Helping Small-Scale and Socially Disadvantaged Growers in Improving Microbial Quality of Irrigation Water in Kentucky
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FSMA | Food Safety Modernization Act |
| PSR | Produce Safety Rule |
| GAP | Good Agricultural Practices |
| GHP | Good Handling Practices |
| U.S. | United States |
| WHO | World Health Organization |
| EU | European Union |
| FDA | Food and Drug Administration |
| CDC | Center for Disease Control and Prevention |
| STEC | Shiga toxin-producing E. coli |
| MWQP | Microbial water quality profile |
| GM | Geometric mean |
| STV | Statistical threshold value |
| CFU | Colony-forming unit |
| ONPG | O-nitrophenyl-beta-D-galactopyranoside |
| MUG | 4-methylumbelliferyl-β-D-glucuronide |
| MPN | Most Probable Number |
References
- Alegbeleye, O.; Sant’Ana, A.S. Microbiological Quality of Irrigation Water Collected from Vegetable Farms in Sao Paulo, Brazil during the Dry and Rainy Season. Agric. Water Manag. 2023, 279, 108190. [Google Scholar] [CrossRef]
- US FDA. Food. FDA. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/foodborne-outbreak-response-improvement-plan (accessed on 12 April 2022).
- Uyttendaele, M.; Jaykus, L.-A.; Amoah, P.; Chiodini, A.; Cunliffe, D.; Jacxsens, L.; Holvoet, K.; Korsten, L.; Lau, M.; McClure, P.; et al. Microbial Hazards in Irrigation Water: Standards, Norms, and Testing to Manage Use of Water in Fresh Produce Primary Production. Compr. Rev. Food Sci. Food Saf. 2015, 14, 336–356. [Google Scholar] [CrossRef]
- CDC Archives. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/ecoli/2018/o157h7-04-18/index.html (accessed on 19 May 2025).
- CDC. Multistate Foodborne Outbreak Notices. Foodborne Outbreaks. Available online: https://www.cdc.gov/foodborne-outbreaks/active-investigations/all-foodborne-outbreak-notices.html (accessed on 19 May 2025).
- Pachepsky, Y.; Shelton, D.R.; McLain, J.E.T.; Patel, J.; Mandrell, R.E. Irrigation Waters as a Source of Pathogenic Microorganisms in Produce: A Review. In Advances in Agronomy; Academic Press: Burlington, ON, USA, 2011; Volume 113, pp. 73–138. [Google Scholar]
- Lopez-Galvez, F.; Allende, A.; Pedrero-Salcedo, F.; Alarcon, J.J.; Gil, M.I. Safety Assessment of Greenhouse Hydroponic Tomatoes Irrigated with Reclaimed and Surface Water. Int. J. Food Microbiol. 2014, 191, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, S.; Marine, S.C.; Micallef, S.A.; Wang, F.; Pahl, D.M.; Melendez, M.; Kline, W.L.; Oni, R.A.; Walsh, C.T.; Everts, K.L.; et al. Assessment of Region, Farming System, Irrigation Source and Sampling Time as Food Safety Risk Factors for Tomatoes. Int. J. Food Microbiol. 2015, 196, 98–108. [Google Scholar] [CrossRef] [PubMed]
- OECD/WHO. Assessing Microbial Safety of Drinking Water: Improving Approaches and Methods; OECD Publishing: Paris, France, 2003. [Google Scholar] [CrossRef]
- USDA/NASS Redirect to AgCensus. Available online: https://www.nass.usda.gov/Publications/AgCensus/2022/Full_Report/ (accessed on 19 May 2025).
- Snell, W. The Ag Census Confirms Geographic and Commodity Shifts in the Kentucky Farm Economy|Agricultural Economics. Available online: https://agecon.ca.uky.edu/ag-census-confirms-geographic-and-commodity-shifts-kentucky-farm-economy (accessed on 19 May 2025).
- World Health Organization. Guidelines for the Safe Use of Wastewater, Excreta and Greywater—Volume 1, Policy and Regulatory Aspects; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Cirelli, G.L.; Consoli, S.; Di Grande, V. Long-Term Storage of Reclaimed Water: The Case Studies in Sicily (Italy). Desalination 2008, 218, 62–73. [Google Scholar] [CrossRef]
- Mujeriego, R.; Hultquist, R. Spanish Regulations for Water Reuse Royal Decree 1620/2007 of 7 December. 2011. Available online: https://www.asersagua.es/Asersa/Documentos/Spanish%20Regulations%20for%20Water%20Reuse%20EN.pdf (accessed on 19 May 2025).
- World Health Organization. Guidelines for Safe Recreational Water Environments—Vol. 1, Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- European Commission. Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, 49, 37–51. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). US Environmental Protection Agency: Recreational Water Quality Criteria. Recreational Water Quality Criteria and Methods. Available online: https://www.epa.gov/wqc/recreational-water-quality-criteria-and-methods (accessed on 19 May 2025).
- Steele, M.; Odumeru, J. Irrigation Water as Source of Foodborne Pathogens on Fruit and Vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Notice on Guidance Document on Addressing Microbiological Risks in Fresh Fruits and Vegetables at Primary Production through Good Hygiene. Off. J. Eur. Union 2017, 60, 1–40. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- European Commission. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 1998, 41, 32–54. [Google Scholar]
- Ministerie van Algemene Zaken. Towards Better Water Quality—Water management—Government.nl. Available online: https://www.government.nl/topics/water-management/water-quality/towards-better-water-quality (accessed on 19 May 2025).
- EPA. National Primary Drinking Water Regulations|US EPA. US EPA. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 19 May 2025).
- U.S. Food and Drug Administration. Requirements for Harvest and Post-Harvest Agricultural Water in Subpart E for Covered Produce Other Than Sprouts; FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
- U.S. Food and Drug Administration. FSMA Final Rule on Pre-Harvest Agricultural Water: Equivalent Testing Methodology for Agricultural Water Produce Safety Rule. U.S. Food and Drug Administration. Available online: https://www.fda.gov/food/laboratory-methods-food/equivalent-testing-methodology-agricultural-water-produce-safety-rule-21-cfr-112 (accessed on 19 May 2025).
- Dieter, C.A. Water Availability and Use Science Program: Estimated Use of Water in the United States in 2015; Geological Survey: Reston, VA, USA, 2018. [Google Scholar]
- CDC. Burden of Foodborne Illness: Findings. CDC. Available online: https://www.cdc.gov/food-safety/php/data-research/foodborne-illness-burden/?CDC_AAref_Val=https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 19 May 2025).
- Shen, C.; Zhang, Y. Total Plate Counts & Coliform Counts of Pond Water. Introd. Microbiol. Lab Ski. Tech. Food Sci. 2022, 1, 143–148. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Jagannathan, B.; Brady, B. The FSMA Produce Safety Rule. 2018. Available online: https://publications.ca.uky.edu/sites/publications.ca.uky.edu/files/FSMA_Water_Introduction_Final_update2.pdf (accessed on 26 April 2025).
- IDEXX. Quanti-Tray System—IDEXX US. Available online: https://www.idexx.com/en/water/water-products-services/quanti-tray-system/ (accessed on 26 April 2025).
- Hach Malaysia. UV Viewing Cabinet|Hach—Overview. Available online: https://my.hach.com/uv-viewing-cabinet/product?id=59428219445 (accessed on 26 April 2025).
- Kentucky State University. IDEXX Trays Indicating Coliform Presence [Photograph]; Kentucky State University: Frankfort, KY, USA, 2024. [Google Scholar]
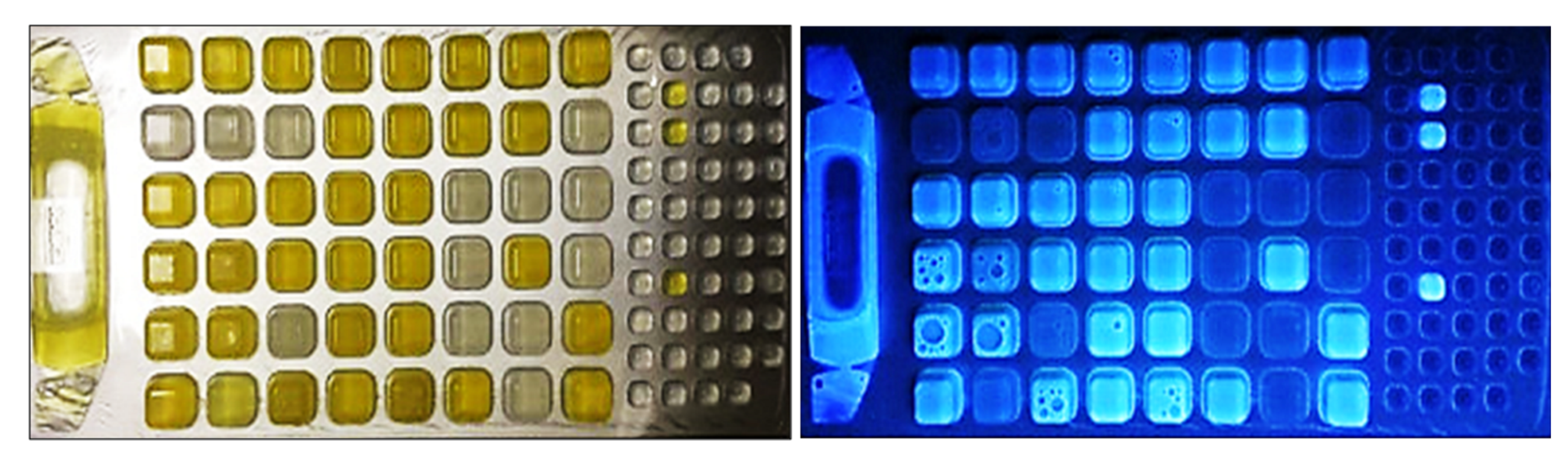
| Country/Region | Criteria (CFU/100 mL) a | Document Type | Reference(s) |
|---|---|---|---|
| Treated Wastewater | |||
| WHO (unrestricted) b | Guideline | [12] | |
| Root crops c | ≤103 E. coli | ||
| Leaf crops d | ≤104 E. coli | ||
| Drip irrigation, high-growing crops e | ≤105 E. coli | ||
| Drip irrigation, low-growing crops f | ≤103 E. coli | ||
| Italy | <10 E. coli and absence of Salmonella | Regulation | [3,13] |
| Spain | <100 E. coli | Regulation | [3,14] |
| Recreational Waters (coastal and fresh waters) | |||
| WHO | <500 enterococci | Guideline | [15] |
| EU g | Inland waters: <330 enterococci or <900 E. coli Coastal and transitional waters: <185 enterococci or <500 E. coli | Guideline | [16] |
| United States | <35 enterococci or <126 E. coli | Guideline | [17] |
| Irrigation water (for all water types) | |||
| Canada | <100 fecal coliforms | Guideline | [3,18] |
| Canada (British Columbia) | 200 fecal coliforms | Guideline | [3,18] |
| EU | Between 100 and 10,000 E. coli | Guideline | [19] |
| Drinking Water | |||
| WHO | <1 E. coli | Guideline | [20] |
| EU | 0 E. coli | Directive | [21] |
| The Netherlands | 0 E. coli | Decree | [22] |
| United States | 0 E. coli | Regulation | [23] |
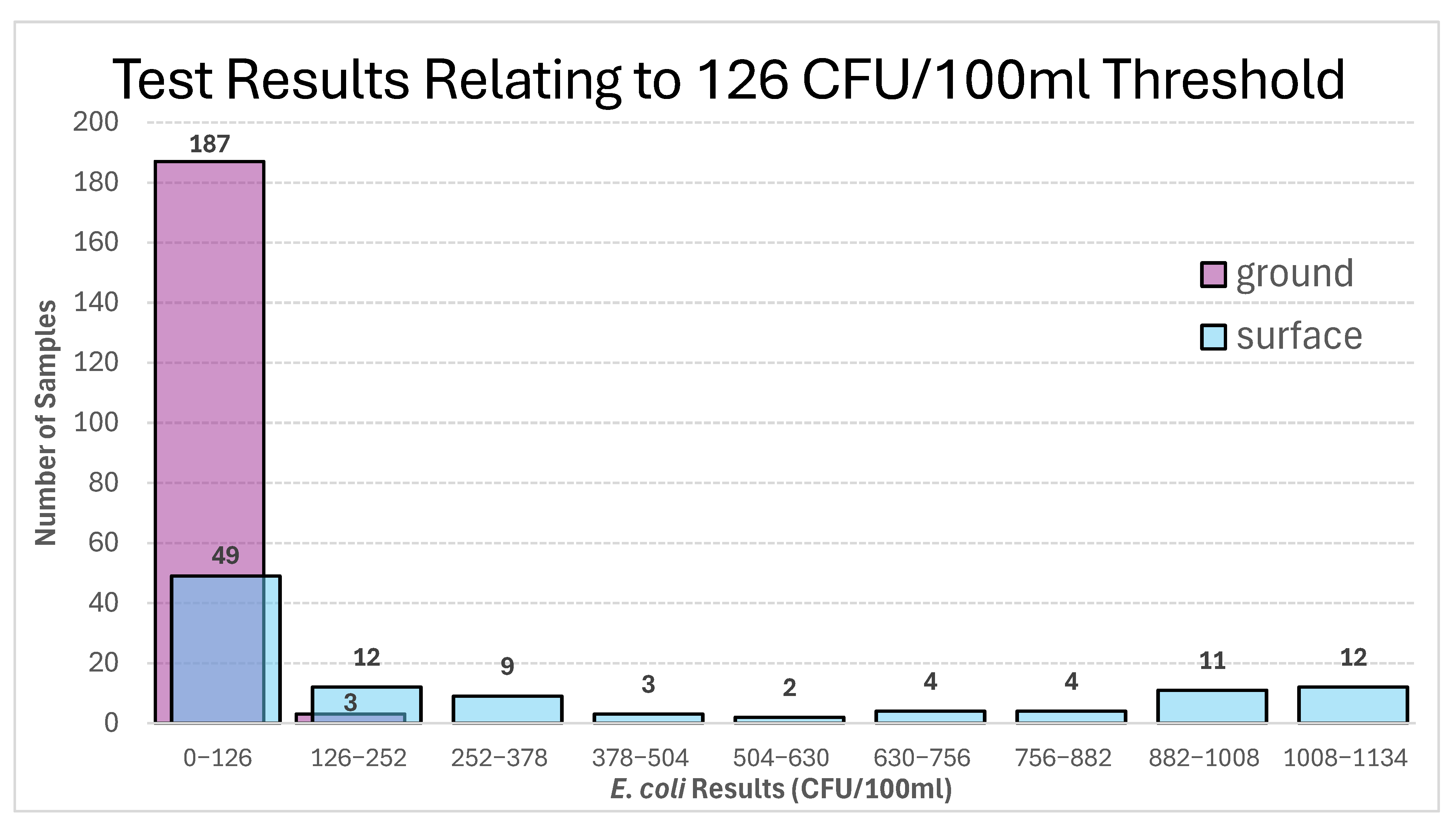
| (a) | ||
| ≤126 CFU/100 mL | ||
| Source | ||
| E. coli results | Ground | Surface |
| <1 | 159 | 4 |
| 1.0 | 6 | 5 |
| 2.0 | 2 | - |
| 3.1 | 2 | - |
| 5.2 | - | 1 |
| 6.3 | 1 | 2 |
| 7.3 | - | 2 |
| 7.5 | 1 | 1 |
| 8.1 | - | 1 |
| 8.6 | 2 | - |
| 10.9 | - | 1 |
| 12.2 | - | 1 |
| 13.4 | 1 | - |
| 16.1 | 1 | 1 |
| 16.6 | - | 1 |
| 17.5 | - | 1 |
| 22.8 | - | 1 |
| 25.0 | - | 1 |
| 25.6 | 1 | - |
| 26.9 | - | 1 |
| 30.1 | 1 | - |
| 30.9 | 1 | - |
| 32.4 | - | 1 |
| 33.1 | - | 1 |
| 36.4 | 1 | - |
| 38.9 | 1 | - |
| 39.3 | 1 | - |
| 43.5 | - | 3 |
| 47.3 | - | 1 |
| 48.0 | - | 1 |
| 55.6 | - | 1 |
| 58.4 | - | 2 |
| 65.0 | 2 | - |
| 67.0 | - | 1 |
| 68.9 | - | 1 |
| 71.7 | - | 2 |
| 79.4 | - | 1 |
| 80.1 | - | 1 |
| 91.0 | - | 1 |
| 95.9 | - | 1 |
| 96.0 | - | 2 |
| 98.3 | - | 1 |
| 105.0 | - | 2 |
| 105.8 | - | 2 |
| 106.3 | - | 1 |
| 108.1 | - | 1 |
| 115.3 | - | 1 |
| 121.1 | - | 1 |
| TOTAL | 183 | 53 |
| (b) | ||
| >126 CFU/100 mL | ||
| Source | ||
| E. coli results | Ground | Surface |
| 142.1 | 1 | 1 |
| 142.3 | - | 1 |
| 165.8 | 1 | - |
| 166.4 | - | 1 |
| 172.2 | - | 2 |
| 172.3 | - | 1 |
| 185.0 | - | 1 |
| 186.0 | - | 1 |
| 198.9 | - | 2 |
| 201.4 | - | 1 |
| 218.7 | - | 2 |
| 260.3 | - | 1 |
| 280.9 | - | 2 |
| 298.7 | - | 2 |
| 328.2 | - | 1 |
| 344.1 | - | 2 |
| 360.9 | - | 1 |
| 378.4 | - | 1 |
| 456.9 | - | 1 |
| 478.6 | - | 1 |
| 524.7 | - | 1 |
| 549.3 | - | 1 |
| 648.6 | - | 1 |
| 686.7 | - | 1 |
| 689.3 | - | 2 |
| 791.5 | - | 1 |
| 829.7 | - | 2 |
| 870.4 | - | 1 |
| 913.9 | - | 6 |
| 960.6 | - | 5 |
| 1011.2 | - | 12 |
| TOTAL | 2 | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tope, A.M.; Thomas, J.; London, T. Helping Small-Scale and Socially Disadvantaged Growers in Improving Microbial Quality of Irrigation Water in Kentucky. Agriculture 2025, 15, 1121. https://doi.org/10.3390/agriculture15111121
Tope AM, Thomas J, London T. Helping Small-Scale and Socially Disadvantaged Growers in Improving Microbial Quality of Irrigation Water in Kentucky. Agriculture. 2025; 15(11):1121. https://doi.org/10.3390/agriculture15111121
Chicago/Turabian StyleTope, Avinash M., John Thomas, and Tyler London. 2025. "Helping Small-Scale and Socially Disadvantaged Growers in Improving Microbial Quality of Irrigation Water in Kentucky" Agriculture 15, no. 11: 1121. https://doi.org/10.3390/agriculture15111121
APA StyleTope, A. M., Thomas, J., & London, T. (2025). Helping Small-Scale and Socially Disadvantaged Growers in Improving Microbial Quality of Irrigation Water in Kentucky. Agriculture, 15(11), 1121. https://doi.org/10.3390/agriculture15111121







