Colloidal Nutrition Improves Parameters of Pecan Tree (Carya illinoinensis) Soil Health Such as Organic Matter, Available Water, and Electrical Conductivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plant Materials and Their Origins
2.3. Data Acquisition
2.4. Data Analysis
3. Results and Discussion
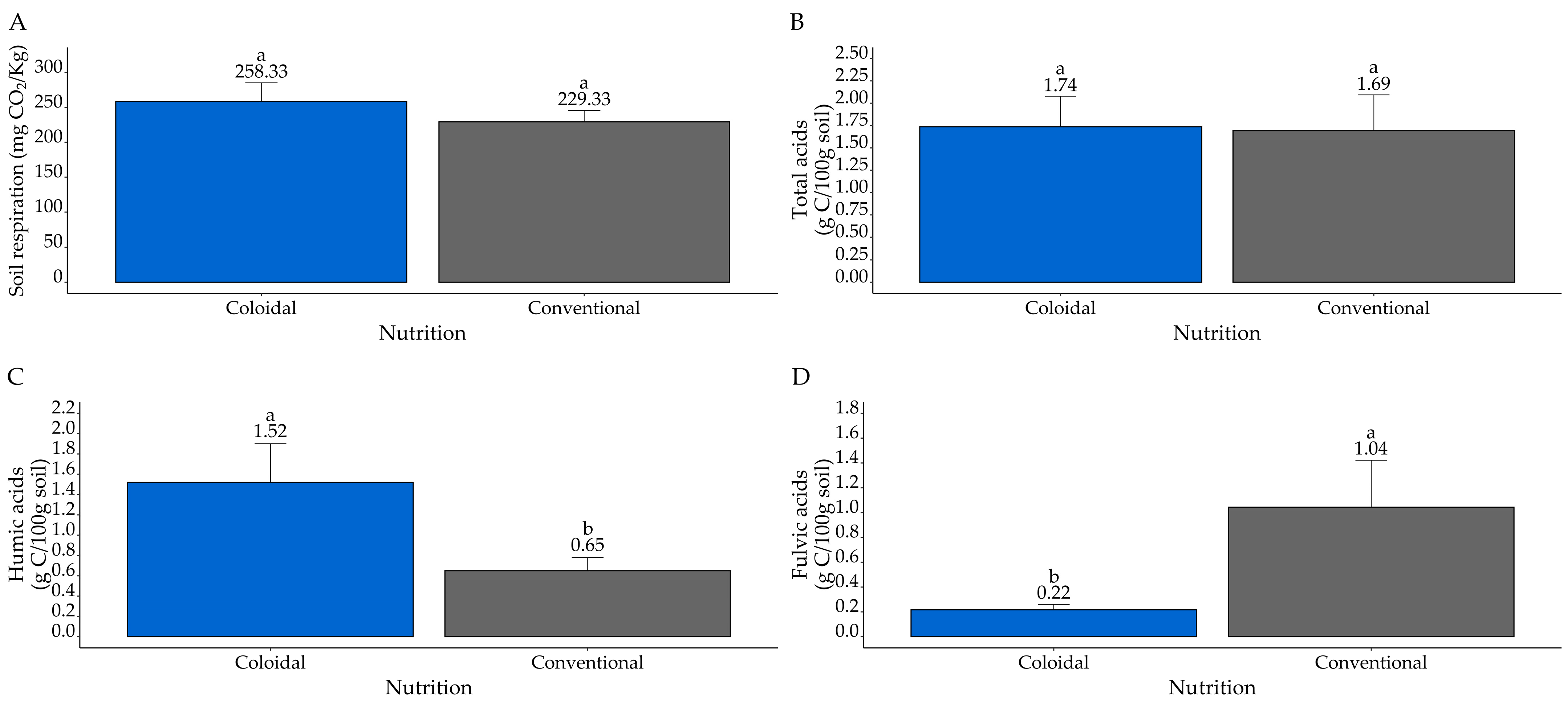
3.1. Soil Health Parameters in 2020
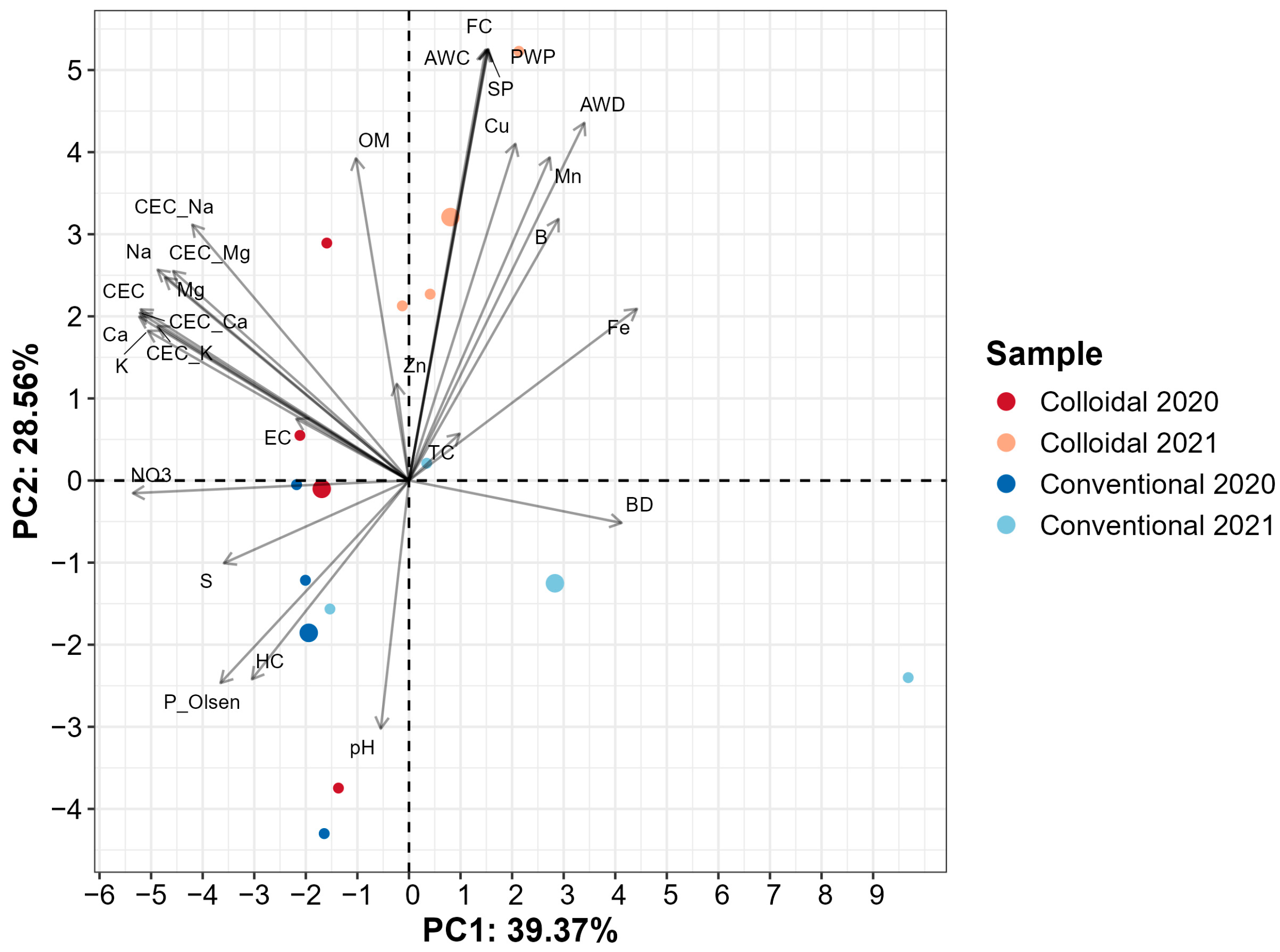
3.2. Soil Health Parameters for theYears 2020 and 2021
3.3. Saturated Paste Extracts for the Years 2020 and 2021
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rüter, B.; Hamrick, J.L.; Wood, B.W. Genetic diversity within provenance and cultivar germplasm collections versus natural populations of pecan (Carya illinoinensis). J. Hered. 1999, 90, 521–528. [Google Scholar] [CrossRef]
- Grauke, L.J.; Wood, B.W.; Harris, M.K. Crop vulnerability: Carya. HortScience 2016, 51, 653–663. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Rodriguez, A.P.M.; Burns, J.A.; Magner, H.N. Carya illinoensis (Pecan). In Trees IV.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 35, pp. 50–75. ISBN 0934-943X. [Google Scholar]
- Orona-Castillo, I.; Sangerman-Jarquín, D.M.; Cervantes-Vázquez, M.G.; Espinoza-Arellano, J.d.J.; Núñez-Moreno, J.H. The production and commercialization of pecan nut in Mexico. Rev. Mex. Cienc. Agric. 2019, 10, 1797–1808. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera, S. Anuario Estadístico de La Producción Agrícola. Available online: https://nube.agricultura.gob.mx/cierre_agricola/ (accessed on 3 February 2025).
- Osman, K.T. Poorly Fertile Soils; Springer: Cham, Switzerland, 2018; ISBN 9783319755274. [Google Scholar]
- Ibragimov, O.; Domuladjanov, I.; Domuladjonova, S. Soil fertility in agriculture: Main tasks. E3S Web Conf. 2023, 431, 01057. [Google Scholar] [CrossRef]
- Warudkar, G.; Dorle, S. Review on sensing the fertility characteristics of agriculture soils. In Proceedings of the 2016 International Conference on Information Communication and Embedded Systems (ICICES), Chennai, India, 25–26 February 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Chandra, H.; Pawar, P.M.; Elakkiya, R.; Tamizharasan, P.S.; Muthalagu, R.; Panthakkan, A. Explainable Al for soil fertility prediction. IEEE Access 2023, 11, 97866–97878. [Google Scholar] [CrossRef]
- Wells, M.L. Pecan nutrient element status and orchard soil fertility in the southeastern coastal plain of the United States. Horttechnology 2009, 19, 432–438. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Gong, D.; Huang, C.; Ma, X.; Ma, X.; Yuan, F.; Fu, S.; Feng, C. Enhancing soil fertility and elevating pecan fruit quality through combined chemical and organic fertilization practices. Horticulturae 2024, 10, 25. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, M.; Liu, H.; Huang, C.; Ma, Y.; Ge, H.; Ge, X.; Fu, S. Pecan agroforestry systems improve soil quality by stimulating enzyme activity. PeerJ 2022, 10, e12663. [Google Scholar] [CrossRef]
- Díaz, G.M. Effect of weeds as coverts in soil fertility and pecanal walnut yield. Rev. Mex. Cienc. Agrícolas Vol. 2019, 10, 123–130. [Google Scholar]
- Rodriguez-Ramos, J.C.; Scott, N.; Marty, J.; Kaiser, D.; Hale, L. Cover crops enhance resource availability for soil microorganisms in a pecan orchard. Agric. Ecosyst. Environ. 2022, 337, 108049. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil. Sci. Plant Anal. 2001, 32, 37–41. [Google Scholar] [CrossRef]
- Adhikari, S.; Anuragi, H.; Chandra, K.; Tarte, S.H.; Dhaka, S.R.; Jatav, H.S.; Hingonia, K. Molecular Basis of Plant Nutrient Use Efficiency—Concepts and Challenges for Its Improvement; Elsevier: Amsterdam, Netherlands, 2022; ISBN 9780443186752. [Google Scholar]
- Dhillon, J.S.; Eickhoff, E.M.; Mullen, R.W.; Raun, W.R. World potassium use efficiency in cereal crops. Agron. J. 2019, 111, 889–896. [Google Scholar] [CrossRef]
- Priya, E.; Sarkar, S.; Maji, P.K. A review on slow-release fertilizer: Nutrient release mechanism and agricultural sustainability. J. Environ. Chem. Eng. 2024, 12, 113211. [Google Scholar] [CrossRef]
- Asadu, C.O.; Ezema, C.A.; Ekwueme, B.N.; Onu, C.E.; Onoh, I.M.; Adejoh, T.; Ezeorba, T.P.C.; Ogbonna, C.C.; Otuh, P.I.; Okoye, J.O.; et al. Enhanced efficiency fertilizers: Overview of production methods, materials used, nutrients release mechanisms, benefits and considerations. Environ. Pollut. Manag. 2024, 1, 32–48. [Google Scholar] [CrossRef]
- Larramendy, M.; Soloneski, S. Organic Fertilizers; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78985-148-9. [Google Scholar]
- Brown, P.H.; Zhao, F.J.; Dobermann, A. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant Soil. 2022, 476, 11–23. [Google Scholar] [CrossRef]
- Zahra, Z.; Habib, Z.; Hyun, H.; Shahzad, H.M.A. Overview on recent developments in the design, application, and impacts of nanofertilizers in agriculture. Sustainability 2022, 14, 9397. [Google Scholar] [CrossRef]
- Chugh, G.; Siddique, K.H.M.; Solaiman, Z.M. Nanobiotechnology for agriculture: Smart technology for combating nutrient deficiencies with nanotoxicity challenges. Sustainability 2021, 13, 1781. [Google Scholar] [CrossRef]
- Alemayehu, B.; Teshome, H. Soil colloids, types and their properties: A review. Open J. Bioinform. Biostat. 2021, 5, 008–113. [Google Scholar] [CrossRef]
- Batsmanova, L.; Taran, N.; Konotop, Y.; Kalenska, S.; Novytska, N. Use of a colloidal solution of metal and metal oxide-containing nanoparticles as fertilizer for increasing soybean productivity. J. Cent. Eur. Agric. 2020, 21, 311–319. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Attia, M.S.; Abdelaziz, A.M. Ferric oxide colloid: Towards green nano-fertilizer for tomato plant with enhanced vegetative growth and immune response against Fusarium wilt disease. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4270–4283. [Google Scholar] [CrossRef]
- Rios, J.J.; Lopez-Zaplana, A.; Bárzana, G.; Martinez-Alonso, A.; Carvajal, M. Foliar application of boron nanoencapsulated in almond trees allows B movement within tree and implements water uptake and transport involving aquaporins. Front. Plant Sci. 2021, 12, 752648. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef]
- Gu, S.; Lian, F.; Yang, H.; Han, Y.; Zhang, W.; Yang, F.; Gao, J. Synergic effect of microorganism and colloidal biochar-based organic fertilizer on the growth and fruit quality of tomato. Coatings 2021, 11, 1453. [Google Scholar] [CrossRef]
- Kubavat, D.; Trivedi, K.; Vaghela, P.; Prasad, K.; Vijay Anand, G.K.; Trivedi, H.; Patidar, R.; Chaudhari, J.; Andhariya, B.; Ghosh, A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Degrad. Dev. 2020, 31, 2734–2746. [Google Scholar] [CrossRef]
- Salas-Leiva, J.; Salas-Leiva, D.E.; Tovar-Ramírez, D.; Herrera-Pérez, G.; Tarango-Rivero, S.; Luna-Velasco, A.; Orrantia-Borunda, E. Copper oxide nanoparticles slightly affect diversity and metabolic profiles of the prokaryotic community in pecan tree (Carya illinoinensis) rhizospheric soil. Appl. Soil. Ecol. 2021, 157, 103772. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía (INEGI) Aspectos Geográficos de Chihuahua. 2022. Available online: https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/889463913375.pdf (accessed on 19 February 2025).
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Chapman, H.D. Cation-exchange capacity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Norman, A.G., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. Determination of organic matter in the soil by chromic acid digestion. Soil. Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Analysis of Organic Fertilizer Products. Available online: https://www.fao.org/faolex/results/details/es/c/LEX-FAOC010152 (accessed on 18 November 2024).
- Anderson, J.P.E. Soil respiration. In Methods of Soil Analysis; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1982; pp. 831–871. ISBN 9780891189770. [Google Scholar]
- Dong, X.Z.; Cai, M.Y. Manual for Systematic Identification of Common Bacteria; Science: Beijing, China, 2001; pp. 348–392. [Google Scholar]
- Ibtissem Ben Salem, N.E.A.; Mahmoud M’Hamdi, M.B.K.; M Hamdi, N.B. Isolation and identification of fungal communities in organic and conventional soils. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1111–1123. [Google Scholar] [CrossRef]
- McSorley, R.; Frederick, J.J. Effect of Extraction method on perceived composition of the soil nematode community. Appl. Soil. Ecol. 2004, 27, 55–63. [Google Scholar] [CrossRef]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.H.; Chakrabartty, I. Nanofertilizers: A smart and sustainable attribute to modern agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef]
- Wu, S.; Li, R.; Peng, S.; Liu, Q.; Zhu, X. Effect of humic acid on transformation of soil heavy metals. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012089. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.; Li, J.; Yang, F.; Dai, W.; Xiang, C.; Zhang, M. The positive effects of humic/fulvic acid fertilizers on the quality of lemon fruits. Agronomy 2022, 12, 1919. [Google Scholar] [CrossRef]
- Rasouli, F.; Nasiri, Y.; Asadi, M.; Hassanpouraghdam, M.B.; Golestaneh, S.; Pirsarandib, Y. Fertilizer type and humic acid improve the growth responses, nutrient uptake, and essential oil content on Coriandrum sativum L. Sci. Rep. 2022, 12, 7437. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, S.; Yuan, L.; Li, Y.; Lin, Z.; Xiong, Q.; Zhao, B. Combining humic acid with phosphate fertilizer affects humic acid structure and its stimulating efficacy on the growth and nutrient uptake of maize seedlings. Sci. Rep. 2020, 10, 17502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, J.; Zhao, Y.G.; Zhai, Y.; Wang, K.; Alva, A.K.; Paramasivam, S. Optimal combination of chemical compound fertilizer and humic acid to improve soil and leaf properties, yield and quality of apple (Malus domestica) in the loess plateau of China. Pak. J. Bot. 2013, 45, 1315–1320. [Google Scholar]
- Wang, D.; Chen, X.; Tang, Z.; Liu, M.; Jin, R.; Zhang, A.; Zhao, P. Application of humic acid compound fertilizer for increasing sweet potato yield and improving the soil fertility. J. Plant Nutr. 2022, 45, 1933–1941. [Google Scholar] [CrossRef]
- Samaniego-Gaxiola, J.A.; Pedroza-Sandoval, A.; Chew-Madinaveitia, Y.; Gaytán-Mascorro, A. Reductive disinfestation, soil desiccation and Trichoderma harzianum to control Phymatotrichopsis omnivora in pecan tree nursery. Mex. J. Phytopathol. 2019, 37, 287–303. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Tarango-Rivero, S.H.; Nevárez-Moorillón, V.G.; Orrantia-Borunda, E. Growth, yield, and nutrient status of pecans fertilized with biosolids and inoculated with rizosphere fungi. Bioresour. Technol. 2009, 100, 1992–1998. [Google Scholar] [CrossRef]
- Rolim, J.M.; Walker, C.; Mezzomo, R.; Muniz, M.F. Antagonism and effect of volatile metabolites of Trichoderma spp. on Cladosporium spp. Floresta E Ambiente 2019, 26, e20170594. [Google Scholar] [CrossRef]
- Schratzberger, M.; Holterman, M.; Van Oevelen, D.; Helder, J. A Worm’s world: Ecological flexibility pays off for free-living nematodes in sediments and soils. Bioscience 2019, 69, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Jones, M.G.K. Nematodes. Encycl. Appl. Plant Sci. 2016, 3, 113–119. [Google Scholar] [CrossRef]
- von Broembsen, S. Pecan diseases: Prevention and control. Okla. Coop. Ext. Serv. 2016, EPP-7642, 7642–7647. [Google Scholar]
- Heerema, R.; Goldberg, N.; Thomas, S. Diseases and Other Disorders of Pecan in New Mexico; New Mexico State University: Las Cruces, NM, USA, 2010; pp. 1–12. [Google Scholar]
- Romagna, I.S.; Sobucki, L.; Santos, J.R.P.; Freiberg, J.A.; Meireles, L.A.; Schardong, I.S.; Somavilla, L.M.; dos Santos Rodrigues, G.L.; Kessler, N.C.H.; Antoniolli, Z.I. Plant-parasite nematodes in soils cultivated with pecans in Rio Grande Do Sul, Brazil. Int. J. Environ. Stud. 2024, 81, 1903–1917. [Google Scholar] [CrossRef]
- WHO. Permissible Limits of Heavy Metals in Soil and Plants; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Neenu, S.; Karthika, K.S. Aluminium toxicity in soil and plants. Harit. Dhara 2019, 2, 15–19. [Google Scholar]
- Reynolds, W.D.; Bowman, B.T.; Drury, C.F.; Tan, C.S.; Lu, X. Indicators of good soil physical quality: Density and storage parameters. Geoderma 2002, 110, 131–146. [Google Scholar] [CrossRef]
- Ummah, M.S. Soil organic matter and water retention. Agron. J. 2020, 112, 3265–3277. [Google Scholar] [CrossRef]
- Cassel, D.K.; Nielsen, D.R. Field capacity and available water capacity. Methods Soil. Anal. 1986, 9, 901–926. [Google Scholar]
- Mutuku, E.A.; Vanlauwe, B.; Roobroeck, D.; Boeckx, P.; Cornelis, W.M. Physico-chemical soil attributes under conservation agriculture and integrated soil fertility management. Nutr. Cycl. Agroecosyst 2021, 120, 145–160. [Google Scholar] [CrossRef]
- Prieto-Méndez, J.; Rubio-Arias, H.; Prieto-García, F.; Roman-Gutiérrez, A.D.; Mendez-Marzo, M.A.; Acevedo-Sandoval, O.A. Soil quality in terms of pyhsical-chemical-metal properties for barely (Hordeum vulgare) production in the state of Hidalgo, Mexico. J. Agric. Environ. Sci. 2011, 10, 230–237. [Google Scholar]
- Sumner, M.E.; Noble, A.D. Soil acidification: The world story. In Handbook of Soil Acidity; Rengel, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–21. ISBN 0824708903. [Google Scholar]
- Arias, M.E.; González-Pérez, J.A.; González-Vila, F.J.; Ball, A.S. Soil health—A new challenge for microbiologists and chemists. Int. Microbiol. 2005, 8, 13–21. [Google Scholar] [PubMed]
- Friedman, S.P. Soil properties influencing apparent electrical conductivity: A review. Comput. Electron. Agric. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Roche, J.R.; Ledgard, S.F.; Sprosen, M.S.; Lindsey, S.B.; Penno, J.W.; Horan, B.; Macdonald, K.A. Increased stocking rate and associated strategic dry-off decision rules reduced the amount of nitrate-N leached under grazing. J. Dairy. Sci. 2016, 99, 5916–5925. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Lin, S.; Wang, Y.; Lian, X.; Zhao, Y.; Li, Y.; Du, J.; Wang, Z.; Wang, J.; Butterbach-Bahl, K. Drip fertigation significantly reduces nitrogen leaching in solar greenhouse vegetable production system. Environ. Pollut. 2019, 245, 694–701. [Google Scholar] [CrossRef]
- Ćirić, V.; Prekop, N.; Šeremešić, S.; Vojnov, B.; Pejić, B.; Radovanović, D.; Marinković, D. The implication of cation exchange capacity (CEC) assessment for soil quality management and improvement. Agric. For. 2023, 69, 113–134. [Google Scholar] [CrossRef]
- Bryson, G.M.; Mills, H.A. Plants Analysis Handbook IV: A Guide to Plant Nutrition and Interpretationof Plant Analysis for Agronomic and Horticultural Crops; Bryson, G.M., Mills, H.A., Eds.; Micro Macro Publishing: Athens, GA, USA, 2014; ISBN 9781878148032. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results; CSIRO Publishing: Melbourne, Australia, 2007; ISBN 978-0-643-09915-9. [Google Scholar]
- Haby, V.A.; Russelle, M.P.; Skogley, E.O. Testing soils for potassium, calcium, and magnesium. In Soil Testing and Plant Analysis; SSSA Book Series; Soil Science of America Inc.: Madison, WI, USA, 1990; pp. 181–227. ISBN 9780891188629. [Google Scholar]
- Chaganti, V.N.; Culman, S.W. Historical perspective of soil balancing theory and identifying knowledge gaps: A review. Crop Forage Turfgrass Manag. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Ertiftik, H.; Zengin, M. Response of maize for grain to potassium and magnesium fertilizers in soils with high contents. J. Plant Nutr. 2017, 40, 93–103. [Google Scholar] [CrossRef]
- Zharare, G.E.; Asher, C.J.; Blamey, F.P.C. Magnesium antagonizes pod-zone calcium and zinc uptake by developing peanut pods. J. Plant Nutr. 2011, 34, 1–11. [Google Scholar] [CrossRef]
- Rhodes, R.; Miles, N.; Hughes, J.C. Interactions between potassium, calcium and magnesium in sugarcane grown on two contrasting soils in South Africa. Field Crops Res. 2018, 223, 1–11. [Google Scholar] [CrossRef]
- Hatten, J.; Liles, G.A. Healthy balance—The role of physical and chemical properties in maintaining forest soil function in a changing world. Dev. Soil Sci. 2019, 36, 373–396. [Google Scholar] [CrossRef]
- Weil, R.; Magdoff, F. Significance of soil organic matter to soil quality and health. In Soil Organic Matter in Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–43. [Google Scholar]
- Kreuser, B.C. Simplifying Soil Test Interpretations for Turf Professionals; University of Nebraska–Lincoln Extension, Institute of Agriculture and Natural Resources: Lincoln, NE, USA, 2015; Volume G2265. [Google Scholar]
- Kargas, G.; Chatzigiakoumis, I.; Kollias, A.; Spiliotis, D.; Massas, I.; Kerkides, P. Soil salinity assessment using saturated paste and mass soil:water 1:1 and 1:5 ratios extracts. Water 2018, 10, 1589. [Google Scholar] [CrossRef]
- Omar, M.M.; Massawe, B.H.J.; Shitindi, M.J.; Pedersen, O.; Meliyo, J.L.; Fue, K.G. Assessment of salt-affected soil in selected rice irrigation schemes in Tanzania: Understanding salt types for optimizing management approaches. Front. Soil. Sci. 2024, 4, 1372838. [Google Scholar] [CrossRef]
- Inzunza-ibarra, M.A.; Monger, H.C. Variation of soil chemical properties in irrigated and non-irrigated areas of the laguna region of Mexico. Terra Latinoam. 2005, 23, 429–436. [Google Scholar]
- Abegunrin, T.P.; Awe, G.O.; Idowu, D.O.; Onigbogi, O.O.; Onofua, O.E. Effect of kitchen wastewater irrigation on soil properties and growth of cucumber (Cucumis sativus). J. Soil. Sci. Environ. Manag. 2013, 4, 139–145. [Google Scholar] [CrossRef]
- Messiga, A.J.; Dyck, K.; Ronda, K.; van Baar, K.; Haak, D.; Yu, S.; Dorais, M. Nutrients leaching in response to long-term fertigation and broadcast nitrogen in blueberry production. Plants 2020, 9, 1530. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]



| Organism | Colloidal | Conventional |
|---|---|---|
| Log10 CFU/g Dried Soil | ||
| Rhizoctonia solani | 2.52 ± 0.14 a | 2.20 ± 0.10 a |
| Verticillium albo-atrum | 2.16 ± 0.16 a | 1.34 ± 0.67 a |
| Cylindrocarpon destructans | 0.00 ± 0.00 a | 0.67 ± 0.67 a |
| Fusarium solani | 1.99 ± 1.00 a | 2.52 ± 0.26 a |
| Fusarium oxysporum | 2.84 ± 0.28 a | 2.55 ± 0.28 a |
| Fusarium longipes | 0.83 ± 0.83 a | 0.00 ± 0.00 a |
| Pythium spp. | 2.91 ± 0.11 a | 2.65 ± 0.19 a |
| Phytophthora spp. | 0.67 ± 0.67 a | 0.00 ± 0.00 a |
| Trichoderma spp. | 2.72 ± 0.24 a | 2.32 ± 0.16 a |
| Organism | Colloidal | Conventional |
|---|---|---|
| Individuals/100 g Dried Soil | ||
| Criconemella spp. | 12.00 ± 7.57 a | 50.33 ± 27.12 a |
| Meloidogyne spp. | 0.00 ± 0.00 a | 26.00 ± 26.00 a |
| Psilenchus spp. | 0.00 ± 0.00 a | 3.33 ± 3.33 a |
| Tylenchus spp. | 0.00 ± 0.00 a | 5.67 ± 2.96 a |
| Tylenchulus spp. | 0.00 ± 0.00 a | 1.67 ± 1.67 a |
| Tylenchorhynchus spp. | 0.00 ± 0.00 a | 4.67 ± 4.67 a |
| Acrobeles spp. | 3.33 ± 3.33 a | 1.67 ± 1.67 a |
| Pratylenchus spp. | 11.33 ± 11.33 a | 0.00 ± 0.00 a |
| Gracilicus spp. | 23.00 ± 23.00 a | 5.00 ± 5.00 a |
| Total plant-parasitic nematode | 49.67 ± 39.67 a | 98.33 ± 32.99 a |
| Free-living nematodes | 45.33 ± 13.30 a | 26.67 ± 6.17 a |
| Properties | Conventional 2020 | Colloidal 2020 | Conventional 2021 | Colloidal 2021 |
|---|---|---|---|---|
| NO3-N (ppm) | 22.47 ± 1.84 a | 18.57 ± 0.88 ab | 9.47 ± 3.66 c | 12.93 ± 1.89 bc |
| P (ppm) | 37.53 ± 0.38 a | 28.63 ± 3.37 b | 19.00 ± 3.14 c | 14.10 ± 1.76 c |
| K (ppm) | 713.33 ± 67.11 a | 634 ± 59.08 a | 571.67 ± 229.90 a | 707.67 ± 27.53 a |
| Ca (ppm) | 4001.00 ± 180.58 a | 4038.67 ± 208.16 a | 2783.33 ± 1078.27 a | 4120.00 ± 113.36 a |
| Mg (ppm) | 341.33 ± 39.16 a | 300.33 ± 31.83 a | 283.33 ± 89.66 a | 329.67 ± 22.33 a |
| S (ppm) | 32.23 ± 5.53 a | 25.07 ± 6.23 a | 2.90 ± 1.45 b | 5.80 ± 4.35 b |
| Zn (ppm) | 0.84 ± 0.58 a | 1.24 ± 0.25 a | 1.03 ± 0.27 a | 1.69 ± 0.31 a |
| Cu (ppm) | 0.34 ± 0.04 a | 0.39 ± 0.05 a | 0.38 ± 0.03 a | 0.49 ± 0.10 a |
| Fe (ppm) | 2.62 ± 0.17 a | 2.82 ± 0.37 a | 4.48 ± 1.60 a | 4.89 ± 2.10 a |
| Mn (ppm) | 1.71 ± 0.12 b | 2.10 ± 0.03 b | 2.15 ± 0.14 b | 2.88 ± 0.34 a |
| B (ppm) | 0.11 ± 0.01 c | 0.18 ± 0.06 c | 0.98 ± 0.05 b | 1.38 ± 0.13 a |
| Na (ppm) | 104.00 ± 9.45 ab | 151.33 ± 10.91 a | 73.97 ± 32.20 b | 141.67 ± 8.76 a |
| CEC (meq/100 g) | 25.05 ± 1.34 a | 24.89 ± 1.47 a | 18.03 ± 6.84 a | 25.70 ± 0.85 a |
| Ca Saturation (%) | 73.73 ± 3.40 a | 74.35 ± 3.87 a | 75.60 ± 1.87 a | 80.03 ± 0.43 a |
| Mg Saturation (%) | 10.36 ± 1.19 b | 9.13 ± 0.96 b | 14.83 ± 2.54 a | 10.53 ± 0.35 b |
| K Saturation (%) | 6.75 ± 0.63 ab | 5.99 ± 0.56 b | 7.71 ± 0.56 a | 7.04 ± 0.18 ab |
| Na Saturation (%) | 1.67 ± 0.16 b | 2.44 ± 0.17 a | 1.73 ± 0.21 b | 2.37 ± 0.08 a |
| Ca:Mg ratio | 7.23 ± 0.53 a | 8.26 ± 0.49 a | 5.41 ± 0.91 b | 7.63 ± 0.32 a |
| Ca:K ratio | 11.13 ± 1.15 ab | 12.56 ± 0.75 a | 9.93 ± 0.54 b | 11.38 ± 0.29 ab |
| Mg:K ratio | 1.56 ± 0.20 a | 1.52 ± 0.09 a | 1.50 ± 0.52 a | 2.01 ± 0.07 a |
| Ca+Mg:K ratio | 12.69 ± 1.33 a | 14.08 ± 0.80 a | 11.93 ± 1.06 a | 12.87 ± 0.33 a |
| Properties | Conventional 2020 | Colloidal 2020 | Conventional 2021 | Colloidal 2021 |
|---|---|---|---|---|
| ECe (dS/m) | 2.11 ± 0.36 a | 0.90 ± 0.04 c | 1.51 ± 0.20 b | 1.41 ± 0.16 bc |
| SAR (dS/m) | 1.41 ± 0.12 c | 1.84 ± 0.04 bc | 2.49 ± 0.24 b | 3.51 ± 0.61 a |
| pH | 8.38 ± 0.07 b | 8.61 ± 0.03 a | 8.56 ± 0.02 a | 8.68 ± 0.05 a |
| NO3-N (ppm) | 18.11 ± 5.53 a | 0.98 ± 0.29 b | 3.64 ± 2.67 b | 4.94 ± 2.70 b |
| PO4-P (ppm) | 3.29 ± 2.67 a | 0.41 ± 0.10 a | 0.31 ± 0.00 a | 0.93 ± 0.47 a |
| SO4-S (ppm) | 463.67 ± 128.24 a | 143.93 ± 38.35 b | 112.23 ± 14.26 b | 73.93 ± 12.44 b |
| Cl (ppm) | 62.10 ± 7.61 b | 30.82 ± 13.48 b | 210.33 ± 13.48 a | 172.33 ± 17.52 a |
| HCO3 (ppm) | 166.33 ± 10.90 bc | 156.30 ± 32.02 c | 287.67 ± 49.74 ab | 330.33 ± 71.85 a |
| CO3 (ppm) | 32.80 ± 14.78 a | 30.70 ± 03.55 a | 14.80 ± 3.70 a | 33.00 ± 0.00 a |
| Ca (ppm) | 245.67 ± 49.56 a | 84.67 ± 3.16 b | 140.67 ± 27.91 b | 110.20 ± 9.11 b |
| Mg (ppm) | 43.23 ± 9.66 a | 14.57 ± 1.65 b | 23.97 ± 2.76 b | 16.03 ± 1.29 b |
| K (ppm) | 49.63 ± 13.71 a | 28.80 ± 3.85 a | 40.00 ± 8.32 a | 36.23 ± 3.89 a |
| Na (ppm) | 91.57 ± 16.71 bc | 69.63 ± 0.30 c | 119.00 ± 11.53 ab | 150.00 ± 28.88 a |
| Fe (ppm) | 4.38 ± 2.87 ab | 9.31 ± 3.43 a | 0.42 ± 0.20 b | 2.31 ± 1.09 b |
| Mn (ppm) | 0.21 ± 0.13 ab | 0.38 ± 0.15 a | 0.05 ± 0.03 b | 0.17 ± 0.10 ab |
| Zn (ppm) | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.37 ± 0.26 a | 0.11 ± 0.06 ab |
| Cu (ppm) | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 ab | 0.02 ± 0.01 a |
| B (ppm) | 0.37 ± 0.03 a | 0.38 ± 0.02 a | 0.28 ± 0.04 a | 0.49 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Chan, R.G.; Morales-Merida, B.E.; Amarillas, L.; Varela-Bojórquez, N.; Lightbourn-Rojas, L.A. Colloidal Nutrition Improves Parameters of Pecan Tree (Carya illinoinensis) Soil Health Such as Organic Matter, Available Water, and Electrical Conductivity. Agriculture 2025, 15, 1201. https://doi.org/10.3390/agriculture15111201
León-Chan RG, Morales-Merida BE, Amarillas L, Varela-Bojórquez N, Lightbourn-Rojas LA. Colloidal Nutrition Improves Parameters of Pecan Tree (Carya illinoinensis) Soil Health Such as Organic Matter, Available Water, and Electrical Conductivity. Agriculture. 2025; 15(11):1201. https://doi.org/10.3390/agriculture15111201
Chicago/Turabian StyleLeón-Chan, Rubén Gerardo, Brandon Estefano Morales-Merida, Luis Amarillas, Nancy Varela-Bojórquez, and Luis Alberto Lightbourn-Rojas. 2025. "Colloidal Nutrition Improves Parameters of Pecan Tree (Carya illinoinensis) Soil Health Such as Organic Matter, Available Water, and Electrical Conductivity" Agriculture 15, no. 11: 1201. https://doi.org/10.3390/agriculture15111201
APA StyleLeón-Chan, R. G., Morales-Merida, B. E., Amarillas, L., Varela-Bojórquez, N., & Lightbourn-Rojas, L. A. (2025). Colloidal Nutrition Improves Parameters of Pecan Tree (Carya illinoinensis) Soil Health Such as Organic Matter, Available Water, and Electrical Conductivity. Agriculture, 15(11), 1201. https://doi.org/10.3390/agriculture15111201









