Abstract
Focusing on the challenges of low utilization of pineapple leaves and the poor stability of phenolic compounds (PCs) in pineapple leaf fiber using traditional solvent extraction methods, the effects of different extraction media (including distilled water, neutral methanol, acidic methanol, alkaline methanol, neutral ethanol, and alkaline ethanol) was systematically investigated on the extraction efficiency of PCs from pineapple leaf fiber. Through response surface methodology (RSM), the optimal extraction conditions were determined. Additionally, the impacts of illumination and pH on the stability of PCs in pineapple leaf fiber were thoroughly examined. The results demonstrated that acidic ethanol outperformed other extraction media, with the optimized extraction conditions as follows: ethanol concentration = 80%, material-to-liquid ratio = 1:40, extraction temperature = 70 °C, and extraction time = 40 min. Under these conditions, the extraction yield of PCs reached 11.55 mg/g. Furthermore, the stability of PCs was significantly enhanced by minimizing light exposure and maintaining a pH range of 3–6. The potential application of PCs extracted from pineapple leaf fiber was also explored, particularly in mango preservation, revealing promising results. This study not only provides an efficient and sustainable approach for the extraction and stabilization of PCs from pineapple leaf fiber but also opens new avenues for the utilization of polyphenols in functional applications, contributing to the valorization of agricultural by-products.
1. Introduction
Pineapple (Ananas comosus L. Merr) is one of the most popular fruits. Pineapple consists of pineapple leaves and pineapple fruits. Pineapple fruits are used for fruit processing, while more than 95% of by-products such as pineapple leaves are crushed andburied, with an effective utilization rate < 1% [1]. With abundant nutrients such as carbohydrates, pineapple leaves are used to prepare silage for dairy cows [2], as well as ethanol, butanol and sugar [3,4].
The fiber content in pineapple leaves is about 1.5%, and pineapple leaf fiber has high contents of antibacterial compounds, including phenylpropanoids, triterpenoids, and amides [5]. These compounds can effectively inhibit microorganism (e.g., E. coli and S. aureus) growth [6,7]. Huang et al. [8] investigated the inhibitory effects of polar extracts (e.g., total extract of pineapple leaf, extract of petroleum ether, extract of ethyl acetate and water phase extract) on bacteria (e.g., B. subtilis and E. coli) and fungi (e.g., A. niger and Penicillium), and found that the extract of ethyl acetate had the optimized antibacterial activity, with the minimum inhibitory concentrations of 6.25 mg/mL for B. subtilis and S. lutea, 3.12 mg/mL for S. aureus and B. cereus, 5 mg/mL for A. niger and 10 mg/mL for Penicillium. The aqueous extracts of pineapple leaves could significantly inhibit the growth of yeast, Staphylococcus aureus, Candida albicans and Bacillus at concentrations of 1.65–4.95 mg/mL, with an inhibition rate of 95% [9]. Therefore, pineapple leaf fiber has been employed to improve the antibacterial performance of textiles and composites [10,11,12,13,14,15]. As typical antibacterial components [16,17,18], phenolic compounds (PCs) exhibit biological activities in reducing the contents of intracellular sugar and lipid, and counteracting fat and lipid accumulations [19,20,21].
Polyphenols can be extracted by ultrasonic-assisted extraction, organic solvent extraction, microwave-assisted extraction, enzyme-assisted extraction and supercritical CO2 extraction [22,23,24]. Ultrasonic extraction has the advantages of short period and high efficiency, but it is not suitable for large-scale production due to high energy consumption. Microwave extraction has high efficiency and low cost, but it is limited by poor temperature control [24]. Supercritical CO2 extraction has high efficiency and high product purity, but it is limited by high cost. Solvent extraction has been widely applied as it is simple, facile, and cost effective. Environmental pollution caused by solvent extraction could be reduced by solvent recovery [25,26,27]. It has been demonstrated that the antioxidant activity of pineapple leaf fiber polyphenols (PLFPs) obtained by traditional solvent extraction was extremely unstable. However, there were few reports on the extraction rate, oxidation stability test and preservation test of PLFPs under different polar conditions. In this study, the effects of different solvent extraction methods on the extraction rate (ER) of PLFPs were investigated, and the optimized parameters of extraction process of PLFPs were determined by the Box–Behnken central composite design, and the storage stability and fresh-keeping effect were explored.
2. Materials and Methods
2.1. Materials and Equipment
The pineapple plants used in this study were a 1.5-year-old Bali variety, sourced from a local plantation affiliated with the Guangdong Agricultural Reclamation Research Institute (110°14′16.811″ E, 21°12′30.694″ N). Fresh pineapple leaves were carefully separated from the plants, cleaned to remove surface impurities, and air-dried under natural sunlight. Subsequently, the dried leaves (7.2%) were finely ground into powder using a high-speed pulverizer (model: WZJ-12B1, Ji’Nan Beili Technology Engineering Co., Ltd. (WZJ-12B1, Jinan Boley Technology Engineering Co., Ltd., China)) for 5 min to obtain pineapple leaf fiber powder, which was packed with a plastic self-sealing bag then stored in room temperature for further analysis.
The chemicals and reagents used in this study were of analytical grade (AR) and sourced from reputable suppliers. Gallic acid was purchased from Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China and anhydrous ethanol from Guangdong Guanghua Technology Co., Ltd., guangdong, China and Folin-Phenol reagent from Beijing Solaibao Technology Co., Ltd., Beijing, China and anhydrous sodium carbonate from Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China and hydrochloric acid from Guangdong Guangshiji Technology Co., Ltd., Guangdong, China. Additionally, kits for measuring total antioxidant capacity, as well as DPPH and hydroxyl radical scavenging capacities, were obtained from Suzhou Grace Biotechnology Co., Ltd., Jiangsu, China. Fresh mangoes of the ‘Tainong’ variety were procured from the Xiashan District Comprehensive Vegetable Market in Zhanjiang for experimental use.
The main instruments and equipment used in this study included the following: a vibration-type pharmaceutical ultrafine pulverizer (WZJ-12B1, Jinan Billion Powder Engineering Technology Co., Ltd., Shandong, China); an electronic analytical balance (CPA225D, Sartorius, Göttingen, Germany); a spectrophotometer (M8 UV, Shanghai Mapada Instruments Co., Ltd., Shanghai, China); a digital display constant temperature water bath (HH-4, Jintan Jinbo Experimental Instrument Factory, China); a centrifuge (JXB, Jiangxi Feige Centrifuge Manufacturing Co., Ltd., China); a numerically-controlled ultrasonic cleaner (KQ5200DE, Kunshan Ultrasonic Instrument Co., Ltd., China); and a freeze dryer (Alpha 1-4 LSC PLUS, Christ, Osterode, Germany).
2.2. Extraction of PLFPs by Different Extraction Media
One g of pineapple leaf powder was mixed with 30 mL of extraction medium in a 250 mL conical flask. The mixture was thoroughly stirred, sealed with polythene wrap to prevent evaporation, and then heated in a water bath at 50 °C for 30 min. After extraction, the solution was centrifuged at 4500 r/min for 10 min to separate the solid residue. The extraction procedure was repeated, and the resulting filtrates and supernatants were combined and transferred into a 100 mL volumetric flask for subsequent determination of pineapple leaf fiber polyphenols (PLFPs) content.
To evaluate the effects of different extraction media on the extraction rate (ER) of polyphenols from PLFPs, the following solvents were tested: distilled water (DiW), neutral methanol (70% methanol solution, NeW), acidic methanol (70% methanol solution with 1% HCl, AcM), acidic ethanol (70% ethanol solution with 1% HCl, AcE), neutral ethanol (70% ethanol solution, NeE), alkaline methanol (1% sodium hydroxide in 70% methanol solution, AlM), and alkaline ethanol (1% sodium hydroxide in 70% ethanol solution (v/v), AlE).
2.3. Determination of Polyphenols Content
Standard curve of gallic acid: the content of pineapple leaf fiber polyphenols (PLFPs) in the samples was quantified using the Folin phenol method [24]. Gallic acid was used as the standard for calibration. Initially, 5 mg of gallic acid was dissolved in 1.25 mL of anhydrous ethanol, and the volume was adjusted to 50 mL with distilled water to prepare a stock solution with a concentration of 100 mg/L. A series of gallic acid standard solutions with concentrations of 10, 20, 30, 40, 50, and 60 mg/L were prepared by diluting the stock solution. For the assay, 2 mL of each gallic acid standard solution was mixed with 1 mL of Folin-phenol reagent, vortexed, and allowed to stand for 4 min. Then, 5 mL of a 7.5% sodium carbonate solution was added, and the final volume was adjusted to 25 mL with distilled water. The mixture was thoroughly mixed and incubated in a water bath at 45 °C for 40 min. The absorbance (A0) of the solution was measured at 765 nm using distilled water as the blank. The standard curve was established by plotting the absorbance values against the corresponding gallic acid concentrations (c0, mg/L), yielding the linear equation:
A0 = 0.0098 × c0 + 0.0085 (R2 = 0.9993)
Measurement of polyphenol content in samples: 2 mL of PLFPs extract solution was mixed with 1 mL of Folin phenol reagent. The mixture was vortexed and allowed to stand for 4 min. Subsequently, 5 mL of a 7.5% sodium carbonate solution was added, and the final volume was adjusted to 25 mL with distilled water. The solution was thoroughly mixed and then incubated in a water bath at 45 °C for 40 min. Using distilled water as the blank control, the absorbance of the solution was measured at 765 nm. The polyphenol concentration (c1, mg/L) in the solution was calculated based on the standard curve of gallic acid concentration. The extraction rate (ER) of polyphenols (y, mg/g) was determined using the following formula: y = (c1 × V)/m; herein, V is the total volume (mL) of the sample extract, and m is the sample mass (g).
2.4. Single Factor Test of Acidified Ethanol Solution Extraction of PLFPs
Effects of Ethanol Concentration on the ER of PLFPs: The effect of acidic ethanol concentration (40%, 50%, 60%, 70%, 80%, and 90%) on the extraction rate (ER) of pineapple leaf fiber polyphenols (PLFPs) was investigated. Specifically, 1 g of pineapple leaf fiber powder was mixed with 30 mL of acidic ethanol, stirred, sealed with plastic wrap, and heated in a water bath at 50 °C for 30 min. The solution was then centrifuged at 4500 r/min for 10 min. The extraction process was repeated, and the resulting filtrates and supernatants were combined for further analysis.
Effect of Extraction Temperature on ER of PLFPs: The effect of extraction temperature (40 °C, 50 °C, 60 °C, 70 °C, and 80 °C) on the ER of PLFPs was examined. A mixture of 1 g pineapple leaf fiber powder and 30 mL of acidified 70% ethanol was stirred, sealed, and heated at the designated temperature for 30 min, followed by centrifugation at 4500 r/min for 10 min. The extraction was repeated, and the filtrates and supernatants were combined for further analysis.
Effect of Extraction Time on ER of PLFPs: The effect of extraction time (30, 40, 50, 60, and 70 min) on the ER of PLFPs was studied. A mixture of 1 g pineapple leaf fiber powder and 30 mL of acidified 70% ethanol was stirred, sealed, and heated at 50 °C for the specified time. After centrifugation at 4500 r/min for 10 min, the extraction was repeated, and the filtrates and supernatants were combined for further analysis.
Effect of Material–Liquid Ratio (MLR) on ER of PLFPs: The effect of MLR (1:20, 1:30, 1:40, 1:50, 1:60, and 1:70) on the ER of PLFPs was evaluated. A mixture of 1 g pineapple leaf fiber powder and acidified 70% ethanol (adjusted to the designated MLR) was stirred, sealed, and heated at 50 °C for 30 min. The solution was centrifuged at 4500 r/min for 10 min, and the extraction was repeated. The filtrates and supernatants were combined for further analysis.
2.5. Box–Behnken (B–B) Central Composite Experimental Design on Acidic Ethanol Solution Extraction of PLFPs
With the ethanol concentration (A), temperature (B), time (C), and MLR (D) as independent variables, and the ER of PLFPs as the response value, the optimized process parameters of the PLFP extraction by acidic ethanol solution were determined by using the B–B central composite experimental design in Design-Expert 8.0.6. Table 1 summarizes the factor level design.

Table 1.
B–B design and results.
2.6. Stability Test of PLFPs
Twenty g of pineapple leaf fiber powders was added into 70% acidified ethanol solution (pH = 4) with an MLR of 1:30, followed by sealing with plastic wrap, sonicated at 50 °C for 30 min, and filtered.
2.6.1. Effect of pH on Stability of PLFPs
Twenty-five mL of PLFPs extract solution was added into a beaker, and the pH of the solutions were adjusted to 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0, respectively, by using 1% hydrochloric acid solution or 7.5% sodium carbonate solution. Then, the solutions were kept in the dark for 24 h at 25 °C, and their absorbance at 666 nm was determined.
2.6.2. Effect of Illumination on Stability of PLFPs
Twenty-five mL of PLFPs extract solution was kept for 1, 2, 3, 4 and 5 h, respectively, in the dark and natural light, and its absorbance at 666 nm was determined.
2.7. Application of PLFPs in Mango Preservation
2.7.1. Preparation of Composite Membrane Solution
Preparation of PLFPs powder: PCs in pineapple leaf fiber were extracted by the 70% ethanol solution (containing 1%HCl, v/v), concentrated to a 5 mL solution, and dried at 40 °C for 72 h.
Preparation of 5% polyvinyl alcohol (PVA) solution: 5 g of PVA was added into 100 mL of distilled water, followed by stirring at 80 °C until complete dissolution.
Preparation of 2% Chitosan (CTS) acetic acid solution: 2 g of CTS was dissolved in 100 mL of 2% acetic acid solution.
Preparation of 5%PVA and 2%CTS solution: 5%PVA solution and 2%CTS solution were mixed by a volume ratio of 4:6, and the solution was heated to 60 °C.
Preparation of 5%PVA, 2%CTS and PLFPs solution: 1.5 g, 3 g and 6 g of PLFPs were dissolved in 5 mL absolute ethanol, and sonicated for 5 min. The PLFPs solutions were added into 45 mL of 5%PVA/2%CTS composite membrane solution, sealed and stirred for 30 min in the dark, and kept untouched. In this way, membrane with fiber mass concentrations of 3%, 6% and 9% were obtained.
2.7.2. Smearing Experiment
Mangoes with no mildew, consistent sizes and undamaged appearance were selected, rinsed, and then dried naturally. Six groups of mangoes were immersed in 2%CTS, 5%PVA, 2%CTS/5%PVA, 3%PLFP/2%CTS/5%PVA, 6%PLFP/2%CTS/5%PVA and 9% PLFP, respectively. The mango samples were stored at 25 °C for 7 d, during which the samples were observed every day, and their weight loss rates (WLRs) were measured.
2.7.3. Calculation of WLR
The WLR of mango was calculated by Zheng et al. [28]:
where m0 represented the initial mass of mango sample, g; Mn represents the weight of mango on the nth day of storage, g.
Weight loss rate (%) = (m0 − mn)/m0 × 100%.
2.8. Statistical Analysis
All experiments were performed in triplicate. Statistical analysis was performed using SPSS 22.0 (IBM, Armonk, NY, USA), with one-way ANOVA followed by LSD post hoc tests (p < 0.05) to determine statistical significance. Experimental data were processed using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA) and visualized using OriginPro 2022 (OriginPro, Northampton, MA, USA) for graphical presentation. This integrated analytical approach ensured rigorous quantification and clear presentation of experimental results.
3. Result and Discussion
3.1. Effects of the Extraction Method on the ER of PLFPs
The effects of the extraction method (DiW, NeW, AcM, AlM, NeE, AcE and AlE) on the extraction of PLFPs are shown in Figure 1. As indicated, The ER of PLFPs by acidic solution was higher than those by neutral and alkaline solutions. The ER of PLFPs by the acidic ethanol solution was higher than those by the acidic methanol solution and distilled water. This is consistent with previous studies [29]. PCs extracted by Xiang et al. [30] were easy to precipitate during storage at 4 °C, and the content of effective components and antioxidant activity significantly decreased, which might be due to the degradation of storage stability of PLFPs under acidity and alkalinity, while the difference in ER of PCs from pineapple leaves was caused by the difference of the two samples. The highest content of PCs in water extracts of silkworm and pea pods was reported by Fendri et al. [18], wherein the pH value was adjusted by the non-extraction method, and the types of PCs were different for different extraction objects. The results of significant analysis showed that the influences of DiW on the ER of PLFPs was significantly different from that of other six extraction methods. The influences of AlE on the ER of PLFPs were basically consistent with that of alkaline ethanol, but significantly different from those of other extraction methods. The influences of AcM and AcE on the ER of PLFPs were significant, and there were significant differences between AcE, AcM, NeE, and NeM. Therefore, AcE was employed as the extraction system to optimize the extraction process of polyphenols.
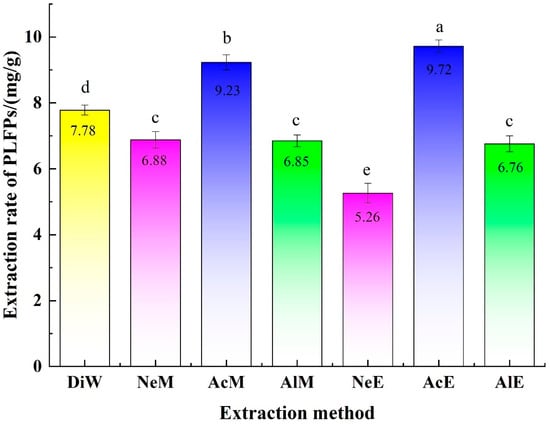
Figure 1.
Influences of the extraction solution on ER of PLFPs. DiW (distilled water), NeW (neutral methanol), AcM (acidic methanol), AlM (alkaline methanol), NeE (neutral ethanol), AcE (acidic ethanol) and AlE (alkaline ethanol). Letters represent significant differences at a level of 0.05.
3.2. Influences of Single Factors on ER of PLFPs
The influences of temperature, ethanol concentration, extraction time, and MLR on the ER of PLFPs by AcE are shown in Figure 2. As shown in Figure 2a, as the ethanol concentration increased, the ER of PLFPs increased and then decreased, and the maximum ER of PLFPs (13.12 ± 0.58 mg/g) was observed at an ethanol concentration of 70%, and the ethanol concentration of 70% was significantly different from that of 40%, 50%, 80% and 90%. As shown in Figure 2b, as the extraction temperature increased, the ER of PLFPs increased and then decreased, and the maximum ER of PLFPs (9.91 ± 0.33 mg/g) was observed at a temperature of 70 °C, and the extraction temperature was 70 °C, which was significantly different from other factors. As shown in Figure 2c, as the MLR increased, the ER of PLFPs increased, while the increasing rate decreased as the MLR exceeded 1:30, and when the ratio of material to liquid was greater than 1:30, the effect on the ER was not significant. As shown in Figure 2d, as the extraction time increased, the ER of PLFPs increased and then decreased, and the maximum ER of PLFPs (9.50 ± 0.20 mg/g) was observed at an extraction time of 50 min. While the 50 min extraction time did not significantly affect the ER of PLFPs relative to the difference between 40 and 70 min, it did demonstrate a significant effect when compared with the difference between 30 and 70 min. Xiang et al. [30] reported that the optimized conditions for extracting polyphenols from pineapple leaf residue with ethanol system were as follows: ethanol concentration = 45%, extraction temperature = 55 °C, extraction time = 55 min, and MLR = 35:1. The differences might be attributed to the fact that the pineapple leaf residue was not specified or the extraction system was different. In addition, different raw materials or varieties would also lead to different extraction temperatures [31,32].
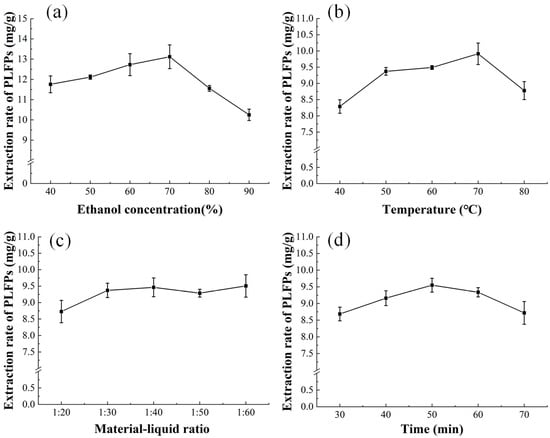
Figure 2.
Influences of extraction factors on ER of PLFPs. (a) = ethanol concentration, (b) = extraction temperature, (c) = material-liquid ratio and (d) = extraction time. Letters represent significant differences at a level of 0.05.
3.3. Results of Response Surface Test
3.3.1. B–B Design Experiment and Regression Model
In the B–B experiment, the ethanol concentration (A), temperature (B), MLR (C) and time (D), were involved in the 29 experiments of PLFPs. As shown in Table 2, p = 0.0001 indicated statistical significance. The correlation coefficient (R2) was 0.9279, and this fitting equation could predict the ER of PLFPs by acidic ethanol under various factors.

Table 2.
ANOVA for Quadratic model.
Table 2 summarizes the 2F-Values. As indicated, the influences of different factors on the ER of PLFPs followed the sequence of temperature > ethanol concentration> time > material-liquid ratio. The extraction ratio of PLFPs was also significantly influenced by the interaction of ethanol concentration and its first term, time, and temperature and its second term. The regression equation of extraction ratio (Y) in PLFPs was obtained by
Y = 10.32 − 0.2008 × A + 0.5833 × B − 0.2950 × A × D + 0.4128 × B2.
3.3.2. Response Surface Analysis of ER of PLFPs
A 3D response surface was developed to analyze the extraction ratio of PLFPs in relation to experimental factors, aiming to optimize the extraction conditions. As shown in Table 2, the interaction between ethanol concentration and extraction time was statistically significant (p < 0.05), while other factor interactions were negligible (p > 0.05). Figure 3 presents the response surface and contour plots for the interaction of ethanol concentration and extraction time, with a fixed MLR of 1:40 and a temperature of 70 °C. The steep slope of the response surface and the elliptical contour lines in Figure 3a,b indicated a strong influence of ethanol concentration and extraction time on the extraction ratio of PLFPs.
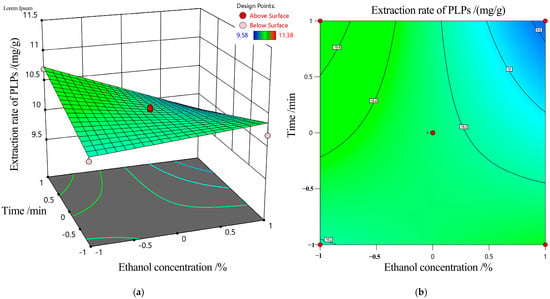
Figure 3.
Response surface (a) and contour map (b) illustrating the effects of ethanol concentration and extraction time on the ER of PLFPs. The red dots represent the actual experimental runs of factor-level combinations in the design: 4 axial points in the Central Composite Design and 1 center point in the Box-Behnken Design.
Based on the analysis, the optimized extraction parameters for AcE extraction were determined as follows: ethanol concentration = 80%, temperature = 70 °C, MLR = 1:40, and extraction time = 40 min. However, it was found that a large amount of ethanol and water condensate was produced in the bottle mouth during the extraction process at 80 °C, and the experimental conditions were difficult to control. Combined with the single factor experimental results, the extraction temperature at 70 °C was more reasonable. Verification experiments conducted under these optimized conditions yielded an extraction ratio of 11.55 ± 0.03 mg/g, confirming the rationality and applicability of the regression model.
3.4. Stability Analysis of PLFPs
Figure 4 illustrates the effects of illumination, pH, and oxidant on the oxidation stability of PLFPs. As shown in Figure 4a, shielding PLFPs from light significantly enhanced their stability. t-test analysis showed that there was a significant difference (p = 0.004, <0.05) between illumination and lucifuge when the storage time was 5 min.
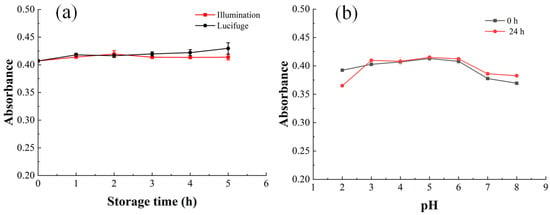
Figure 4.
Influences of storage conditions on stability of PLFPs, and (a,b), respectively, represent the effects of illumination and pH on the absorbance of PLFPs.
Figure 4b demonstrates that the stability of PLFPs first increased and subsequently declined with increasing pH, reaching optimal storage stability in the pH range of 3–6, and the statistical analysis (t-test) indicated no significant difference between 0 h and 24 h storage under these pH conditions. However, the absorbance of PLFPs decreased sharply when the storage time exceeded 3 h.
These findings align with previous studies. For instance, Gan et al. [33] reported that polyphenols from water chestnut peel exhibited relative stability under pH = 3 and light exposure, while Yu et al. [34] found that polyphenols in partridge tea were stable under conditions of pH < 5, weak light, and low temperature. These results suggested that the stability of PCs might vary depending on their source and extraction conditions
3.5. Effect Analysis of PLFPs on Mango Preservation
The influences of PLFPs/CTS/PVA composite film on mango preservation are shown in Figure 5 and Figure 6. Compared with the Blank group, the 2%CTS group, the 5%PVA group and the 2%CTS/5%PVA group, PLFPs led to an improved fresh-keeping effect of the composite films on mangoes. As the content of PLFPs increased, the spots of mango skin anthracnose decreased and the gloss increased, which might be attributed to the antibacterial activity of polyphenols and inhibition of microbial growth by polyphenols [6,7,9]. On the third day of preservation, anthracnose spots appeared on the surface of mango samples without PLFPs. When the dosage of PLFPs was 9%, small lesions appeared after storage at 25 °C for 6 d, while the peel color remained green, indicating that PLFPs can decelerate the growth of anthracnose on mango epidermis and delayed the ripening of the mango.
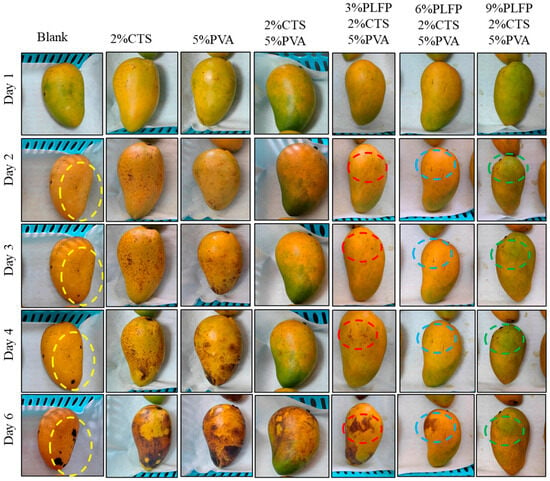
Figure 5.
Effect of PLFPs/CTS/PVA film on mango preservation.
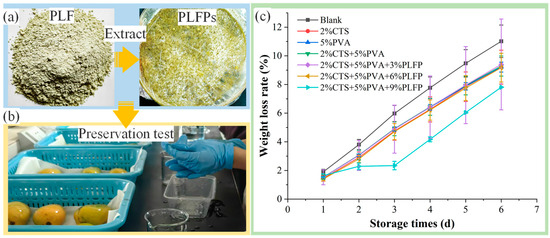
Figure 6.
Extraction (a), preservation test (b), and weight loss rate curve (c) of PLFPs.
Compared with the Blank group, the 2%CTS group, the 5%PVA group, the 2%CTS/5%PVA group and the PLFP/2%CTS/5%PVA co group exhibited reduced water evaporation and decreased WLR of mango, and the WLR of mango decreased as the content of PLFPs increased; when the content of PLFPs was 9%, the WLR of mango samples decreased drastically, and the WLR was lower in the first three days of preservation. After that, water loss increased and the WLR increased owing to fruit metabolism. In addition, the films could not only reduce water evaporation, but also inhibit the growth of microorganisms, thereby extending the preservation period [35,36].
4. Conclusions
The ER of PLFPs was investigated using different solvent systems, and the optimal extraction parameters were determined through a Box–Behnken (B–B) central composite design. Additionally, the storage stability and fresh-keeping performance of PLFPs were explored first. Results indicated that acidic extraction methods were superior to alkaline methods for extracting PCs from pineapple leaf fiber. Among all tested extraction media, acidic ethanol yielded the highest PLFPs content. The optimized extraction conditions for acidic ethanol were as follows: ethanol concentration = 80%, extraction temperature = 70 °C, material–liquid ratio = 1:40, and extraction time = 40 min. Under these conditions, the maximum ER of PLFPs reached 11.55 ± 0.03 mg/g. Furthermore, the stability of PLFPs was significantly enhanced by minimizing light exposure and maintaining a pH range of 3–6. In practical applications, PLFPs demonstrated potential in fruit preservation by inhibiting the growth of anthracnose on mango epidermis. This treatment improved the appearance quality, reduced the weight loss rate, and extended the shelf life of mangoes. These findings highlight the promising application of PLFPs in fruit preservation technologies.
Author Contributions
Y.L. and H.M.: writing—original, methodology, data curation and formal analysis. Y.W.: supervision, visualization, and formal analysis. Q.L.: methodology, data curation and funding acquisition. M.S. and G.C.: visualization, funding acquisition and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the opening subject of Hainan key laboratory of fruit and vegetable storage and processing (No. HNGS202301 and HNGS202303), Hainan Provincial Natural Science Foundation of China (No. 325QN435), Central Public-interest Scientific Institution Basal Research Fund (1630012025203, 1630062025016).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful for the technical guidance in data detection provided by the Beijing Biotech-Pack Scientific Co., Ltd.
Conflicts of Interest
Author Qiangyou Li was employed by the company Science and Technology Department, Zhanjiang Agribusiness Modern Agriculture Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, Y.J.; Qian, Y.Y.; Wang, C.Y.; He, Y.Y.; Zhu, C.X.; Chen, G.; Lin, L.J.; Chen, Y.L. Study of the metabolite changes in Ganoderma lucidum under pineapple leaf residue stress via LC-MS/MS coupled with a non-targeted metabolomics approach. Metabolites 2023, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Du, J.H.; Zhang, J.; Han, J.C.; Gong, P.; Lian, W.W.; Li, M.F. Analysis on change of nutritional ingredients in ensiling process of pineapple leaf residue. Adv. Mater. Res. 2014, 3514, 1033–1034. [Google Scholar] [CrossRef]
- Sajjanshetty, R.; Kulkarni, S.; Shankar, K.; Jayalakshmi, S.K.; Sreeramulu, K. Enhanced production and in-situ removal of butanol during the fermentation of lignocellulosic hydrolysate of pineapple leaves. Ind. Crops Prod. 2021, 173, 114147. [Google Scholar] [CrossRef]
- Nashiruddin, N.I.; Abd, R.N.H.; Rahman, R.; IIlias, R.; Ghazali, N.F.; Abomoelak, B.; Enshasy, H.A. Improved sugar recovery of alkaline pre-treated pineapple leaf fibres via enzymatic hydrolysis and its enzymatic kinetics. Fermentation 2022, 8, 640. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liu, J.; Zhu, L.; Lin, L.; Zhuang, Z.; He, J.; Li, T.; Chen, G.; Yao, S. Separation and identification of terpenoids in three pineapple fibers using ultra-high performance liquid chromatography-tandem mass spectrometry. J. Nat. Fibers 2024, 21, 2315596. [Google Scholar] [CrossRef]
- Wang, J.L.; Jiang, J.M.; Lian, W.W.; Huang, T.; Zhang, J.; Deng, Y.G. Bacteria resistant property of pineapple leaf fiber. Chin. J. Trop. Crops 2009, 30, 1694–1697. [Google Scholar]
- Zhuang, Z.K.; Du, J.H.; Qian, Y.Y.; Wang, Y.H.; Jiao, J.; Liu, Y.J.; Chen, G. Composition, distribution, and bioactivity of polyphenols in pineapple fibers: Insights from UHPLC-MS analysis for the development of antibacterial materials. Curr. Res. Food. Sci. 2025, 10, 101069. [Google Scholar] [CrossRef]
- Huang, Y.J.; Ji, M.H.; Shu, H.M.; Guo, F.Y.; Dong, L.W.; Zhai, F.R. Antibacterial activities and stability of the ethanolic extract from the leaves of Ananas comosus. Sci. Technol. Food Ind. 2014, 35, 166–169. Available online: https://api.semanticscholar.org/CorpusID:88220706 (accessed on 24 December 2024).
- Sangita, D.; Debasish, B. Enzymatic, antimicrobial and toxicity studies of the aqueous extract of Ananas comosus (pineapple) crown leaf. J. Ethnopharmacol. 2013, 150, 451–457. [Google Scholar] [CrossRef]
- Santosh, S.T.; Suresh, A.P. Review on mechanical properties evaluation of pineapple leaf fibre (PALF) reinforced polymer composites. Compos. Part B 2019, 174, 106927. [Google Scholar] [CrossRef]
- Ridzuan, M.J.M.; Abdul Majid, M.S.; Khasri, A.; Gan, E.H.D.; Razlan, Z.M.; Syahrullail, S. Effect of pineapple leaf (PALF), napier, and hemp fibres as filler on the scratch resistance of epoxy composites. J. Mater. Res. Technol. 2019, 8, 5384–5395. [Google Scholar] [CrossRef]
- Kueh, A.; Razali, A.W.; Hamdan, S.; Yakub, L.; Suhaili, N. Acoustical and mechanical characteristics of mortars with pineapple leaf fiber and silica aerogel infills—Measurement and modeling. Mater. Today Commun. 2023, 35, 105540. [Google Scholar] [CrossRef]
- Liao, S.Y.; Chen, J.M.; Li, L.; Li, P.W.; Wang, X.G. Stepwise degumming of pineapple leaf fibers with tunable fineness and excellent antibacterial property. Ind. Crops Prod. 2025, 225, 120490. [Google Scholar] [CrossRef]
- Najeeb, M.I.; Sultan, M.T.H.; Shah, A.U.M.; Safri, S.N.A.; Jawaid, M.; Abu, T.; Basri, A.A. Flexural, dynamic and thermo-mechanical analysis of pineapple leaf fiber/epoxy composites. J. Nat. Fibers 2022, 19, 15930–15947. [Google Scholar] [CrossRef]
- Zeleke, Y.; Feleke, T.; Tegegn, W.; Atinaf, Y. Design and development of false ceiling board composite material using pineapple leaf fibre reinforcement in unsaturated polyester matrix. Int. J. Sustain. Eng. 2022, 15, 144–152. [Google Scholar] [CrossRef]
- Huang, Y.J.; Chen, W.H.; Ji, M.H.; Guo, F.Y.; Shu, H.M.; Zheng, C.J. Chemical constituents from leaves of Ananas comosus and their biological activities. Chin. Tradit. Herb. Drugs 2015, 46, 949–954. [Google Scholar] [CrossRef]
- Chaudhry, F.; Ahmad, M.L.; Hayat, Z.; Ranjha, M.M.; Chaudhry, K.; Elboughdiri, N.; Asmari, M.; Uddin, J. Extraction and evaluation of the antimicrobial activity of polyphenols from banana peels employing different extraction techniques. Separations 2022, 9, 165. [Google Scholar] [CrossRef]
- Fendri, L.B.; Chaari, F.; Kallel, F.; Koubaa, M.; Zouari, E.S.; Kacem, I.; Chaabouni, S.E.; Ghribi, A.D. Antioxidant and antimicrobial activities of polyphenols extracted from pea and broad bean pods wastes. J. Food Meas. Charact. 2022, 16, 4822–4832. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, Z.G.; Wu, Y.L.; Xiao, L.P.; Zhuang, Y.H.; Ding, N.S.; Huang, B.Q. non-radical synthesis of amide chitosan with p-coumaric acid and caffeic acid and its application in pork preservation. Int. J. Biol. Macromol. 2022, 222, 1778–1788. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, Y.; Hao, W.; Zhang, W.; Lu, J.; Zhou, J.; Du, L.; Xie, W. Pineapple leaf phenols attenuate dss-induced colitis in mice and inhibit inflammatory damage by targeting the NF-κB pathway. Molecules 2021, 26, 7656. [Google Scholar] [CrossRef]
- Xie, W.D.; Zhang, S.B.; Lei, F.; Ouyang, X.X.; Du, L.J. Ananas comosus L. Leaf Phenols and p-coumaric acid regulate liver fat metabolism by upregulating cpt-1 expression. Evid. Based Complement. Altern. Med. eCAM 2014, 2014, 903258. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Sahar, A.; Usman, M.; Sameen, A.; Azhar, M.; Tahir, R.; Younas, R.; Issa, K. Extraction of dietary fiber and polyphenols from mango peel and its therapeutic potential to improve gut health. Food Biosci. 2023, 53, 102669. [Google Scholar] [CrossRef]
- Huang, W.J.; Tian, F.L.; Wang, H.; Wu, S.; Jin, W.P.; Shen, W.Y.; Hu, Z.Z.; Cai, Q.Y.; Liu, G. Comparative assessment of extraction, composition, and in vitro antioxidative properties of wheat bran polyphenols. LWT 2023, 180, 114706. [Google Scholar] [CrossRef]
- Zhang, X.J.; Liu, Z.T.; Chen, X.Q.; Zhang, T.T.; Zhang, Y. Deep eutectic solvent combined with ultrasound technology: A promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food Chem. 2023, 422, 136224. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and recovery of bioactive soluble phenolic compounds from brocade orange (Citrus sinensis) peels: Effect of different extraction methods thereon. LWT 2023, 173, 114337. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The research progress of extraction, purification and analysis methods of phenolic compounds from blueberry: A comprehensive review. Molecules 2023, 28, 3610. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, M.; Mujumda, A.S.; Wang, H. Inhibition of nitrite in prepared dish of Brassica chinensis L. during storage via non-extractable phenols in hawthorn pomace: A comparison of different extraction methods. Food Chem. 2022, 393, 133344. [Google Scholar] [CrossRef]
- Zheng, M.; Su, H.; Xiao, R.; Chen, J.; Chen, H.; Tan, K.B.; Zhu, Y. Effects of Polygonatum cyrtonema extracts on the antioxidant ability, physical and structure properties of carboxymethyl cellulose-xanthan gum-flaxseed gum active packaging films. Food Chem. 2023, 403, 134320. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, L.H.; Tang, B.; Wei, X.Y.; Fu, T.K.; Zhang, J. Optimization of extraction process for polyphenols from pineapple leaves fiber by response surface methodology. Sci. Technol. Food Ind. 2017, 38, 219–223. [Google Scholar] [CrossRef]
- Xiang, B.P.; Geng, X.L.; Zhu, P. Extraction of polyphenols from pineapple leaf. Sichuan Chem. Ind. 2014, 17, 1–4. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Haris, S.; Baba, W.N.; Akhter, R.; AlMarzouqi, A.H.; Masoodi, F.A. Supercritical fluid extraction tandem responsive polarity modifier separation of hydroxytyrosol rich phenolic raffinates from olive oil. Int. J. Food Sci. Technol. 2023, 58, 5701–5710. [Google Scholar] [CrossRef]
- Gan, J. Purification, antioxidation and stability of eleocharis tuberosa peel polyphenols. Food Res. Dev. 2022, 43, 27–33. [Google Scholar] [CrossRef]
- Yu, S.S.; Xie, H.; Xin, T.; Wang, S.P.; Wu, Y.C.; Duan, Y.Z. Stability and bioactivity of polyphenols purified from mallotus oblongifolius. Food Sci. Technol. 2023, 48, 173–179. [Google Scholar] [CrossRef]
- Yuan, Y.; Tian, H.; Huang, R.; Liu, H.; Wu, H.; Guo, G.; Xiao, J. Fabrication and characterization of natural polyphenol and ZnO nanoparticles loaded protein-based biopolymer multifunction electrospun nanofiber films, and application in fruit preservation. Food Chem. 2023, 418, 135851. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, M.; Tan, K.B.; Lin, J.; Chen, M.; Zhu, Y. Development of xanthan gum/hydroxypropyl methyl cellulose composite films incorporating tea polyphenol and its application on fresh-cut green bell peppers preservation. Int. J. Biol. Macromol. 2022, 211, 198–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).