńska, B.; Puppel, K.; Gołębiewski, M.; Wiśniewski, K.; Przysucha, T. Metabolic Profile According to the Parity and Stage of Lactation of High-Performance Holstein-Friesian Cows. Anim. Biosci. 2021, 34, 575–583. [Google Scholar] [CrossRef]
- Lee, W.-C.; Hsiao, H.-C.; Wu, Y.-L.; Lin, J.-H.; Lee, Y.-P.; Fung, H.-P.; Chen, H.-H.; Chen, Y.-H.; Chu, R.-M. Serum C-Reactive Protein in Dairy Herds. Can. J. Vet. Res. 2003, 67, 102–107. [Google Scholar]
- Ali, A.; Rehman, M.U.; Mushtaq, S.; Ahmad, S.B.; Khan, A.; Karan, A.; Bashir Wani, A.; Ganie, S.A.; Mir, M.U.R. Biochemical and Computational Assessment of Acute Phase Proteins in Dairy Cows Affected with Subclinical Mastitis. Curr. Issues Mol. Biol. 2023, 45, 5317–5346. [Google Scholar] [CrossRef]
- Toscano, A.; Giannuzzi, D.; Pegolo, S.; Vanzin, A.; Bisutti, V.; Gallo, L.; Trevisi, E.; Cecchinato, A.; Schiavon, S. Associations between the Detailed Milk Mineral Profile, Milk Composition, and Metabolic Status in Holstein Cows. J. Dairy Sci. 2023, 106, 6577–6591. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.A.; Bruckmaier, R.M.; Schwarz, F.J. Performance and Metabolic Profile of Dairy Cows during a Lactational and Deliberately Induced Negative Energy Balance with Subsequent Realimentation. J. Dairy Sci. 2011, 94, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Tsukano, K.; Hirata, H.; Suzuki, K. Evaluation of Blood Serum Iron Concentration as an Alternative Biomarker for Inflammation in Dairy Cows. Biol. Trace Elem. Res. 2023, 201, 4710–4717. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.; Bell, R. Acute Phase Proteins: Biomarkers of Infection and Inflammation in Veterinary Medicine. Vet. J. Lond. Engl. 1997 2010, 185, 23–27. [Google Scholar] [CrossRef]
- Murata, H.; Shimada, N.; Yoshioka, M. Current Research on Acute Phase Proteins in Veterinary Diagnosis: An Overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Tanai, S.; Endo, N.; Tanaka, T. Quantifying the C-Reactive Protein Concentrations of Uterine Lavage Samples in Postpartum Dairy Cows. Anim. Reprod. Sci. 2020, 217, 106455. [Google Scholar] [CrossRef]
- Trevisi, E.; Jahan, N.; Bertoni, G.; Ferrari, A.; Minuti, A. Pro-Inflammatory Cytokine Profile in Dairy Cows: Consequences for New Lactation. Ital. J. Anim. Sci. 2015, 14, 3862. [Google Scholar] [CrossRef]
- Ceciliani, F.; Lecchi, C.; Urh, C.; Sauerwein, H. Proteomics and Metabolomics Characterizing the Pathophysiology of Adaptive Reactions to the Metabolic Challenges during the Transition from Late Pregnancy to Early Lactation in Dairy Cows. J. Proteom. 2018, 178, 92–106. [Google Scholar] [CrossRef]
- Ability of Diagnostic Tests to Predict Subclinical Mastitis and Intramammary Infections in Quarters from Lactating Dairy Cows. Available online: https://www.researchgate.net/publication/359257331_Ability_of_Diagnostic_Tests_to_Predict_Subclinical_Mastitis_and_Intramammary_Infections_in_Quarters_from_Lactating_Dairy_Cows (accessed on 15 April 2025).
- Thomsen, P.T.; Munksgaard, L.; Tøgersen, F.A. Evaluation of a Lameness Scoring System for Dairy Cows. J. Dairy Sci. 2008, 91, 119–126. [Google Scholar] [CrossRef]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A Lameness Scoring System That Uses Posture and Gait to Predict Dairy Cattle Reproductive Performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Gantner, V.; Jožef, I.; Popović, V.; Solić, D.; Popović, J.; Potočnik, K. The Effect of Age of Cows on Variability in Mastitis Prevalence Risk and Its Concomitant Impacts on the Successive Daily Milk Yield. Contemp. Agric. 2023, 72, 170–174. [Google Scholar] [CrossRef]
- Nutrient Requirements of Dairy Cattle: Eighth Revised Edition; National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-67777-6.
- Mekuriaw, Y. Negative Energy Balance and Its Implication on Productive and Reproductive Performance of Early Lactating Dairy Cows: Review Paper. J. Appl. Anim. Res. 2023, 51, 220–228. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Ospina, P.A.; Capel, M.B.; Nydam, D.V. The Association of Subclinical Hypocalcemia, Negative Energy Balance and Disease with Bodyweight Change during the First 30 Days Post-Partum in Dairy Cows Milked with Automatic Milking Systems. Vet. J. 2015, 204, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Prion, S.; Haerling, K.A. Making Sense of Methods and Measurement: Pearson Product-Moment Correlation Coefficient. Clin. Simul. Nurs. 2014, 10, 587–588. [Google Scholar] [CrossRef]
- Puth, M.-T.; Neuhäuser, M.; Ruxton, G.D. Effective Use of Pearson’s Product–Moment Correlation Coefficient. Anim. Behav. 2014, 93, 183–189. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, M.; Wang, Z.; Liu, H.; Bai, X.; Cui, S.; Guo, X.; Gao, L.; Gao, L.; Liao, A.; et al. Predictive Model for Early Functional Outcomes Following Acute Care after Traumatic Brain Injuries: A Machine Learning-Based Development and Validation Study. Injury 2023, 54, 896–903. [Google Scholar] [CrossRef]
- SciELO Brazil—Breed and Season Influence on Milk Quality Parameters and in Mastitis Occurrence Breed and Season Influence on Milk Quality Parameters and in Mastitis Occurrence. Available online: https://www.scielo.br/j/pvb/a/F3yVJs5mxmJZGdynmQBb6tk/?lang=en (accessed on 24 February 2025).
- Comparison of Hepatic Adaptation in Extreme Metabolic Phenotypes Observed in Early Lactation Dairy Cows on-farm-Dorland-2014-Journal of Animal Physiology and Animal Nutrition-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jpn.12125 (accessed on 3 March 2025).
- Batista, C.P.; Gonçalves, R.S.; Contreras, L.V.; de Faria Valle, S.; González, F. Correlation between Liver Lipidosis, Body Condition Score Variation, and Hepatic Analytes in Dairy Cows. Braz. J. Vet. Med. 2022, 44, e005121. [Google Scholar] [CrossRef]
- Pandey, V.; Aditi, A.; Pratiksha, P.; Gupta, S.K.; Sharma, N.; Sharma, D. Impact of Subclinical Mastitis on Blood Biochemistry of Dairy Cows. Indian J. Anim. Sci. 2012, 82, 477. [Google Scholar]
- Cui, L.; Wang, H.; Ding, Y.; Li, J.; Li, J. Changes in the Blood Routine, Biochemical Indexes and the pro-Inflammatory Cytokine Expressions of Peripheral Leukocytes in Postpartum Dairy Cows with Metritis. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Sattler, T.; Fürll, M. Creatine Kinase and Aspartate Aminotransferase in Cows as Indicators for Endometritis. J. Vet. Med. Ser. A 2004, 51, 132–137. [Google Scholar] [CrossRef]
- El-Shafey, Y. Effect of Some Strains of Mycoplasma on Serum and Milk Biochemistry of Dairy Cows. Egypt J. Comp. Pathol. Clin. Pathol. 2008, 21, 230–249. [Google Scholar]
- Andjelić, B.; Djoković, R.; Cincović, M.; Bogosavljević-Bošković, S.; Petrović, M.; Mladenović, J.; Čukić, A. Relationships between Milk and Blood Biochemical Parameters and Metabolic Status in Dairy Cows during Lactation. Metabolites 2022, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Tsukano, K.; Suzuki, K. Serum Iron Concentration Is a Useful Biomarker for Assessing the Level of Inflammation That Causes Systemic Symptoms in Bovine Acute Mastitis Similar to Plasma Haptoglobin. J. Vet. Med. Sci. 2020, 82, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Tsukano, K.; Shimamori, T.; Suzuki, K. Serum Iron Concentration in Cattle with Endotoxaemia. Acta Vet. Hung. 2020, 68, 53–58. [Google Scholar] [CrossRef]
- Shimamori, T.; Noda, J.; Tsukano, K.; Sera, K.; Yokota, H.; Koiwa, M.; Suzuki, T.; Suzuki, K. Particle-Induced X-Ray Emission Analysis of Zierum Trace and Major Elements in Cattle with Acute Coliform Mastitis. Jpn. J. Vet. Res. 2017, 65, 29–37. [Google Scholar]
- Tsukano, K.; Shimamori, T.; Fukuda, T.; Nishi, Y.; Otsuka, M.; Kitade, Y.; Suzuki, K. Serum Iron Concentration as a Marker of Inflammation in Young Cows That Underwent Dehorning Operation. J. Vet. Med. Sci. 2019, 81, 626–628. [Google Scholar] [CrossRef]
- Televičius, M.; Juozaitiene, V.; Malašauskienė, D.; Antanaitis, R.; Rutkauskas, A.; Urbutis, M.; Baumgartner, W. Inline Milk Lactose Concentration as Biomarker of the Health Status and Reproductive Success in Dairy Cows. Agriculture 2021, 11, 38. [Google Scholar] [CrossRef]
- Šematoviča, I.; Eihvalde, I.; Kairiša, D. Reticulo-Ruminal pH and Temperature Relationship between Dairy Cow Productivity and Milk Composition. Agron. Res. 2017, 15, 576–584. [Google Scholar]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status. Sensors 2020, 20, 1022. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Rutkauskas, A.; Televičius, M.; Stasiulevičiūtė, I. Reticulorumen Temperature and pH as Indicators of the Likelihood of Reproductive Success. J. Dairy Res. 2018, 85, 23–26. [Google Scholar] [CrossRef]
- Puppel, K.; Kuczyńska, B. Metabolic Profiles of Cow’s Blood; a Review. J. Sci. Food Agric. 2016, 96, 4321–4328. [Google Scholar] [CrossRef]
- Li, L.; Bai, S.; Zhao, H.; Tan, J.; Wang, Y.; Zhang, A.; Jiang, L.; Zhao, Y. Dietary Supplementation with Naringin Improves Systemic Metabolic Status and Alleviates Oxidative Stress in Transition Cows via Modulating Adipose Tissue Function: A Lipid Perspective. Antioxidants 2024, 13, 638. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, C.; Nagy, O.; Kovác, G. Changes in the Concentrations of Selected Acute Phase Proteins and Variables of Energetic Profile in Dairy Cows after Parturition. J. Appl. Anim. Res. 2014, 42, 278–283. [Google Scholar] [CrossRef]
- Džermeikaitė, K.; Krištolaitytė, J.; Sutkevičienė, N.; Vilkonienė, T.; Vaičiulienė, G.; Rekešiūtė, A.; Girdauskaitė, A.; Arlauskaitė, S.; Bajcsy, Á.C.; Antanaitis, R. Relationships Among In-Line Milk Fat-to-Protein Ratio, Metabolic Profile, and Inflammatory Biomarkers During Early Stage of Lactation in Dairy Cows. Vet. Sci. 2025, 12, 187. [Google Scholar] [CrossRef]
- Luo, Z.; Yong, K.; Du, Z.; Huang, Y.; Zhou, T.; Ma, L.; Yao, X.; Shen, L.; Yu, S.; Yan, Z.; et al. Association between Tryptophan Metabolism and Inflammatory Biomarkers in Dairy Cows with Ketosis. Metabolites 2023, 13, 333. [Google Scholar] [CrossRef]
- Stiehler, T.; Heuwieser, W.; Pfützner, A.; Burfeind, O. Serum Haptoglobin and C-Reactive Protein Concentration in Relation to Rectal and Vaginal Temperature of Early Postpartum Sows. Theriogenology 2016, 86, 862–867. [Google Scholar] [CrossRef]
- Schmitt, R.; Pieper, L.; Gonzalez-Grajales, L.A.; Swinkels, J.; Gelfert, C.-C.; Staufenbiel, R. Evaluation of Different Acute-Phase Proteins for Herd Health Diagnostics in Early Postpartum Holstein Friesian Dairy Cows. J. Dairy Res. 2021, 88, 33–37. [Google Scholar] [CrossRef]
- Yogeshpriya, S.; Selvaraj, P. C-Reactive Protein in Veterinary Practice. J. Dairy Vet. Sci. 2019, 13, 1–3. [Google Scholar]
- Beauchemin, K.A. Invited Review: Current Perspectives on Eating and Rumination Activity in Dairy Cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef]
- Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Trevisi, E. Inflammatory Status and Metabolic Changes at Dry-off in High-Yield Dairy Cows. Ital. J. Anim. Sci. 2020, 19, 51–65. [Google Scholar] [CrossRef]
- Tufarelli, V.; Colonna, M.A.; Losacco, C.; Puvača, N. Biological Health Markers Associated with Oxidative Stress in Dairy Cows during Lactation Period. Metabolites 2023, 13, 405. [Google Scholar] [CrossRef]
- Mohsin, M.A.; Zhou, X.; Huiru, Y.; Shen, W.; He, B.; Sobiech, P.; Pierzchała, M.; Ogłuszka, M.; Starzyński, R.; Kalra, G.; et al. Effect of β-Hydroxybutyrate Acid on Gene Expression Levels of Antioxidant Biomarkers and Growth Hormone–Related Genes in Liver Cell Culture. J. Vet. Res. 2024, 68, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Yehia, S.G.; Ramadan, E.S.; Megahed, E.A.; Salem, N.Y. Effect of Parity on Metabolic and Oxidative Stress Profiles in Holstein Dairy Cows. Vet. World 2020, 13, 2780–2786. [Google Scholar] [CrossRef]
| TMR Component | Value (%) |
|---|---|
| Corn silage | 25% |
| Alfalfa grass hay | 5% |
| Grass silage | 20% |
| Sugar beet pulp silage | 15% |
| Grain concentrates mash | 30% |
| Mineral mix | 5% |
| Dry matter (DM) | 48.8% |
| Neutral detergent fibre | 28.2% of DM |
| Net energy lactation | 1.6 Mcal/kg |
| Crude protein | 15.8% of DM |
| Non-fibre carbohydrates | 38.7% of DM |
| Acid detergent fibre | 19.8% of DM |
| Calcium | 0.80% |
| Phosphorus | 0.45% |
| Ether extract | 3.50% |
| Traits | Lactose (%) | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | Significance | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||
| CRP (mg/L) | <4.5 | 30 | 14.15 | 3.94 | 0.72 | 12.68 | 15.62 | 4.41 | 22.30 | 0.399 |
| ≥4.5 | 41 | 14.94 | 3.83 | 0.60 | 13.73 | 16.15 | 5.13 | 22.73 | ||
| AST (U/L) | <4.5 | 30 | 94.95 | 31.53 | 5.76 | 83.17 | 106.72 | 48.10 | 196.00 | 0.042 |
| ≥4.5 | 41 | 83.35 | 14.68 | 2.29 | 78.72 | 87.98 | 58.60 | 124.40 | ||
| Fe (µg/dL) | <4.5 | 30 | 97.88 | 21.17 | 3.87 | 89.97 | 105.78 | 51.00 | 135.40 | 0.013 |
| ≥4.5 | 41 | 110.02 | 18.9 | 2.95 | 104.06 | 115.99 | 66.30 | 141.00 | ||
| GGT (U/L) | <4.5 | 30 | 32.03 | 16.56 | 3.02 | 25.85 | 38.22 | 18.00 | 88.00 | 0.085 |
| ≥4.5 | 41 | 26.27 | 11.24 | 1.76 | 22.72 | 29.82 | 17.00 | 88.00 | ||
| NEFA (mmol/L) | <4.5 | 30 | 0.38 | 0.17 | 0.03 | 0.32 | 0.45 | 0.13 | 0.91 | 0.359 |
| ≥4.5 | 41 | 0.34 | 0.19 | 0.03 | 0.28 | 0.40 | 0.10 | 0.84 | ||
| SAA (μg/mL) | <4.5 | 30 | 65.27 | 23.83 | 4.35 | 56.37 | 74.17 | 23.81 | 100.00 | 0.125 |
| ≥4.5 | 41 | 74.18 | 23.99 | 3.75 | 66.61 | 81.76 | 23.81 | 100.00 | ||
| CREA (g/L) | <4.5 | 31 | 74.10 | 10.48 | 1.91 | 70.19 | 78.01 | 56.00 | 98.00 | 0.161 |
| ≥4.5 | 41 | 77.51 | 9.69 | 1.51 | 74.45 | 80.57 | 61.00 | 98.00 | ||
| Traits | Lactose (%) | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | Significance | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||
| Milk yield (kg) | <4.5 | 30 | 40.82 | 8.02 | 1.46 | 37.82 | 43.82 | 23.32 | 56.85 | 0.588 |
| >4.5 | 41 | 39.82 | 7.39 | 1.15 | 37.49 | 42.15 | 14.31 | 54.53 | ||
| Milk fat | <4.5 | 30 | 4.00 | 0.76 | 0.14 | 3.72 | 4.28 | 2.64 | 5.52 | 0.705 |
| ≥4.5 | 41 | 3.94 | 0.58 | 0.09 | 3.76 | 4.12 | 2.98 | 5.32 | ||
| Milk protein | <4.5 | 30 | 3.27 | 0.31 | 0.06 | 3.15 | 3.38 | 2.64 | 3.95 | 0.472 |
| ≥4.5 | 41 | 3.22 | 0.23 | 0.04 | 3.15 | 3.29 | 2.66 | 3.82 | ||
| Milk fat-to-protein ratio | <4.5 | 30 | 1.24 | 0.27 | 0.05 | 1.14 | 1.34 | 0.79 | 1.86 | 0.882 |
| ≥4.5 | 41 | 1.23 | 0.20 | 0.03 | 1.17 | 1.29 | 0.88 | 1.95 | ||
| Reticulorumen pH | <4.5 | 30 | 5.99 | 0.22 | 0.04 | 5.91 | 6.07 | 5.51 | 6.36 | 0.03 |
| ≥4.5 | 41 | 6.10 | 0.18 | 0.03 | 6.04 | 6.15 | 5.50 | 6.49 | ||
| Reticulorumen temperature (°C) | <4.5 | 30 | 38.94 | 0.15 | 0.03 | 38.88 | 39.00 | 38.69 | 39.20 | 0.579 |
| ≥4.5 | 41 | 38.97 | 0.18 | 0.03 | 38.91 | 39.02 | 38.59 | 39.60 | ||
| Temperature without drink cycles (°C) | <4.5 | 30 | 39.63 | 0.17 | 0.03 | 39.57 | 39.70 | 39.37 | 39.99 | 0.456 |
| ≥4.5 | 41 | 39.66 | 0.14 | 0.02 | 39.62 | 39.70 | 39.44 | 40.14 | ||
| Normal temperature (°C) | <4.5 | 30 | 39.79 | 0.15 | 0.03 | 39.73 | 39.85 | 39.5 | 40.00 | 0.963 |
| ≥4.5 | 41 | 39.79 | 0.12 | 0.02 | 39.75 | 39.83 | 39.59 | 40.00 | ||
| Rumination time (min./day) | <4.5 | 30 | 436.89 | 76.17 | 13.91 | 408.45 | 465.34 | 253.2 | 549.39 | 0.541 |
| ≥4.5 | 41 | 447.36 | 66.77 | 10.43 | 426.28 | 468.43 | 224.55 | 567.10 | ||
| Cow activity (sec/h) | <4.5 | 30 | 1295.90 | 429.44 | 78.40 | 1135.54 | 1456.25 | 555.91 | 2445.83 | 0.497 |
| ≥4.5 | 41 | 1370.30 | 470.33 | 73.45 | 1221.89 | 1518.8 | 323.16 | 2418.33 | ||
| Water intake (L/day) | <4.5 | 30 | 130.01 | 18.11 | 3.31 | 123.24 | 136.77 | 95.59 | 168.94 | 0.618 |
| ≥4.5 | 41 | 132.11 | 17.03 | 2.66 | 126.74 | 137.49 | 95.00 | 166.93 | ||
| Traits | Pearson Correlation | Significance | N |
|---|---|---|---|
| Milk temperature (°C) | −0.058 | 0.733 | 71 |
| Lactose (%) | 0.043 | 0.802 | 71 |
| AST (U/L) | 0.189 | 0.262 | 71 |
| Fe (µg/dL) | −0.177 | 0.294 | 71 |
| GGT (U/L) | −0.087 | 0.608 | 71 |
| NEFA (mmol/L) | 0.335 | 0.043 | 71 |
| SAA (μg/mL) | −0.32 | 0.053 | 71 |
| CREA (g/L) | 0.059 | 0.624 | 71 |
| Milk yield (kg/day) | 0.147 | 0.386 | 71 |
| Milk fat | 0.161 | 0.343 | 71 |
| Milk protein | −0.264 | 0.115 | 71 |
| Milk fat-to-protein ratio | 0.244 | 0.145 | 71 |
| Reticulorumen pH | 0.498 | 0.002 | 71 |
| Reticulorumen temperature (°C) | 0.140 | 0.408 | 71 |
| Temperature without drink cycles (°C) | 0.275 | 0.100 | 71 |
| Normal temperature (°C) | 0.372 | 0.023 | 71 |
| Rumination time (min/day) | 0.429 | 0.008 | 71 |
| Cow activity (seconds/hour) | −0.099 | 0.558 | 71 |
| Water intake (L/day) | −0.007 | 0.969 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Džermeikaitė, K.; Krištolaitytė, J.; Anskienė, L.; Girdauskaitė, A.; Arlauskaitė, S.; Šertvytytė, G.; Lembovičiūtė, G.; Baumgartner, W.; Antanaitis, R. Milk Lactose and Inflammatory Marker Changes: Early Indicators of Metabolic and Inflammatory Stress in Early Lactation Dairy Cattle. Agriculture 2025, 15, 1205. https://doi.org/10.3390/agriculture15111205
Džermeikaitė K, Krištolaitytė J, Anskienė L, Girdauskaitė A, Arlauskaitė S, Šertvytytė G, Lembovičiūtė G, Baumgartner W, Antanaitis R. Milk Lactose and Inflammatory Marker Changes: Early Indicators of Metabolic and Inflammatory Stress in Early Lactation Dairy Cattle. Agriculture. 2025; 15(11):1205. https://doi.org/10.3390/agriculture15111205
Chicago/Turabian StyleDžermeikaitė, Karina, Justina Krištolaitytė, Lina Anskienė, Akvilė Girdauskaitė, Samanta Arlauskaitė, Greta Šertvytytė, Gabija Lembovičiūtė, Walter Baumgartner, and Ramūnas Antanaitis. 2025. "Milk Lactose and Inflammatory Marker Changes: Early Indicators of Metabolic and Inflammatory Stress in Early Lactation Dairy Cattle" Agriculture 15, no. 11: 1205. https://doi.org/10.3390/agriculture15111205
APA StyleDžermeikaitė, K., Krištolaitytė, J., Anskienė, L., Girdauskaitė, A., Arlauskaitė, S., Šertvytytė, G., Lembovičiūtė, G., Baumgartner, W., & Antanaitis, R. (2025). Milk Lactose and Inflammatory Marker Changes: Early Indicators of Metabolic and Inflammatory Stress in Early Lactation Dairy Cattle. Agriculture, 15(11), 1205. https://doi.org/10.3390/agriculture15111205









