The Mechanism of Seed Priming with Abscisic Acid for Enhancing Cuticle Deposition Under Drought Stress: Phenotypic and Transcriptomic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions, Seed Priming Treatments, Stress Treatments, and Exogenous Hormone Treatments
2.2. Leaf Cuticle Composition and Structure Analysis
Leaf Cutin and Wax Extraction
2.3. GC/MS Analysis
2.4. Leaf Growth and Physiological Characteristic Analysis
2.4.1. Leaf Relative Water Content
2.4.2. Leaf Water Loss Rate
2.5. Biomass Analysis
2.6. RNA Quantification and Qualification
2.7. Quantitative Real-Time PCR Validation
2.8. Statistical Analysis
3. Results
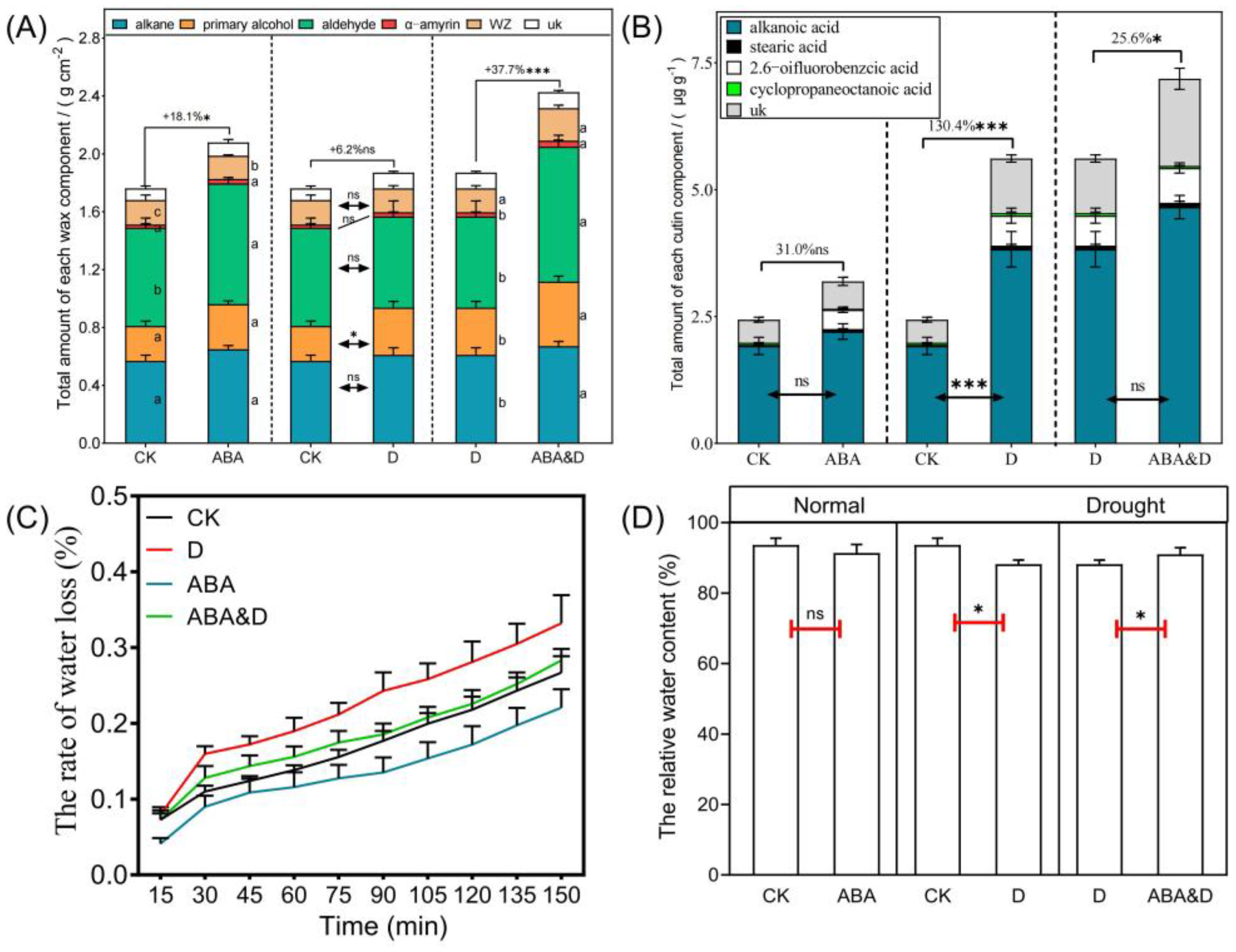
3.1. Seed Priming Improved Leaf Cuticular Wax Deposition and Drought Tolerance of Sorghum Seedlings
3.2. ABA Priming Influenced Plant Cutin and Wax Biosynthesis
3.3. Co-Expression Network Analysis Identified Photosynthesis and Wax-Related DEGs
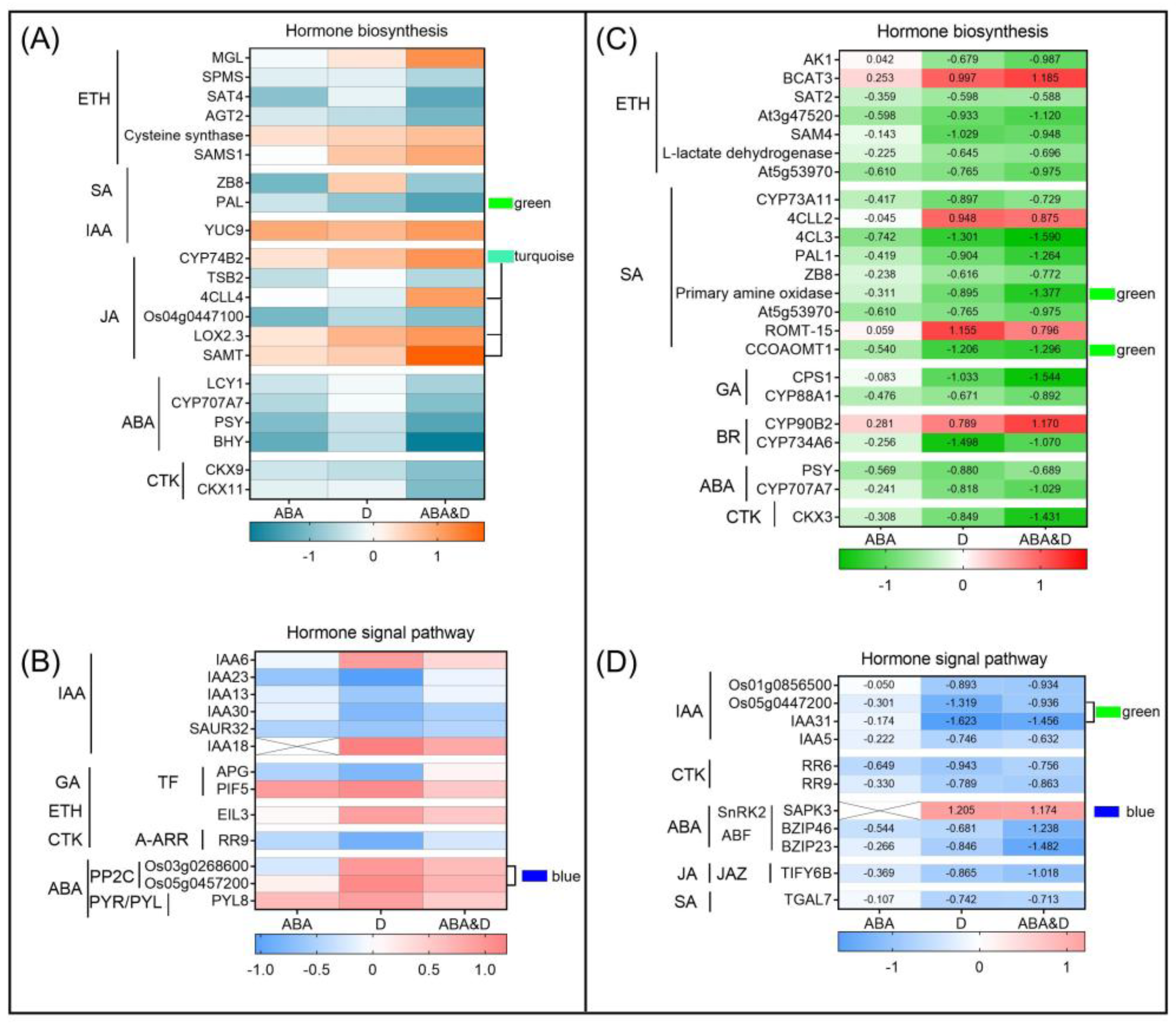
3.4. Co-Expression Network Analysis Identified Hormone- and Wax-Related DEGs
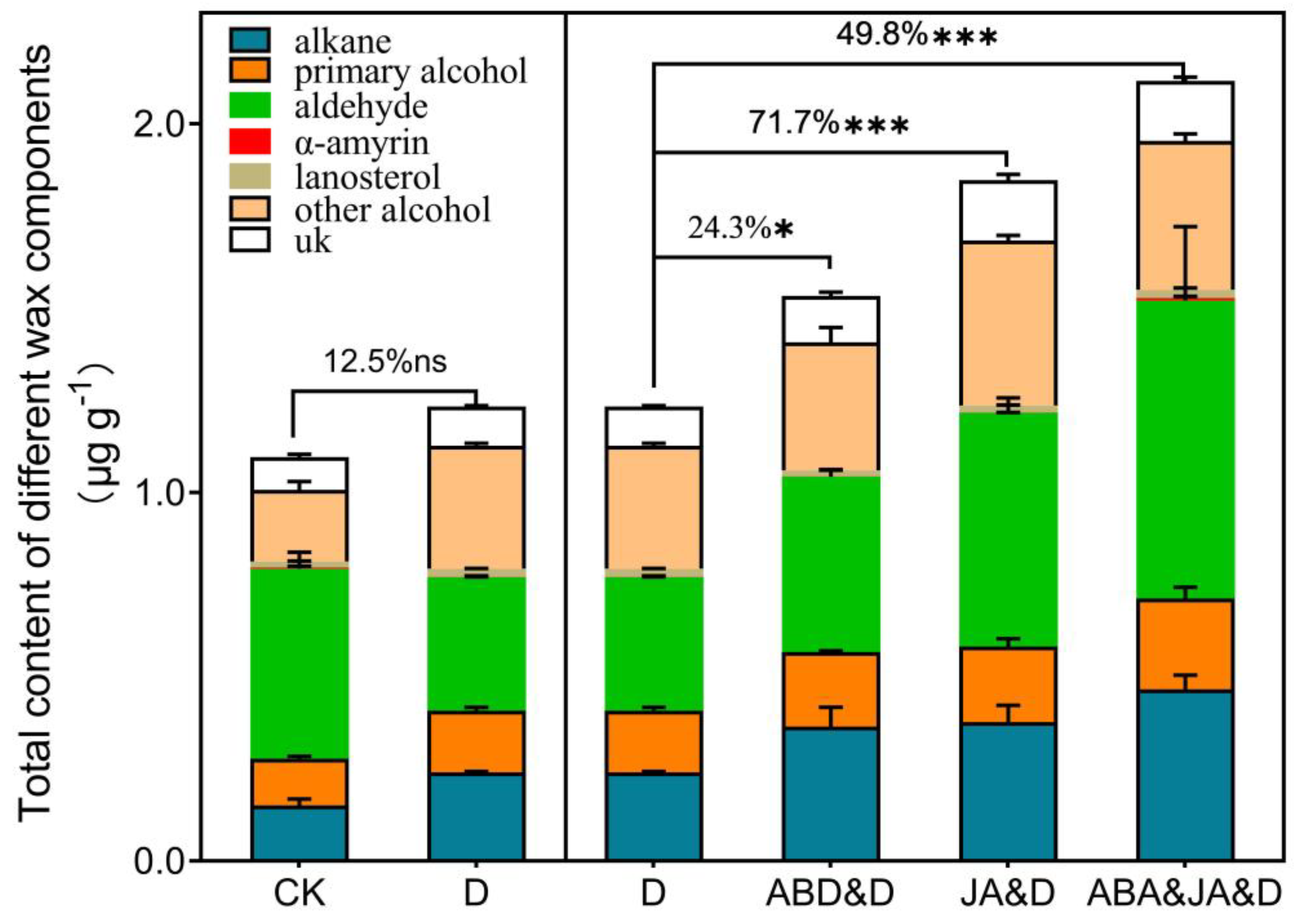
3.5. Verification Using Other Sweet Sorghum Cultivars (DLS and ML8000)
4. Discussion
4.1. Seed Priming with ABA Influenced Endogenous Hormone Accumulation
4.2. Seed Priming with ABA Improved Cuticle Tolerance by Endogenous Hormone Adjustment Under Drought Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| DLS | Hunnigreen |
| ML8000 | Mule8000 |
| CK | control |
| D | drought stress |
| ABA | abscisic acid |
| JA | Jasmonic acid |
| GC | Gas chromatography |
| MS | Mass Spectrometry |
| FID | flame ionization detector |
| RWC | relative water content |
| FW | fresh weight |
| SW | saturated weight |
| DEGs | differentially expressed genes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DW | dry weight |
| WGCNA | Weighted gene co-expression network analysis |
| qRT-PCR | Quantitative real-time PCR |
References
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.B.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture-recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Wojtyla, L.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds-increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; De Man, W.; De Proft, M.; Prinsen, E.; Delcour, J.A. The impact of hydro-priming and osmo-priming on seedling characteristics, plant hormone goncentrations, activity of selected hydrolytic enzymes, and cell wall and phytate hydrolysis in sprouted wheat (Triticum aestivum L.). Acs Omega 2019, 4, 22089–22100. [Google Scholar] [CrossRef]
- Murungu, F.S.; Chiduza, C.; Nyamugafata, P.; Clark, L.J.; Whalley, W.R.; Finch-Savage, W.E. Effects of ‘on-farm seed priming’ on consecutive daily sowing occasions on the emergence and growth of maize in semi-arid Zimbabwe. Field Crops Res. 2004, 89, 49–57. [Google Scholar] [CrossRef]
- Harris, D. The effects of manure, genotype, seed priming, depth and date of sowing on the emergence and early growth of Sorghum bicolor (L) Moench in semi-arid Botswana. Soil Tillage Res. 1996, 40, 73–88. [Google Scholar] [CrossRef]
- Tu, K.l.; Cheng, Y.; Pan, T.; Wang, J.; Sun, Q. Effects of seed priming on vitality and preservation of pepper seeds. Agriculture 2022, 12, 603. [Google Scholar] [CrossRef]
- Iqbal, S.; Bano, A.; Ilyas, N. Abscisic acid (ABA) seed soaking induced changes in physiology of two wheat cultivars under water stress. Pak. J. Bot. 2012, 44, 51–56. [Google Scholar] [CrossRef]
- Abid, M.; Hakeem, A.; Shao, Y.; Liu, Y.; Zahoor, R.; Fan, Y.; Jiang, S.; Ata-Ul-Karim, S.T.; Tian, Z.; Jiang, D.; et al. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2018, 145, 12–20. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A.; Shahid, M. Terminal drought and seed priming improves drought tolerance in wheat. Physiol. Mol. Biol. Plants 2018, 24, 845–856. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Bargel, H.; Neinhuis, C. Altered tomato (Lycopersicon esculentum Mill.) fruit cuticle biomechanics of a pleiotropic non ripening mutant. J. Plant Growth Regul. 2004, 23, 61–75. [Google Scholar] [CrossRef]
- Bernard, A.; Joubes, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef]
- Kim, H.; Choi, D.; Suh, M.C. Cuticle ultrastructure, cuticular lipid composition, and gene expression in hypoxia-stressed Arabidopsis stems and leaves. Plant Cell Rep. 2017, 36, 815–827. [Google Scholar] [CrossRef]
- Cui, F.; Brosche, M.; Lehtonen, M.T.; Amiryousefi, A.; Xu, E.; Punkkinen, M.; Valkonen, J.P.T.; Fujii, H.; Overmyer, K. Dissecting abscisic acid signaling pathways involved in cuticle formation. Mol. Plant 2016, 9, 926–938. [Google Scholar] [CrossRef]
- Mackova, J.; Vaskova, M.; Macek, P.; Hronkova, M.; Schreiber, L.; Santrucek, J. Plant response to drought stress simulated by ABA application: Changes in chemical composition of cuticular waxes. Environ. Exp. Bot. 2013, 86, 70–75. [Google Scholar] [CrossRef]
- Martin, L.B.B.; Romero, P.; Fich, E.A.; Domozych, D.S.; Rose, J.K.C. Cuticle biosynthesis in tomato leaves is developmentally regulated by abscisic acid. Plant Physiol. 2017, 174, 1384–1398. [Google Scholar] [CrossRef]
- Thomas, T.T.D.; Puthur, J.T. UV-B priming enhances specific secondary metabolites in Oryza sativa (L.) empowering to encounter diverse abiotic stresses. Plant Growth Regul. 2020, 92, 169–180. [Google Scholar] [CrossRef]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Fu, Y.; Hu, Q.; Nawaz, A.; Guan, Y.; Li, Z.; Huang, Y.; Hu, J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ. Sci. Pollut. Res. 2016, 23, 19989–20002. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition and physiological function of the wax layers coating Arabidopsis leaves: β-amyrin negatively affects the intracuticular water barrier. Plant Physiol. 2012, 160, 1120–1129. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- Sarkar, B.; Bandyopadhyay, P.; Das, A.; Pal, S.; Hasanuzzaman, M.; Adak, M.K. Abscisic acid priming confers salt tolerance in maize seedlings by modulating osmotic adjustment, bond energies, ROS homeostasis, and organic acid metabolism. Plant Physiol. Biochem. 2023, 202, 107980. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.; Voytenko, L.; Vasyuk, V.; Shcherbatiuk, M.; Kholodny, M.G. ABA-induced alterations in cytokinin homeostasis of Triticum aestivum and Triticum spelta under heat stress. Plant Stress 2024, 11, 100353. [Google Scholar] [CrossRef]
- Cong, L.; Wu, T.; Liu, H.; Wang, H.; Zhang, H.; Zhao, G.; Wen, Y.; Shi, Q.; Xu, L.; Wang, Z. CPPU may induce gibberellin-independent parthenocarpy associated with PbRR9 in ‘Dangshansu’ pear. Hortic. Res. 2020, 7, 68. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.-J.; Go, Y.S.; Park, C.-M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Li, C.; Haslam, T.M.; Krueger, A.; Schneider, L.M.; Mishina, K.; Samuels, L.; Yan, H.; Kunst, L.; Schaffrath, U.; Nawrath, C.; et al. The β-Ketoacyl-CoA Synthase HvKCS1, Encoded by Cer-zh, plays a key role in synthesis of barley leaf wax and germination of barley powdery mildew. Plant Cell Physiol. 2018, 59, 811–827. [Google Scholar] [CrossRef]
- Graca, J.; Lamosa, P. Linear and branched poly(ω-hydroxyacid) esters in plant cutins. J. Agric. Food Chem. 2010, 58, 9666–9674. [Google Scholar] [CrossRef]
- Polichuk, D.R.; Zhang, Y.; Reed, D.W.; Schmidt, J.F.; Covello, P.S. A glandular trichome-specific monoterpene alcohol dehydrogenase from Artemisia annua. Phytochemistry 2010, 71, 1264–1269. [Google Scholar] [CrossRef]
- Cheeld, H.; Bhutada, G.; Beaudoin, F.; Eastmond, P.J. DES2 is a fatty acid Δ11 desaturase capable of synthesizing palmitvaccenic acid in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Febs Lett. 2020, 594, 1770–1777. [Google Scholar] [CrossRef]
- Millar, A.A.; Clemens, S.; Zachgo, S.; Giblin, E.M.; Taylor, D.C.; Kunst, L. CUT1, an arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 1999, 11, 825–838. [Google Scholar] [CrossRef]
- Lue, S.; Song, T.; Kosma, D.K.; Parsons, E.P.; Rowland, O.; Jenks, M.A. Arabidopsis CER8 encodes Long-chain acyl-CoA synthetase 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J. 2009, 59, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yogendra, K.N.; Karre, S.; Kushalappa, A.C.; Dion, Y.; Choo, T.M. Wax INDUCER1 (HvWIN1) transcription factor regulates free fatty acid biosynthetic genes to reinforce cuticle to resist Fusarium head blight in barley spikelets. J. Exp. Bot. 2016, 67, 4127–4139. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Balfagon, D.; Sengupta, S.; Gomez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Li, S.; Zhou, N.; Guo, Y. The Mechanism of Seed Priming with Abscisic Acid for Enhancing Cuticle Deposition Under Drought Stress: Phenotypic and Transcriptomic Insights. Agriculture 2025, 15, 1124. https://doi.org/10.3390/agriculture15111124
Yao L, Li S, Zhou N, Guo Y. The Mechanism of Seed Priming with Abscisic Acid for Enhancing Cuticle Deposition Under Drought Stress: Phenotypic and Transcriptomic Insights. Agriculture. 2025; 15(11):1124. https://doi.org/10.3390/agriculture15111124
Chicago/Turabian StyleYao, Luhua, Sennan Li, Nana Zhou, and Yanjun Guo. 2025. "The Mechanism of Seed Priming with Abscisic Acid for Enhancing Cuticle Deposition Under Drought Stress: Phenotypic and Transcriptomic Insights" Agriculture 15, no. 11: 1124. https://doi.org/10.3390/agriculture15111124
APA StyleYao, L., Li, S., Zhou, N., & Guo, Y. (2025). The Mechanism of Seed Priming with Abscisic Acid for Enhancing Cuticle Deposition Under Drought Stress: Phenotypic and Transcriptomic Insights. Agriculture, 15(11), 1124. https://doi.org/10.3390/agriculture15111124



