Leaf Plasticity and Biomass Allocation of Arundo donax Under Combined Irrigation and Nitrogen Conditions in Salinized Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Material
2.2. Characteristics of Leaf Morphology
2.3. Characteristics of Leaf Water Content
2.4. Characteristics of Leaf Relative Chlorophyll Content and Photosynthesis
2.5. Characteristics of Biomass Accumulation and Allocation
2.6. Statistical Analysis
3. Results
3.1. Effects of Irrigation-Nitrogen-Salinity Coupling on Leaf Morphology in A. donax
3.2. Effects of Irrigation-Nitrogen-Salinity Coupling on the Photosynthetic Characteristics of A. donax
3.2.1. SPAD Values
3.2.2. Photosynthetic Characteristics
3.3. Effects of Irrigation-Nitrogen-Salinity Coupling on Water-Related Physiological Parameters of Fully Expanded A. donax Leaves
3.4. Effects of Irrigation-Nitrogen-Salinity Coupling on Biomass Accumulation in A. donax
3.5. Effects of Irrigation-Nitrogen-Salinity Coupling on Biomass Accumulation and Allocation in A. donax
3.6. Regression and Path Analysis of Irrigation-Nitrogen-Salinity Coupling Effects on Biomass Accumulation in A. donax
4. Discussion
4.1. Leaf Plasticity of A. donax Under Irrigation-Nitrogen-Salinity Coupling
4.2. Water Physiology of A. donax Under Irrigation-Nitrogen-Salinity Coupling
4.3. Biomass Accumulation and Allocation of A. donax Under Irrigation-Nitrogen-Salinity Coupling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jámbor, A.; Török, Á. The Economics of Arundo donax—A Systematic Literature Review. Sustainability 2019, 11, 4225. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Ngernsaengsaruay, C.; Puangsin, B.; Leksungnoen, N.; Khantayanuwong, S.; Chanton, P.; Thaepthup, T.; Wessapak, P.; Meeboonya, R.; Yimlamai, P.; Wanitpinyo, K.; et al. Morphology, Taxonomy, Culm Internode and Leaf Anatomy, and Palynology of the Giant Reed (Arundo donax L.), Poaceae, Growing in Thailand. Plants 2023, 12, 1850. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivera-Vargas, P.; Serrat, X.; Nogués, S. Arundo donax L.: How High Photosynthetic Capacity Is Maintained under Water Scarcity Conditions. Agronomy 2021, 11, 1089. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Moran, P.J.; Martínez Jiménez, M.; Yang, C.; Canavan, K.; Paynter, Q.; Ota, N.; Kriticos, D.J. Biology of Invasive Plants 4. Arundo donax L. Invasive Plant Sci. Manag. 2023, 16, 81–109. [Google Scholar] [CrossRef]
- Lino, G.; Espigul, P.; Nogués, S.; Serrat, X. Arundo donax L. growth potential under different abiotic stress. Heliyon 2023, 9, e15521. [Google Scholar] [CrossRef]
- Ceotto, E.; Di Candilo, M. Shoot cuttings propagation of giant reed (Arundo donax L.) in water and moist soil: The path forward? Biomass Bioenergy 2010, 34, 1614–1623. [Google Scholar] [CrossRef]
- Amaducci, S.; Perego, A. Field evaluation of Arundo donax clones for bioenergy production. Ind. Crops Prod. 2015, 75, 122–128. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Cosentino, S.L. Perennial grasses as lignocellulosic feedstock for second-generation bioethanol production in Mediterranean environment. Ital. J. Agron. 2014, 9, 84–92. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Serra, P.; Pilla, M.; Marcone, A.; Amaducci, S. Belowground biomass C outweighs soil organic C of perennial energy crops: Insights from a long-term multispecies trial. Glob. Change Biol. Bioenergy 2021, 13, 459–472. [Google Scholar] [CrossRef]
- Rodriguez, L.D.; Confalone, A.E.; Lazaro, L.; Pimentel, R.M.; Lyra, G.B.; de Oliveira, J.F.; Singh, S.K.; Pereira, C.R. Growth of the energy crop giant reed (Arundo donax L.) and optimization of the ARMIDA model in the south-central region of Buenos Aires, Argentina. Ind. Crops Prod. 2024, 211, 118190. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; di Mola, I.; Ottaiano, L.; Mori, M.; Cenvinzo, V.; Fagnano, M. Use of giant reed (Arundo donax L.) to control soil erosion and improve soil quality in a marginal degraded area. Ital. J. Agron. 2020, 15, 1764. [Google Scholar] [CrossRef]
- Siri Prieto, G.; Bustamante, M.; Battaglia, M.; Ernst, O.; Seleiman, M.F.; Sadeghpour, A. Effects of perennial biomass yield energy grasses and fertilization on soil characteristics and nutrient balances. Agron. J. 2021, 113, 4292–4305. [Google Scholar] [CrossRef]
- Ceotto, E.; Vasmara, C.; Marchetti, R.; Cianchetta, S.; Galletti, S. Biomass and methane yield of giant reed (Arundo donax L.) as affected by single and double annual harvest. Glob. Change Biol. Bioenergy 2021, 13, 393–407. [Google Scholar] [CrossRef]
- Arias, C.; Lino, G.; Sánchez, E.; Nogués, S.; Serrat, X. Drought Impact on the Morpho-Physiological Parameters of Perennial Rhizomatous Grasses in the Mediterranean Environment. Agriculture 2023, 13, 1233. [Google Scholar] [CrossRef]
- Pompeiano, A.; Guglielminetti, L.; Bargiacchi, E.; Miele, S. Responses in chemical traits and biomass allocation of Arundo donax L. to deficit resources in the establishment year. Chil. J. Agric. Res. 2013, 73, 377–384. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Baraza, E.; Cifre, J.; Achir, C.; Gulías, J. Leaf plasticity and stomatal regulation determines the ability of Arundo donax plantlets to cope with water stress. Photosynthetica 2018, 56, 698–706. [Google Scholar] [CrossRef]
- Scordia, D.; D’Agosta, G.M.D.; Mantineo, M.; Testa, G.; Cosentino, S.L. Life Cycle Assessment of Biomass Production from Lignocellulosic Perennial Grasses under Changing Soil Nitrogen and Water Content in the Mediterranean Area. Agronomy 2021, 11, 988. [Google Scholar] [CrossRef]
- Angelini, L.; Ceccarini, L.; Bonari, E. Biomass yield and energy balance of giant reed (Arundo donax L.) cropped in central Italy as related to different management practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Wang, A.; Wu, T.; Guo, J.; Shi, G.; Tian, B.; Wei, F.; Cao, G. Integrated physiological and transcriptomic analysis reveals the involvement of photosynthesis and redox homeostasis in response of Arundo donax to low and high nitrogen supply. Ind. Crop. Prod. 2024, 221, 119377. [Google Scholar] [CrossRef]
- Lloyd, L.; Nackley, S.K. A salt on the bioenergy and biological invasions debate: Salinity tolerance of the invasive biomass feedstock Arundo donax. Glob. Change Biol. Bioenergy 2015, 7, 752–762. [Google Scholar]
- Brilli, F.; Pignattelli, S.; Baraldi, R.; Neri, L.; Pollastri, S.; Gonnelli, C.; Giovannelli, A.; Loreto, F.; Cocozza, C. Root Exposure to 5-Aminolevulinic Acid (ALA) Affects Leaf Element Accumulation, Isoprene Emission, Phytohormonal Balance, and Photosynthesis of Salt-Stressed Arundo donax. Int. J. Mol. Sci. 2022, 23, 4311. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Arcoverde Cerveira Sterner, V.; Papp, L.; May, Z.; Orlóci, L.; Gyuricza, C.; Sági, L.; Solti, Á.; Fodor, F. Alkaline Salt Tolerance of the Biomass Plant Arundo donax. Agronomy 2022, 12, 1589. [Google Scholar] [CrossRef]
- Ayub, M.A.; Ahmad, H.R.; Ali, M.; Rizwan, M.; Ali, S.; Zia Ur Rehman, M.; Waris, A.A. Chapter 3-Salinity and its tolerance strategies in plants. In Plant Life Under Changing Environment; Tripathi, D.K., Pratap Singh, V., Chauhan, D.K., Sharma, S., Prasad, S.M., Dubey, N.K., Ramawat, N., Eds.; Academic Press: NewYork, NY, USA, 2020; pp. 47–76. [Google Scholar]
- Triana, F.; Di Nasso, N.N.O.; Ragaglini, G.; Roncucci, N.; Bonari, E. Evapotranspiration, crop coefficient and water use efficiency of giant reed (Arundo donax L.) and miscanthus (Miscanthusxgiganteus Greef et Deu.) in a Mediterranean environment. Glob. Change Biol. Bioenergy 2015, 7, 811–819. [Google Scholar] [CrossRef]
- Cosentino, S.C.S.L.; Patanè, C.P.C.; Sanzone, E.S.E.; Testa, G.T.G.; Scordia, D.S.D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Riggi, E.; Avola, G.; Marino, G.; Haworth, M.; Cosentino, S.L.; Centritto, M. Open field experiment for the evaluation of Arundo donax ecotypes ecophysiology and yield as affected by soil water content. Ind. Crops Prod. 2019, 140, 9. [Google Scholar] [CrossRef]
- Pompeiano, A.; Landi, M.; Meloni, G.; Vita, F.; Guglielminetti, L. Allocation pattern, ion partitioning, and chlorophyll a fluorescence in Arundo donax L. in responses to salinity stress. Plant Biosyst. 2017, 151, 613–622. [Google Scholar] [CrossRef]
- Cano-Ruiz, J.; Sanz, M.; Curt, M.D.; Plaza, A.; Lobo, M.C.; Mauri, P.V. Fertigation of Arundo donax L. with different nitrogen rates for biomass production. Biomass Bioenergy 2020, 133, 105451. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Riggi, E.; Avola, G.; Brunetti, C.; Scordia, D.; Testa, G.; Thiago, G.G.M.; Loreto, F.; Luciano, C.S.; et al. The effect of summer drought on the yield of Arundo donax is reduced by the retention of photosynthetic capacity and leaf growth later in the growing season. Ann. Bot. 2019, 124, 567–580. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Chapter Two-Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Pathum Thani, Thailand, 2019; Volume 156, pp. 109–157. [Google Scholar]
- Pompeiano, A.; Remorini, D.; Vita, F.; Guglielminetti, L.; Miele, S.; Morini, S. Growth and physiological response of Arundo donax L. to controlled drought stress and recovery. Plant Biosyst. 2017, 151, 906–914. [Google Scholar] [CrossRef]
- Galal, T.M.; Shehata, H.S. Growth and nutrients accumulation potentials of giant reed (Arundo donax L.) in different habitats in Egypt. Int. J. Phytoremediation 2016, 18, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Nackley, L.; Hough-Snee, N.; Kim, S. Competitive traits of the invasive grass Arundo donax are enhanced by carbon dioxide and nitrogen enrichment. Weed Res. 2017, 57, 67–71. [Google Scholar] [CrossRef]
- De Battisti, D. Plant biomass allocation advances our understanding of plant adaptation to environmental gradients: A commentary on ‘Contrasting biomass allocations explain adaptations to cold and drought in the world’s highest-growing angiosperms’. Ann. Bot. 2024, 134, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Di Nasso, N.N.O.; Roncucci, N.; Bonari, E. Giant reed (Arundo donax L.) as energy crop in Central Italy: A review. Ital. J. Agron. 2013, 8, 10–17. [Google Scholar] [CrossRef]
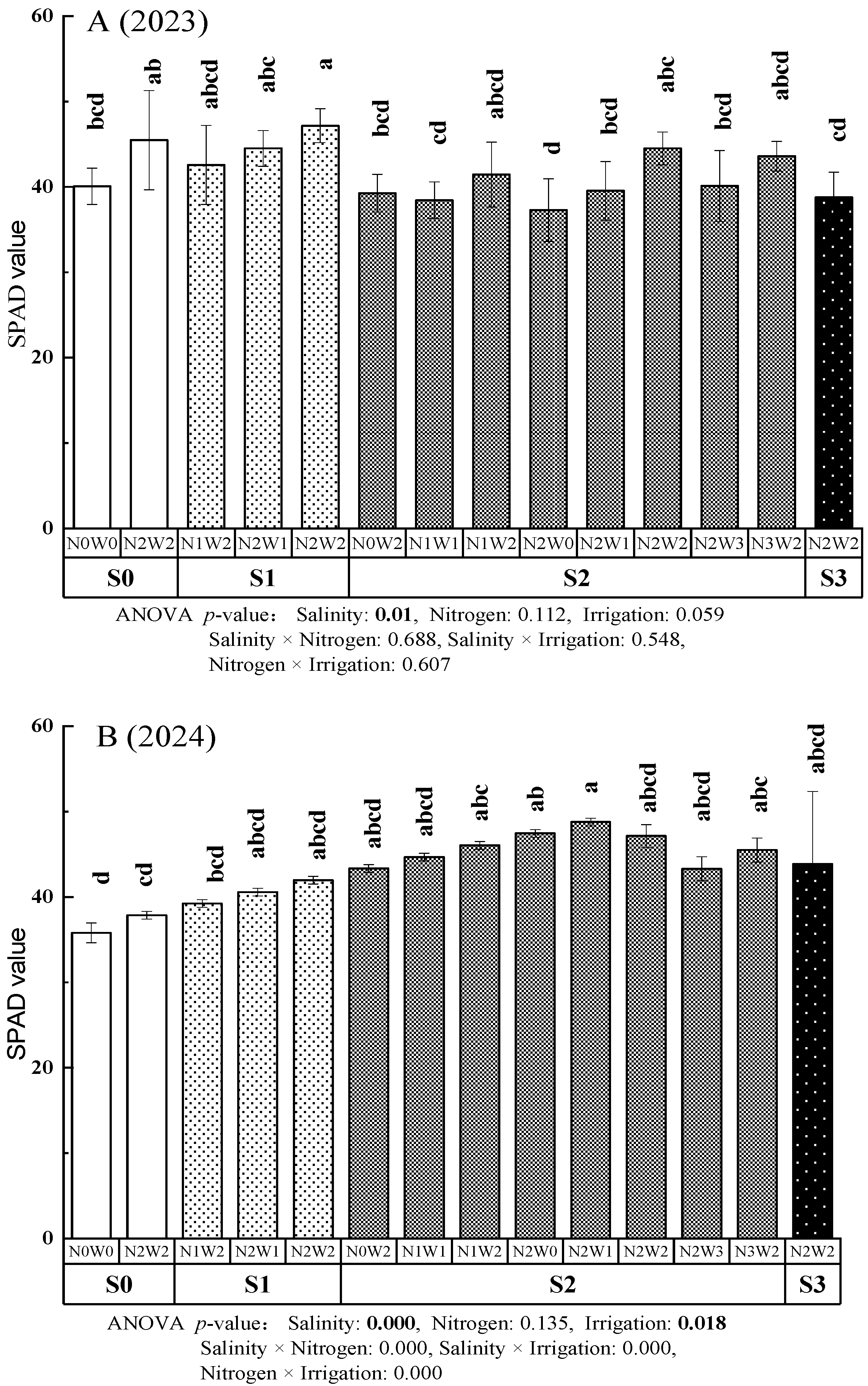

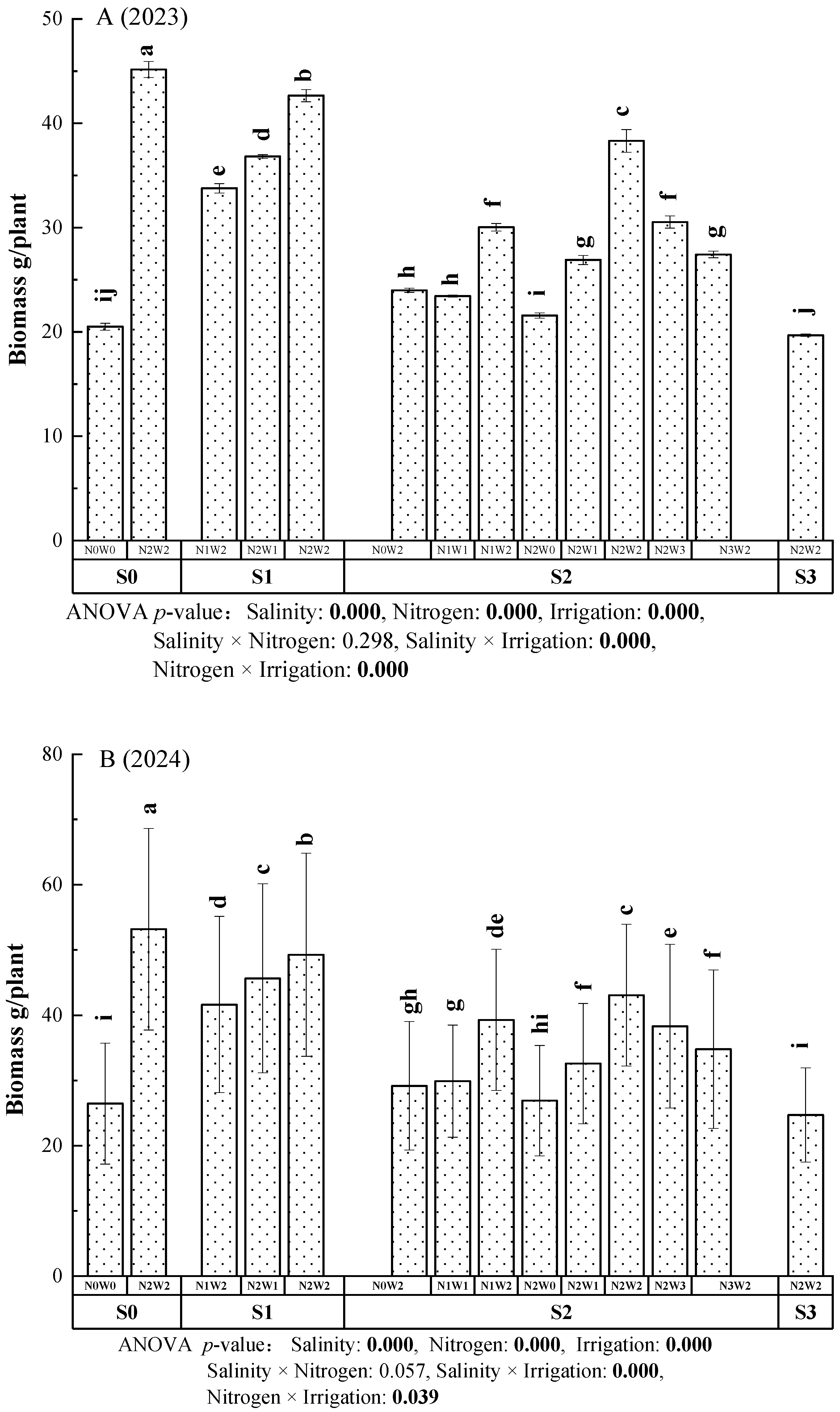
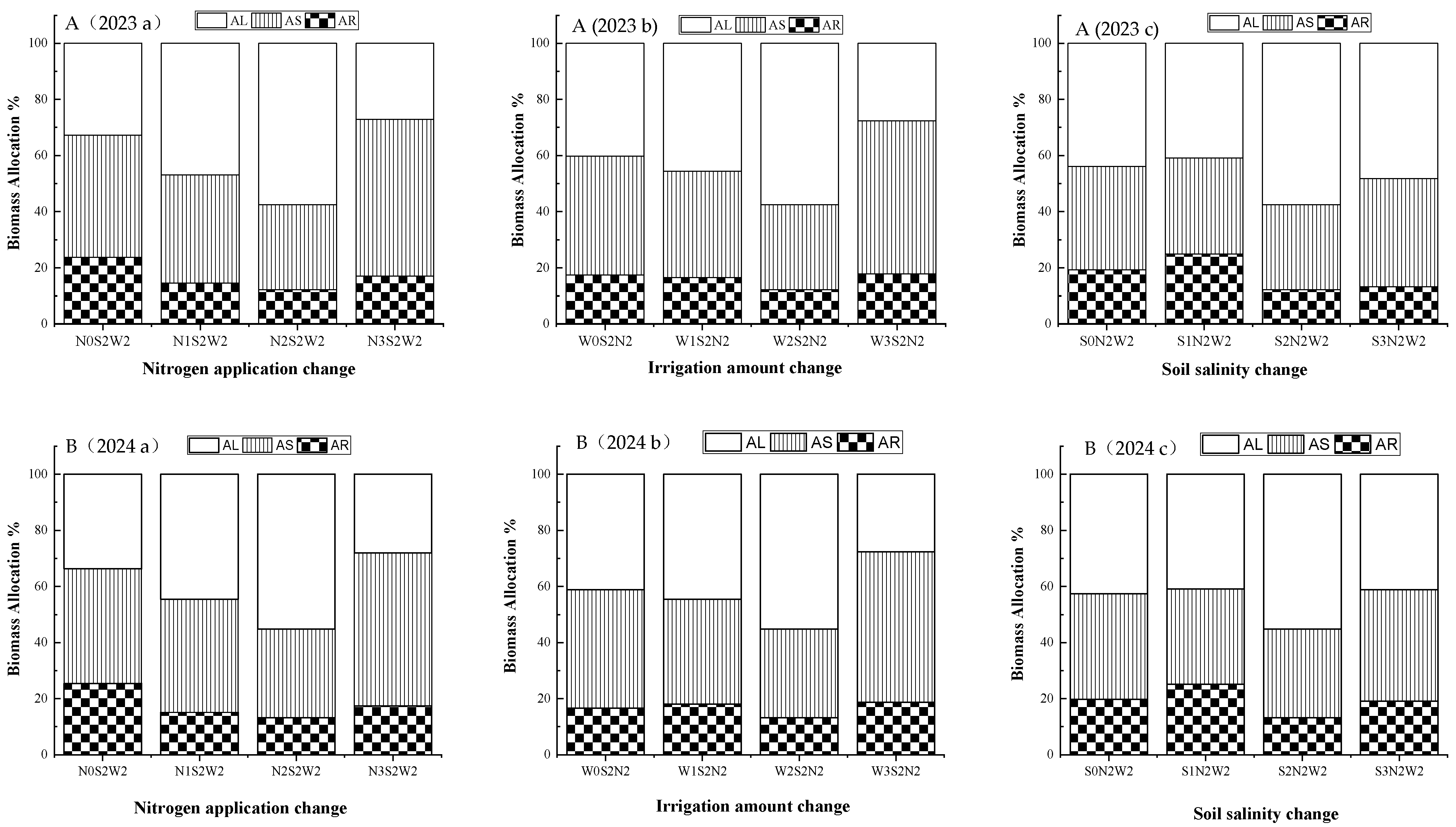
| Treatment | Leaf Length (cm) | Leaf Width (cm) | Leaf Length-to-Width Ratio | Leaf Area (cm2) |
|---|---|---|---|---|
| S0N0W0 | 15.95 ± 0.53 gh | 0.64 ± 0.01 f | 24.92 ± 0.87 b | 8.36 ± 0.32 fg |
| S0N2W2 | 23.75 ± 0.25 a | 1.03 ± 0.05 b | 23.09 ± 1.10 bc | 20.06 ± 1.11 a |
| S1N1W2 | 19.33 ± 0.58 c | 0.84 ± 0.04 cde | 22.97 ± 1.79 bc | 13.35 ± 0.25 cd |
| S1N2W1 | 18.43 ± 1.12 cd | 0.94 ± 0.04 bc | 19.66 ± 1.87 d | 13.40 ± 1.28 cd |
| S1N2W2 | 20.74 ± 1.87 b | 1.06 ± 0.04 b | 19.57 ± 1.60 d | 17.08 ± 2.88 b |
| S2N0W2 | 16.65 ± 0.18 efgh | 0.84 ± 0.05 cde | 19.77 ± 0.93 d | 10.84 ± 0.23 def |
| S2N1W1 | 16.30 ± 0.51 fgh | 0.83 ± 0.03 cde | 19.48 ± 0.25 d | 10.23 ± 0.68 ef |
| S2N1W2 | 17.04 ± 0.81 defg | 1.32 ± 0.30 a | 13.22 ± 2.30 e | 15.64 ± 4.16 bc |
| S2N2W0 | 15.17 ± 0.55 h | 0.75 ± 0.03 def | 20.23 ± 0.21 cd | 9.33 ± 0.66 efg |
| S2N2W1 | 16.94 ± 0.68 defg | 0.91 ± 0.04 bcd | 18.73 ± 1.56 d | 11.85 ± 0.46 de |
| S2N2W2 | 24.27 ± 0.49 a | 0.78 ± 0.06 cdef | 31.22 ± 2.27 a | 15.52 ± 1.16 bc |
| S2N2W3 | 17.83 ± 1.01 de | 0.95 ± 0.07 bc | 18.81 ± 2.38 d | 13.11 ± 0.65 cd |
| S2N3W2 | 17.55 ± 0.27 def | 0.82 ± 0.04 cde | 21.36 ± 1.35 cd | 11.49 ± 0.17 de |
| S3N2W2 | 13.44 ± 0.92 i | 0.69 ± 0.05 ef | 19.45 ± 2.70 d | 7.16 ± 0.39 g |
| Treatment | Leaf Length (cm) | Leaf Width (cm) | Leaf Length-to-Width Ratio | Leaf Area (cm2) |
|---|---|---|---|---|
| S0N0W0 | 15.24 ± 1.41 de | 0.61 ± 0.02 f | 25.02 ± 2.76 ab | 6.13 ± 0.52 f |
| S0N2W2 | 25.73 ± 1.77 a | 1.04 ± 0.07 b | 24.80 ± 3.23 ab | 18.74 ± 0.60 a |
| S1N1W2 | 20.55 ± 2.94 bc | 0.87 ± 0.06 cde | 23.94 ± 4.90 b | 12.39 ± 1.04 cd |
| S1N2W1 | 19.65 ± 1.64 bcd | 0.94 ± 0.04 bc | 20.81 ± 1.09 b | 13.00 ± 1.55 cd |
| S1N2W2 | 22.42 ± 1.99 ab | 1.07 ± 0.01 b | 21.00 ± 1.63 b | 16.75 ± 1.68 ab |
| S2N0W2 | 18.31 ± 1.81 cde | 0.83 ± 0.04 cde | 22.07 ± 3.22 b | 10.56 ± 0.80 de |
| S2N1W1 | 16.73 ± 0.36 cde | 0.83 ± 0.05 cde | 20.13 ± 1.34 bc | 9.48 ± 0.47 def |
| S2N1W2 | 17.14 ± 3.27 cde | 1.28 ± 0.19 a | 13.45 ± 2.22 c | 15.49 ± 4.64 bc |
| S2N2W0 | 14.68 ± 0.71 e | 0.73 ± 0.03 def | 20.11 ± 0.63 bc | 7.29 ± 0.58 ef |
| S2N2W1 | 16.88 ± 1.31 cde | 0.91 ± 0.04 bcd | 18.54 ± 2.12 bc | 10.68 ± 0.60 de |
| S2N2W2 | 23.01 ± 1.87 ab | 0.77 ± 0.07 cde | 30.11 ± 4.33 a | 12.37 ± 0.86 cd |
| S2N2W3 | 17.59 ± 1.73 cde | 0.95 ± 0.08 bc | 18.61 ± 3.17 bc | 11.70 ± 1.10 d |
| S2N3W2 | 17.16 ± 1.39 cde | 0.81 ± 0.08 cde | 21.26 ± 1.95 b | 9.59 ± 1.68 def |
| S3N2W2 | 13.58 ± 0.32 e | 0.70 ± 0.07 ef | 19.53 ± 2.07 bc | 6.34 ± 0.67 f |
| Source of Variation | 2023 | 2024 | ||||||
|---|---|---|---|---|---|---|---|---|
| Length | Width | L:W | Area | Length | Width | L:W | Area | |
| S | ** | ** | ** | ** | ** | ** | ** | ** |
| N | ** | ** | * | ** | * | ** | ns | ** |
| I | ** | ** | ** | ** | ** | ** | * | ** |
| S × N | ** | ** | ** | * | ns | ** | ** | ** |
| S × I | ** | ** | ** | ns | ns | ** | ** | ns |
| N × I | ** | ** | ** | ns | ** | ** | ** | * |
| Treatment | Pn (μmol(CO2) m−2·s−1) | Tr (mmol·m−2·s−1) | Gs (mmol·m−2·s−1) | Ci (μmol·mol−1) |
|---|---|---|---|---|
| S0N0W0 | 8.86 ± 0.59 bcd | 3.53 ± 0.64 c | 10.68 ± 5.49 ab | 414.80 ± 17.26 ab |
| S0N2W2 | 14.62 ± 1.74 a | 5.53 ± 0.64 ab | 20.68 ± 10.54 a | 352.80 ± 8.80 c |
| S1N1W2 | 10.05 ± 2.12 bcd | 3.90 ± 1.14 c | 9.81 ± 5.60 ab | 414.2 ± 24.46 ab |
| S1N2W1 | 12.22 ± 2.54 ab | 4.04 ± 1.08 bc | 13.96 ± 6.64 ab | 374.67 ± 24.97 bc |
| S1N2W2 | 14.72 ± 1.58 a | 5.64 ± 0.60 a | 20.61 ± 10.55 a | 352.67 ± 6.11 c |
| S2N0W2 | 8.68 ± 1.49 bcd | 3.06 ± 0.78 c | 9.86 ± 5.46 ab | 404.37 ± 16.74 ab |
| S2N1W1 | 8.59 ± 1.55 bcd | 2.98 ± 0.75 c | 9.61 ± 5.21 ab | 394.73 ± 17.55 ab |
| S2N1W2 | 10.24 ± 2.16 bcd | 3.53 ± 0.96 c | 11.82 ± 6.25 ab | 389.2 ± 21.81 abc |
| S2N2W0 | 7.17 ± 2.44 d | 2.72 ± 0.43 c | 6.40 ± 3.73 b | 425.63 ± 27.77 a |
| S2N2W1 | 8.93 ± 2.56 bcd | 3.29 ± 0.59 c | 9.62 ± 5.47 ab | 402.5 ± 27.84 ab |
| S2N2W2 | 12.12 ± 2.51 abc | 4.10 ± 1.05 bc | 13.95 ± 7.01 ab | 374.17 ± 20.73 bc |
| S2N2W3 | 9.73 ± 2.09 bcd | 3.70 ± 1.10 c | 9.42 ± 5.29 ab | 394.63 ± 23.97 ab |
| S2N3W2 | 10.52 ± 1.67 bcd | 3.79 ± 1.03 c | 11.46 ± 6.27 ab | 386.87 ± 18.23 abc |
| S3N2W2 | 8.25 ± 2.22 cd | 3.70 ± 1.04 c | 9.28 ± 4.37 ab | 409.30 ± 23.83 ab |
| Treatment | Pn (μmol(CO2) m−2·s−1) | Tr (mmol·m−2·s−1) | Gs (mmol·m−2·s−1) | Ci (μmol·mol−1) |
|---|---|---|---|---|
| S0N0W0 | 7.33 ± 1.06 d | 3.17 ± 0.12 c | 13.34 ± 1.76 cd | 401.00 ± 17.62 abc |
| S0N2W2 | 8.97 ± 0.58 cd | 3.65 ± 0.52 bc | 23.03 ± 1.67 a | 348.83 ± 12.07 c |
| S1N1W2 | 10.23 ± 0.36 bcd | 3.96 ± 0.47 bc | 13.17 ± 1.69 cd | 419.30 ± 16.04 a |
| S1N2W1 | 11.55 ± 0.53 abcd | 4.42 ± 0.48 b | 17.28 ± 2.09 bc | 384.97 ± 27.73 abc |
| S1N2W2 | 13.29 ± 0.58 abc | 5.53 ± 0.31 a | 20.58 ± 3.41 ab | 360.53 ± 6.61 bc |
| S2N0W2 | 15.16 ± 0.65 a | 3.55 ± 0.37 bc | 13.03 ± 1.95 cd | 413.03 ± 18.34 ab |
| S2N1W1 | 9.75 ± 5.78 bcd | 3.24 ± 0.38 c | 12.96 ± 2.07 cd | 396.03 ± 23.11 abc |
| S2N1W2 | 7.64 ± 0.54 d | 3.76 ± 0.35 bc | 15.17 ± 2.04 bcd | 395.80 ± 17.01 abc |
| S2N2W0 | 9.30 ± 0.55 cd | 3.15 ± 0.21 c | 10.79 ± 1.65 d | 428.23 ± 25.84 a |
| S2N2W1 | 10.96 ± 0.55 abcd | 3.61 ± 0.37 bc | 12.98 ± 2.06 cd | 405.53 ± 23.69 abc |
| S2N2W2 | 12.65 ± 0.55 abc | 4.21 ± 0.36 bc | 17.25 ± 2.28 bc | 377.80 ± 15.65 abc |
| S2N2W3 | 14.30 ± 0.55 ab | 4.18 ± 0.35 bc | 13.10 ± 2.24 cd | 396.10 ± 36.03 abc |
| S2N3W2 | 7.13 ± 1.71 d | 3.91 ± 0.76 bc | 14.81 ± 2.36 cd | 394.70 ± 19.80 abc |
| S3N2W2 | 10.61 ± 0.96 abcd | 3.67 ± 0.16 bc | 11.30 ± 1.16 cd | 396.40 ± 13.15 abc |
| Source of Variation | Leaf Photosynthetic Parameters (2023) | Leaf Photosynthetic Parameters (2024) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pn | Tr | Gs | Ci | Pn | Tr | Gs | Ci | |
| S | ** | * | ns | * | ** | ** | ** | ** |
| N | * | ns | ns | ns | ** | ** | ** | ns |
| I | * | ns | ns | ns | ** | ** | ** | * |
| S × N | ns | ns | ns | ns | ns | * | ns | ns |
| S × I | ns | ns | ns | ns | ns | ns | ns | ns |
| N × I | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | LER (mm/d) | LFW (g) | LDW (g) | SLW (g cm−2) | RWC (%) |
|---|---|---|---|---|---|
| S0N0W0 | 1.507 ± 0.057 cd | 0.091 ± 0.010 ef | 0.019 ± 0.003 efg | 445.39 ± 54.30 cd | 95.99 ab |
| S0N2W2 | 1.827 ± 0.085 a | 0.118 ± 0.007 cd | 0.020 ± 0.002 efg | 986.24 ± 103.78 a | 97.20 ab |
| S1N1W2 | 1.643 ± 0.025 b | 0.160 ± 0.024 b | 0.026 ± 0.002 cd | 509.83 ± 51.48 cd | 97.76 a |
| S1N2W1 | 1.837 ± 0.116 a | 0.171 ± 0.020 b | 0.031 ± 0.004 b | 441.07 ± 74.72 cd | 98.33 a |
| S1N2W2 | 1.837 ± 0.025 a | 0.080 ± 0.018 fg | 0.019 ± 0.005 efg | 937.69 ± 374.06 a | 96.91 ab |
| S2N0W2 | 1.437 ± 0.021 d | 0.063 ± 0.009 g | 0.014 ± 0.002 h | 783.33 ± 98.47 ab | 95.86 ab |
| S2N1W1 | 1.433 ± 0.025 d | 0.134 ± 0.007 c | 0.022 ± 0.002 def | 460.44 ± 53.77 cd | 97.66 a |
| S2N1W2 | 1.553 ± 0.021 c | 0.114 ± 0.009 cd | 0.025 ± 0.003 d | 618.89 ± 96.30 bc | 95.96 ab |
| S2N2W0 | 1.443 ± 0.015 d | 0.133 ± 0.005 c | 0.030 ± 0.001 bc | 307.70 ± 14.88 d | 94.84 b |
| S2N2W1 | 1.507 ± 0.021 cd | 0.123 ± 0.002 cd | 0.026 ± 0.002 d | 463.31 ± 36.57 cd | 97.76 a |
| S2N2W2 | 1.820 ± 0.036 a | 0.081 ± 0.004 fg | 0.016 ± 0.003 gh | 982.07 ± 255.23 a | 96.77 ab |
| S2N2W3 | 1.640 ± 0.026 b | 0.216 ± 0.008 a | 0.038 ± 0.001 a | 342.76 ± 9.67 d | 97.43 ab |
| S2N3W2 | 1.570 ± 0.010 bc | 0.106 ± 0.004 de | 0.019 ± 0.002 fg | 619.00 ± 59.82 bc | 96.64 ab |
| S3N2W2 | 0.983 ± 0.006 e | 0.085 ± 0.002 f | 0.023 ± 0.001 de | 305.28 ± 13.41 d | 95.01 b |
| Treatment | LER (mm/d) | LFW (g) | LDW (g) | SLW (g cm−2) | RWC (%) |
|---|---|---|---|---|---|
| S0N0W0 | 1.323 ± 0.220 e | 0.094 ± 0.010 bcd | 0.045 ± 0.005 bcd | 136.49 ± 6.08 d | 96.27 ab |
| S0N2W2 | 1.810 ± 0.114 ab | 0.121 ± 0.006 abcd | 0.058 ± 0.003 abcd | 322.83 ± 23.65 abc | 97.31 ab |
| S1N1W2 | 1.607 ± 0.032 bcd | 0.162 ± 0.024 abc | 0.078 ± 0.012 abc | 159.93 ± 12.39 cd | 97.75 ab |
| S1N2W1 | 1.817 ± 0.064 ab | 0.174 ± 0.020 ab | 0.083 ± 0.010 ab | 158.16 ± 32.61 cd | 97.91 a |
| S1N2W2 | 1.760 ± 0.098 abc | 0.082 ± 0.018 cd | 0.039 ± 0.009 cd | 445.53 ± 144.32 a | 96.48 ab |
| S2N0W2 | 1.437 ± 0.015 de | 0.064 ± 0.010 d | 0.031 ± 0.005 d | 349.45 ± 71.66 ab | 95.60 ab |
| S2N1W1 | 1.457 ± 0.047 de | 0.136 ± 0.007 abcd | 0.065 ± 0.003 abcd | 145.15 ± 10.20 d | 95.95 ab |
| S2N1W2 | 1.557 ± 0.025 cde | 0.116 ± 0.009 abcd | 0.056 ± 0.004 abcd | 275.62 ± 64.31 bcd | 95.41 ab |
| S2N2W0 | 1.553 ± 0.125 cde | 0.133 ± 0.009 abcd | 0.064 ± 0.004 abcd | 115.20 ± 17.45 d | 94.17 b |
| S2N2W1 | 0.510 ± 0.036 de | 0.114 ± 0.022 abcd | 0.055 ± 0.011 abcd | 202.00 ± 55.73 bcd | 96.77 ab |
| S2N2W2 | 1.853 ± 0.031 a | 0.123 ± 0.074 abcd | 0.059 ± 0.036 abcd | 251.46 ± 104.38 bcd | 96.37 ab |
| S2N2W3 | 1.627 ± 0.040 abcd | 0.185 ± 0.066 a | 0.089 ± 0.031 a | 146.34 ± 62.91 d | 97.08 ab |
| S2N3W2 | 1.550 ± 0.030 cde | 0.100 ± 0.012 abcd | 0.048 ± 0.006 bcd | 200.91 ± 25.75 bcd | 96.84 ab |
| S3N2W2 | 0.977 ± 0.015 f | 0.087 ± 0.001 cd | 0.042 ± 0.001 cd | 152.63 ± 14.72 cd | 94.51 ab |
| Source of Variation | Leaf Water-Related Parameters (2023) | Leaf Water-Related Parameters (2024) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LER | LFW | LDW | SLW | RWC | LER | LFW | LDW | SLW | RWC | |
| S | ** | ** | ** | ** | ns | ** | ns | ns | ** | * |
| N | ** | ** | ** | ** | ns | ** | * | ** | ** | ns |
| I | ** | ** | ** | ** | * | * | * | ** | ** | * |
| S × N | ns | ** | ns | ns | ns | ns | * | * | ** | ns |
| S × I | ** | ** | ns | ns | ns | ** | * | ** | ** | ns |
| N × I | ** | ns | ** | * | ns | * | ns | ns | ns | ns |
| Treatment | Height (cm) | Number of Leaves | Root Dry Weight (g/plant) | Stem Dry Weight (g/plant) | Leaf Dry Weight (g/plant) |
|---|---|---|---|---|---|
| S0N0W0 | 83.12 ± 5.08 e | 9.33 ± 0.58 fg | 2.43 ± 0.11 f | 11.50 ± 0.51 h | 9.23 ± 0.41 j |
| S0N2W2 | 141.23 ± 5.25 a | 15.33 ± 1.53 a | 5.00 ± 0.08 d | 23.65 ± 0.37 a | 18.97 ± 0.30 b |
| S1N1W2 | 108.48 ± 4.44 c | 13.00 ± 1.00 bcd | 3.85 ± 0.11 d | 18.20 ± 0.51 e | 14.60 ± 0.41 d |
| S1N2W1 | 122.12 ± 3.14 b | 11.67 ± 0.58 cde | 3.00 ± 0.07 b | 14.20 ± 0.33 d | 11.39 ± 0.27 e |
| S1N2W2 | 137.35 ± 4.00 a | 13.67 ± 1.53 ab | 4.70 ± 0.09 a | 22.23 ± 0.42 c | 17.83 ± 0.33 c |
| S2N0W2 | 78.24 ± 5.47 ef | 11.00 ± 1.00 ef | 2.92 ± 0.15 e | 13.82 ± 0.71 g | 11.09 ± 0.57 hi |
| S2N1W1 | 76.65 ± 5.49 ef | 11.33 ± 1.15 df | 2.81 ± 0.06 gh | 13.29 ± 0.3 h | 10.66 ± 0.24 g |
| S2N1W2 | 92.84 ± 6.35 d | 11.00 ± 1.00 ef | 3.52 ± 0.09 g | 16.63 ± 0.42 f | 13.34 ± 0.33 de |
| S2N2W0 | 75.22 ± 3.05 f | 9.00 ± 1.00 g | 2.46 ± 0.18 i | 11.64 ± 0.83 i | 9.34 ± 0.67 gh |
| S2N2W1 | 83.64 ± 3.28 e | 11.67 ± 0.58 cde | 3.03 ± 0.11 gh | 14.33 ± 0.52 g | 11.50 ± 0.42 f |
| S2N2W2 | 122.12 ± 2.13 b | 11.67 ± 0.58 cde | 4.39 ± 0.18 g | 20.75 ± 0.86 f | 16.64 ± 0.69 a |
| S2N2W3 | 102.91 ± 4.24 c | 12.33 ± 0.58 bcde | 3.42 ± 0.06 f | 16.15 ± 0.29 a | 12.96 ± 0.23 h |
| S2N3W2 | 94.64 ± 11.03 d | 13.33 ± 1.15 bc | 3.07 ± 0.05 g | 14.52 ± 0.25 b | 11.65 ± 0.20 i |
| S3N2W2 | 62.66 ± 4.17 g | 8.00 ± 1.00 g | 2.26 ± 0.07 j | 10.67 ± 0.32 j | 8.56 ± 0.26 g |
| Treatment | Height (cm) | Number of Leaves | Root Dry Weight (g/plant) | Stem Dry Weight (g/plant) | Leaf Dry Weight (g/plant) |
|---|---|---|---|---|---|
| S0N0W0 | 97.91 ± 9.82 de | 10.00 ± 1.00 cd | 5.57 ± 0.04 ef | 10.08 ± 0.23 f | 5.50 ± 0.16 j |
| S0N2W2 | 140.54 ± 2.20 a | 14.67 ± 1.53 ab | 8.79 ± 0.10 c | 16.67 ± 0.89 a | 18.91 ± 0.90 b |
| S1N1W2 | 111.30 ± 1.50 c | 12.00 ± 1.00 abc | 7.49 ± 0.38 d | 12.36 ± 0.76 de | 14.80 ± 0.52 d |
| S1N2W1 | 127.80 ± 7.89 b | 11.00 ± 1.00 bcd | 9.64 ± 0.58 b | 14.20 ± 0.10 bc | 13.65 ± 0.32 e |
| S1N2W2 | 140.18 ± 3.94 a | 15.00 ± 2.00 a | 10.26 ± 0.08 a | 13.89 ± 1.04 bcd | 16.69 ± 0.33 c |
| S2N0W2 | 79.10 ± 0.37 gh | 12.00 ± 2.65 abc | 5.99 ± 0.36 e | 9.64 ± 0.83 fg | 7.95 ± 0.13i |
| S2N1W1 | 77.37 ± 1.17 gh | 11.33 ± 0.58 abcd | 5.35 ± 0.26 ef | 9.58 ± 0.27 fg | 9.82 ± 0.18 fg |
| S2N1W2 | 93.76 ± 1.15 ef | 12.00 ± 1.00 abc | 4.95 ± 0.19 f | 13.25 ± 1.03 cde | 14.60 ± 0.58 de |
| S2N2W0 | 76.84 ± 0.58 gh | 8.67 ± 0.58 cd | 3.68 ± 0.36 g | 9.41 ± 0.55 fg | 9.15 ± 0.32 gh |
| S2N2W1 | 84.51 ± 0.80 fg | 11.33 ± 0.58 abcd | 4.94 ± 0.10 f | 10.27 ± 0.06 f | 12.26 ± 0.25 f |
| S2N2W2 | 125.30 ± 3.04 b | 11.67 ± 1.15 abc | 4.93 ± 0.12 f | 11.81 ± 0.38 e | 20.59 ± 0.58 a |
| S2N2W3 | 104.71 ± 2.02 cd | 12.33 ± 1.53 abc | 5.71 ± 0.05 e | 16.49 ± 0.72 a | 8.46 ± 0.48 hi |
| S2N3W2 | 93.73 ± 0.87 ef | 12.00 ± 1.00 abc | 4.88 ± 0.13 f | 15.26 ± 0.07 ab | 7.87 ± 0.44 i |
| S3N2W2 | 68.64 ± 5.52 h | 8.00 ± 1.00 d | 3.93 ± 0.15 g | 8.17 ± 0.16 g | 8.48 ± 0.08 hi |
| Source of Variation | 2023 | 2024 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Height | Leaves Number | Root Dry Weight | Stem Dry Weight | Leaf Dry Weight | Height | Leaves Number | Root Dry Weight | Stem Dry Weight | Leaf Dry Weight | |
| S | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| N | ** | * | ** | ** | ** | ** | ns | ** | ** | ** |
| I | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| S × N | ns | ns | ** | ** | ** | ns | * | ** | ** | ** |
| S × I | ** | ns | ** | ns | ** | ** | * | ** | * | ** |
| N × I | ** | ns | ns | ** | ** | ** | ns | ns | ** | ** |
| Variation | Simple Correlation Coefficient with Biomass Y | Path Coefficient (Direct Function) | Correlation Coefficient Between Independent Variables | Indirect Path Coefficient | |||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X1-Y | X2-Y | X3-Y | SUM | |||
| X1 | −0.477 | 0.707 ** | --- | 0.255 | 0.255 | --- | 0.100 | 0.130 | 0.230 |
| X2 | 0.342 | 0.391 ** | 0.255 | --- | 0.258 | −0.180 | ---- | 0.132 | −0.048 |
| X3 | 0.431 | 0.511 ** | 0.255 | 0.258 | --- | −0.180 | 0.101 | ---- | −0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Fan, Y.; Chen, T.; Duan, Z.; Liu, S.; Gao, X. Leaf Plasticity and Biomass Allocation of Arundo donax Under Combined Irrigation and Nitrogen Conditions in Salinized Soil. Agriculture 2025, 15, 1166. https://doi.org/10.3390/agriculture15111166
Jia Y, Fan Y, Chen T, Duan Z, Liu S, Gao X. Leaf Plasticity and Biomass Allocation of Arundo donax Under Combined Irrigation and Nitrogen Conditions in Salinized Soil. Agriculture. 2025; 15(11):1166. https://doi.org/10.3390/agriculture15111166
Chicago/Turabian StyleJia, Yamin, Yaqiong Fan, Tingyu Chen, Zhiwen Duan, Shuhui Liu, and Xiaoli Gao. 2025. "Leaf Plasticity and Biomass Allocation of Arundo donax Under Combined Irrigation and Nitrogen Conditions in Salinized Soil" Agriculture 15, no. 11: 1166. https://doi.org/10.3390/agriculture15111166
APA StyleJia, Y., Fan, Y., Chen, T., Duan, Z., Liu, S., & Gao, X. (2025). Leaf Plasticity and Biomass Allocation of Arundo donax Under Combined Irrigation and Nitrogen Conditions in Salinized Soil. Agriculture, 15(11), 1166. https://doi.org/10.3390/agriculture15111166





