Abstract
Cover crops are becoming widely integrated into many farms as tools for improving sustainability. However, the decisions by growers for planting follow several objectives/criteria, many of which overlap. This review orders these sowing rationales into a practical framework for land management guidance. Prioritised by cover crop performance objectives, the optimal species and their environmental requirements are discussed. A key consideration of this review is that cover crops are used as part of a rotation strategy. Here, farmers’ primary objectives are to maintain or enhance biomass not of the cover plants themselves but for the following commercial crop. For example, a large cover crop biomass may be beneficial for reducing field-nutrient losses but are counterproductive if nutrient immobilisation or offtake then results in subsequent nutrition stresses and yield declines. Furthermore, species selection and management practices must be integrated if these negative impacts are to be mitigated. This review has found a strong research focus on cover crop nitrogen dynamics but limited research on nutrient recycling more broadly. Moreover, there is growing evidence that regionality plays a critical role in cover crop and land management partnering due to variations in edaphic and climatic influences, but there is a shortfall in research to inform strategies for many important agricultural centres such as Northern Ireland.
1. Introduction
Intensive farming with rigorous tillage, limited crop rotation combined with extensive reliance on monocultures, along with the overuse of synthetic fertiliser has the potential to degrade soil and negatively impact sustainability [1]. Grown in winter or spring between the main/cash crop, cover crops diversify rotations and enhance land resilience and soil health [2]. Their first documented use, on farms, dates back to the 18th century and became prevalent in rotations, but by 1960 their use had diminished due to the increasing use of synthetic fertiliser and herbicides [3]. Today, cover crops serve multiple simultaneous functions. These include soil erosion control [4] and soil improvement (e.g., N fixation, adding OM, enhancing soil structure) [5] They also act as climate change mitigators (e.g., reduce GHG emissions, sequester C), improve water quality (e.g., reduce water runoff, increase water infiltration) [4,6] and aid nutrient management [7]. Biologically, cover crops play roles in controlling weeds [8], pests and diseases [9]. The objective of this review is to summarise these sowing objectives to provide guidance on how to prioritise species and management regimes whilst critically evaluating the supporting evidence for this and what the limitations are for these practices.
2. Methodology
A systematic literature search was conducted on the Web of Science, ScienceDirect, Scopus and Google Scholar databases with a combination of subject headings, abstracts and keywords including “cover crops”, “cover crops” yield, “cover crops” biofumigation. “cover crops” weeds, “cover crops” pests, “cover crops” nitrogen uptake, “cover crops” leaching reduction “cover crops” water, “cover crops” soil structure, “cover crops” soil health, “cover crops” soil biology. The search of “cover crop” returns 6111 articles in Web of Science and 11,295 in ScienceDirect.
In total, 730 peer-reviewed research articles, books and reports published from 1991 to 2024 were collected and imported to Endnote software (Endnote X9). These articles were further screened based on the title and abstract, followed by a full-text review. The final selection of articles fulfilled the following criteria: (1) the study included both “cover crop” and keyword, (2) the study reported the information based on the search words and (3) the full text must be available.
3. Cover Crops and Biological Soil Health
Soil health is “the capacity of a soil to function as a living system, with ecosystem and use boundaries, to sustain plant and animal productivity, maintain or enhance water air quality, and promote plant and animal health” [10,11]. Multiple authors agree that modern agriculture has largely ignored stewardship of this most important asset [12,13,14]. Cover cropping provides the means to improve or at least maintain soil health. However, soil health requires a range of biological, chemical and physical measurements, and in a commercial setting, this needs to be evaluated alongside the impacts on the main crop’s yield and quality [14]. Examples of cover crop systems are shown in Figure 1.

Figure 1.
Examples of cover crop systems suitable for Northern Ireland. (A) Westerwolds grass growing as a cover crop. (B) Forage rape providing total soil coverage over winter. (C) Phacelia growing, which was sown at the same time as the cover crops in the picture. (D,E) Cover crops of phacelia, westerwolds and tillage radish sown mid-September 2018 and pictured mid-October. (E) An aerial shot of two fully randomised and replicated field trials investigating cover crop response to the slurry and the effect of sowing date on cover crop performance. (F) The final sowing date is 27 September 2018 and being sown using a plot sower.
3.1. Improving Soil Organic Matter
Organic matter contains the living and functional medium of the soil and is arguably the predominant fraction of soil that is key to determining its quality, productivity and functionality [15]. The benefits of increasing and maintaining soil organic matter levels are globally accepted for both soil health and to help mitigate against the anthropogenic release of carbon dioxide through sequestration into soil C [2,16]. Increasing soil organic C stocks annually by 0.4% would help offset the anthropogenic release of carbon dioxide into the atmosphere, which is the aim of a global initiative [17].
Cover crops add C and N to the soil through its residue being returned to the soil [2,18] and thus contribute to the sequestration of C [2]. However, Sapkota, Askegaard [19] found that cover crops alone were unable to offset the C loss caused by tillage in the continuous production of spring crops in Denmark. However, Peng and Van Eerd [20] found significant increases in soil C below 15 cm in response to long-term cover cropping. Stable organic matter is relatively slow to change, whereas microbial biomass C responds very quickly to management practices. This means that biological indicators, including microbial biomass and enzymatic activity, are more effective measures that could signal long-term effects [21].
Soil biological activity in tillage crops is difficult to maintain due to the detrimental effect of soil disturbance [22]. Sapkota, Askegaard [19] compared N applied as inorganic fertiliser (conventional practice) and organic (N applied as pig slurry 56–65 kg N/ha) along with the effect of cover crops on soil C changes. This was conducted over two years, with results simulated over 30 years to estimate long-term changes in soil total carbon (C) with spring barley (Hordeum vulgare L.). Mineral fertiliser without cover crops had the highest rate of C loss (342 kg C/ha/year). This loss was reduced to 92 kg C/ha/year when fodder radish (Raphanus sativus oleiformis L.) was grown. While a ryegrass (Lolium perenne L.) cover crop increased total soil C by 25 kg C/ha/year, the organic/pig slurry fertiliser treatment produced a much smaller reduction in soil C % of 91 kg C/ha/year [19]. When cover crops were incorporated, soil C accumulated faster than with the inorganic fertilisers. Slurry and fodder radish management added 183 kg C/ha/year, while ryegrass added 407 C/ha/year. Furthermore, the experiment conducted by Sapkota, Askegaard [19] applied slurry at relatively low rates (supplying between 56–65 kg/ha N in each trial year), which could be sustainably increased to maximise C inputs [23].
3.2. Soil Microbial Activity and Microbial Biomass Enhancement Through the Use of Cover Crops
Soil microbes play an important role in nutrient cycling. Many are highly dependent on the symbiotic relationship with plants directly through root exudates and indirectly from root hair cells, sloughed-off root cells, mucilage and decomposing plant residue/rhizodeposition, which can be as high as 50% of net photosynthesis [24,25,26]. Haney, Haney [27] found that microbes sequester C, N and P in a ratio of 100:10:1, where their growth response to labile C inputs can cause them to be significant sinks of nutrients such as N [28]. This can cause immobilisation as they can compete with roots for nutrients [29]. Therefore, replacing fallow with actively growing plants could be an effective way to support and diversify the microbial community in comparison to fallow land.
Soil microbial biomass is affected by many factors: soil type, rainfall, temperature, soil pH, cation exchange capacity and underlying organic matter of the soil [30,31]. It is also affected in the short term by species of plants growing and energy-rich plant residues [32]. The input of organic matter has been well documented to influence soil food web dynamics and, thus, microbial biomass [33,34]. Cover crops influence the soil microbial community through individual species/family capabilities to modify the rhizosphere during the growing phase due to root structure and root exudates [35]. Upon breakdown and nutrient release, soil microbes can also modify the microbial community diversity and size [36]. Brennan and Acosta-Martinez [26] speculated that the primary reason cover crops were found to increase microbial biomass was due to the added input of labile C from root exudates in the cover crop compared to bare fallow. Furthermore, the C:N ratio of the residue has been noted to change the specific community structure [37]. Bossuyt, Denef [38] found that fungi favour lower quality (high) C:N ratio residue, whereas bacteria are better at decomposing low C:N ratio materials. Brennan and Acosta-Martinez [26] found that a rye (Secale cereale L.) cover crop with a C:N ratio of 31 increased the saprophytic fungi, whereas the other systems/crops had no effect as the C:N ratio was much lower, such as mustard (Sinapis alba L.) with a ratio of 22, and a legume mixture with rye with a ratio of C:N = 21.
Plants are dependent on microbes for nutrient acquisition and form symbiotic relationships through the infection of roots, e.g., mycorrhizal fungi [39]. Other mechanisms include plant release of root exudates tailored to culture-specific/beneficial microfauna. These organisms can mineralise unavailable soil-bound nutrients through enzymatic processes and alteration of soil pH [27]. Plant species have an effect due to their root architecture/structure, associations with the microbiome and the quantity and quality of the organic matter returned to the soil [40]. This suggests that cover crop selection could be a beneficial way to improve the microbial community function for enhancing the performance of subsequent commercial crops [35]. Chavarría, Verdenelli [41] experimented using cover crops of oats, vetch and radish in mixtures, oat/radish and oat/radish/vetch (Avena sativa L./Raphanus sativus L./Viciasativa L.) and determined microbial activity through phospholipid fatty acid measurements. The phospholipid bilayer is a component of all cellular membranes and allows the determination of the abundance of bacterial, fungal and protozoan groups [42]. The legume (vetch) produced a significantly higher concentration of phospholipid bilayer extract compared to the oat/radish mix and the control. Furthermore, these treatments significantly (p < 0.05) increased the enzymatic activity of esterase, dehydrogenase and acid phosphatase over the control. These enzymes are important for supplying both N and P to plants. Chavarría, Verdenelli [41] found that, on average, cover crops increased the overall microbial biomass by 19.3% whilst also increasing enzyme activity by 14.6–20.4% compared to a control of bare stubble. Similarly, Torabian, Kim [43] found that cover crops increased MBC by up to 41% in unfumigated soil. A meta-analysis found that, on average, cover crops increased soil microbial biomass C by 19.5% [40]. Piotrowska and Wilczewski [21] conducted an experiment to investigate the enzyme activity under two types of cover crops, oilseed radish (Raphanus sativus var. oleiferus L.), field pea (Pisum sativum L.) and an unplanted control (fallow). Subsequently, five rates of N fertiliser (0, 40, 80, 120, 160 kg/ha) were applied to the following crop of spring wheat (Triticum aestivum L.). The sampling period was prior to the sowing of spring wheat (after the cover crop) and again after the harvest of the spring wheat. Enzyme activity decreased by 10–26% at 160 kg N/ha and was highest in the range of 40–80 kg N/ha in the spring wheat. The field pea also produced significantly higher enzymatic activity compared to that of oilseed radish. Cover crops have significant effects on soil enzymes, which can represent overall biological activity. Furthermore, there is an interaction with N-fertiliser. Piotrowska and Wilczewski [21] concluded that cover crops and moderate mineral fertilisation of nitrogen are effective ways to increase the soil’s biological activity.
3.3. Arbuscular Mycorrhizal Fungi (AMF)
Intensive tillage practices, monocultures and seasonal/over-winter fallow periods combined with inorganic fertiliser applications have been reported to reduce arbuscular mycorrhizal fungi (AMF) [44]. These obligate mutualists are of high agronomic value and importance to such host plants through providing protection from pathogens, reducing abiotic stress and facilitating nutrient uptake like potassium [45] and particularly P through maximising the volume of soil contact with the roots through the beneficial fungal hyphae infection of the roots [44,46,47]. The AMF interface allows for the exchange of nutrients, signalling molecules and other protective chemical compounds, which, in turn, benefits the host plant [48]. In addition, AMF can increase antioxidant levels and assist the host plant in the protection from environmental stress [25,49]. As AMF requires a host plant, fallow can reduce AMF populations [44]. Therefore, cover crops provide a mechanism for reducing the winter fallow period and would help plants to sustain and propagate AMF development through the supply of organic exudates to the obligate symbionts, which could, in turn, lead to higher associations in the subsequent crop [25,46]. García-González, Quemada [50] reported that a barley cover crop increased the spores of AMF in a subsequent maize crop by 42–72%, but vetch was not significantly different from the control (fallow).
Lehman, Taheri [44] experimented with three types of cover crops, i.e., forage oats, canola and vetch, along with mixture combinations on the propagules of AMF across three sites in South Dakota, USA. They measured the effect of three cover crops and their subsequent cover crop mixture combinations on the amounts of compound C16:1 cis 11 (fatty acid group contained by AMF), which was recovered in the two years following a winter cover crop as opposed to a fallow (none). Oats, either as a monoculture or included in any mixture, had a significant (p < 0.05) and consistent effect of increasing AMF. The authors concluded that, where the mean of the two years was combined at all different sites, cover crops led to significantly higher (p < 0.01) AMF propagules per gram of soil compared to that of a fallow (1.19 vs. 0.42, respectively). They also found at other sites using other Brassicaceae species (turnip, forage radish), which are non-host species of AMF, that it did not have any negative effect on AMF.
3.4. Improving Earthworm Numbers (Macro-Fauna)
Earthworm numbers are a simple, visual method of assessing soil structure and biological activity pillars of soil health. Stobart, Morris [51] found that a cover crop of brassica mixes increased earthworm numbers from 187/m3 under a fallow stubble to 487/m3 when in a plough-based system. Lumbricus terrestris is an anecic worm that creates vertical channels in the soil by coming up to the surface to feed at night. This species is important for water infiltration through soil pore creation [52]. It has been found that tillage, in particular, deep inversive tillage, can devastate this population more than others. Stroud, Irons [53] hypothesised that the use of oilseed radish as a cover crop would be associated with higher abundances of L. terrestris middens compared to non-cover cropping. The oilseed radish numerically reduced the worms’ abundance under all tillage regimes, although it was not significantly different. In another experiment, Stroud, Irons [52] found that the application of organic manures and/or straw significantly increased the abundance (p > 0.01 and p = 0.05). This concurs with other authors’ findings that manures can have a positive impact on earthworm numbers [54].
Roarty, Hackett and Schmidt [55] found that only the pea legume cover crop significantly (p < 0.05) increased earthworm abundance and biomass. However, the peas only had a crop biomass range between 56 and 80 g/m2 between the two experimental years, demonstrating that despite low growth, the peas had considerable effects on the soil fauna. Schmidt, Clements and Donaldson [56] also found that legumes supported a much higher earthworm biomass and abundance compared to a wheat cover crop. Legumes are thought to support higher populations, as the residue returned to the soil provides a better quality and continuity of food for the earthworms.
4. Physical Aspect of Soil Health
Physical aspects of soil relate to how soil is structured, the correct mix of air, water and soil. These are important to maximise soil function and productivity, sustainability and minimise soil erosion and nutrient leaching [2,57].
4.1. Enhancing Soil Bulk Density Using Cover Crops
The use of heavy machinery, intensive cropping, short rotations and inappropriate soil management has led to compaction becoming a global problem [58,59]. Soil compaction is defined as “the process by which soil grains are rearranged to decrease void space and bring them into closer contact with one another, thereby increasing bulk density” [60]. This reduces air porosity and air permeability, resulting in impeded plant growth and water drainage. Soil strength and aeration are dynamic parameters affected by soil structure, texture and water content [61]. Furthermore, compacted soil is less able to drain due to pore channels being squeezed together [59]. This increases the risk of further degradation of soil structure. Lund and Elkins [62] found that Bahia grass (Paspalum notatum L.) pastures helped relieve soil compaction and led to an 80% increase in cotton productivity. The beneficial effect of the cover crops comes from both the roots and the organic matter, which favour increased biological activity modifying soil structure through better aggregation [63]. Root morphology is important; when growing through soils with unfavourable bulk densities (compaction), thicker roots can exert a higher penetrative pressure [64,65]. However, [62] observed that plants with extensive fine roots were better able to deal with compacted soil.
Chen, Weil and Hill [66] investigated how forage radish (Daikon), rye, rapeseed and a control of no cover crop affected air permeability at three different levels of compaction (bulk densities)—high 1.7 g/cm3, low compaction 1.55–1.54 g/cm3 and medium compaction between these two values. Significant differences were found (p < 0.05) between treatments, where cover crops that developed a large tap root (forage radish and rapeseed) could grow through the compacted layers and positively modify the soil structure by increasing air permeability, as found by Chen and Weil [59] in a prior experiment. At high compaction, the brassicas (tillage radish and rapeseed) facilitated significantly higher soil air permeability compared to rye or no cover crop, demonstrating a considerable species effect. At medium and low compaction, the rye was the only species to significantly increase air permeability over the control. This was due to its extensive rooting.
The process of roots growing can loosen the soil and decrease bulk density and penetration resistance [66]. Cover crops could play a vital role in relieving compaction and reducing risk in a high-rainfall climate. A living, growing cover crop over winter will modify the soil structure through roots loosening the soil, as well as upon death, to leave channels through the profile, especially those species with taproots as a process of bio-drilling as they are least affected by soil compaction compared to oilseed rape (Brassica napus L.) and rye when used as a cover crop [67]. Forage/Daikon radish is a widely used cover crop in America because it is renowned for its effect on soil structure. It has a large taproot that can grow to between 12 and 20 inches deep, effectively bio-drilling through the compacted soil [66]. It is effective in the long term in mitigating against compaction as these tap roots rot and can leave a channel down below the ploughpan. However, Welch, Behnke [68] investigated the effect of cover crops on soil physical qualities, e.g., bulk density, penetrative resistance and water aggregate stability, between headlands and non-headlands of fields and found that cover crops did not change any soil physical structure parameters on the more compacted headland (1.32 Mg/m3 vs. 1.28 Mg/m3 and 1891 kPa vs. 1625 kPa) on the non-compacted headland areas. The species used were forage radish and combined mixtures with buckwheat (Fagopyrum esculentum L.), hairy vetch (Vicia villosa L.) and cereal rye. In addition, a meta-analysis of 33 papers found that cover crops had a weak effect in terms of reducing soil bulk density [40].
4.2. Enhancing Aggregate Stability
Aggregate stability is important to maintain a porous structure [22,69]. Aggregates are crumbs of soil particles enmeshed together by roots and their exudates, e.g., glomalin. Resistant aggregates are more able to resist physical stress, e.g., moisture (slaking) traffic from farm machinery, and reduce the soil’s susceptibility to surface crusting as well as compaction [70]. Oades [71] found that roots, root hairs and fungal hyphae create an extensive network, which physically enmeshes fine particles of soil into aggregates even after their death. As part of nutrient acquisition, both roots and fungi exude mucilaginous polysaccharide materials that act as gluing agents, which bind soils to form aggregates [22]. The total amount of organic C released by plant roots is highly dependent on root mass [72]. The rhizosphere bacteria also exude polysaccharide and phenolic compounds which, when combined with fungal hyphae, are highly effective in binding soil [22,73]. Arbuscular mycorrhizal fungi exude glomulin that acts as a glue to bind soil aggregates. It has been found that there is a positive linear relationship between the amount of glomulin produced and the stability of soil aggregates [74].
Stone and Buttery [5] found amongst nine grasses and forages that those with the most extensive roots exhibited the greatest improvement in aggregate stability. Haynes and Beare [22] found in a pot experiment that Italian ryegrass had a significantly greater root mass (48 kg/m3), root length (cm3) and microbial biomass C (µg C/g) than any other treatment (control, wheat, barley prairie grass, white clover (Trifolium repens L.) and lupin (Lupinus luteus L.)). In terms of aggregate stability, Italian ryegrass was the only species to significantly (p < 0.05) increase this parameter over the control. The lupin had the greatest overall effect on aggregate stability, but there was no difference in aggregate stability when measured at field moisture at the time of sampling (35% moisture content) compared to when the samples were dried. Dai, Feng [75] found that cover crops of peas and rye improved aggregate stability. Abiven, Menasseri and Chenu [70] found in a meta-analysis that the addition of manures and organic material had significant positive effects on aggregate stability, which provided longer-lasting benefits compared to cover crops. Manures increased aggregate stability over the long term, e.g., 4 years in an experiment by Celik, Ortas and Kilic [76]. Integrating manure/slurry and cover crops with extensive roots could be very beneficial to soil structure.
5. Enhancing Soil Fertility
Soil fertility can be defined as the capability of soil to supply nutrients to crops. In agricultural systems, soil fertility is highly reliant on synthetic fertilisers, lime and manures [77]. It is estimated that between 50% and 70% of applied N is lost to the surrounding environment [78]. N is the prevalent macronutrient in short supply and, along with its low nutrient efficiency, is, therefore, a key target in nutrient management to enhance overall soil fertility and, thus, sustainability. Other highly limiting macronutrients are phosphorous and potassium.
5.1. Increasing Total N Supply to the Soil Using Legumes
The beneficial symbiotic relationship legumes exploit through supplying carbon (C) compounds to proteobacteria allows additional plant-available N to be derived [79]. It is estimated that 22.8 g of glucose is required for each gram of N2 reduced to plant-available nitrogen for both the reaction including construction and maintenance of the root nodules. This equates to 25–33% of all C fixed through photosynthesis [80]. Büchi, Gebhard [81] planted 19 legumes as cover crops at two sites in Switzerland at the beginning of August after a crop of wheat and subsequently harvested the biomass at the beginning of November. They recorded significant differences in growth amongst species (p < 0.001) and, importantly, atmospheric-derived N (p < 0.001) across the two experimental sites. This demonstrates that, despite declining heat and light conditions, some species are better suited to those unique conditions than others. It is important to choose the best cover crop if this is the objective. The Faba bean (field bean) (Vicia faba L.) fixed considerable amounts of N, averaging 150 kg N/ha, and had the highest biomass yield of 5.49 t/ha (averaged across the two sites). The mean temperature over the three-month growing period was 15.8 °C for Changins (C) and 13.9 °C for Zollikofen. The Faba bean derived 172 kg N/ha from atmospheric N2 at the site with the highest mean temperature compared to the other site of 129 kg N/ha. However, this research was undertaken in Switzerland and will have limited transferability to many other regions due to climatic variation. Regional research to find the best-suited legume is paramount. A study conducted by Roarty, Hackett and Schmidt [55] in Carlow (Ireland) found that peas had the lowest biomass yield at 56.7 g/m2 (0.567 t/ha) compared to mustard, which yielded 360 g/m2 (3.6 t/ha). However, in the experiment by Büchi, Gebhard [81], peas yielded 5.52 t/ha and 4.46 t/ha, i.e., almost a tenfold difference. This is important as Li, Sorensen.P. [82] found that 24 kg of N was fixed per tonne of DM in a mixture of red clover (Trifolium pratense L.), winter vetch and perennial ryegrass-clover. Carlsson and Huss-Danell [83] indicated similar rates, and Herridge, Peoples and Boddey [84] found that biological N fixation was around 15–25 kg N/t of dry matter.
Leguminous cover crops should be planted in a mixture with a non-legume to protect against leaching [85]. Such mixtures have resulted in greater N fixation, possibly because of greater competition for N [86,87,88]. This competition has been reported to increase the productivity of the cover crop [89]. Legumes do not reduce leaching potential due to increasing residual nutrients [85], but, when sown in a mixture, this leaching risk is reduced as a result of absorption by a non-legume crop [90]. For this reason, a sole legume cover crop is prohibited by Danish regulations, and a maximum percentage of legumes in mixes is stipulated [85]. The considerable potential of legumes to fix N and accumulate biomass requires the suitability of these species to soil type and regional weather conditions.
5.2. Increasing N Efficiency Through Reducing N Losses from Leaching
A review for the region of Finland and Southern Scandinavia found that cover crops reduce N leaching by a mean of 43% [85]. A red clover cover crop, however, increased N leaching by 62% due to it being a legume and fixing additional N. A perennial ryegrass cover crop on clay soil led to a reduction in N leaching by 85–89% or 36–51 kg/ha [85]. Oilseed radish has been reported to reduce leaching by up to 96–97% [91]. Other studies reported leaching reductions of 50% [92] and 59% when legumes and crucifers were integrated [87]. Meisinger, Hargrove [93] found in a literature review of studies using lysimeters that cover crops, on average, reduce NO3− leachate concentrations by 20–80% compared to where no cover crop is planted. In addition, those authors stipulated that brassicas are two- to threefold more efficient in terms of N leaching reduction compared to legumes. This literature review focused on studies conducted in the USA. However, in Europe (Ireland), Hooker, Coxon [94] found similar results as [93], whereby a mustard (brassica) cover crop reduced NO3− concentrations in soil solution by between 38% and 70%, in comparison to fallow. Later studies from the same authors found that mustard reduced NO3− concentration in soil solution, whereby the authors stipulated that tilling of soils in autumn to encourage natural regeneration of weeds also reduced NO3− concentration in soil solution by 70% [95]. Additionally, the cover crop of mustard reduced NO3− concentrations in groundwater [96].
The extent of N leaching reduction is dependent on the N uptake potential of the cover crops [28,88], which is a combination of management practices and the species used [2]. Delayed cover crop sowing can decrease their effectiveness in reducing N leaching [92,97]. To reduce N leaching, it is, therefore, critical to maximise cover crop biomass to increase total sequestered N [28]. Kumar, Thomsen [98] found that per day, delayed sowing of covers reduced their NO3− leaching reduction potential by 0.8 kg N/ha. Delayed sowing is also significantly affected by N uptake of the cover crops. The cover crops used were fodder radish, phacelia and oats. The authors found that early sowing of fodder radish was the best option to reduce leaching of NO3−.
5.3. N Accumulation by Cover Crops
Over winter, low evapotranspiration potential combined with high precipitation leads to excess water percolating and draining through the soil profile and the loss of leachable nutrients [85]. Cover crops deplete the soil N reserves and trap nutrients in physical biomass, thus offering a strategic and effective way to reduce leaching. Maximising cover crop N uptake and retention in biomass have real potential to reduce leaching and thus retain nutrients within the plant-soil system for the subsequent crop. Deep, extensive roots maximise the volume of soil the plant is in contact with, which allows greater nutrient extraction and better potential to reduce nutrient leaching. Lost nutrients can be defined as those below root level where species with deeper roots can effectively lift these nutrients back into the rhizosphere of a shallower-rooted commercial crop [99].
Teixeira, Johnstone [92] outlined the following factors that influence N uptake in a cover crop:
- Species
- Nutrients available (Soil mineral N)
- Light and temperature
- Biomass potential
- Planting date.
Species differ in growth habit, biomass accumulation and relative N content. Table 1 shows the variability in biomass accumulation and, thus, N accumulation in a number of studies. The brassica family appears to have the greatest potential for N uptake, with reported accumulation from studies ranging between 30 and 127 kg N/ha [7].
The brassica family typically exhibits larger biomass yields and N uptakes compared to many other species or families due to greater growth and N acquisition rates [100]. It is suggested that many studies may be inaccurate in estimating N sequestration/uptake by cover crops as a result of roots not being analysed [87]. Kanders, Berendonk [101] found that brassicas can leave 4.6–10.3% of total N as rhizodeposits in fractions including root hair cells, root exudates, border cells and sloughed-off root cells and suggested that many of the preexisting studies do not take this into account under-estimating uptake of N. Furthermore, In ’t Zandt, Fritz and Wichern [28] found a considerable N uptake within the microbial biomass due to the roots of the cover crop supplying C in comparison to fallow despite not finding increased microbial biomass.

Table 1.
A summary showing results of multiple studies on species investigated and biomass, N uptake and effect on N leaching.
Table 1.
A summary showing results of multiple studies on species investigated and biomass, N uptake and effect on N leaching.
| Species | Biomass (t/ha) | N Uptake (kg/ha) | % N Leaching Reduction | Notes | Region | Author |
|---|---|---|---|---|---|---|
| Mustard | 2.05 | 36.9 | Switzerland | [102] | ||
| Pea | 2.68 | 76.5 | ||||
| Oat | 2.38 | 31.3 | ||||
| Phacelia | 1.78 | 31.1 | ||||
| Chicory | 1.39 | 25 | 71 | The deep roots were effective in depleting soil N from further down the soil profile | Switzerland | [102] |
| Fodder radish | 2.00 | 45 | 79 | |||
| Ryegrass | 1.05 | 22 | 67 | |||
| Oilseed radish | 2.6 | 57.3 | 96–97 | Starter fertiliser resulted in larger K uptakes 76.8 vs. 90 kg/ha (p < 0.05) | Switzerland | [102] |
| Oilseed radish + starter fertiliser | 2.8 | 79.4 | ||||
| Hairy vetch | 154 | Multiple sources | Switzerland | [102] | ||
| Rye | 30–61 | |||||
| Crimson clover | 28 | |||||
| White mustard | 57–116 | |||||
| Oilseed radish | 70–127 | |||||
| Mustard | 46 | 63 | Initial soil mineral nitrogen (SMN) 20 (kg/ha) | France | [103] | |
| Ryegrass | 44 | 59 | ||||
| Vetch | 61 | 32 | ||||
| Mustard | 62 | 51 | Initial soil mineral nitrogen (SMN) 60 (kg/ha) | |||
| Ryegrass | 56 | 47 | ||||
| Vetch | 56 | 24 | ||||
| Mustard | 80 | 48 | Initial soil mineral nitrogen (SMN) 80 (kg/ha) | |||
| Ryegrass | 70 | 43 | ||||
| Vetch | 54 | 22 | ||||
| Stubble | English site (Telford) | England | [104] | |||
| Oilseed radish | 66 | Equivalent to applying 40–60 kg N/ha | ||||
| White mustard | 57 | |||||
| Winter rye | 48 |
5.4. Integrating Organic Manures to Increase Biomass and Nutrient Assimilation
Low soil mineral N (specifically nitrate) is critical to ensure a high N retention when integrating cover crops into an arable system [7]. This is not always possible when slurry is integrated into arable systems. The increasing abundance of organic manures will require more measures to minimise their environmental risk whilst maximising their economic potential [105,106]. Parkin, Kaspar and Singer [107] found that using three rates of pig slurry (0, low and high) in a pot experiment improved growth in the cover crop of rye, increased N uptake and subsequently reduced N leachate compared to relative fallow (unplanted) controls. Thilakarathna, Serran [108] found biomass increases from applications of pig slurry in perennial ryegrass (130–110%), oilseed radish (130%) and oats (90–110%), with legumes exhibiting a lower (25%) response. Biomass ranged from 0.08 to 2 t/ha across the autumn cover crops between two different sites. All species, apart from oilseed radish at one site, significantly (p < 0.05) increased N uptake in response to the 0, 134 and 266 kg N/ha supplied through the slurry. N recovery from the slurry was between 20% and 25% for perennial ryegrass, 15% for oilseed radish and 0–3% for red clover. Only one of the two experimental sites exhibited significant effects, with the site that did not respond producing 6–44% less biomass than the responsive site due to dry weather causing poor establishment. Cottney, Black [109] found that species including tillage radish, oilseed radish, brown mustard and forage rye could significantly increase their N uptake in response to slurry due to increased biomass but also concentration of N percentage. In Italy, it was found that prep-planting N increased the biomass and N uptake of the cover crops [110].
Everett, Wilson [111] found in an experiment, in Minnesota (USA) across 19 test sites, that when either pig or dairy slurry was injected into a cereal rye cover crop in the autumn, soil nitrate (NO3−) was reduced on average by 36% compared to fallow where N in biomass ranged from 5 to 114 kg N/ha and that the rye did not reduce, the commercial crop, maize (Zea mays. L) grain yield. Singer, Cambardella and B. Moorman [112] found that by incorporating slurry at 3 rates (112, 224 and 336 kg N/ha) and a cover crop of 70/30 rye/oats, N uptake was unaffected. They acknowledged that this was because of low biomass growth primarily caused by the slurry being applied post-establishment of the cover crop (causing damage) and that the control (no slurry) accumulated the most biomass. Cover crop growth was very low in this experiment, ranging between 373 and 256 kg DM/ha. In the second year’s replication, growth was higher, which caused significant (p < 0.05) increases in N uptake in response to slurry, whereas the high rates (224 and 336 kg N/ha) of slurry increased biomass over a low rate (112 kg/ha) (p < 0.05). The cover crops in this study were established prior to the cash crop harvest and the applications of deep-injected slurry resulted in damage to the cover crop. Strategies to avoid damaging the cover crop are important. In regions or rotations with longer growing periods, applying the slurry pre-planting rather than post-planting could be a better practice, reducing damage and increasing biomass and nutrient uptake.
5.5. Enhancing N Supply from the Cover Crops to Commercial Crops
The rate of residue decomposition dictates the nutrient availability to the subsequent commercial crop. Mineralisation of N is the process whereby organic matter is transformed into plant-available N species, including ammonium and nitrate, by soil microbes [113]. The multifactorial process is dependent on many factors, as shown in Figure 2 [7,114,115,116].
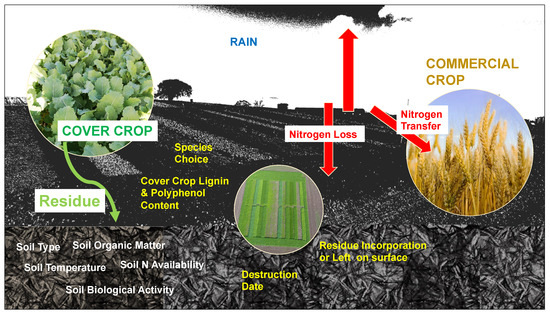
Figure 2.
Factors affecting nitrogen (N) supply from cover crop to commercial crop.
Rapid nutrient release from the breakdown of cover crop residue could lead to reduced fertiliser expenditure in the subsequent crop [12]. Mineralisation of N is related to the amount of organic N within residues, where the C:N ratio is the simplest method to compare breakdown [117]. Slow mineralisation could result in the immobilisation of N due to increased microbial activity sequestering N in the microbes and reducing soil-available N to the subsequent crop. This could decrease yield, as was found when using a rye cover crop over winter followed by a commercial crop of corn [113,118].
The rate of mineralisation can be summarised as follows: Total N accumulated x Speed of breakdown = Total N supplied [7]. It is dependent on a range of factors, such as soil temperature, soil moisture, soil texture, C:N ratio of the residue, SMN, particle size of residue, proportion of structural compounds and amount of soil contact [119]. This causes considerable difficulty in estimating supply to the commercial crop to effectively supplement its nutrient requirement. N mineralisation models of cover crops do concur that C:N ratio is the most indicative measure to estimate breakdown [87,117,120]. Couëdel, Alletto [87] used the following equation to estimate N mineralisation:
N mineralised = N acquired × [0.72 − (26.57 × C:N/1000)]
where [0.72 − (26.57 × C:N/1000)] = N % avail
However, the equation only compares the breakdown of N between species and is relatively inconclusive to indicate N fertiliser strategies on-farm. This arises from the inability to predict the weather over the growing season, which affects many dynamic parameters that influence mineralisation [121]. This limits knowledge of the N fertiliser regime to implement post-cover crops and how to manage the commercial crop for maximal yield without overusing N fertiliser. Therefore, cover crop N mineralisation could be estimated using field trial plots to obtain the commercial crop N offtake following different cover crops and in comparison to plots that have remained fallow over winter. Poffenbarger, Mirsky [122] found that hairy vetch, a leguminous species, reduced the subsequent corn N requirement considerably, but the cover crop was unable to meet the N requirement of the subsequent corn crop unless it received additional N in the form of pelletised poultry litter. Similarly, De Notaris, Peixoto [88] found that in Denmark, legume cover crops could reduce subsequent crop N requirement by 50–100 kg/ha and were significantly correlated to crop biomass.
Wagger [123] found that when the C:N ratio of the cover crop biomass increased from 27 to 38, mineralisation of the residue N dropped from 33 to 12%. Ranells and Wagger [124] found that N release rates for the following species followed the order rye < rye/crimson clover (Trifolium incarnatum L.) < rye/hairy vetch< = crimson clover < hairy vetch. The N release (kg/ha) in an 8-week period amongst these species was 24 (rye), 48 (rye/crimson clover), 60 (crimson clover), 108 (rye / hairy vetch) and 132 kg N/ha (hairy vetch). Wagger [123] found that rye released 50% of N accumulated compared to hairy vetch, with crimson clover releasing 75–80% due to its higher N % combined with its lower C:N ratio. Silgram and Harrison [120] in a study using winter rye, barley and forage rape, white mustard, stubble turnips (Brassica rapa oleifera L.) and phacelia (Phacelia tanacetifolia L.) drilled between August and October and destroyed from December to March, found that net mineralisation occurred within 8 weeks. It was estimated that between 25% and 33% of the total cover crop N was released into PAN. Silgram and Harrison [120] concluded that a C:N ratio of the cover crop biomass must be below 25–30 and N % of 1.4–1.8 for net mineralisation in the first year. Silgram, Williams [104] found that rye, mustard and fodder radish took up between 48 and 66 kg N/ha, and release to the subsequent crop of potatoes (Solanum tuberosum L.) was found to be equivalent to 40–60 kg N/ha. This large release of N as a percentage of total uptake could be due to the intense tillage required for potatoes, with residue being finely chopped and mixed with soil. Specific nutrient releases from cover crops from research findings have limited transferability due to specific edaphic and climatic interactions, as well as seasonal effects. However, the fundamentals of management practices and strategies to enhance nutrient release are transferrable to various climates.
Early destruction of the cover crop will reduce increased lignin, hemicellulose and pectin through the plant reaching maturity, which increases the C:N ratio [103]. The most important factor affecting the C:N ratio and nutrient breakdown, apart from species, is the percentage N of the cover crop [117]. Species high in N are more desirable to increase nutrient cycling for the subsequent crop by maintaining a lower C:N ratio [87]. Supply of N from the cover crop to the commercial crop is the function of total cover crop N uptake multiplied by the release coefficient [87], where both factors can be managed. Therefore, taking steps to maximise N uptake and the identification of optimum species (large growth potential, low C:N ratio) will help increase breakdown. Furthermore, management practices must be conducive to facilitating the increased breakdown of residue and, thus, nutrient supply. This will provide the commercial crop with a significant amount of N from the cover crop and could reduce the total inorganic N requirement of the commercial crop.
5.6. Phosphorous (P) Accumulation in Cover Crops and Enhancing Availability to Commercial Crops
Cover crops are net accumulators of P, storing it in their biomass. However, the effect of this differs depending on whether the soil is enriched or depleted/low in P [125,126,127]. The latter is of more interest to soil fertility as P will be a limiting nutrient, with potentially adverse yield implications for the cash crops. The first scenario relates more closely to environmental considerations as high P soils increase runoff potential, i.e., pose a pollution risk to waterways. P becomes rapidly fixed to soil particles, forming unavailable complexes for uptake by plants [128]. Roots absorb P as H2PO4− which only accounts for a small quantity of soil total P, organic P (P0) representing 30–80% of the total soil P, depending on soil type [129]. P0 usually contains a single covalent bond to a C atom, mainly through an oxygen atom ester link, requiring enzymatic hydrolysis to form plant-available P. The enzymes involved are phosphatase enzymes and are classified into two groups: alkaline and acid phosphatases. Plants produce acid phosphatases and microbes produce both acid and alkaline types [128,130].
Reynolds, Ritz [128] investigated the effect of five cover crops (vetch, radish, buckwheat, oats and phacelia) compared to bare stubble on levels of alkaline and acid phosphatases during the growth of the cover crop and in the subsequent cash crop in a P index 2- soil (Defra classification). Cover crops exhibited significant effects on acid phosphatase activity (p < 0.001) during growth, with oats having the highest value, followed by phacelia. Vetch lowered enzyme activity below that of bare stubble. There was no effect on soil alkaline phosphatase within the cover crop. Measurements taken in the subsequent cash crop found significant differences in both alkaline phosphatase activity (p < 0.01) and acid phosphatase activity (p < 0.001). Phacelia and oats increased activity to the greatest extent because the vetch reduced the activity of these enzymes both during and after the growth of the vetch cover crop. Biomass of the cover crops did differ but was not the cause of the variance in enzyme activity [128]. Oats, phacelia and radish had similar levels of biomass but had contrasting effects on enzymes. The authors concluded that cover crops have positive effects on P cycling. Cover crops exuding organic acids to hydrolyse P0 may have caused the increased acid phosphatase [131]. There is a definite species effect not associated with biomass. Potentially, these can be attributed to differences in rooting structure where large, more vigorous roots in contact with a greater rhizosphere could exude more organic acids, or the species effect of the microbial and fungal community in the rhizosphere creating differences in their size/mass, which affect mineralisation and release of phosphatase.
Other mechanisms shown by cover crops to increase P cycling include some species, such as grasses, which can increase the amount of mycelia in mycorrhizal fungi in the succeeding commercial crop. This can create a larger volume of soil in direct contact with the roots of the commercial crop [41,127,132]. Forage radish (daikon) can acquire labile soil P and concentrate it in the soil surrounding the roots (max 3 cm from roots), probably due to its large taproot compared to that of grass species, which typically exhibit a multitude of fine roots. Therefore, it is hypothesised that cover crops increase overall P availability to plants as the sorption capacity of the soil is filled to a greater extent, making it more readily released to the soil solution [133]. These authors hypothesised that if the cover crop (daikon radish) was planted in rows and if the following crop was planted within close proximity to the cover crop, then increased P availability could result in increased crop yield. Cavigelli and Thien [132] reported that P uptake in a Sorghum (Sorghum bicolor L.) crop was positively correlated to the P uptake in the previous cover crop on a low P site. They concluded that the Brassicaceae species had a greater potential for total P uptake through the mechanisms of high tissue P concentration (%) and a higher biomass yield potential than many other families of species.
Cover crops, therefore, can have beneficial immediate and legacy effects on P cycling, both during growth and upon decomposition and also from the legacy of microbial community and structure [134]. The overall effects and benefits will be dependent on the underlying soil P availability being classed as either in excess or in undersupply and also the C:P ratio of the residue [134,135].
5.7. Cover Crops Can Enhance Soil Potassium Cycling
In plants, potassium (K) is commonly found in the cytoplasm and exists as either free ions or weak complexes, which release relatively quickly from decomposing tissues (40–45 days) [136,137]. Leached K can end up deep in soil (below 1.0 m in tropical sandy soils), whereby deep-rooted cover crops pull soil K up to the top of the soil profile and access both soil exchangeable and non-exchangeable pools [137,138]. This aids K fertilisation of the commercial crop due to cover crops improving nutrient cycling in soil and improving soil pools/sinks of available K [137,139]. The rate and amount of K released from cover crops is dependent on leaf tissue concentration and crop biomass [137]. Therefore, high biomass and total K uptake is important. There is currently limited work on how cover crops influence the K dynamics and its cycling in cropping systems (Echer et al. 2020) [139].
5.8. Using Cover Crops to Enhance Micronutrient Supply to Commercial Crops
Human malnutrition, called hidden hunger, from Zn deficiency alone is estimated to affect one-sixth of the global population [140]. Crops are also at risk of micronutrient deficiencies due to low total concentrations and availability. Availability is dependent on a number on a number of factors, e.g., soil type, pH, clay content, agronomic practice, moisture content and presence of other nutrients [141]. Addition of organic matter is beneficial in increasing total micronutrient concentrations and availability. Grüter, Costerousse [142] investigated the use of farmyard manure and green manures (cover crops) to increase total Zn content in wheat. The rationale is that when organic matter decomposes, soluble organic ligands, e.g., citrate, oxalate and malate, are released, as also found by Jones, Dennis [141]. These compounds can form complexes with trace elements, making them more available for plant uptake, e.g., Zn, which is bound to soil particles and unavailable. Other mechanisms include increasing beneficial soil microbes that have a symbiotic association with the host, e.g., arbuscular mycorrhizal fungi increase the volume of exploration in the soil profile by roots [143].
Cover crops can significantly increase Zn concentration in the subsequent crops when grown in Zn-deficient soils, as demonstrated by using red clover, sunflowers (Helianthus annuus L.), Sudan grass (Sorghum sudanense L.) and safflower (Carthamus tinctorius L. [144,145,146]. Grüter, Costerousse [142] found that red clover had a significantly (p < 0.05) higher total uptake of Zn compared to a white mustard cover crop (211.2 vs. 73.6 g/ha, respectively). This was because red clover had a significantly (p < 0.05) higher biomass yield and concentration of Zn within the plant. Organic manures and standard inorganic fertiliser were also investigated. There were significant differences observed in treatments using cattle manure (organic manure). In the subsequent crop, Zn uptake was higher in wheat treated with organic manures due to a lower yield and higher plant-available Zn determined by DGT (diffusive gradient in thin films). Cover crops did not influence wheat grain Zn concentration, nor was there any difference between species.
6. Weed, Pest and Disease Suppression via Cover Crops
Escalation of pesticide resistance, product removal from the marketplace and threat of chemicals entering waterways mean that there is a greater need for better-integrated crop management [8,147].
6.1. Weed Suppression by Using Cover Crops
Cover crops have the ability to suppress weeds through competition for resources such as nutrients, light and water. Certain species also release allelopathic chemicals from living or decomposing plant tissue, which creates unfavourable conditions for weed germination and subsequent competition [148]. Weed suppression requires fast-growing and deep-rooted plants [8,149]. Brust, Weber and Gerhards [8] found that the species phacelia reduced weed dry matter by 77%, whilst buckwheat led to a reduction of 96% relative to fallow, but forage radish was found to be the only species to consistently reduce weed dry matter in the eight experiments across three sites by an average of 81% (Ihinger Hof, Meiereihof and Trossin, Germany) in two different years (2010 and 2011). Corti, Bechini [110] found that legumes did not suppress weeds whilst white mustard and black oats did. Brust, Weber and Gerhards [8] outlined that mustard and oilseed radish are the predominant cover crops in Germany, with their fast emergence of 4.1 and 4.5 days, respectively, compared to the less prevalent cover crop of grain amaranth, which took 7 days. The rapid growth of the brassicas continued after germination, with quick growth and biomass accumulation being the reasons given for consistent weed reduction [8].
Brust, Weber and Gerhards [8] identified weeds in the trials, including volunteer barley and wheat, common chickweed (Stellaria media L.), red deadnettle (Lamium purpureum L.), scented mayweed (Matricaria chamomilla L.) and Persian speedwell (Veronica persica L.). These weeds are all listed as “common” in a report by the AHDB, Managing Weeds in Arable Rotations, which broadly covers the United Kingdom (UK) [150]. Cover crops susceptible to frost may die back, allowing weeds to grow and impact the subsequent commercial crop. Alternatively, in some situations, frost can be an effective and cheap strategy to terminate a cover crop.
6.2. Soil Pest Suppression by Cover Crops
Chemical control of nematodes and soil pests relies on the application of nematocides derived from organophosphates, carbamates and other fumigants. As they are extremely toxic, they can pose a risk to human and animal health. Consequently, many are withdrawn from the world market [151,152]. In response, farmers and researchers are turning to the identification of biological control, using antagonistic nematodes and plants with bio-fumigant properties [9]. The use of cover crops for pest suppression involves growing non-host species to break lifecycles and/or species high in natural compounds that can kill or reduce the viability of pests when incorporated into the soil. The brassica family, in particular, is high in sulphur (S)-containing compounds called glucosinolates that act as bio-fumigants when incorporated into the soil [9]. The glucosinolates hydrolyse in the soil to form isothiocyanates and other volatile compounds toxic to pests, which include harmful nematodes like potato cyst nematodes (PCNs) Globodera rostochiensis and Globodera pallida [153,154].
Table 2 displays a section of a synopsis filtered by research that uses field studies to investigate the effects of using cover crops on pests. The synopsis by Fourie, Ahuja [152] reports pot experiments and using the grain meal and the effects on pests. Many of the experiments were conducted using a refined seed meal applied to pot experiments. These are excluded on the basis that cover crops in the initial stage will just be incorporated into the soil. Table 2 only includes practices of green manuring, which is the incorporation of the cover crop back into the soil. The studies, given in Table 2, use species from the brassicas because they contain glucosinolates that can release toxic compounds which can affect pests, when incorporated into the soil [155]. In three separate studies, white mustard had mixed effects on the potato cyst nematode, varying from a 74% reduction in population [154], a 16% reduction in juvenile cyst levels [156] and no effects recorded by Valdes, Viaene and Moens [157] (Table 2).
Degraded soils, which are predominant in many crop production areas worldwide, do not favour a build-up of beneficial soil nematodes that contribute to soil quality and so are at an increased risk of problematic nematodes [158]. Cover crops could improve soil quality by increasing soil microbial diversity and beneficial populations and be a sustainable method of improving soil resilience. Gruver, Weil [158] found that the effect on soil nematodes when investigating brassica cover crops did not result from the allelopathic effects but rather the quality of the organic matter (C:N ratio) added back into the soil. Valdes, Viaene and Moens [157] investigated the effect of yellow mustard cover crops on Globodera rostochiensis (potato cyst nematode) and found that, whilst it did not reduce this particular nematode, it did reduce overall plant parasitic nematodes. They observed an increase in overall soil bacterivores (p < 0.001) and a decrease in herbivores (p < 0.001).
The chemicals released from glucosinolates can be toxic to entomopathogenic nematodes (EPN). EPN enter the host through natural openings and release a bacterium that often kills the host, which they then feed on [159]. Ramirez, Henderson [159] found that mustard biofumigant can affect beneficial nematodes, as was proven in the genera Steinernema. This suggests that Brassicaceae cover crops could have negative impacts on soil biology. However, a similar study by Jaffuel, Blanco-Pérez [160] found no effect on the persistence of EPN when using a pea (legume) or Indian mustard (brassica) cover crop.
Another benefit of integrating cover crops is that they may be a non-host of the pests affecting the commercial crop. This can also alter the pests’ life cycle and potentially population. However, some species are susceptible to pests, e.g., hairy vetch is a host to root-knot nematodes, and so appropriate rotations must be adopted to reduce any potential adversity to the commercial crop [161].

Table 2.
Synopsis of multiple studies investigating biofumigant potential by various cover crops and on various pests.
Table 2.
Synopsis of multiple studies investigating biofumigant potential by various cover crops and on various pests.
| Cover Crop | Application | Pest | Result | Location | Author |
|---|---|---|---|---|---|
| Brassica juncea L. (brown mustard) | Green manure | Glodera pallida (Potato cyst nematode) | Reduction in viable egg count | UK | [153] |
| Brassica juncea L. (brown mustard) | Green manure | Glodera pallida (Potato cyst nematode) | 50–95% reduction in juvenile population levels | UK | [154] |
| Brassica napus L. (Fodder rape) | Green manure | Meloidogyne chitwoodi (Root-knot nematode) | 89% reduction in population levels | USA | [162] |
| Raphanus sativus L. (Fodder radish) | Green manure | Glodera pallida (Potato cyst nematode) | Up to 100% in juvenile population levels and >95% reduction in viability of encysted eggs | UK | [154] |
| Raphanus sativus L. (Fodder radish) | Green manure | Glodera pallida (Potato cyst nematode) | 50% reduction in cyst population | USA | [163] |
| Sinapsis alba L. (White Mustard) | Green Manure | Glodera pallida (Potato cyst nematode) | 74% reduction in population levels | UK | [154] |
| Sinapsis alba L. (White Mustard) | Incorporated, fallow and unincorporated | Globodera rotochiensis (Potato cyst nematode) | No decrease | Belgium | [157] |
| Sinapsis alba L. (White Mustard) | Trap crop | Globodera rotochiensis and Glodera pallida (Potato cyst nematode) | 16% reduction in juvenile cyst levels | [156] |
Source: Adapted from Fourie, Ahuja, Lammers and Daneel [148].
6.3. Disease Suppression in the Commercial Crop by the Previous Cover Crop
It is desirable for cover crops to support mechanisms that reduce plant disease in the subsequent commercial crop through the colonisation of symbiotic biota or through the release of chemicals, which act as anti-microbial (biocides) harmful to pathogens [161]. Mycorrhizae are symbiotic fungi that live in association with the majority of plants, with the primary benefit of increased nutrient and water supply to plants. They can also contribute to pathogen suppression and, thus, host disease resistance [39]. Martinez [164] found that ryegrass (Westerwolds—Lolium multiflorum L.) was a useful tool in the management of Scleotinia sclerotiorum (White mould) as it suppressed the pathogen growth rate. This disease can affect up to 400 different species, but the important agronomic species are potatoes, oilseed rape, carrots, lettuce, peas and beans. Brassica cover crops have been reported to reduce mycorrhizae colonisation as well as being a host of Sclerotinia sclerotiorum [161]. Furthermore, brassicas should not be used as cover crops in oilseed rape rotations due to accepted yield reductions [7].
As mentioned above, glucosinolates are S-containing compounds that hydrolyse to produce toxic compounds, namely, isothiocyanate, nitrile and thiocyanate, that act as biocides in the soil [165,166]. Considerable research has been conducted to investigate the effects of brassicas on soil-borne pathogens. Results conflict with some that report decreases in both disease and crop yields, whilst others report increases [167]. The brassica genera have been studied for glucosinolate (GSL) content as it is thought that the highest concentrations would be the most effective. However, a study by Larkin and Griffin [165] showed that for Rhizoctonia solani (a soil-borne disease of many crops including potatoes, pasture and cereals), fodder rape was the most effective despite being relatively low in GSL. Brown mustard, high in GSL, was most effective for powdery scab (Spongospora subterranea) and common scab (Streptomyces scabies) control in potatoes. Contrastingly, Ref. [168] found that brassica cover crops were ineffective in reducing the abundance of Pythium spp., R. solani and S. rolfsii prior to planting red bell pepper crops. However, they did observe reduced plant seedling stunting of various cover crops compared to that of fallow.
GSL concentration and total production can be influenced by supplemented S levels and N [169]. Animal wastes, slurries and manures are a source of both N and S. Therefore, integrating cover crops and slurries could both increase concentrations and total production of glucosinolates, but no research has been conducted to confirm this.
Kirkegaard, Sarwar [170] found that mustard, in particular, Indian (brown) mustard (Brassica juncea L.), was as effective as canola (oilseed rape) at reducing Gaeumannomyces graminis var. triciti (take all) inoculum levels in the subsequent wheat crop. This was attributed to the bio-fumigant effects of the glucosinolate content in these brassicas. However, it did not affect the overall yield in the subsequent crop of wheat. Many of these studies were conducted in pots and greenhouse experiments, but field trials to examine relevance to the field situation are needed [161]. Table 3 shows a summary of some studies on cover crops and their effect on specific diseases and on the yield of the commercial crop.
Cover crops can themselves host diseases: White clover and buckwheat increased the infection and severity of bean rot, Fusarium, Pythium and Rhizotonia [161]. Management should include termination of the growing cover crop in sufficient time before the subsequent commercial crop is sown to reduce the green bridge potential. However, this is a trade-off with the brassicas, as the green material should be incorporated fresh to maximise bio-fumigant properties.

Table 3.
Summary of studies conducted on cover crops, effect on diseases and commercial crop yield.
Table 3.
Summary of studies conducted on cover crops, effect on diseases and commercial crop yield.
| Species | Pot/Greenhouse or Field Trial | Cash Crop | Diseases Observed | Effect | Author |
|---|---|---|---|---|---|
| Brassica Napus Brassica Juncea Linum usitatissimum L. (Linola) | Both | Wheat (cereals) | Gaeumannomyces graminis var. triciti (Take-all) | Greater reduction in inoculum by brassica than by non-brassica (p = 0.05). | [170] |
| Brassica juncea | Field | Sugarbeet (Beta vulgaris L.) | Rhizoctnia solani | Reduction in disease incidence 2006, 2007 and 2008 found to be p < 0.001 | [166] |
| Avena sativa (Oats) Lolium multiflorum (Ryegrass) Brassica napus Raphanus sativa Sinapsis alba Brassica juncea | Pot and field trials | Potatoes | (Field trials) Powdery scab (Spongospora subterranea) | p = 0.05 | [165] |
| Black scurf (Rhizoctnia ultium) | p = 0.05 | ||||
| Common scab (Streptomycies scabiebi) | p = NS | ||||
| Brassica napus B. juncea S. alba Vicia dasycarpa (vetch) Pisum sativum (pea) Avena sativa (oat) | Field | Tomatoes (Solanum lycopersicum L.) | Fusarium wilt (Verticillium dahliae Fusarium spp.) | NS | [167] |
| Fallow was actually better for disease reduction. |
7. Environmental Considerations for Using Cover Crops
The intensification of agriculture has been widely documented and accepted to have caused unintentional negative effects on the environment, including nutrient enrichment of waterways, and loss of wildlife abundance and diversity [12,171,172,173]. Cover crops potentially have a place as a mitigation factor to reduce soil erosion [174,175] and nutrient enrichment of waterways [85], whilst providing habitats and alternative food sources for wildlife [173].
7.1. Improvement of Wildlife Diversity in Arable Fields
The effect of cover crops on wildlife diversity has not been extensively researched. White, Holmes [7] forecast that they were likely to increase farmland biodiversity through the provision of habitat. A study in Illinois, USA, by Wilcoxen, Walk and Ward [173] found that cover crops better supported bird communities of high conservation concern by providing a more ideal habitat than bare stubble. Cover crops were found to have, on average, five times larger vegetation density compared to either maize or soybean (Glycine max L.) stubbles, thus providing greater vegetation height, which was found to increase grassland bird nesting success [173,176,177]. Cover crops increased species abundance on average from 3.9 birds/100 m (experimental field strip) to 2.3 birds/100 m (experimental field strip) under the control (fallow) treatment (p < 0.05).
Wilcoxen, Walk and Ward [173] presented three hypotheses as to why birds might have a greater preference for cover crops:
- Areas to forage
- Shelter from adverse weather
- Areas to breed.
Wilcoxen, Walk and Ward [173] concluded that extra habitat structure added to agricultural fields is of higher value for bird use, which was a similar finding in a study by Bryan and Best [178]. The sample size was too small to look at the individual or group species effect. The fact that the authors found differences in abundance between both stubbles and cover crops raises the possibility that there is also a cover crop species interaction.
7.2. Mitigating Against Pollution from Soil Erosion, Runoff and Nutrient Leaching Using Cover Crops
Soil erosion is a significant global problem that represents both a loss of nutrients and a medium critical to support all terrestrial life [4,179,180]. Soil erosion arises from both wind and rain, and the risk is escalated by modern intensive agriculture, which means that mitigation factors are essential. In the UK, the maritime climate means that rainfall is the largest soil erosion factor, and cover crops could contribute in order to provide effective control mitigation [4,175,181]. Boardman [4] found in the UK that post-harvest (fallow) fields were at greater risk of soil erosion than winter cereals or fodder turnips during the most prone winter period. The soil was most prone to rill erosion, whereby water moves in concentrated streams, dislodging soil particles and creating channels in the soil. Cover crops act as a physical barrier to rainfall by diffusing the hydraulic force from raindrops and reducing surface capping. This, combined with cover crop roots aiding the formation of soil pores, will result in reduced surface runoff and increased water infiltration and drainage [6,182].
7.3. Reducing Nutrient Loss of Phosphorous (P)
The effect of cover crops on leachate of N has already been covered. However, P represents an arguably larger problem in terms of water quality due to its eutrophication potential. P loss from agricultural land is a non-point source of pollution and represents a major environmental problem [6,183], as elevated P levels in waterways cause eutrophication and reduce water quality and biota diversity [184]. Cover crops could help reduce P loss not only through alteration of soil structure to reduce water runoff but also by increasing microbial and enzymatic activity in low P sites. This would, therefore, reduce the annual requirement of P from inorganic sources [6,183,185]. Furthermore, cover crop roots and biomass can absorb and deplete dissolved P from the rhizosphere, trapping it in biomass, which will be broken down during the growth of the commercial crop. Consequently, this would be more synchronised with the supply and demand of this nutrient for the commercial crop [85].
Zhang, Tan [6] investigated mitigation practices on how cover crops altered P loss in a field-scale study in Canada using a wheat cover crop after a soybean or maize crop. The soil had a water extractable P result of 11.3 mg/kg and a 7.49% degree of P saturation determined using the Melich-3 procedure. The soil would be classified as “low” in P, where crop yield responses would be observed if investigated on higher P sites. Amendments of either yard leaf waste compost or liquid pig compost were added to the trial areas (rates or years not given). The authors found that cover crops significantly reduced the surface water run-off volume (mm/year) in three of the four years (1999–2000 p < 0.001; 2000–2001 p < 0.001; 2001–2002 N.S; 2002–2003 p < 0.05). The cover crops reduced surface runoff volume by 33% on average. However, the opposite effect was observed in the field drains flow volume (mm/year), where cover crops significantly (p < 0.05) increased the flow in three of the four years. Averages over the four trial years showed that cover crops significantly reduced particulate P (PP) and total P (TP) compared to fallow land in the surface runoff discharge water. PP loss, overall, was reduced due to the decrease in surface runoff, minimising the amount of soil sediment eroded and displaced into waterways.
Cover crops significantly (p < 0.05) reduced PP and, more importantly, total P concentrations (mg P/L/year) in the field drainage waters. Between years, non-significant effects were also observed on dissolved P concentrations; although in some years, significant effects (p < 0.05) were observed, where P concentrations of the drainage water increased. This resulted from lower volumes of water that left the drains, which caused the increased concentration.
Zhang, Tan [6] quantified total P loss taking into account tile drainage and surface run-off volume to calculate a total P loss per hectare (kg/ha/year). Cover crops produced a significant (p < 0.05) reduction in total P loss from surface runoff of 18% compared to bare fallow. The total P loss from cover crops was reduced by 10% compared to fallow. The site used in that study had a low availability of P. Fallow land with higher soil P availability may, therefore, demonstrate larger reductions in P loss in response to cover crops. The findings on the effects of cover crops on P losses are contradictory, with a recorded change in P leachate from +86% to −43% due to plant destruction and release of P that is susceptible to freeze-thawing [85]. However, thorough consideration and selection of frost-tolerant species could overcome this problem.
Wendling, Büchi [125] hypothesised that, in high fertility systems, cover crop species with high root length and density should be used. In low fertility systems, species that display an enhanced ability to solubilise immobile inorganic P through higher root exudates should be used, e.g., buckwheat or white lupin [186,187]. Wendling, Büchi [125] found that out of twenty species, daikon radish had the highest biomass, accumulating over 42 kg P/ha. This could be a useful cover crop species in NI to store nutrients from manures. P availability to the subsequent crop after application through manures is only 50% available [188] This means that the uptake of 42 kg/ha from the daikon radish could store all nutrients provided from the highest legal application of any organic manure [188].
Trapping P in biomass suggests that cover crops have considerable potential to better synchronise P demand in the subsequent crop through increasing supply in both low and high soil P conditions. The growing cover crop can extract P as well as capture excess that may otherwise be liable to loss over winter. Therefore, they could be a valuable asset to NI’s unique conditions. This requires investigation to find species best able to accumulate large amounts of P.
8. Cover Crops and Commercial Crop Yield Improvement
Many studies have found little to no benefit of cover crops on the yield of the subsequent commercial crop [68,189]. A meta-analysis found that maize yield increased by an average of 13% following a cover crop mixture versus no cover crop [190]. However, Peng, Wang [191] found only a 2.6% increase in main crop yield following cover crops in a global review. The authors also found that legumes increased maize yields by 30–33% when N rates were reduced or the tillage system changed to a less intensive version [192]. However, Qin, Guan [193] found that non-legumes resulted in a 3.9% yield reduction in maize in the Midwestern USA. Using cover crops of forage radish and hairy vetch prior to spring barley in Estonia increased grain yields [194]. Similarly, Holland, Brown [195] found that brassica-dominated cover crops increased both spring barley grain yield and grain N %. Stobart and Morris [196] found that a legume and legume mix cover crop increased wheat yields in comparison to a radish cover crop. Abdalla, Hastings [2] found a 4% decrease in grain yields due to cover crops in a meta-analysis but found that legume or legume + non-legume mixes increased grain yield by 13% as a result of increased N supply compared to other cover crops, which can reduce availability to the commercial crop. Maize yield has been found to be negatively correlated with cover crop C:N ratio (R2 = 0.134, p < 0.0001) when investigating single species with a C:N ratio range between 12 and 40 [197]. The authors of this study found that biomass under 2.5 t/ha and C:N under 25 did not negatively affect yield. Therefore, cover crops must be managed to maintain a low C:N ratio. This can be achieved through species choice [100,198], sowing date [199] and underlying soil mineral nitrogen [117].
Cottney, Black [109] found that species of cover crops such as Ethiopian mustard (Brassica carinata L.), stubble turnips, oilseed radish, forage rape and tillage radish increased spring barley yield over the control (p < 0.05). However, it was found that when slurry was integrated with the cover crops, whilst it did not significantly increase spring barley yield, it did significantly enhance N cycling and commercial crop N offtake. Furthermore, slurry applied to oilseed radish, forage rape and tillage radish resulted in a greater apparent recovery of the N coming from the slurry in comparison to applying slurry to the control of fallow land.
9. Conclusions
This review demonstrates the numerous benefits cover crops have for the environment, sustainability of cropping and the main aspects of soil health. Unfortunately, the limitations of cover crops not only include their cost of integration into rotations but also that numerous studies demonstrate a reduction in commercial crop yield following cover crops. However, this is usually due to incorrect species choice, causing a rotational conflict and management practices not encouraging early destruction, which results in low nutrient transfer from the cover crop to the commercial crop. In addition, the region has a strong influence on the impact of cover crops on commercial crop yield. Regions of low rainfall, which limits commercial crop yields, often cite that cover crops reduce cash crop yields due to reductions in soil water availability. However, temperate climates with high over-winter rainfall, which also suffer from nutrient leaching, mean that transpiration from a cover crop and its nutrient uptake are often a benefit to reduce leaching and improve soil resilience to high rainfall and excess moisture. Correct species choice is region-dependent. Whereby each climatic area requires its own research to identify the best species and management practices to resolve their specific objectives. Therefore, regional research is warranted to get an accurate effect on cover crops.
Regardless of the objective of sowing cover crops, the biggest impact on their success is the production of high biomass. High biomass increases nutrient uptake, directly reducing nutrient leaching, resulting in greater weed suppression and more C being returned to the soil. However, the effects that cover crops have on reducing soil parasitic nematodes are currently limited to glasshouse studies. Benefits demonstrated in field scenarios are not fully integratable as it uses seed meal as opposed to specific cover crop biomass. Despite this, studies have demonstrated the pathway of how glucosinolates in brassicas break down and affect soil nematodes. Again, the transparent message is glucosinolate quantity is important, which is influenced by the concentration and biomass quantity being paramount, along with the best possible management practices of direct incorporation.
This review has found limited research on how cover crops affect soil potassium availability compared to the extensive research on N. Cover crops accumulate large amounts of potassium, whereby this is released quickly as it is held as free ions or weak complexes and could be highly beneficial for subsequent crops. There are also limited numbers of studies on how cover crops affect wildlife and the diversity of the ecosystems.
Author Contributions
P.C.: Conceptulization, Investigation, Data Curation, Writing—original draft; P.N.W.: Conceptulization, Supervision, Review and editing; L.B.: Conceptulization, Supervision, Review and editing; E.W.: Conceptulization, Supervision, Review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support was received from the Department of Agriculture, Environment and Rural Affairs: Grant No 17/1/01.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, B. Are Nitrogen Fertilizers Deleterious to Soil Health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, B.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Change Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef]
- Groff, S. The past, present, and future of the cover crop industry. J. Soil Water Conserv. 2015, 70, 130A–133A. [Google Scholar] [CrossRef]
- Boardman, J. Soil Erosion in Britain: Updating the Record. Agriculture 2013, 3, 418–442. [Google Scholar] [CrossRef]
- Stone, J.A.; Buttery, B.R. Nine forages and the aggregation of a clay loam soil. Can. J. Soil Sci. 1989, 69, 165–169. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Tan, C.S.; Zheng, Z.M.; Welacky, T.; Wang, Y.T. Drainage water management combined with cover crop enhances reduction of soil phosphorus loss. Sci. Total Environ. 2017, 586, 362–371. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Holmes, H.F.; Morris, N.L.; Stobart, R.M. A Review of the Benefits, Optimal Crop Management Practices and Knowledge Gaps Associated with Different Cover Crop Species; Agriculture and Horticulture Development Board: Cambridge, UK, 2016. [Google Scholar]
- Brust, J.; Weber, J.; Gerhards, R. Do cover crop mixtures have the same ability to suppress weeds as competitive monoculture cover crops? Julius-Kühn-Archiv 2014, 443, 422–430. [Google Scholar] [CrossRef]
- Henderson, D.R.; Riga, E.; Ramirez, R.A.; Wilson, J.; Snyder, W.E. Mustard biofumigation disrupts biological control by Steinernema spp. nematodes in the soil. Biol. Control 2009, 48, 316–322. [Google Scholar] [CrossRef]
- FAO. An international technical workshop. Investing in sustainable crop intensification The case for improving soil health. In Integrated Crop Management; Food and Agricuture Organization of the United Nations: Rome, Italiy, 2008; Volume 6. [Google Scholar]
- Doran, J. Soil health and global sustainability: Translating science into practice. Agric. Ecosyst. Environ. 2002, 88, 119–127. [Google Scholar] [CrossRef]
- Wittwer, R.; Dorn, B.; Jossi, W.; Van der Heijden, M. Cover crops support ecological intensification of arable cropping systems. Sci. Rep. 2017, 7, 41911. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Büchi, L.; Gebhard, C.; Liebisch, F.; Sinaj, S.; Ramseier, H.; Charles, R. Importance of cover crops in alleviating negative effects of reduced soil tillage and promoting soil fertility in a winter wheat cropping system. Agric. Ecosyst. Environ. 2018, 256, 92–104. [Google Scholar] [CrossRef]
- Gurjar, G.; Swami, S.; Telkar, S.; Meena, N.; Kant, K.; Kumar, R. Soil Biological Properties and Their Importance in Agricultural Production. Biomol. Rep. 2017, BR/10/17/03. [Google Scholar]
- FAO. Soil carbon sequestration. In SOLAW Background Thematic Report—TR04B; FAO: Rome, Italy, 2006. [Google Scholar]
- COP 21. 4 pour 1000 4 per 1000 Initiative 2018. Available online: https://www.4p1000.org/ (accessed on 20 February 2025).
- Sainju, U.; Whitehead, W.; Singh, A. Cover crops and nitrogen fertilization effects on soil aggregation and carbon and nitrogen pools. Can. J. Soil Sci. 2003, 83, 155–165. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Askegaard, M.; Lægdsmand, M.; Olesen, J.E. Effects of catch crop type and root depth on nitrogen leaching and yield of spring barley. Field Crops Res. 2012, 125, 129–138. [Google Scholar] [CrossRef]
- Peng, Y.; Van Eerd, L.L. Surface soil sampling underestimates soil carbon and nitrogen storage of long-term cover cropping. Geoderma Reg. 2024, 39, e00885. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wilczewski, E. Effects of catch crops cultivated for green manure and mineral nitrogen fertilization on soil enzyme activities and chemical properties. Geoderma 2012, 189–190, 72–80. [Google Scholar] [CrossRef]
- Haynes, R.J.; Beare, M.H. Influence of six crop species on aggregate stability and some labile organic matter fractions. Soil Biol. Biochem. 1997, 29, 1647–1653. [Google Scholar] [CrossRef]
- Yost, J.L.; Leytem, A.B.; Bjorneberg, D.L.; Dungan, R.S.; Schott, L.R. The use of winter forage crops and dairy manure to improve soil water storage in continuous corn in Southern Idaho. Agric. Water Manag. 2023, 277, 108074. [Google Scholar] [CrossRef]
- Dazzo, F.; Garoutte, A. Rhizosphere. In Reference Module in Life Science, 3rd ed.; Elsevier: Amsterdam, The Netherland, 2017. [Google Scholar]
- Benitez, M.-S.; Taheri, W.I.; Lehman, R.M. Selection of fungi by candidate cover crops. Appl. Soil Ecol. 2016, 103, 72–82. [Google Scholar] [CrossRef]
- Brennan, E.B.; Acosta-Martinez, V. Cover cropping frequency is the main driver of soil microbial changes during six years of organic vegetable production. Soil Biol. Biochem. 2017, 109, 188–204. [Google Scholar] [CrossRef]
- Haney, R.L.; Haney, E.B.; Smith, D.R.; Harmel, R.D.; White, M.J. The soil health tool—Theory and initial broad-scale application. Appl. Soil Ecol. 2018, 125, 162–168. [Google Scholar] [CrossRef]
- In ’t Zandt, D.; Fritz, C.; Wichern, F. In the land of plenty: Catch crops trigger nitrogen uptake by soil microorganisms. Plant Soil 2017, 423, 549–562. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Chauhan, P.S.; Bhatia, C.R. Changes in soil physico-chemical properties and microbial functional diversity due to 14 years of conversion of grassland to organic agriculture in semi-arid agroecosystem. Soil Tillage Res. 2010, 109, 55–60. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Eyakub, A.M.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef]
- Barel, J.M.; Kuyper, T.W.; Paul, J.; de Boer, W.; Cornelissen, J.H.C.; De Deyn, G.B. Winter cover crop legacy effects on litter decomposition act through litter quality and microbial community changes. J. Appl. Ecol. 2019, 56, 132–143. [Google Scholar] [CrossRef]
- Briar, S.S.; Fonte, S.J.; Park, I.; Six, J.; Scow, K.; Ferris, H. The distribution of nematodes and soil microbial communities across soil aggregate fractions and farm management systems. Soil Biol. Biochem. 2011, 43, 905–914. [Google Scholar] [CrossRef]
- Overstreet, L.F.; Hoyt, G.D.; Imbriani, J. Comparing nematode and earthworm communities under combinations of conventional and conservation vegetable production practices. Soil Tillage Res. 2010, 110, 42–50. [Google Scholar] [CrossRef]
- Finney, D.; Buyer, J.S.; Kaye, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Frasier, I.; Noellemeyer, E.; Figuerola, E.; Erijman, L.; Permingeat, H.; Quiroga, A. High quality residues from cover crops favor changes in microbial community and enhance C and N sequestration. Glob. Ecol. Conserv. 2016, 6, 242–256. [Google Scholar] [CrossRef]
- Bossuyt, H.; Denef, K.; Six, J.; Frey, S.D.; Merckx, R.; Paustian, K. Influence of microbial populations and residue quality on aggregate stability. Appl. Soil Ecol. 2001, 16, 195–208. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef]
- Hao, X.; Abou Najm, M.; Steenwerth, K.L.; Nocco, M.A.; Basset, C.; Daccache, A. Are there universal soil responses to cover cropping? A systematic review. Sci. Total Environ. 2023, 861, 160600. [Google Scholar] [CrossRef]
- Chavarría, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. Eur. J. Soil Biol. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Torabian, S.; Kim, E.; Qin, R.; Sathuvalli, V.; Gollany, H.T.; Kleber, M. Soil microbial biomass influenced by cover crop after fumigation of potato fields. Sci. Total Environ. 2025, 958, 177910. [Google Scholar] [CrossRef]
- Lehman, R.M.; Taheri, W.I.; Osborne, S.L.; Buyer, J.S.; Douds, D.D. Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil Ecol. 2012, 61, 300–304. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Kabir, Z.; Koide, R.T. Effect of autumn and winter mycorrhizal cover crops on soil properties, nutrient uptake and yield of sweet corn in Pennsylvania, USA. Plant Soil 2002, 238, 205–215. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae and terrestrial ecosystems processes. Ecology 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Burak, K.; Yanardağ, İ.H.; Gómez-López, M.D.; Faz, Á.; Yalçin, H.; Sakin, E.; Ramazanoğlu, E.; Orak, A.B.; Yanardağ, A. The effect of arbuscular mycorrhizal fungi on biological activity and biochemical properties of soil under vetch growing conditions in calcareous soils. Heliyon 2024, 10, e24820. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C(3) (Leymus chinensis) and C(4) (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef]
- García-González, I.; Quemada, M.; Gabriel, J.L.; Hontoria, C. Arbuscular mycorrhizal fungal activity responses to winter cover crops in a sunflower and maize cropping system. Appl. Soil Ecol. 2016, 102, 10–18. [Google Scholar] [CrossRef]
- Stobart, R.; Morris, N.L.; Fielding, H.; Leake, A.; Egan, J.; Burkinshaw, R. Developing the use of cover crops on farm through the Kellogg’s OriginsTM grower programme. Asp. Appl. Biol. 2015, 129, 27–34. [Google Scholar]
- Stroud, J.L.; Irons, D.E.; Watts, C.W.; Storkey, J.; Morris, N.L.; Stobart, R.M.; Fielding, H.A.; Whitmore, A.P. Population collapse of Lumbricus terrestris in conventional arable cultivations and response to straw applications. Appl. Soil Ecol. 2016, 108, 72–75. [Google Scholar] [CrossRef]
- Stroud, J.L.; Irons, D.E.; Watts, C.W.; Storkey, J.; Morris, N.L.; Stobart, R.M.; Fielding, H.A.; Whitmore, A.P. Cover cropping with oilseed radish (Raphanus sativus) alone does not enhance deep burrowing earthworm (Lumbricus terrestris) midden counts. Soil Tillage Res. 2017, 165, 11–15. [Google Scholar] [CrossRef]
- Curry, P. Some effects of animal manures on earthworms in grassland. Pedobiologia 1976, 16, 425–438. [Google Scholar] [CrossRef]
- Roarty, S.; Hackett, R.; Schmidt, O. Earthworm populations in twelve cover crop and weed management combinations. Appl. Soil Ecol. 2017, 114, 142–151. [Google Scholar] [CrossRef]
- Schmidt, O.; Clements, R.O.; Donaldson, G. Why do cereal–legume intercrops support large earthworm populations? Appl. Soil Ecol. 2003, 22, 181–190. [Google Scholar] [CrossRef]
- Chen, G. Alleviation of Soil Compaction by Brassica Cover Crops. In Geography; University of Maryland: College Park, MD, USA, 2009. [Google Scholar]
- Servadio, P.; Marsili, A.; Vignozzi, A.; Pellegrini, S.; Pagliai, M. Effects on some clay soil qualities following the passage of rubber-tracked and wheeled tractors in central Italy. Soil Tillage Res. 2001, 61, 143–155. [Google Scholar] [CrossRef]
- Chen, G.H.; Weil, R.R. Root growth and yield of maize as affected by soil compaction and cover crops. Soil Tillage Res. 2010, 117, 17–27. [Google Scholar] [CrossRef]
- Soil Science Society of America. Glossary of Soil Science Terms; Soil Science Society of America: Madison, WI, USA, 1997. [Google Scholar]
- Vaz, C.M.P.; Manieri, J.M.; de Maria, I.C.; Tuller, M. Modeling and correction of soil penetration resistance for varying soil water content. Geoderma 2011, 166, 92–101. [Google Scholar] [CrossRef]
- Lund, Z.F.; Elkins, C.B. Creating soil conditions favourable to rooting. Proc. Beltwide Cotton Prod. 1978, 91–92. [Google Scholar]
- Ren, L.; Nest, T.V.; Ruysschaert, G.; D’Hose, T.; Cornelis, W.M. Short-term effects of cover crops and tillage methods on soil physical properties and maize growth in a sandy loam soil. Soil Tillage Res. 2019, 192, 76–86. [Google Scholar] [CrossRef]
- Materechera, S.A.; Alston, A.M.; Kirby, J.M.; Dexter, A.R. Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant Soil 1991, 144, 297–303. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Foloni, J.S.S.; Tiritan, C.S. Root growth and nutrient accumulation in cover crops as affected by soil compaction. Soil Tillage Res. 2002, 85, 109–115. [Google Scholar] [CrossRef]
- Chen, G.; Weil, R.R.; Hill, R.L. Effects of compaction and cover crops on soil least limiting water range and air permeability. Soil Tillage Res. 2014, 136, 61–69. [Google Scholar] [CrossRef]
- Chen, G.; Weil, R. Penetration of cover crop roots through compacted soil. Plant Soil 2010, 331, 31–43. [Google Scholar] [CrossRef]
- Welch, R.Y.; Behnke, G.D.; Davis, A.S.; Masiunas, J.; Villamil, M.B. Using cover crops in headlands of organic grain farms: Effects on soil properties, weeds and crop yields. Agric. Ecosyst. Environ. 2016, 216, 322–332. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Oades, J.M. Mucilages at the root surface. J. Soil Sci. 1972, 29, 1–16. [Google Scholar] [CrossRef]
- Ali, W.; Hussain, S.; Chen, J.; Hu, F.; Liu, J.; He, Y.; Yang, M. Cover crop root-derived organic carbon influences aggregate stability through soil internal forces in a clayey red soil. Geoderma 2023, 429, 116271. [Google Scholar] [CrossRef]
- Haynes, R.J.; Beare, M.H. Aggregation and organic matter storage in meso-thermal, humid soils. In Advances in Soil Science: Structure and Organic Matter Storage in Agricultural Soils; Carter, B.A.S., Ed.; CRC Lewis: Boca Raton, FL, USA, 1995; pp. 213–263. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Dai, W.; Feng, G.; Huang, Y.; Adeli, A.; Jenkins, J.N. Influence of cover crops on soil aggregate stability, size distribution and related factors in a no-till field. Soil Tillage Res. 2024, 244, 106197. [Google Scholar] [CrossRef]
- Celik, I.; Ortas, L.; Kilic, S. Effects of compost, mycorrhiza, manure and fertiliser on some physical properties of a chromoxerert soil. Soil Tillage Res. 2004, 78, 59–67. [Google Scholar] [CrossRef]
- Hartemink, A. Soil Fertility Decline: Definitions and Assessment. In Encyclopedia of Soil Science, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Ladha, J.; Padre, A.; K Reddy, C.; Cassman, K.; Verma, S.; Powlson, D.S.; Kessel, C.; Richter, D.; Chakraborty, D.; Pathak, S. Global nitrogen budgets in cereals: A 50-year assessment for maize, rice, and wheat production systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef]
- Silgram, M.; Williams, D.; Wale, S.; Griffin-Walker, R.; Baddeley, J.A.; Jones, S.; Topp, C.F.E.; Watson, C.A.; Helming, J.; Stoddard, F.L. Biological Nitrogen Fixation (BNF) by Legume Crops in Europe. Legume Futures Report 1.5. 2014. Available online: https://www.legumehub.eu/wp-content/uploads/2021/06/Legume-Futures-Report-1.5.pdf (accessed on 20 February 2025).
- Thomas, R.B.; Van Bloem, S.J.; Schlesinger, W.H. Climate change and symbiotic nitrogen fixation in agroecosystems. In Agroecosystems in a Changing Climate; CRC Press: Boca Raton, FL, USA, 2006; pp. 85–116. [Google Scholar] [CrossRef]
- Büchi, L.; Gebhard, C.; Liebisch, F.; Sinaj, S.; Ramseier, H.; Charles, R. Accumulation of biologically fixed nitrogen by legumes cultivated as cover crops in Switzerland. Plant Soil 2015, 393, 273–287. [Google Scholar] [CrossRef]
- Li, X.; Sorensen, P.; Li, F.; Petersen, S.O.; Olesen, J.E. Quantifying biological nitrogen fixation of different catch crops, and residual effects of roots and tops on nitrogen uptake in barley using in-situ 15N labelling. Plant Soil 2015, 395, 273–287. [Google Scholar] [CrossRef]
- Carlsson, G.; Huss-Danell, K. Nitrogen fixation in perennial forage legumes in the field. Plant Soil 2003, 253, 353–372. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Aronsson, H.; Hansen, E.; Thomsen, I.K.; Liu, J.; Øgaard, A.; Kankanen, H.; Ulén, B. The ability of cover crops to reduce nitrogen and phosphorus losses from arable land in southern Scandinavia and Finland. J. Soil Water Conserv. 2016, 71, 41–55. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Alonso-Ayuso, M.; García-González, I.; Hontoria, C.; Quemada, M. Nitrogen use efficiency and fertiliser fate in a long-term experiment with winter cover crops. Eur. J. Agron. 2016, 79, 14–22. [Google Scholar] [CrossRef]
- Couëdel, A.; Alletto, L.; Tribouillois, H.; Justes, É. Cover crop crucifer-legume mixtures provide effective nitrate catch crop and nitrogen green manure ecosystem services. Agric. Ecosyst. Environ. 2018, 254, 50–59. [Google Scholar] [CrossRef]
- De Notaris, C.; Peixoto, L.; Mortensen, E.Ø.; Rasmussen, J. Cover crop biomass production as a predictor of nitrogen fertilizer replacement value—Legumes secure positive effects. Agric. Ecosyst. Environ. 2025, 381, 109446. [Google Scholar] [CrossRef]
- Moreno-Cadena, P.; Salmeron, M.; Canisares, L.P.; Poffenbarger, H.J. Productivity benefits of cereal-legume cover crop mixtures under variable soil nitrogen and termination times. Eur. J. Agron. 2024, 155, 127114. [Google Scholar] [CrossRef]
- Couëdel, A.; Alletto, L.; Justes, E. Crucifer-legume cover crop mixtures provide effective sulphate catch crop and sulphur green manure services. Plant Soil 2018, 426, 61–76. [Google Scholar] [CrossRef]
- Cooper, R.J.; Hama-Aziz, Z.; Hiscock, K.M.; Lovett, A.A.; Dugdale, S.J.; Sünnenberg, G.; Noble, L.; Beamish, J.; Hovesen, P. Assessing the farm-scale impacts of cover crops and non-inversion tillage regimes on nutrient losses from an arable catchment. Agric. Ecosyst. Environ. 2017, 237, 181–193. [Google Scholar] [CrossRef]
- Teixeira, E.I.; Johnstone, P.; Chakwizira, E.; Ruiter, J.d.; Malcolm, B.; Shaw, N.; Zyskowski, R.; Khaembah, E.; Sharp, J.; Meenken, E.; et al. Sources of variability in the effectiveness of winter cover crops for mitigating N leaching. Agric. Ecosyst. Environ. 2016, 220, 226–235. [Google Scholar] [CrossRef]
- Meisinger, J.J.; Hargrove, W.; Mikkelsen, R.; Williams, J.; Benson, V. Effects of cover crops on groundwater quality. In Cover Crops for Clean Water; 1991; Available online: https://www.swcs.org/static/media/cms/CCCW4ground_D30E9A7F53D56.pdf (accessed on 20 February 2025).
- Hooker, K.; Coxon, C.; Hackett, R.; Kirwan, L.; O’Keeffe, E.; Richards, K. Evaluation of Cover Crop and Reduced Cultivation for Reducing Nitrate Leaching in Ireland. J. Environ. Qual. 2008, 37, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Premrov, A.; Coxon, C.E.; Hackett, R.; Kirwan, L.; Richards, K.G. Effects of over-winter green cover on soil solution nitrate concentrations beneath tillage land. Sci. Total Environ. 2014, 470–471, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Premrov, A.; Coxon, C.E.; Hackett, R.; Kirwan, L.; Richards, K.G. Effects of over-winter green cover on groundwater nitrate and dissolved organic carbon concentrations beneath tillage land. Sci. Total Environ. 2012, 438, 144–153. [Google Scholar] [CrossRef]
- Hashemi, M.; Farsad, A.; Sadeghpour, A.; Weis, S.; Herbert, S. Cover-crop seeding-date influence on fall nitrogen recovery. J. Plant Nutr. Soil Sci. 2013, 176, 69–75. [Google Scholar] [CrossRef]
- Kumar, U.; Thomsen, I.; Eriksen, J.; Vogeler, I.; Mäenpää, M.; Hansen, E. Delaying sowing of cover crops decreases the ability to reduce nitrate leaching. Agric. Ecosyst. Environ. 2023, 355, 108598. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant Soil 2001, 230, 185–195. [Google Scholar] [CrossRef]
- Tribouillois, H.; Fort, F.; Cruz, P.; Charles, R.; Flores, O.; Garnier, E.; Justes, E. A Functional Characterisation of a Wide Range of Cover Crop Species: Growth and Nitrogen Acquisition Rates, Leaf Traits and Ecological Strategies. PLoS ONE 2015, 10, e0122156. [Google Scholar] [CrossRef]
- Kanders, M.J.; Berendonk, C.; Fritz, C.; Watson, C.; Wichern, F. Catch crops store more nitrogen below-ground when considering Rhizodeposits. Plant Soil 2017, 417, 287–299. [Google Scholar] [CrossRef]
- Wendling, M.; Büchi, L.; Amossé, C.; Jeangros, B.; Walter, A.; Charles, R. Specific interactions leading to transgressive overyielding in cover crop mixtures. Agric. Ecosyst. Environ. 2017, 241, 88–99. [Google Scholar] [CrossRef]
- Constantin, J.; Le Bas, C.; Justes, E. Large-scale assessment of optimal emergence and destruction dates for cover crops to reduce nitrate leaching in temperate conditions using the STICS soil–crop model. Eur. J. Agron. 2015, 69, 75–87. [Google Scholar] [CrossRef]
- Silgram, M.; Williams, D.; Wale, S.; Griffin-Walker, R. Managing Cultivations and Cover Crops for Improved Profitability and Envionmental Benefits in Potatoes; Agriculture and Horticulture Development Board: Warwickshire, UK, 2015. [Google Scholar]
- Frost, J.P.; Bailey, J.S.; Stevens, R.J. Making Best on-Farm Use of Plant Nutrients in Livestock Manures; 78th Annual Report; Agricultural Research Institute of Northern Ireland: Hillsborough, UK, 2004. [Google Scholar]
- Cambardella, C.A.; Moorman, T.B.; Singer, J.W. Soil nitrogen response to coupling cover crops with manure injection. Nutr. Cycl. Agroecosyst. 2010, 87, 383–393. [Google Scholar] [CrossRef]
- Parkin, T.B.; Kaspar, T.; Singer, J.W. Cover crop effects on the fate of N following soil application of swine manure. Plant Soil 2006, 289, 141–152. [Google Scholar] [CrossRef]
- Thilakarathna, M.; Serran, S.; Lauzon, J.; Janovicek, K.; Deen, B. Management of Manure Nitrogen Using Cover Crops. Agron. J. 2015, 107, 1595–1607. [Google Scholar] [CrossRef]
- Cottney, P.; Black, L.; White, E.; Williams, P.N. The Correct Cover Crop Species Integrated with Slurry Can Increase Biomass, Quality and Nitrogen Cycling to Positively Affect Yields in a Subsequent Spring Barley Rotation. Agronomy 2020, 10, 1760. [Google Scholar] [CrossRef]
- Corti, M.; Bechini, L.; Cavalli, D.; Ben Hassine, M.; Michelon, L.; Cabassi, G.; Pricca, N.; Perego, A.; Marino Gallina, P. Early sowing dates and pre-plant nitrogen affect autumn weed control and nitrogen content of winter cover crops in rotation with spring crops. Eur. J. Agron. 2024, 155, 127140. [Google Scholar] [CrossRef]
- Everett, L.; Wilson, M.; Pepin, R.; Coulter, J. Winter Rye Cover Crop with Liquid Manure Injection Reduces Spring Soil Nitrate but Not Maize Yield. Agronomy 2019, 9, 852. [Google Scholar] [CrossRef]
- Singer, J.W.; Cambardella, C.A.; Moorman, T.B. Enhancing Nutrient Cycling by Coupling Cover Crops with Manure Injection. Agron. J. 2008, 100, 1735–1739. [Google Scholar] [CrossRef]
- Janusauskaite, D.; Arlauskienė, A.; Maikštėnienė, S. Soil mineral nitrogen and microbial parameters as influenced by catch crops and straw management. Zemdirb. Agric. 2013, 100, 9–18. [Google Scholar] [CrossRef]
- Rosenfield, A.; Raynes, F. Sort out your soil: A practical guide to Green Manures. In Cotswold Grass Seeds Direct; Cotswold Seeds: Gloucestershire, UK, 2011. [Google Scholar]
- Justes, E.; Beaudoin, N.; Bertuzzi, P.; Charles, R.; Constantin, J.; Dürr, C.; Hermon, C.; Joannon, C.; Le Bas, B.; Mary, C.; et al. The use of cover crops to reduce nitrate leaching: Effect on the water and nitrogen balance and other ecosystem services. Synop. Study Rep. 2012, 68, 1–8. [Google Scholar]
- Fillery, I.R.P. The fate of biologically fixed nitrogen in legume-based dryland farming systems: A review. Aust. J. Exp. Agric. 2001, 41, 361–381. [Google Scholar] [CrossRef]
- Justes, E.; Mary, B.; Nicolardot, B. Quantifying and modelling C and N mineralization kinetics of catch crop residues in soil: Parameterization of the residue decomposition module of STICS model for mature and non mature residues. Plant Soil 2009, 325, 171–185. [Google Scholar] [CrossRef]
- Nevins, C.J.; Nakatsu, C.; Armstrong, S. Characterization of microbial community response to cover crop residue decomposition. Soil Biol. Biochem. 2018, 127, 39–49. [Google Scholar] [CrossRef]
- Kremen, A.; Weil, R. Nitrogen Mineralization from Brassica Cover Crops. Master’s Thesis, University of Maryland, College, College Park, MD, USA, 2006; p. 115. [Google Scholar]
- Silgram, M.; Harrison, R. The Mineralisation of Nitrogen in Cover Crops: A Review; NT1508; Department of Environment and Rural Affairs: London, UK, 1998.
- Melkonian, J.; Poffenbarger, H.J.; Mirsky, S.; Ryan, M.R.; Moebius-Clune, B. Estimating Nitrogen Mineralization from Cover Crop Mixtures Using the Precision Nitrogen Management Model. Agron. J. 2017, 109, 1944–1959. [Google Scholar] [CrossRef]
- Poffenbarger, H.; Mirsky, S.; Kramer, M.; Weil, R.; Meisinger, J.J.; Cavigelli, M.; Spargo, J. Cover Crop and Poultry Litter Management Influence Spatiotemporal Availability of Topsoil Nitrogen. Soil Sci. Soc. Am. J. 2015, 79, 1660–1673. [Google Scholar] [CrossRef]
- Wagger, M.G. Time of desiccation effects on plant composition and subsequent nitrogen release from several winter annual cover crops. Agron. J. 1989, 81, 236–241. [Google Scholar] [CrossRef]
- Ranells, N.N.; Wagger, M.G. Nitrogen Release from Grass and Legume Cover Crop Monocultures and Bicultures. Agron. J. 1996, 88, 777–882. [Google Scholar] [CrossRef]
- Wendling, M.; Büchi, L.; Amosse, C.; Sinaj, S.; Walter, A.; Charles, R. Nutrient accumulation by cover crops with different root systems. Asp. Appl. Biol. 2015, 129, 91–95. [Google Scholar]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners—The roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2018, 434, 7–45. [Google Scholar] [CrossRef]
- Dube, E.; Chiduza, C.; Muchaonyerwa, P. High biomass yielding winter cover crops can improve phosphorous availability in soil. S. Afr. J. Sci. 2014, 110, 1–4. [Google Scholar]
- Reynolds, S.H.; Ritz, K.; Crotty, F.H.; Stoate, C.; West, H.; Neal, A.L. Effects of cover crops on phosphatase activity in a clay arable soil in the UK. Asp. Appl. Biol. 2017, 136, 215–220. [Google Scholar]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.; Bol, R.; Mackay, R.L.; Richardson, A.E.; Condron, L.M.; Turner, B.L.; Haygarth, P.M. Recovering phosphorous from soil: A root solution? Environ. Sci. Technol. 2012, 46, 1977–1978. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Williams, P.; Zhang, H.; Yang, Y.; Yin, D.; Liu, Z.; Sun, H.; Luo, J. Combining Multiple High-Resolution In Situ Techniques to Understand Phosphorous Availability Around Rice Roots. Environ. Sci. Technol. 2021, 55, 13082–13092. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhou, Y.; Gu, Z.; Zhu, H.; Fu, S.; Yao, Q. The combined effects of cover crops and symbiotic microbes on phosphatase gene and organic phosphorus hydrolysis in subtropical orchard soils. Soil Biol. Biochem. 2015, 82, 119–126. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Thien, S.J. Phosphorous bioavailability following incorporation of green manure crops. Soil Sci. Soc. Am. 2003, 67, 1186–1194. [Google Scholar] [CrossRef]
- White, C.; Weil, R. Forage Radish Cover Crops Increase Soil Test Phosphorus Surrounding Radish Taproot Holes. Soil Sci. Soc. Am. J. 2011, 75, 121. [Google Scholar] [CrossRef]
- Crespo, C.; Wyngaard, N.; Sainz Rozas, H.R.; Pizzuto, A.; Barbagelata, P.; Barraco, M.; Gudelj, V.; Barbieri, P.A. Cover crops affect phosphorus fractions in soybean-based sequences with different phosphorus availability in Mollisols. Soil Tillage Res. 2024, 240, 106096. [Google Scholar] [CrossRef]
- Valadares, R.; Ávila-Silva, L.; Teixeira, R.; de Sousa, R.; Vergutz, L. Green Manures and Crop Residues as Source of Nutrients in Tropical Environment. In Organic Fertilizers—From Basic Concepts to Applied Outcomes; IntechOpen: London, UK, 2016; pp. 51–84. [Google Scholar]
- Murrell, T.S.; Mikkelsen, R.; Sulewski, G.; Norton, R.; Thompson, M. Improving Potassium Recommendations for Agricultural Crops; Springer: Cham, Swizerland, 2021. [Google Scholar]
- Rosolem, C.; Mallarino, A.; Nogueira, T. Considerations for Unharvested Plant Potassium; Springer: Cham, Swizerland, 2021; pp. 147–162. [Google Scholar]
- Rosolem, C.A.; Steiner, F. Effects of soil texture and rates of K input on potassium balance in tropical soil. Eur. J. Soil Sci. 2017, 68, 658–666. [Google Scholar] [CrossRef]
- Echer, F.; Peres, V.; Rosolem, C. Potassium application to the cover crop prior to cotton planting as a fertilization strategy in sandy soils. Sci. Rep. 2020, 10, 20404. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. Sci. Total Environ. 2012, 7, 1330–1343. [Google Scholar] [CrossRef]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behavior in soils—Misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Grüter, R.; Costerousse, B.; Bertoni, A.; Mayer, J.; Thonar, C.; Frossard, E.; Schulin, R.; Tandy, S. Green manure and long-term fertilization effects on soil zinc and cadmium availability and uptake by wheat (Triticum aestivum L.) at different growth stages. Sci. Total Environ. 2017, 599–600, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Tringovska, L. Beneficial soil microorganisms, an ecological alternative for soil fertility management. Sustain. Agric. Res. Educ. 2011, 7, 161–214. [Google Scholar]
- Aghili, F.; Gamper, H.A.; Eikenberg, J.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Schulin, R.; Jansa, J. Green manure addition to soil increases grain zinc concentration in bread wheat. PLoS ONE 2014, 9, 101487. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Shrivani, M.; Schulin, R. The effect of preceding crop on wheat grain zinc concentration and its relationship to total amino acids and dissolved organic carbon in rhizosphere soil solution. Biol. Fertil. Soils 2014, 50, 239–247. [Google Scholar] [CrossRef]
- Habiby, H.; Afyuni, M.; Khoshgoftarmanesh, A.H.; Schulin, R. Effect of preceding crops and their residues on availability of zinc in a calcareous Zn-deficient soil. Biol. Fertil. Soils 2014, 50, 1061–1067. [Google Scholar] [CrossRef]
- Baraibar, B.; Hunter, M.; Schipanski, M.; Hamilton, A.; Mortensen, D. Weed Suppression in Cover Crop Monocultures and Mixtures. Weed Sci. 2017, 66, 121–133. [Google Scholar] [CrossRef]
- Bezuidenhout, S.R.; Reinhardt, C.F.; Whitwell, M.I. Cover crops of oats, stooling rye and three annual ryegrass cultivars influence maize and Cyperus esculentus growth. Weed Res. 2012, 52, 153–160. [Google Scholar] [CrossRef]
- Masilionyte, L.; Maiksteniene, S.; Kriauciuniene, Z.; Jablonskyte-Rasce, D.; Zou, L.; Sarauskis, E. Effect of cover crops in smothering weeds and volunteer plants in alternative farming systems. Crop Prot. 2017, 91, 74–81. [Google Scholar] [CrossRef]
- Gosling, P.; Nicholls, C. Managing Weeds in an Arable Rotation; Agriculture and Horticulture Development Board: Warwickshire, UK, 2014. [Google Scholar]
- U.S. Environmental Protection Agency. Ozone Layer Depletion—Regulatory Programs, Methyl Bromide Alternatives; U.S. Environmental Protection Agency: Washington, DC, USA, 2008.
- Fourie, H.; Ahuja, P.; Lammers, J.; Daneel, M. Brassicacea-based management strategies as an alternative to combat nematode pests: A synopsis. Crop Prot. 2016, 80, 21–41. [Google Scholar] [CrossRef]
- Ngala, B.M.; Haydock, P.P.J.; Woods, S.; Back, M.A. Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest Manag. Sci. 2014, 71, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.S.; Lazzeri, L.; Atkinson, A.H.; Urwin, P.E. Biofumigation for control of pale potato cyst nematodes: Activity of Brassica leaf extracts and green manures on Globodera pallida in vitro and in soil. J. Agric. Food Chem. 2011, 59, 7882–7890. [Google Scholar] [CrossRef]
- Kruger, D.; Fourie, J.; Malan, A. Cover Crops with Biofumigation Properties for the Suppression of Plant-Parasitic Nematodes: A Review. S. Afr. J. Enol. Vitic. 2013, 34, 287. [Google Scholar] [CrossRef]
- Scholte, K.; Vos, J. Effects of potential trap crops and planting date on soil infestation with potato cyst nematodes and root-knot nematodes. Ann. Appl. Biol. 2000, 137, 153–164. [Google Scholar] [CrossRef]
- Valdes, Y.; Viaene, N.; Moens, M. Effects of yellow mustard amendments on the soil nematode community in a potato field with focus on Globodera rostochiensis. Appl. Soil Ecol. 2012, 59, 39–47. [Google Scholar] [CrossRef]
- Gruver, L.S.; Weil, R.R.; Zasada, A.I.; Sardanelli, S.; Momen, B. Brassicaceae and rye cover crops altered free-living soil nematode community composition. Appled Soil Ecol. 2010, 10, 1–12. [Google Scholar]
- Ramirez, R.A.; Henderson, D.R.; Riga, E.; Lacey, L.A.; Snyder, W.E. Harmful effects of mustard bio-fumigants on entomopathogenic nematodes. Biol. Control 2009, 48, 147–154. [Google Scholar] [CrossRef]
- Jaffuel, G.; Blanco-Pérez, R.; Büchi, L.; Mäder, P.; Fließbach, A.; Charles, R.; Degen, T.; Turlings, T.C.J.; Campos-Herrera, R. Effects of cover crops on the overwintering success of entomopathogenic nematodes and their antagonists. Appl. Soil Ecol. 2017, 114, 62–73. [Google Scholar] [CrossRef]
- Everts, K. Soil Biology: Cover Crops and Disease Suppression; Sustainable Agriculture Research and Education: Griffin, GA, USA, 2016. [Google Scholar]
- Mojtahedi, H.; Santo, G.S.; Wilson, J.H.; Hang, A.N. Managing Meloidogyne chitwoodi on potato with rapeseed as green manure. Plant Dis. 1993, 77, 42–46. [Google Scholar] [CrossRef]
- Dossey, Z. Potential of Green Manure Biofumigants and Seed Exudates in the Control of Globodera pallida, the White Potato Cyst Nematode. Master’s Thesis, Washington State University, Pullman, WA, USA, 2010. [Google Scholar]
- Martinez, M.S. Influence of Cover Crops on the Development of Some Soil-Borne Plant Pathogens; Swedish University of Agricultural Science of Crop Production Ecology: Uppsala, Sweden, 2009; Volume 30. [Google Scholar]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Motisi, N.; Montfort, F.; Faloya, V.; Lucas, P.; Doré, T. Growing Brassica juncea as a cover crop, then incorporating its residues provide complementary control of Rhizoctonia root rot of sugar beet. Field Crops Res. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R.; Miyao, E.M.; Davis, R.M. Mustard Cover Crops Are Ineffective in Suppressing Soilborne Disease or Improving Processing Tomato Yield. Hortic. Sci. 2005, 40, 2016–2019. [Google Scholar] [CrossRef]
- Hansen, Z.R.; Keinath, A.P. Increased pepper yields following incorporation of biofumigation cover crops and the effects on soilborne pathogen populations and pepper diseases. Appl. Soil Ecol. 2013, 63, 67–77. [Google Scholar] [CrossRef]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The Effect of Sulfur Nutrition on Plant Glucosinolate Content: Physiology and Molecular Mechanisms. Plant Biol. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M.; Wong, P.T.W.; Mead, A.; Howe, G.; Newell, M. Field studies on the biofumigation of take-all by Brassica break crops. Aust. J. Agric. Res. 2000, 51, 445–456. [Google Scholar] [CrossRef]
- Holland, J.M.; Storkey, J.; Wutman, P.J.W.; Henderson, I.; Orson, J. The Farm4Bio project: Managing uncropped land for biodiversity. Asp. Appl. Biol. 2013, 118, 89–99. [Google Scholar]
- Heaney, I.; Foy, F.B.; Kennedy, J.A.; Crozier, G.; Walter, K.; O’ Connor, W.C.K. Impacts of agriculture on aquatic systems: Lessons learnt and new unknowns in Northern Ireland. Biol. Mar. Freshw. Res. 2001, 52, 151–163. [Google Scholar] [CrossRef]
- Wilcoxen, C.A.; Walk, J.W.; Ward, M.P. Use of cover crop fields by migratory and resident birds. Agric. Ecosyst. Environ. 2018, 252, 42–50. [Google Scholar] [CrossRef]
- Storr, T.; Simmons, R.W.; Hannam, J.A. A UK survey of the use and management of cover crops. Ann. Appl. Biol. 2019, 174, 179–189. [Google Scholar] [CrossRef]
- Gómez, J.A.; Sobrinho, T.A.; Giráldez, J.V.; Fereres, E. Soil management effects on runoff, erosion and soil properties in an olive grove of Southern Spain. Soil Tillage Res. 2009, 102, 5–13. [Google Scholar] [CrossRef]
- Granfors, D.A.; Church, K.E.; Smith, L.M. Eastern meadowlarks nesting in rangelands and conservation reserve program fields in Kansas. J. Field Ornithol. 1996, 67, 222–235. [Google Scholar]
- Hubbard, R.D.; Althoff, D.P.; Blecha, K.A.; Bruvold, B.A.; Japuntich, R.D. Nest site characteristics of eastern meadowlarks and grasshopper sparrows in tallgrass prairie at the Fort Riley military installation, Kansas. Trans. Kans. Acad. Sci. 2006, 109, 168–174. [Google Scholar] [CrossRef]
- Bryan, G.G.; Best, L.B. Avian nest density and success in grassed waterways in Iowa rowcrop fields. Wildl. Soc. Bull. 1994, 22, 583–592. [Google Scholar]
- EC. Soil Erosion Costs European Farmers €1.25 Billion a Year. EU Science Hub. 2018. Available online: https://european-union.europa.eu/index_en (accessed on 13 February 2020).
- Eurostat. Agri-Environmental Indicators—Soil Cover. 2012. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_soil_cover (accessed on 20 February 2025).
- Fleskens, L.; Stroosnijder, L. Is soil erosion in olive groves as bad as often claimed? Geoderma 2007, 141, 260–271. [Google Scholar] [CrossRef]
- Joyce, B.A.; Wallender, W.W.; Mitchell, J.P.; Huyck, L.M.; Temple, S.R.; Brostrom, P.N. Infiltration and soil water storage under winter cover cropping in California’s Sacramento Valley. Am. Soc. Agric. Eng. 2002, 45, 315–326. [Google Scholar]
- Smith, R.V.; Jordan, C.; Annett, J.A. A phosphorus budget for Northern Ireland: Inputs to inland and coastal waters. J. Hydrol. 2005, 304, 193–202. [Google Scholar] [CrossRef]
- Foy, B.; Bailey, J.; Lennox, S.D. Mineral balances for the use of phosphorus and other nutrients by agriculture in Northern Ireland from 1925 to 2000—Methodology, trends and impacts of losses to water. Ir. J. Agric. Food Res. 2002, 41, 247–263. [Google Scholar]
- Kataoka, R.; Nagasaka, K.; Tanaka, Y.; Yamamura, H.; Shinohara, S.; Haramoto, E.; Hayakawa, M.; Sakamoto, Y. Hairy vetch (Vicia villosa), as a green manure, increases fungal biomass, fungal community composition, and phosphatase activity in soil. Appl. Soil Ecol. 2017, 117–118, 16–20. [Google Scholar] [CrossRef]
- Teboh, J.M.; Franzen, D.W. Buckwheat (Flagopyrum esculentum Monch) potential to contribute solubilized soil phosphorous to subsequent crops. Commun. Soil Sci. Plant Anal. 2011, 42, 1544–1550. [Google Scholar] [CrossRef]
- Kamh, M.; Horst, W.J.; Amer, F. Mobilization of soil fertilizer phosphate by cover crops. Plant Soil 1999, 211, 19–27. [Google Scholar] [CrossRef]
- AHDB. Nutrient Management Guide (RB209); AHDB: Warwickshire, UK, 2017. [Google Scholar]
- Smith, R.G.; Atwood, L.W.; Warren, N.D. Increased Productivity of a Cover Crop Mixture Is Not Associated with Enhanced Agroecosystem Services. PLoS ONE 2014, 9, e97351. [Google Scholar] [CrossRef] [PubMed]
- Marcillo, G.S.; Miguez, F.E. Corn yield response to winter cover crops: An updated meta-analysis. J. Soil Water Conserv. 2017, 72, 226–239. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Jacinthe, P.-A.; Ren, W. Global synthesis of cover crop impacts on main crop yield. Field Crops Res. 2024, 310, 109343. [Google Scholar] [CrossRef]
- Miguez, F.E.; Bollero, G.A. Review of Corn Yield Response under Winter Cover Cropping Systems Using Meta-Analytic Methods. Crop Sci. 2005, 45, 2318–2329. [Google Scholar] [CrossRef]
- Qin, Z.; Guan, K.; Zhou, W.; Peng, B.; Villamil, M.B.; Jin, Z.; Tang, J.; Grant, R.; Gentry, L.; Margenot, A.J.; et al. Assessing the impacts of cover crops on maize and soybean yield in the U.S. Midwestern agroecosystems. Field Crops Res. 2021, 273, 108264. [Google Scholar] [CrossRef]
- Toom, M.; Tamm, S.; Talgre, L.; Tamm, I.; Tamm, Ü.; Narits, L.; Hiiesalu, I.; Mae, A.; Lauringson, E. The Effect of Cover Crops on the Yield of Spring Barley in Estonia. Agriculture 2019, 9, 172. [Google Scholar] [CrossRef]
- Holland, J.; Brown, J.L.; MacKenzie, K.; Neilson, R.; Piras, S.; McKenzie, B.M. Over winter cover crops provide yield benefits for spring barley and maintain soil health in northern Europe. Eur. J. Agron. 2021, 130, 126363. [Google Scholar] [CrossRef]
- Stobart, M.R.; Morris, N.L. The impact of cover crops on yield and soils in the New Farming Systems programme. Asp. Appl. Biol. 2014, 127, 223–232. [Google Scholar]
- Hunter, M.C.; Schipanski, M.E.; Burgess, M.H.; LaChance, J.C.; Bradley, B.A.; Barbercheck, M.E.; Kaye, J.P.; Mortensen, D.A. Cover Crop Mixture Effects on Maize, Soybean, and Wheat Yield in Rotation. Agric. Environ. Lett. 2019, 4, 180051. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. The effect of nitrogen catch crop species on the nitrogen nutrition of succeeding crops. Fertil. Res. 1994, 37, 227–234. [Google Scholar] [CrossRef]
- Lawson, A.; Cogger, C.; Bary, A.; Fortuna, A.-M. Influence of Seeding Ratio, Planting Date, and Termination Date on Rye-Hairy Vetch Cover Crop Mixture Performance under Organic Management. PLoS ONE 2015, 10, e0129597. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).