Combined Effects of Biochar and Rhamnolipid on Phenanthrene Biodegradation in Agricultural Soil: Bioavailability and Microbial Community Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Biochar
2.2. Rhamnolipid and Chemicals
2.3. Incubation Experiments
2.4. Extraction and Analysis of Total Phenanthrene in Soil
2.5. Determination of Bioavailable Phenanthrene in Soil
2.6. Determination of Enzyme Activity
2.7. Soil Microbial Community Analysis
2.8. Quantitative PCR Analysis of Functional Genes
2.9. Data Analysis
3. Results and Discussion
3.1. Phenanthrene Bioavailability and Microbial Degradation in Soil Affected by Biochar and Rhamnolipid
3.2. Soil Microbial Abundance and Enzyme Activity Affected by Biochar and Rhamnolipid
3.3. Diversity and Structure of Soil Bacterial Communities Affected by Biochar and Rhamnolipid
3.4. Abundances of PAH-Degrading Bacteria and Associated Functional Genes Affected by Biochar and Rhamnolipid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soukarieh, B.; Hawari, K.E.; Husseini, M.E.; Budzinski, H.; Jaber, F. Impact of Lebanese practices in industry, agriculture and urbanization on soil toxicity. Evaluation of the Polycyclic Aromatic Hydrocarbons (PAHs) levels in soil. Chemosphere 2018, 210, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Gao, M.; Sun, X.H.; Wang, Y.; Yuan, C.L.; Sun, H.W. Nationwide distribution of polycyclic aromatic hydrocarbons in soil of China and the association with bacterial community. J. Environ. Sci. 2023, 128, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Rocha, B.A.; Souza, M.C.O.; Bocato, M.Z.; Azevedo, L.F.; Adeyemi, J.A.; Santana, A.; Campiglia, A.D. Polycyclic aromatic hydrocarbons (PAHs): Updated aspects of their determination, kinetics in the human body, and toxicity. J. Toxiocol. Environ. Heal. B 2023, 26, 28–65. [Google Scholar] [CrossRef]
- Ansari, F.; Momina; Ahmad, A.; Rafatullah, M. Review on bioremediation technologies of polycyclic aromatic hydrocarbons (PAHs) from soil: Mechanisms and future perspective. Int. Biodeter. Biodegr. 2023, 179, 105582. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwall, J.D.; Yuan, Z.Z.; Wang, M.; Wang, F.M.; Li, S.; Yin, Z.G.; Huang, L.; Fu, Y.H.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef]
- Lu, Y.; Gu, K.; Shen, Z.T.; Tang, C.S.; Shi, B.; Zhou, Q.Y. Biochar implications for the engineering properties of soils: A review. Sci. Total Environ. 2023, 888, 164185. [Google Scholar] [CrossRef]
- Ren, X.Y.; Zeng, G.M.; Tang, L.; Wang, J.J.; Wan, J.; Feng, H.P.; Song, B.; Huang, C.; Tang, X. Effect of exogenous carbonaceous materials on the bioavailability of organic pollutants and their ecological risks. Soil Biol. Biochem. 2018, 116, 70–81. [Google Scholar] [CrossRef]
- Valizadeh, S.; Lee, S.S.; Choi, Y.J.; Baek, K.; Jeon, B.H.; Lin, K.Y.A.; Park, Y.K. Biochar application strategies for polycyclic aromatic hydrocarbons removal from soils. Environ. Res. 2022, 213, 113599. [Google Scholar] [CrossRef]
- Bao, H.Y.; Wang, J.F.; Zhang, H.; Li, J.; Li, H.; Wu, F.Y. Effects of biochar and organic substrates on biodegradation of polycyclic aromatic hydrocarbons and microbial community structure in PAHs-contaminated soils. J. Hazard. Mater. 2020, 385, 121595. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Y.Q.; Zhu, Y.T.; Zhang, H.Y.; Wang, X.L.; Li, W.; Li, P.P.; Han, J.G. Insights into the mechanisms underlying the biodegradation of phenanthrene in biochar-amended soil: From bioavailability to soil microbial communities. Biochar 2023, 5, 14. [Google Scholar] [CrossRef]
- Zhang, G.X.; He, L.X.; Guo, X.F.; Han, Z.W.; Ji, L.; He, Q.S.; Han, L.F.; Sun, K. Mechanism of biochar as a biostimulation strategy to remove polycyclic aromatic hydrocarbons from heavily contaminated soil in a coking plant. Geoderma 2020, 375, 114497. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Miao, R.H.; Guo, M.X.; Shang, X.T.; Zhou, Y.M.; Zhu, J.W. Biochar enhanced polycyclic aromatic hydrocarbons degradation in soil planted with ryegrass: Bacterial community and degradation gene expression mechanisms. Sci. Total Environ. 2022, 838 Pt 2, 156076. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Dai, Z.M.; Xiong, X.Q.; Zhu, H.; Xu, H.J.; Leng, P.; Li, J.H.; Tang, C.; Xu, J.M. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Kong, L.L.; Gao, Y.Y.; Zhou, Q.X.; Zhao, X.Y.; Sun, Z.W. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mater. 2018, 343, 276–284. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.B.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.L.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Cao, Q.F.; An, T.Y.; Xie, J.X.; Liu, Y.X.; Xing, L.; Ling, X.L.; Chen, C.J. Insight to the physiochemical properties and DOM of biochar under different pyrolysis temperature and modification conditions. J. Anal. Appl. Pyrol. 2022, 166, 105590. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, F.; Gong, H.F.; Sun, N.; Huang, J.J.; Chi, J. Responses of phenanthrene degradation to the changes in bioavailability and microbial community structure in soils amended with biochars pyrolyzed at low and high temperatures. J. Hazard. Mater. 2021, 410, 124584. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenerg. 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Li, S.M.; Barreto, V.; Li, R.W.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrol. 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Joseph, S.; Kammann, C.I.; Shepherd, J.G.; Conte, P.; Schmidt, H.P.; Hagemann, N.; Rich, A.M.; Marjo, C.E.; Allen, J.; Munroe, P.; et al. Microstructural and associated chemical changes during the composting of a high temperature biochar: Mechanisms for nitrate, phosphate and other nutrient retention and release. Sci. Total Environ. 2018, 618, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, W.; Zhu, L.Z. Effects of mixed surfactants on the bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in crops and the bioremediation of contaminated farmlands. Sci. Total Environ. 2019, 646, 1211–1218. [Google Scholar] [CrossRef]
- Bolan, S.; Padhye, L.P.; Mulligan, C.N.; Alonso, E.R.; Saint-Fort, R.; Jasemizad, T.; Wang, C.S.; Zhang, T.; Rinklebe, J.; Wang, H.L.; et al. Surfactant-enhanced mobilization of persistent organic pollutants: Potential for soil and sediment remediation and unintended consequences. J. Hazard. Mater. 2023, 443 Pt A, 130189. [Google Scholar] [CrossRef]
- Shah, A.; Shahzad, S.; Munir, A.; Nadagouda, M.N.; Khan, G.S.; Shams, D.F.; Dionysiou, D.D.; Rana, U.A. Micelles as soil and water decontamination agents. Chem. Rev. 2016, 116, 6042–6074. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, W.X.; Chuan, X.Y.; Guo, X.Y.; Shen, X.F.; Zhang, H.Y.; Wu, F.; Hu, J.; Wu, Z.P.; Wang, X.L. Remediation of heavily PAHs-contaminated soil with high mineral content from a coking plant using surfactant-enhanced soil washing. Sci. Total Environ. 2024, 909, 168499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.B.; Aitken, M.D. Surfactant-enhanced desorption and biodegradation of polycyclic aromatic hydrocarbons in contaminated soil. Environ. Sci. Technol. 2010, 44, 7260–7265. [Google Scholar] [CrossRef]
- Liu, J.W.; Wei, K.H.; Xu, S.W.; Cui, J.; Ma, J.; Xiao, X.L.; Xi, B.D.; He, X.S. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Yesankar, P.J.; Pal, M.; Patil, A.; Qureshi, A. Microbial exopolymeric substances and biosurfactants as ‘bioavailability enhancers’ for polycyclic aromatic hydrocarbons biodegradation. Int. J. Environ. Sci. Technol. 2023, 20, 5823–5844. [Google Scholar] [CrossRef]
- Ogunmokun, F.A.; Wallach, R. Effect of surfactant surface and interfacial tension reduction on infiltration into hydrophobic porous media. Geoderma 2024, 441, 116735. [Google Scholar] [CrossRef]
- Wang, X.X.; Sun, L.N.; Wang, H.; Wu, H.; Chen, S.; Zheng, X.H. Surfactant-enhanced bioremediation of DDTs and PAHs in contaminated farmland soil. Environ. Technol. 2017, 39, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Posada-Baquero, R.; Grifoll, M.; Ortega-Calvo, J.J. Rhamnolipid-enhanced solubilization and biodegradation of PAHs in soil after conventional bioremediation. Sci. Total Environ. 2019, 668, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.T.; Wu, H.Z.; Yan, B.; Zhang, Y.X.; Wei, C.H. Enhancement of PAHs biodegradation in biosurfactant/phenol system by increasing the bioavailability of PAHs. Chemosphere 2021, 266, 128941. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Peng, C. Shift of soil polycyclic aromatic hydrocarbons (PAHs) dissipation pattern and microbial community composition due to rhamnolipid supplementation. Water Air Soil Pollut. 2019, 230, 107. [Google Scholar] [CrossRef]
- Rong, L.G.; Zheng, X.H.; Oba, B.T.; Shen, C.B.; Wang, X.X.; Wang, H.; Luo, Q.; Sun, L.N. Activating soil microbial community using bacillus and rhamnolipid to remediate TPH contaminated soil. Chemosphere 2021, 275, 130062. [Google Scholar] [CrossRef]
- Thomas, G.E.; Brant, J.L.; Campo, P.; Clark, D.R.; Coulon, F.; Gregson, B.H.; McGenity, T.J.; McKew, B.A. Effects of dispersants and biosurfactants on Crude-Oil Biodegradation and bacterial community succession. Microorganisms 2021, 9, 1200. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Khan, S.; Qazi, M.A. Insights into rhamnolipid-assisted bioelectrochemical system for remediating soil pollution: A promising green approach towards the sustainable environment. Int. Biodeter. Biodegr. 2024, 191, 105808. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, F.; Zhan, Y.; Zhu, L.Z. Shifts in microbial community structure during in situ surfactant-enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 14451–14461. [Google Scholar] [CrossRef]
- Wang, J.F.; Bao, H.Y.; Pan, G.D.; Zhang, H.; Li, J.; Li, J.; Cai, J.; Wu, F.Y. Combined application of rhamnolipid and agricultural wastes enhances PAHs degradation via increasing their bioavailability and changing microbial community in contaminated soil. J. Environ. Manag. 2021, 294, 112998. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, L.V.; Guimarães, C.R.; da Silva Marquita, R.L.; Santiago, V.M.J.; de Souza, M.P.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.C.; Sukumaran, V.; Park, S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kadiri, F.; Ezaouine, A.; Blaghen, M.; Bennis, F.; Chegdani, F. Antibiofilm potential of biosurfactant produced by Bacillus aerius against pathogen bacteria. Biocatal. Agric. Biotech. 2024, 56, 102995. [Google Scholar] [CrossRef]
- Marecik, R.; Wojtera-Kwiczor, J.; Lawniczak, L.; Cyplik, P.; Szulc, A.; Piotrowska-Cyplik, A.; Chrzanowski, L. Rhamnolipids increase the phytotoxicity of diesel oil towards four common plant species in a terrestrial environment. Water Air Soil Pollut. 2012, 223, 4275–4282. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Meng, Y.L.; Li, J.B.; Gaston, L.A.; Fultz, L.M.; DeLaune, R.D. Potential use of biochar and rhamnolipid biosurfactant for remediation of crude oil-contaminated coastal wetland soil: Ecotoxicity assessment. Chemosphere 2020, 253, 126617. [Google Scholar] [CrossRef]
- Wolf, D.C.; Cryder, Z.; Gan, J. Soil bacterial community dynamics following surfactant addition and bioaugmentation in pyrene-contaminated soils. Chemosphere 2019, 231, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Kasprzyk, A.; Galvez, R.; Ghoshal, S. A rhamnolipid biosurfactant increased bacterial population size but hindered hydrocarbon biodegradation in weathered contaminated soils. Sci. Total Environ. 2021, 778, 145441. [Google Scholar] [CrossRef]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, X.J.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. Concentrations, sources, and spatial distribution of individual polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in the Eastern part of the EU: Poland as a case study. Sci. Total Environ. 2009, 407, 3746–3753. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wang, C.H.; Li, H.X.; Xu, S.D.; Du, J.; Chen, Y.J.; Ma, C.; Tang, J.H. Concentration, distribution, source apportionment, and risk assessment of surrounding soil PAHs in industrial and rural areas: A comparative study. Ecol. Indic. 2021, 125, 107513. [Google Scholar] [CrossRef]
- Dean, S.M.; Jin, Y.; Cha, D.K.; Wilson, S.V.; Radosevich, M. Phenanthrene degradation in soils co-inoculated with phenanthrene-degrading and biosurfactant-producing bacteria. J. Environ. Qual. 2001, 30, 1126–1133. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Semple, K.T. Biodegradation of phenanthrene-nitrogen-containing analogues in soil. Water Air Soil Pollut. 2015, 226, 252. [Google Scholar] [CrossRef]
- Li, X.N.; Song, Y.; Yao, S.; Bian, Y.R.; Gu, C.G.; Yang, X.L.; Wang, F.; Jiang, X. Can biochar and oxalic acid alleviate the toxicity stress caused by polycyclic aromatic hydrocarbons in soil microbial communities? Sci. Total Environ. 2019, 695, 133879. [Google Scholar] [CrossRef]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a means to improve soil fertility and crop productivity: A review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd Allah, E.F.; et al. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: A review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Song, Y.; Bian, Y.R.; Gu, C.G.; Yang, X.L.; Wang, F.; Jiang, X. Insights into the mechanisms underlying efficient rhizodegradation of PAHs in biochar-amended soil: From microbial communities to soil metabolomics. Environ. Int. 2020, 144, 105995. [Google Scholar] [CrossRef]
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant-soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Gaston, L.A.; Li, J.F.; Fultz, L.M.; DeLaune, R.D.; Dodla, S.K. Remediation of crude oil-contaminated coastal marsh soil: Integrated effect of biochar, rhamnolipid biosurfactant and nitrogen application. J. Hazard. Mater. 2020, 396, 122595. [Google Scholar] [CrossRef]
- van Noort, P.C.M.; Poot, A.; Koelmans, A.A. Analysis of organic contaminant desorption kinetic data for sediments and soils: Implications for the Tenax extraction time for the determination of bioavailable concentrations. Sci. Total Environ. 2014, 490, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, Z.X.; Xu, X.Y.; Zhou, H.; Yao, X.W.; Ji, F.Y. Effect of Tenax addition amount and desorption time on desorption behaviour for bioavailability prediction of polycyclic aromatic hydrocarbons. Sci. Total Environ. 2019, 651 Pt 1, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Mukherji, S. Desorption kinetics of soil sorbed carbazole, fluorene, and dibenzothiophene by P. aeruginosa RS1 from single and multicomponent systems and elucidation of their interaction effects. Biochem. Eng. J. 2022, 180, 108367. [Google Scholar] [CrossRef]
- Li, X.; Zheng, R.; Bu, Q.H.; Cai, Q.H.; Liu, Y.F.; Lu, Q.; Cui, J.Z. Comparison of PAH content, potential risk in vegetation, and bare soil near Daqing oil well and evaluating the effects of soil properties on PAHs. Environ. Sci. Pollut. Res. 2019, 26, 25071–25083. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K.; Gałązka, A.; Gawryjołek, K. Effect of coapplication of biochar and nutrients on microbiocenotic composition, dehydrogenase activity index and chemical properties of sandy soil. Waste Biomass Valori. 2020, 11, 3911–3923. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Li, X.N.; Song, Y.; Wang, F.; Bian, Y.R.; Jiang, X. Combined effects of maize straw biochar and oxalic acid on the dissipation of polycyclic aromatic hydrocarbons and microbial community structures in soil: A mechanistic study. J. Hazard. Mater. 2019, 364, 325–331. [Google Scholar] [CrossRef]
- Zhang, F.S.; Zhang, G.X.; Liao, X.Y. Negative role of biochars in the dissipation and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) in an agricultural soil: Cautions for application of biochars to remediate PAHs-contaminated soil. Ecotox. Environ. Saf. 2021, 213, 112075. [Google Scholar] [CrossRef]
- Lu, J.F.; Liu, Y.X.; Zhang, R.L.; Hu, Z.Y.; Xue, K.; Dong, B.Y. Biochar inoculated with Pseudomonas putida alleviates its inhibitory effect on biodegradation pathways in phenanthrene-contaminated soil. J. Hazard. Mater. 2024, 461, 132550. [Google Scholar] [CrossRef] [PubMed]
- Bielská, L.; Škulcová, L.; Neuwirthová, N.; Cornelissen, G.; Hale, S.E. Sorption, bioavailability and ecotoxic effects of hydrophobic organic compounds in biochar amended soils. Sci. Total Environ. 2018, 624, 78–86. [Google Scholar] [CrossRef]
- Zhu, X.M.; Wang, Y.S.; Zhang, Y.C.; Chen, B.L. Reduced bioavailability and plant uptake of polycyclic aromatic hydrocarbons from soil slurry amended with biochars pyrolyzed under various temperatures. Environ. Sci. Pollut. Res. 2018, 25, 16991–17001. [Google Scholar] [CrossRef]
- Tomczyk, B.; Siatecka, A.; Jedruchniewicz, K.; Sochacka, A.; Bogusz, A.; Oleszczuk, P. Polycyclic aromatic hydrocarbons (PAHs) persistence, bioavailability and toxicity in sewage sludge- or sewage sludge-derived biochar-amended soil. Sci. Total Environ. 2020, 747, 141123. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierz, M.; Oleszczuk, P.; Kraska, P.; Pałys, E.; Andruszczak, S. Persistence of polycyclic aromatic hydrocarbons (PAHs) in biochar-amended soil. Chemosphere 2016, 146, 272–279. [Google Scholar] [CrossRef]
- Ogbonnaya, O.U.; Adebisi, O.O.; Semple, K.T. The impact of biochar on the bioaccessibility of 14C-phenanthrene in aged soil. Environ. Sci. Proc. Imp. 2014, 16, 2635–2643. [Google Scholar]
- Anyika, C.; Abdul Majid, Z.; Ibrahim, Z.; Zakaria, M.P.; Yahya, A. The impact of biochars on sorption and biodegradation of polycyclic aromatic hydrocarbons in soils-A review. Environ. Sci. Pollut. Res. 2015, 22, 3314–3341. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Zhou, C.H.; Huang, J.H.; Wang, R.; Xia, N. Mineralization of phenanthrene sorbed on multiwalled carbon nanotubes. Environ. Toxicol. Chem. 2013, 32, 894–901. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, X.F.; Zhang, H.Y.; Werner, D.; Wang, B.; Yang, Y.; Tao, S.; Wang, X.L. Humic acid can enhance the mineralization of phenanthrene sorbed on biochars. Environ. Sci. Technol. 2019, 53, 13201–13208. [Google Scholar] [CrossRef]
- Johnson, M.D.; Keinath, T.M.; Weber, W.J. A distributed reactivity model for sorption by sails and sediments. 14. Characterization and modeling of phenanthrene desorption rates. Environ. Sci. Technol. 2001, 35, 1688–1695. [Google Scholar] [CrossRef]
- Congiu, E.; Ortega-Calvo, J.J. Role of desorption kinetics in the rhamnolipid-enhanced biodegradation of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2014, 48, 10869–10877. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.N.; Wang, W.; Li, F.; Zhu, L.Z. Mixed-surfactant-enhanced phytoremediation of PAHs in soil: Bioavailability of PAHs and responses of microbial community structure. Sci. Total. Environ. 2019, 653, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Zhen, M.N.; Tang, J.C.; Li, C.; Sun, H.W. Rhamnolipid-modified biochar-enhanced bioremediation of crude oil-contaminated soil and mediated regulation of greenhouse gas emission in soil. J. Soil Sediment 2021, 21, 123–133. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. The role of lipopeptide biosurfactant on microbial remediation of aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil. Chem. Eng. J. 2017, 309, 563–576. [Google Scholar] [CrossRef]
- Giagnoni, L.; Renella, G. Effects of biochar on the C use efficiency of soil microbial communities: Components and mechanisms. Environments 2022, 9, 138. [Google Scholar] [CrossRef]
- Gao, L.; Wang, R.; Shen, G.M.; Zhang, J.X.; Meng, G.X.; Zhang, J.G. Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils. J. Soil Sci. Plant Nut. 2017, 17, 884–896. [Google Scholar] [CrossRef]
- Ennis, C.J.; Evans, A.G.; Islam, M.; Ralebitso-Senior, T.K.; Senior, E. Biochar: Carbon sequestration, land remediation, and impacts on soil microbiology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2311–2364. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Hamzah, N.; Kasmuri, N.; Tao, W.; Singhal, N.; Padhye, L.; Swift, S. Effect of rhamnolipid on the physicochemical properties and interaction of bacteria and fungi. Braz. J. Microbiol. 2020, 51, 1317–1326. [Google Scholar] [CrossRef]
- Kaparaju, P.; Felby, C. Characterization of lignin during oxidative and hydrothermal pre-treatment processes of wheat straw and corn stover. Bioresour. Technol. 2010, 101, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Hansen, V.; Müller-Stöver, D.; Imparato, V.; Krogh, P.H.; Jensen, L.S.; Dolmer, A.; Hauggaard-Nielsen, H. The effects of straw or straw-derived gasification biochar applications on soil quality and crop productivity: A farm case study. J. Environ. Manag. 2017, 186, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Nie, X.; Wei, J.; Gu, M.Y.; Wu, W.P.; Chen, M.F. Effects of feedstock biopolymer compositions on the physiochemical characteristics of dissolved black carbon from lignocellulose-based biochar. Sci. Total Environ. 2021, 751, 141491. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Ma, Z.L.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Bioremediation of polycyclic aromatic hydrocarbon contaminated soil by a microbial consortium through supplementation of biosurfactant produced by Pseudomonas aeruginosa strain. Polycycl. Aromat. Comp. 2016, 36, 848–872. [Google Scholar] [CrossRef]
- Wolf, D.C.; Gan, J. Influence of rhamnolipid biosurfactant and Brij-35 synthetic surfactant on 14C-Pyrene mineralization in soil. Environ. Pollut. 2018, 243 PT B, 1846–1853. [Google Scholar] [CrossRef]
- Schnee, L.S.; Knauth, S.; Hapca, S.; Otten, W.; Eickhorst, T. Analysis of physical pore space characteristics of two pyrolytic biochars and potential as microhabitat. Plant Soil 2016, 408, 357–368. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Maiti, S.K. Different soil factors influencing dehydrogenase activity in mine degraded lands—State-of-art review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Lu, Q.; Jiang, Z.W.; Feng, W.X.; Yu, C.J.; Jiang, F.Z.; Huang, J.Y.; Cui, J.Z. Exploration of bacterial community-induced polycyclic aromatic hydrocarbons degradation and humus formation during co-composting of cow manure waste combined with contaminated soil. J. Environ. Manag. 2023, 326, 116852. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, J.W.; Kang, Y.K.; Ran, J.; Song, J.C.; Jiang, M.Q.; Li, W.; Zhang, M. Effects of wheat straw-derived biochar on soil microbial communities under phenanthrene stress. Agriculture 2025, 15, 77. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Johns, D.L. Life in the ‘charosphere’–Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Dai, Z.; Barberán, A.; Li, Y.; Brookes, P.C.; Xu, J. Bacterial community composition associated with pyrogenic organic matter (Biochar) varies with pyrolysis temperature and colonization environment. mSphere 2017, 2, e00085-17. [Google Scholar] [CrossRef]
- Pettersson, B.; Lembke, F.; Hammer, P.; Stackebrandt, E.; Priest, F.G. Bacillus sporothermodurans, a new species producing highly heat-resistant endospores. Int. J. Syst. Bacteriol. 1996, 46, 759–764. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Zhu, T.; Xiao, Q.X.; An, N. The addition of biochar and hyper-thermal inoculum can regulate the fate of heavy metals resistant bacterial communities during the livestock manure composting. Fermentation 2022, 8, 207. [Google Scholar] [CrossRef]
- Wu, Y.C.; Ding, Q.M.; Zhu, Q.H.; Zeng, J.; Ji, R.; Dumont, M.G.; Lin, X.G. Contributions of ryegrass, lignin and rhamnolipid to polycyclic aromatic hydrocarbon dissipation in an arable soil. Soil Biol. Biochem. 2018, 118, 27–34. [Google Scholar] [CrossRef]
- Yi, S.W.; Li, F.; Wu, C.; Ge, F.; Feng, C.; Zhang, M.; Liu, Y.; Lu, H.N. Co-transformation of HMs-PAHs in rhizosphere soils and adaptive responses of rhizobacteria during whole growth period of rice (Oryza sativa L.). J. Environ. Sci. 2023, 132, 71–82. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, T.X.; Luo, Y.Q.; Zhang, H.Y.; Li, W.; Wang, X.L.; Han, J.G. Impact mechanisms of various surfactants on the biodegradation of phenanthrene in soil: Bioavailability and microbial community responses. Sci. Total Environ. 2024, 950, 175225. [Google Scholar] [CrossRef]
- Hussain, B.; Ma, H.; Wu, Y.; Ganesan, S.; Yu, C.L.; Dixit, S.; Singh, S.; Pu, S.Y. Efficient immobilization of enzyme on covalent organo-framework for remediation of pyrene-contaminated soil and degradation mechanism. Int. J. Biol. Macromol. 2025, 305 Pt 2, 141234. [Google Scholar] [CrossRef] [PubMed]
- Omoni, V.T.; Baidoo, P.K.; Fagbohungbe, M.O.; Semple, K.T. The impact of enhanced and non-enhanced biochars on the catabolism of 14C-phenanthrene in soil. Environ. Technol. Innov. 2020, 20, 101146. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Selvam, A.; Wong, J.W.C. Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene. Bioresour. Technol. 2011, 102, 3999–4007. [Google Scholar] [CrossRef]
- Zeng, Z.T.; Liu, Y.; Zhong, H.; Xiao, R.; Zeng, G.M.; Liu, Z.F.; Cheng, M.; Lai, C.; Zhang, C.; Liu, G.S.; et al. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds. Sci. Total Environ. 2018, 634, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, A.; Smułek, W.; Pacholak, A.; Dudzinska-Bajorek, B.; Kaczorek, E. Surfactant addition in diesel oil degradation–how can it help the microbes? J. Environ. Health Sci. Eng. 2020, 18, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, R.; Nakata, H.; Ohta, H.; Niidome, T.; Takikawa, K.; Morimura, S. Isolation and evaluation of PAH degrading bacteria. J. Bioremed. Biodeg. 2015, 6, 3. [Google Scholar]
- Zheng, T.Y.; Liu, R.; Chen, J.J.; Gu, X.J.; Wang, J.; Li, L.M.; Hou, L.Q.; Li, N.; Wang, Y.J. Fire Phoenix plant mediated microbial degradation of pyrene: Increased expression of functional genes and diminishing of degraded products. Chem. Eng. J. 2021, 407, 126343. [Google Scholar] [CrossRef]
- Xia, X.H.; Xia, N.; Lai, Y.J.; Dong, J.W.; Zhao, P.J.; Zhu, B.T.; Li, Z.H.; Ye, W.; Yuan, Y.; Huang, J.X. Response of PAH-degrading genes to PAH bioavailability in the overlying water, suspended sediment, and deposited sediment of the Yangtze River. Chemosphere 2015, 128, 236–244. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
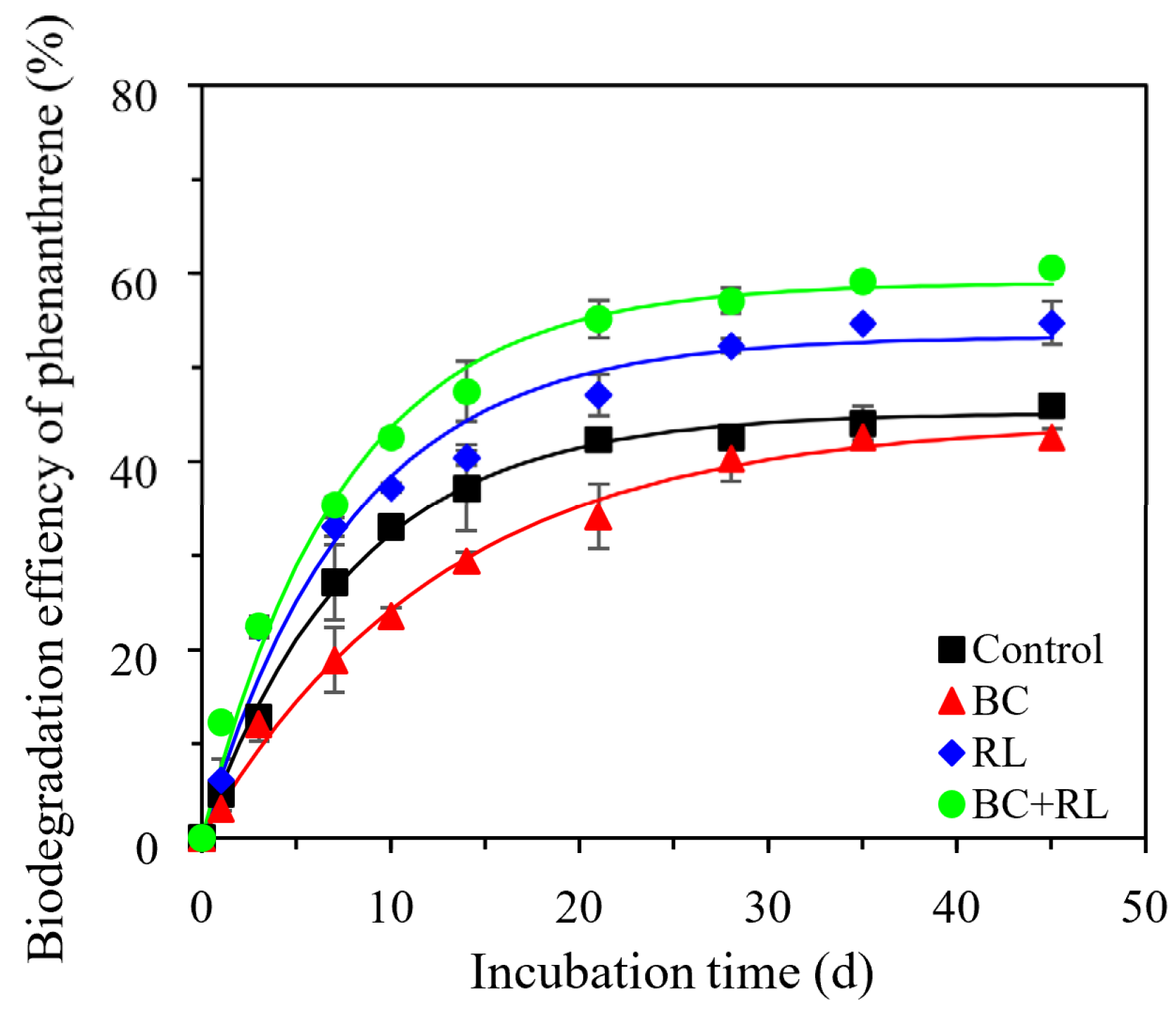
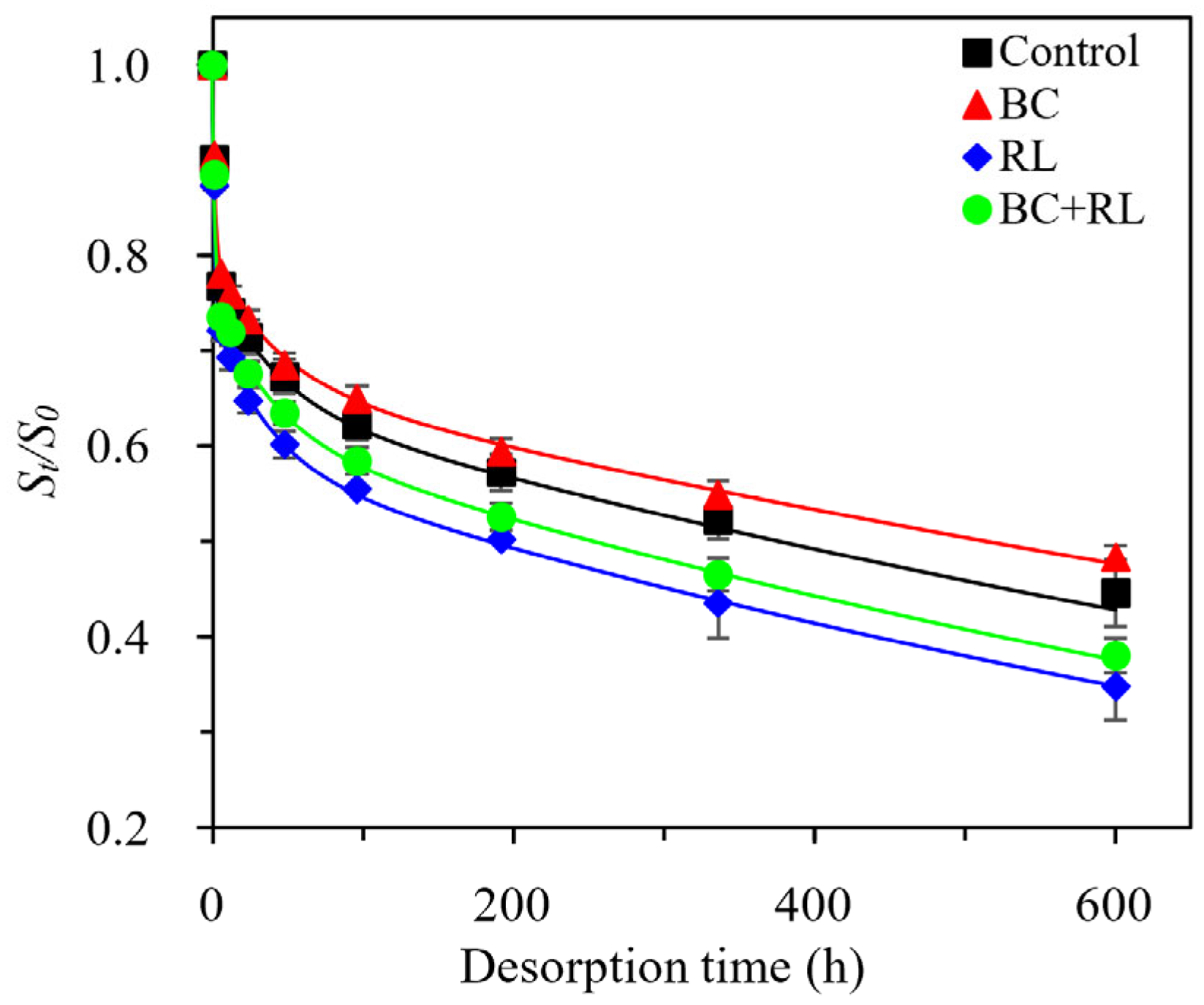
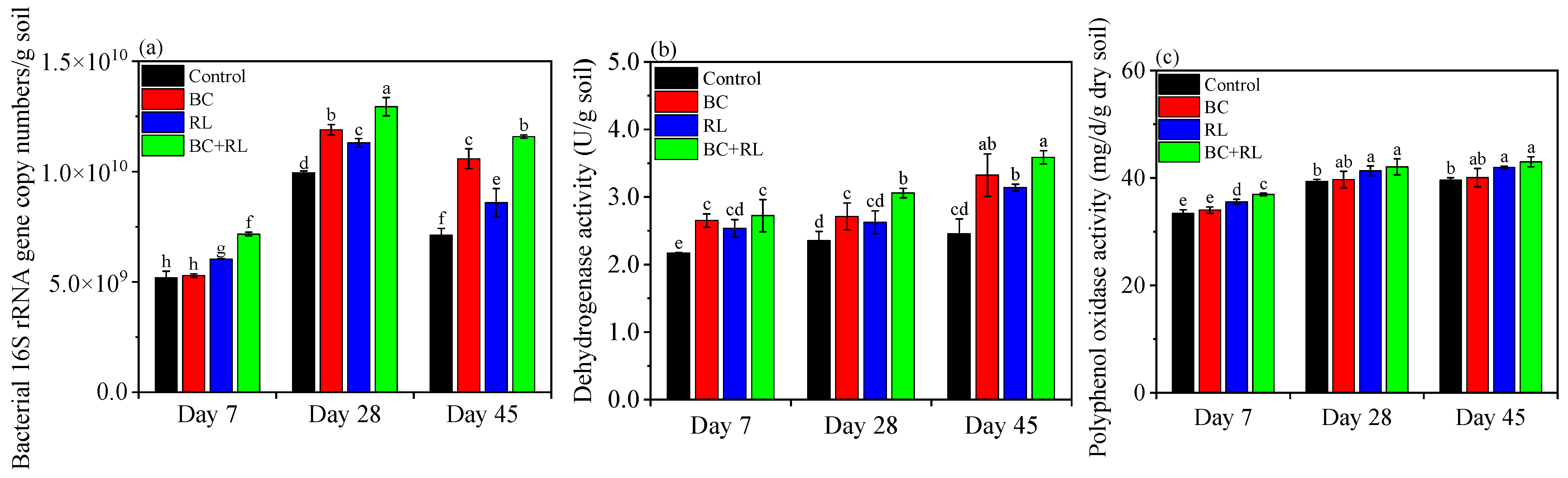

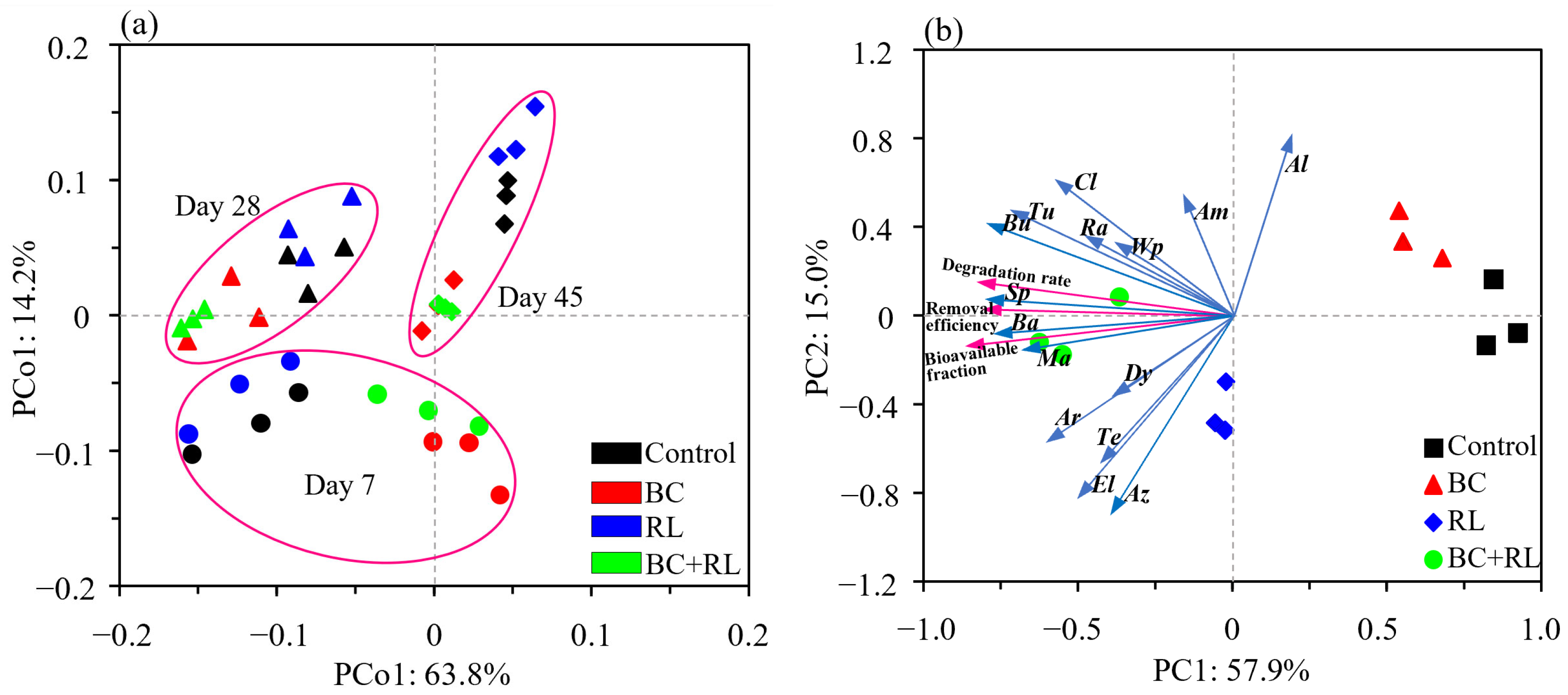

| Treatments | 45 d Biodegradation Percentage (%) | First-Order Kinetic Parameters | Maximum Biodegradation Rate 1 (ng/g/d) | ||
|---|---|---|---|---|---|
| Pmax (%) | k (10−2 d−1) | R2 | |||
| Control | 45.98 ± 0.72 | 45.16 ± 0.54 | 12.55 ± 0.51 | 0.998 | 281.0 ± 0.1 |
| BC | 42.55 ± 0.97 | 44.29 ± 1.16 | 7.91 ± 0.55 | 0.994 | 173.7 ± 0.3 |
| RL | 54.74 ± 2.29 | 53.32 ± 1.71 | 12.76 ± 1.38 | 0.982 | 337.4 ± 1.2 |
| BC + RL | 60.63 ± 0.69 | 58.97 ± 1.41 | 13.46 ± 1.12 | 0.989 | 393.6 ± 0.8 |
| Treatments | Triphasic First-Order Parameters | Desorption Amount at 600 h (%) | Frapid + Fslow (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frapid (%) | krapid (10−1 h−1) | Fslow (%) | kslow (10−2 h−1) | Fvslow (%) | kvslow (10−4 h−1) | R2 | |||
| Control | 21.5 ± 2.5 | 5.76 ± 0.19 | 13.5 ± 1.9 | 2.51 ± 0.03 | 65.0 ± 4.6 | 6.92 ± 1.51 | 0.991 | 55.3 ± 3.5 | 35.0 ± 3.2 |
| BC | 20.6 ± 1.6 | 5.40 ± 0.15 | 12.5 ± 1.3 | 2.24 ± 0.65 | 66.9 ± 3.9 | 5.64 ± 0.53 | 0.986 | 51.7 ± 1.3 | 33.1 ± 2.0 |
| RL | 25.9 ± 2.4 | 6.31 ± 0.21 | 15.6 ± 2.5 | 2.84 ± 0.04 | 58.5 ± 5.0 | 8.64 ± 0.04 | 0.986 | 65.2 ± 3.5 | 41.5 ± 3.5 |
| BC + RL | 23.8 ± 2.0 | 5.92 ± 0.29 | 14.6 ± 1.0 | 2.63 ± 0.27 | 61.6 ± 3.7 | 8.23 ± 0.04 | 0.985 | 61.9 ± 1.8 | 38.4 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Kang, Y.; Ran, J.; Song, J.; Wang, Z.; Li, J.; Chen, L. Combined Effects of Biochar and Rhamnolipid on Phenanthrene Biodegradation in Agricultural Soil: Bioavailability and Microbial Community Dynamics. Agriculture 2025, 15, 1116. https://doi.org/10.3390/agriculture15111116
Zhang M, Kang Y, Ran J, Song J, Wang Z, Li J, Chen L. Combined Effects of Biochar and Rhamnolipid on Phenanthrene Biodegradation in Agricultural Soil: Bioavailability and Microbial Community Dynamics. Agriculture. 2025; 15(11):1116. https://doi.org/10.3390/agriculture15111116
Chicago/Turabian StyleZhang, Meng, Yuke Kang, Jie Ran, Jichao Song, Zhongyi Wang, Jiawang Li, and Liyuan Chen. 2025. "Combined Effects of Biochar and Rhamnolipid on Phenanthrene Biodegradation in Agricultural Soil: Bioavailability and Microbial Community Dynamics" Agriculture 15, no. 11: 1116. https://doi.org/10.3390/agriculture15111116
APA StyleZhang, M., Kang, Y., Ran, J., Song, J., Wang, Z., Li, J., & Chen, L. (2025). Combined Effects of Biochar and Rhamnolipid on Phenanthrene Biodegradation in Agricultural Soil: Bioavailability and Microbial Community Dynamics. Agriculture, 15(11), 1116. https://doi.org/10.3390/agriculture15111116






