Organic–Inorganic Fertilization Sustains Crop Yields While Mitigating N2O and NO Emissions in Subtropical Wheat–Maize Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site and Experimental Design
2.2. Measurement of N2O and NO Fluxes
2.3. Auxiliary Data
2.4. Calculations and Statistical Analysis
3. Results
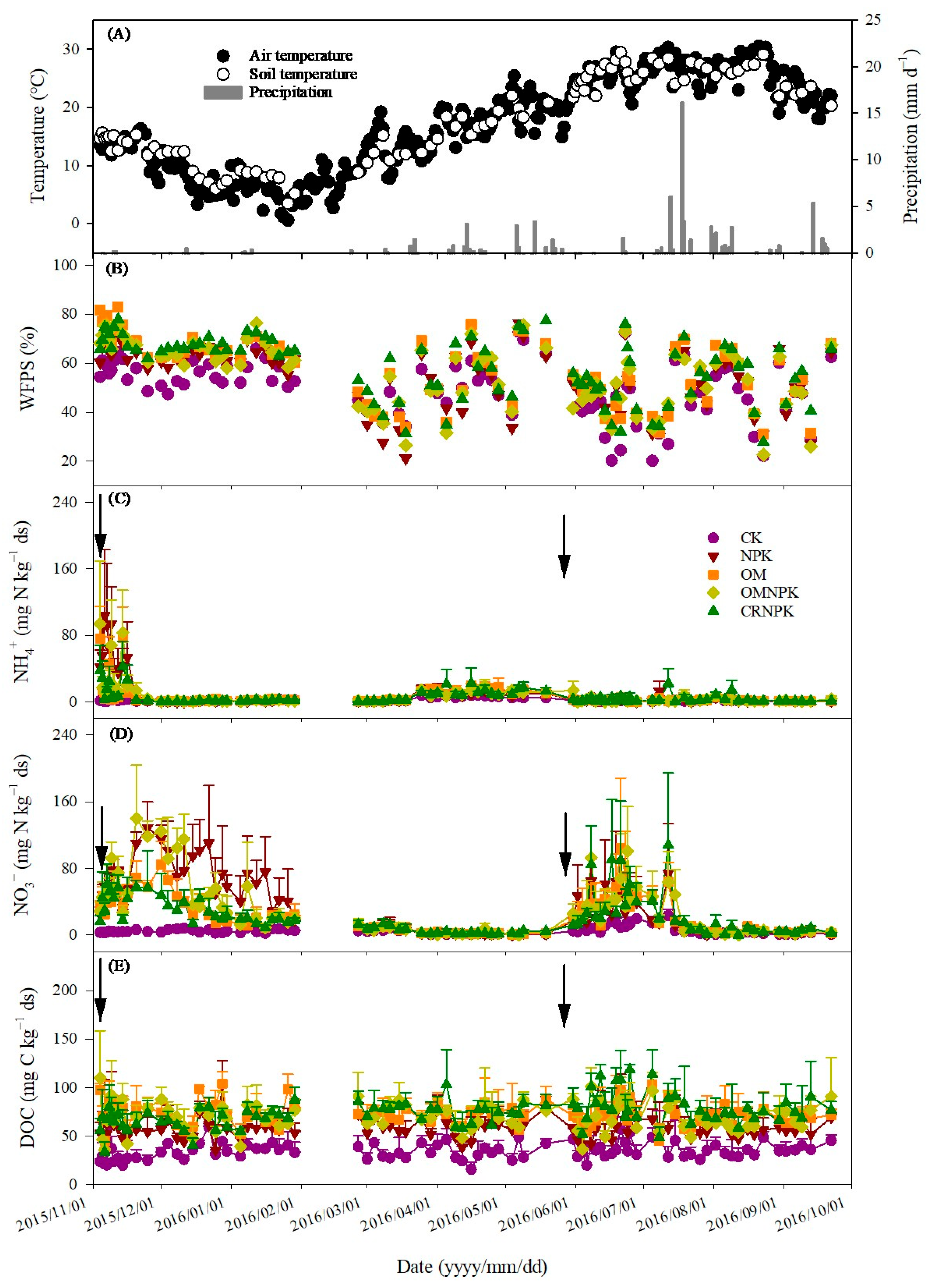
3.1. Environmental Factors
3.2. Crop Yields and Nitrogen Use Efficiency (NUE)
3.3. N2O Flux Dynamics
3.4. NO Flux Dynamics
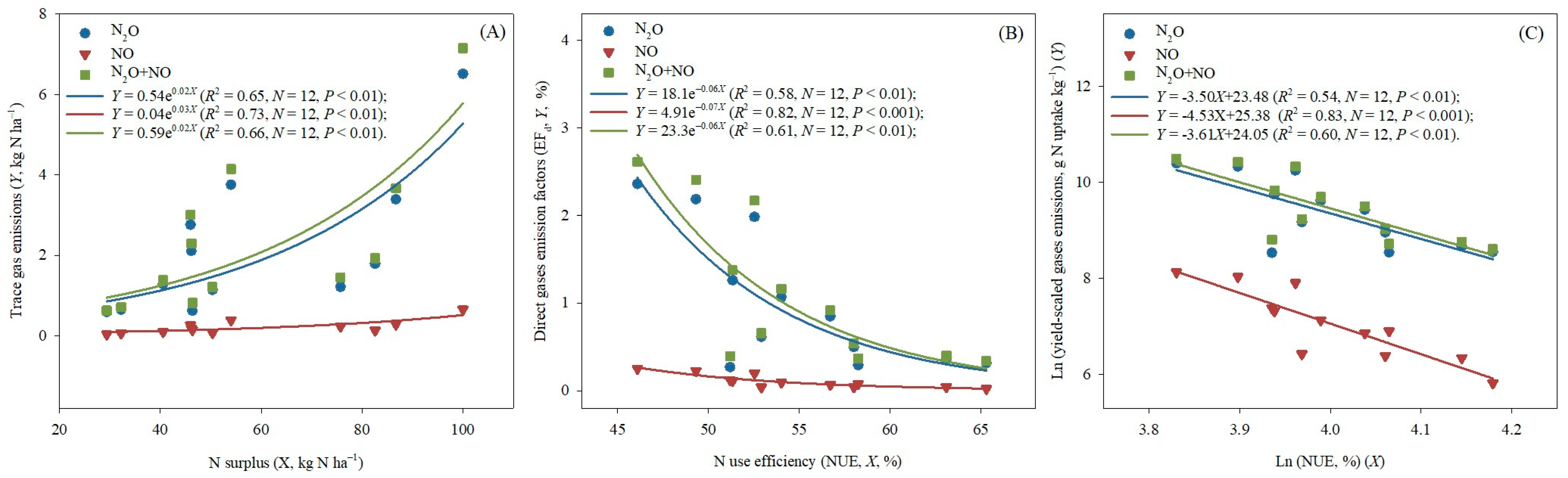
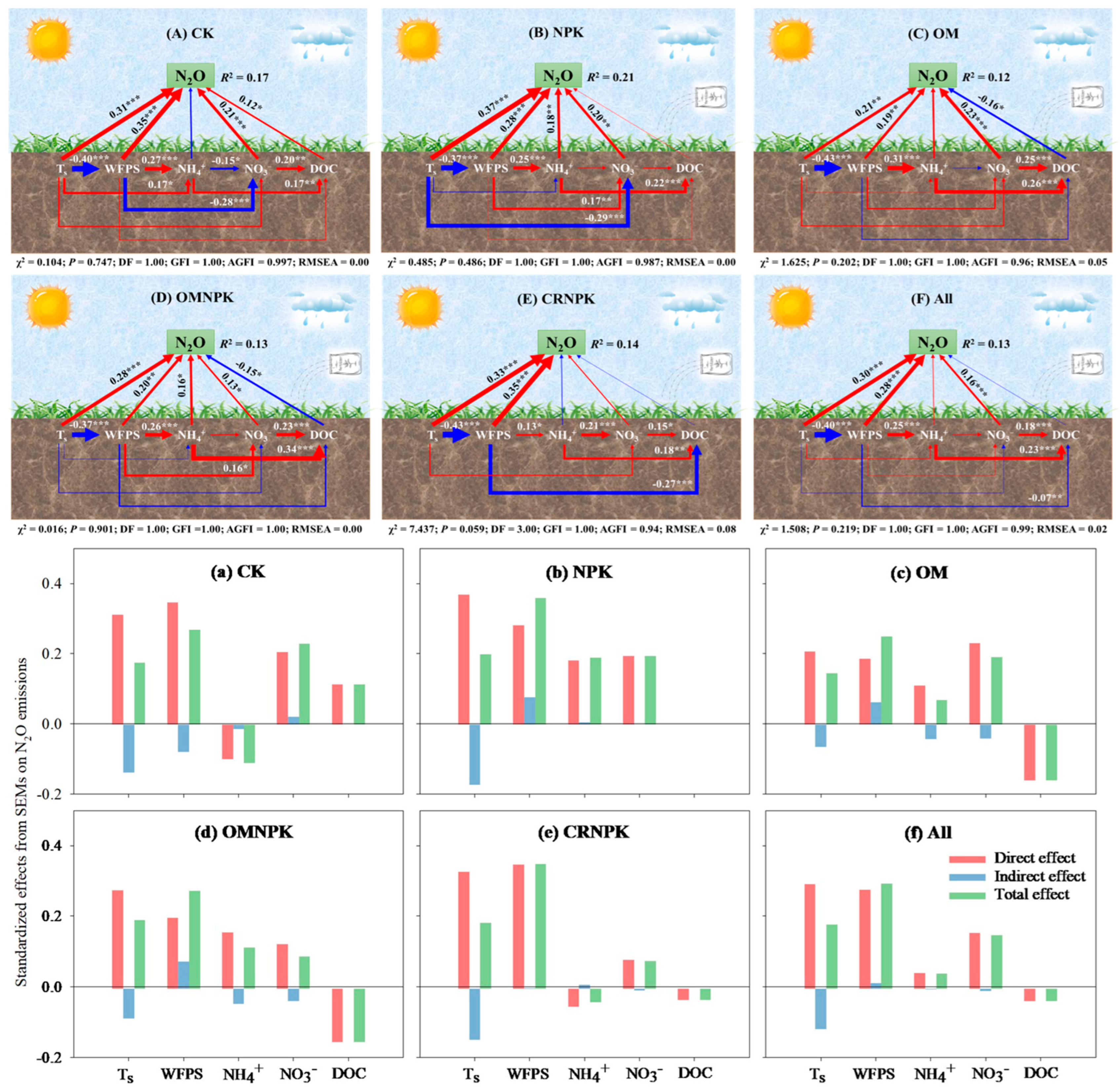
3.5. Soil Drivers of N2O and NO Emissions
4. Discussion
4.1. Crop Yields and NUE
4.2. N2O and NO Emissions
4.3. A Promising Strategy to Mitigate N2O and NO Emissions While Maintaining Crop Yields
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 26 June 2022).
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Garnier, J.; Bouwman, L.; Velazquez, E.; Mueller, N.D.; Gerber, J.S. Nitrogen use in the global food system: Past trends and future trajectories of agronomic performance, pollution, trade, and dietary demand. Environ. Res. Lett. 2016, 11, 095007. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; p. 1535. [Google Scholar]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 1058. [Google Scholar] [CrossRef]
- Yao, Z.; Zhou, Z.; Zheng, X.; Xie, B.; Liu, C.; Butterbach-Bahl, K.; Zhu, J. Effects of tillage during the nonwaterlogged period on nitrous oxide and nitric oxide emissions in typical Chinese rice–wheat rotation ecosystems. J. Geophys. Res. Biogeosci. 2010, 115, G00H12. [Google Scholar] [CrossRef]
- Fracetto, F.J.C.; Fracetto, G.G.M.; Bertini, S.C.B.; Cerri, C.C.; Feigl, B.J.; Siqueira Neto, M. Effect of agricultural management on N2O emissions in the Brazilian sugarcane yield. Soil Biol. Biochem. 2017, 109, 205–213. [Google Scholar] [CrossRef]
- Foltz, M.E.; Zilles, J.L.; Koloutsou-Vakakis, S. Prediction of N2O emissions under different field management practices and climate conditions. Sci. Total Environ. 2019, 646, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Hei, Z.; Chen, Y.; Zhang, F.; de Vries, W. Full substitution of chemical fertilizer by organic manure decreases soil N2O emissions driven by ammonia oxidizers and gross nitrogen transformations. Glob. Change Biol. 2023, 30, e16957. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, H.; He, S.; Zhao, Q.; Liu, Y.; Liu, H. Partial substitution of chemical fertilizer by organic fertilizer increases yield, quality and nitrogen utilization of Dioscorea polystachya. PLoS ONE 2024, 19, e0301108. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Wuta, M.; Nyamangara, J.; Smith, J.L.; Rees, R.M. Nitrous oxide and methane emissions from cultivated seasonal wetland (dambo) soils with inorganic, organic and integrated nutrient management. Nutr. Cycl. Agroecosyst. 2014, 100, 161–175. [Google Scholar] [CrossRef]
- Yuan, J.; Sha, Z.; Hassani, D.; Zhao, Z.; Cao, L. Assessing environmental impacts of organic and inorganic fertilizer on daily and seasonal Greenhouse gases effluxes in rice field. Atmos. Environ. 2017, 155, 119–128. [Google Scholar] [CrossRef]
- Zhao, Z.; Yue, Y.; Sha, Z.; Li, C.; Deng, J.; Zhang, H.; Gao, M.; Cao, L. Assessing impacts of alternative fertilizer management practices on both nitrogen loading and greenhouse gas emissions in rice cultivation. Atmos. Environ. 2015, 119, 393–401. [Google Scholar] [CrossRef]
- Graham, R.F.; Wortman, S.E.; Pittelkow, C.M. Comparison of organic and integrated nutrient management strategies for reducing soil N2O emissions. Sustainability 2017, 9, 510. [Google Scholar] [CrossRef]
- Abalos, D.; Sanz-Cobena, A.; Garcia-Torres, L.; van Groenigen, J.W.; Vallejo, A. Role of maize stover incorporation on nitrogen oxide emissions in a non-irrigated Mediterranean barley field. Plant Soil 2013, 364, 357–371. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Y.; Wang, R.; Wang, X.; Wang, Y.; Zheng, X.; Liu, C.; Zhu, B.; Zhou, M.; Liu, Y.; et al. Long-term straw return to a wheat-maize system results in topsoil organic C saturation and increased yields while no stimulating or reducing yield-scaled N2O and NO emissions. Agric. For. Meteorol. 2024, 349, 109937. [Google Scholar] [CrossRef]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.W.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef]
- Ma, R.; Yu, K.; Xiao, S.; Liu, S.; Ciais, P.; Zou, J. Data-driven estimates of fertilizer induced soil NH3, NO and N2O emissions from croplands in China and their climate change impacts. Glob. Change Biol. 2022, 28, 1008–1022. [Google Scholar] [CrossRef]
- Yan, X.; Gong, W. The role of chemical and organic fertilizers on yield, yield variability and carbon sequestration- results of a 19-year experiment. Plant Soil 2010, 331, 471–480. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Zheng, X.; Subramaniam, L.; Butterbach-Bahl, K. A synthesis of nitric oxide emissions across global fertilized croplands from crop-specific emission factors. Glob. Change Biol. 2022, 28, 4395–4408. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, W.; Huang, R.; Yan, X.; Wang, S. Extremely high N2O but unexpectedly low NO emissions from a highly organic and chemical fertilized peach orchard system in China. Agric. Ecosyst. Environ. 2017, 246, 202–209. [Google Scholar] [CrossRef]
- Zhou, M.; Butterbach-Bahl, K. Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 2014, 374, 977–991. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, G.; Zheng, X.; Wang, R.; Liu, C.; Butterbach-Bahl, K. Straw return reduces yield-scaled N2O plus NO emissions from annual winter wheat-based cropping systems in the North China Plain. Sci. Total Environ. 2017, 590–591, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yao, Z.; Hu, D.; Bar, H. Effects of substitution of mineral nitrogen with organic amendments on nitrogen loss from sloping cropland of purple soil. Front. Agric. Sci. Eng. 2022, 9, 396–406. [Google Scholar] [CrossRef]
- Brentrup, F.; Lammel, J. Nitrogen use efficiency, nitrogen balance, and nitrogen productivity–A combined indicator system to evaluate nitrogen use in crop production systems. In Proceedings of the International Nitrogen Initiative Conference: Solutions to Improve Nitrogen Use Efficiency for the World, Melbourne, Australia, 4–8 December 2016. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.; You, X.; Gao, M.R. Nutrient release from weathering of purplish rocks in the Sichuan Basin, China. Pedosphere 2008, 18, 257–264. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Brüggemann, N.; Dannenmann, M.; Wang, Y.; Butterbach-Bahl, K. Sustaining crop productivity while reducing environmental nitrogen losses in the subtropical wheat-maize cropping systems: A comprehensive case study of nitrogen cycling and balance. Agric. Ecosyst. Environ. 2016, 231, 1–14. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Li, J.; Wang, L. Sustainable agricultural practices in subtropical China: Balancing productivity and environmental impacts. Nat. Food 2022, 3, 197–206. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, B.; Li, Z.; Zhao, C.; Qian, R.; Huang, F.; Zhang, P.; Li, H.; Jia, Z. Ameliorating C and N balance without loss of productivity by applying mulching measures in rainfed areas. Agric. Ecosyst. Environ. 2023, 343, 108267. [Google Scholar] [CrossRef]
- Zheng, X.; Mei, B.; Wang, Y.; Xie, B.; Wang, Y.; Dong, H.; Xu, H.; Chen, G.; Cai, Z.; Yue, J. Quantification of N2O fluxes from soil-plant systems may be biased by the applied gas chromatograph methodology. Plant Soil 2008, 311, 211–234. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Xie, B.; Mei, B.; Wang, R.; Butterbach-Bahl, K.; Zhu, J.G.; Yin, R. Tillage and crop residue management significantly affects N-trace gas emissions during the non-rice season of a subtropical rice–wheat rotation. Soil Biol. Biochem. 2009, 41, 2131–2140. [Google Scholar] [CrossRef]
- Kroon, P.S.; Hensen, A.; van den Bulk, W.C.M.; Jongejan, P.A.C.; Vermeulen, A.T. The importance of reducing the systematic error due to non-linearity in N2O flux measurements by static chambers. Nutr. Cycl. Agroecosyst. 2008, 82, 175–186. [Google Scholar] [CrossRef]
- Gu, B.J.; Ju, X.T.; Chang, J.; Ge, Y.; Vitousek, P.M. Integrated reactive nitrogen budgets and future trends in China. Proc. Natl. Acad. Sci. USA 2015, 112, 8792–8797. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Kuang, F.; Skiba, U.; Zhu, B.; Liu, X.; Levy, P.; Dore, A.; Fowler, D. Bulk deposition of organic and inorganic nitrogen in southwest China from 2008 to 2013. Environ. Pollut. 2017, 227, 157–166. [Google Scholar] [CrossRef]
- Firestone, M.; Davidson, E. Microbiological Basis of NO and N2O Production and Consumption in Soils. In Exchange of Trace Gases Between Terrestrial Ecosystems and the Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; John Willey and Sons: New York, NY, USA, 1989; pp. 7–21. [Google Scholar]
- Davidson, E.A.; Verchot, L.V. Testing the hole-in-the-pipe model of nitric and nitrous oxide emissions from soils using the TRAGNET database. Glob. Biogeochem. Cycles 2000, 14, 1035–1043. [Google Scholar] [CrossRef]
- Yan, G.; Yao, Z.; Zheng, X.; Liu, C. Characteristics of annual nitrous and nitric oxide emissions from major cereal crops in the North China Plain under alternative fertilizer management. Agric. Ecosyst. Environ. 2015, 207, 67–78. [Google Scholar] [CrossRef]
- Miao, Y.; Stewart, B.A.; Zhang, F. Long-term experiments for sustainable nutrient management in China. A review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Du, H.; Wang, Y.; Zhang, L.; Shi, X. Carbon sequestration and soil microbes in purple paddy soil as affected by long-term fertilization. Toxicol. Environ. Chem. 2015, 97, 464–476. [Google Scholar] [CrossRef]
- Abdalla, K.; Sun, Y.; Zarebanadkouki, M.; Gaiser, T.; Seidel, S.; Pausch, J. Long-term continuous farmyard manure application increases soil carbon when combined with mineral fertilizers due to lower priming effects. Geoderma 2022, 428, 116216. [Google Scholar] [CrossRef]
- Amanullah, H. Influence of organic and inorganic nitrogen on grain yield and yield components of hybrid rice in Northwestern Pakistan. Rice Sci. 2016, 23, 326–333. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Y.; Tian, Y.; Ceng, K.; Zhao, M.; Zhao, M.; Yin, B. Increasing yield and N use efficiency with organic fertilizer in Chinese intensive rice cropping systems. Field Crops Res. 2018, 227, 102–109. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Till. Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Silva, A.; Oliveira, B.; Santos, C.; Pereira, D.; Costa, E.; Ferreira, F.; Rodrigues, G.; Martins, H.; Almeida, I.; Carvalho, J.; et al. Synergistic effects of molybdenum and plant growth-promoting rhizobacteria on sugarcane performance. J. Soil Sci. Plant Nutr. 2025, 25, 512–525. [Google Scholar] [CrossRef]
- Dawe, D.; Dobermann, A.; Ladha, J.K.; Yadav, R.L.; Bao, L.; Gupta, R.K.; Lal, P.; Panaullah, G.; Sariam, O.; Singh, Y.; et al. Do organic amendments improve yield trends and profitability in intensive rice systems? Field Crops Res. 2003, 83, 191–213. [Google Scholar] [CrossRef]
- Bi, L.; Zhang, B.; Liu, G.; Li, Z.; Liu, Y.; Ye, C.S.; Yu, X.; Lai, T.; Zhang, J.; Yin, J.; et al. Long-term effects of organic amendments on the rice yields for double rice cropping systems in subtropical China. Agric. Ecosyst. Environ. 2009, 129, 534–541. [Google Scholar] [CrossRef]
- Davidson, E.A.; Samanta, S.; Caramori, S.S.; Savage, K. The dual Arrhenius and Michaelis–Menten (DAMM) kinetics model for decomposition of soil organic matter at hourly to seasonal time scale. Glob. Change Biol. 2012, 18, 371–384. [Google Scholar] [CrossRef]
- Wang, R.; Pan, Z.; Zheng, X.; Ju, X.; Yao, Z.; Butterbach-Bahl, K.; Zhang, C.; Wei, H.; Huang, B. Using field-measured soil N2O fluxes and laboratory scale parameterization of N2O/(N2O+N2) ratios to quantify field-scale soil N2 emissions. Soil Biol. Biochem. 2020, 148, 107904. [Google Scholar] [CrossRef]
- Pan, Z.; He, P.; Fan, D.; Jiang, R.; Song, D.; Song, L.; Zhou, W.; He, W. Global impact of enhanced-efficiency fertilizers on vegetable productivity and reactive nitrogen losses. Sci. Total Environ. 2024, 918, 170632. [Google Scholar] [CrossRef]
- Skiba, U.; Smith, K.A.; Fowler, D. Nitrification and denitrification as sources of nitric-oxide and nitrous-oxide in a sandy loam soil. Soil Biol. Biochem. 1993, 25, 1527–1536. [Google Scholar] [CrossRef]
- Conrad, R. Microbiological and biochemical background of production and consumption of NO and N2O in soil. In Trace Gas Exchange in Forest Ecosystems; Tree Physiology; Gasche, R., Papen, H., Rennenberg, H., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 3, pp. 3–33. [Google Scholar] [CrossRef]
- Grant, R.F.; Pattey, E.; Goddard, T.W.; Kryzanowski, L.M.; Puurveen, H. Modeling the effects of fertilizer application rate on nitrous oxide emissions. Soil Sci. Soc. Am. J. 2006, 70, 235–248. [Google Scholar] [CrossRef]
- Zheng, X.; Han, S.; Huang, Y.; Wang, Y.; Wang, M. Re-quantifying the emission factors based on field measurements and estimating the direct N2O emission from Chinese croplands. Glob. Biogeochem. Cycles 2004, 18, GB2018. [Google Scholar] [CrossRef]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies: Hayama, Japan, 2006; Volume 4. [Google Scholar]
- Huang, Y.; Li, D. Soil nitric oxide emissions from terrestrial ecosystems in China: A synthesis of modeling and measurements. Sci. Rep. 2014, 4, 7406. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, F.; Wu, S.; Ji, C.; Sun, Y.; Jin, Y.; Li, S.; Li, Z.; Zou, J. A meta-analysis of fertilizer-induced soil NO and combined NO+N2O emissions. Glob. Change Biol. 2017, 23, 2520–2532. [Google Scholar] [CrossRef]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2019; Volume 4, Available online: https://www.ipcc.ch/report/2019-refinement-to-the-2006-ipcc-guidelines/ (accessed on 26 June 2022).
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Change Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Weslien, P.; Kasimir Klemedtsson, Å.; Borjesson, G.; Klemedtsson, L. Strong pH influence on N2O and CH4 fluxes from forested organic soils. Eur. J. Agron. 2009, 60, 311–320. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; Van Groenigen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Brüggemann, N.; Bergmann, J.; Wang, Y.; Butterbach-Bahl, K. N2O and CH4 Emissions, and NO3⁻ Leaching on a Crop-Yield Basis from a Subtropical Rain-fed Wheat–Maize Rotation in Response to Different Types of Nitrogen Fertilizer. Ecosystems 2014, 17, 286–301. [Google Scholar] [CrossRef]
- Baral, K.R.; Labouriau, R.; Olesen, J.E.; Petersen, S.O. Nitrous oxide emissions and nitrogen use efficiency of manure and digestates applied to spring barley. Agric. Ecosyst. Environ. 2017, 239, 188–198. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, G.; Zheng, X.; Wang, R.; Liu, C.; Butterbach-Bahl, K. Reducing N2O and NO emissions while sustaining crop productivity in a Chinese vegetable-cereal double cropping system. Environ. Pollut. 2017, 231, 929–941. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, Y.; Yu, Y.; Xue, L.; Yang, L.; Zhong, L.; Delgado-Baquerizo, M.; He, S. Closing the gap between climate regulation and food security with nano iron oxides. Nat. Sustain. 2024, 7, 758–765. [Google Scholar] [CrossRef]


| Crop | Sowing and Harvesting Date | Fertilization Date | N Fertilizer Types and Rates (kg N ha−1) 1 | ||||
|---|---|---|---|---|---|---|---|
| CK | NPK | OM | OMNPK | CRNPK | |||
| Winter wheat | 3 November 2015, 23 May 2016 | 3 November 2015 | 0 | AB (130) | OM (130) | AB (91) + OM (39) | AB (110) + MR (20) |
| Summer maize | 30 May, 25 September 2016 | 30 May 2016 | 0 | AB (150) | OM (150) | AB (105) + OM (45) | AB (127) + WR (23) |
| Annual total N rate 2 | 0 | 280 | |||||
| Code 1 | Equations 3 | N | R2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| (1) | ||||||||
| A | k1 | k2 | B | C | ||||
| N2O | ||||||||
| CK | 0.527 | 0.223 | 6.881 | 0.053 | 0.050 | 88 | 0.49 | <0.01 |
| NPK | 0.077 | 0.999 | 59.26 | 0.087 | 0.131 | 88 | 0.83 | <0.01 |
| OM | 0.124 | 0.125 | 40.88 | 0.043 | 0.125 | 88 | 0.55 | <0.01 |
| OMNPK | 0.067 | 0.545 | 8.386 | 0.048 | 0.119 | 88 | 0.43 | <0.01 |
| CRNPK | 9.1 × 10−11 | 9.570 | 85.89 | 0.338 | 0.263 | 88 | 0.96 | <0.01 |
| All 2 | 0.145 | 0.678 | 35.43 | 0.087 | 0.109 | 440 | 0.29 | <0.01 |
| NO | ||||||||
| CK | 0.124 | 0 | 9.778 | 0.018 | 0.070 | 88 | 0.17 | <0.01 |
| NPK | 4.660 | 1.157 | 6.306 | 0 | 0.034 | 88 | 0.05 | <0.05 |
| OM | 6.990 | 0.380 | 24.08 | 0.021 | 0.016 | 88 | 0.16 | <0.01 |
| CRNPK | 42.49 | 0.925 | 165.4 | 0 | 0 | 88 | 0.31 | <0.01 |
| All 2 | 4.070 | 0.726 | 19.63 | 0.016 | 0.030 | 440 | 0.11 | <0.01 |
| N2O+NO | ||||||||
| CK | 0.623 | 0.192 | 7.590 | 0.051 | 0.051 | 88 | 0.49 | <0.01 |
| NPK | 0.130 | 1.060 | 42.57 | 0.080 | 0.123 | 88 | 0.80 | <0.01 |
| OM | 12.64 | 0 | 31.75 | 0.041 | 0.063 | 88 | 0.30 | <0.01 |
| OMNPK | 0.010 | 1.834 | 30.90 | 0.117 | 0.147 | 88 | 0.35 | <0.01 |
| CRNPK | 3.3 × 10−10 | 8.120 | 85.56 | 0.325 | 0.249 | 88 | 0.95 | <0.01 |
| All 2 | 0.257 | 0.606 | 34.12 | 0.081 | 0.103 | 440 | 0.28 | <0.01 |
| Code 1 | Equations 3 | N | R2 | p | ||||
| (2) | ||||||||
| A | B | C | D | |||||
| CK | 0.298 | 0.044 | 0.065 | −2.610 | 88 | 0.14 | <0.01 | |
| NPK | 0.944 | 0.006 | 0.090 | −2.770 | 88 | 0.47 | <0.01 | |
| OM | 1.080 | 0.056 | 0.119 | −5.470 | 88 | 0.51 | <0.01 | |
| OMNPK | 0.865 | 0.032 | 0.101 | −3.440 | 88 | 0.49 | <0.01 | |
| CRNPK | 1.000 | 0.055 | 0.134 | −6.451 | 88 | 0.51 | <0.01 | |
| All 2 | 0.835 | 0.042 | 0.099 | −4.100 | 440 | 0.44 | <0.01 | |
| CK | NPK | OM | OMNPK | CRNPK | |
|---|---|---|---|---|---|
| Winter wheat 1 | |||||
| Crop yield | 1.02 [0.21] c | 4.71 [0.44] ab | 3.89 [0.55] b | 4.17 [0.51] ab | 5.28 [0.85] a |
| Biomass | 3.01 [0.62] c | 9.48 [0.07] ab | 8.47 [0.62] b | 8.78 [1.08] b | 10.7 [1.07] a |
| N uptake | 29.7 [6.2] c | 115 [6.5] ab | 98 [10.9] b | 103 [12.7] ab | 129 [17.4] a |
| N surplus | 29.4 [6.5] a | 46.0 [10.9] a | 40.5 [12.7] a | 15.5 [17.4] a | |
| NUE | 65 [5] a | 53 [8] a | 57 [10] a | 76 [13] a | |
| Summer maize 1 | |||||
| Crop yield | 2.14 [0.17] b | 6.77 [0.45] a | 6.14 [1.41] a | 6.43 [1.24] a | 7.98 [1.85] a |
| Biomass | 4.69 [0.25] b | 11.0 [0.93] a | 10.5 [2.00] a | 11.6 [1.58] a | 13.2 [2.60] a |
| N uptake | 46.0 [2.9] b | 123 [1.0] a | 115 [23.8] a | 123 [20.2] a | 145 [28.1] a |
| N surplus | 46.4 [1.0] a | 54.0 [23.8] a | 46.1 [20.2] a | 23.9 [28.1] a | |
| NUE | 51 [1] a | 46 [16] a | 51 [13] a | 66 [19] a | |
| Annual 1 | |||||
| Crop yield | 3.16 [0.39] b | 11.5 [0.90] a | 10.0 [1.96] a | 10.6 [1.75] a | 13.3 [2.71] a |
| Biomass | 7.70 [0.87] b | 20.4 [1.00] a | 19.0 [2.62] a | 20.4 [2.65] a | 23.9 [3.67] a |
| N uptake | 76 [9.1] b | 237 [7.5] a | 213 [34.6] a | 226 [32.8] a | 274 [45.5] a |
| N surplus | 75.7 [7.5] a | 100 [34.6] a | 86.7 [32.8] a | 39.3 [45.5] a | |
| NUE | 58 [3] a | 49 [12] a | 54 [12] a | 71 [16] a | |
| CK | NPK | OM | OMNPK | CRNPK | |
|---|---|---|---|---|---|
| Winter wheat 1 | |||||
| N2O | 0.18 [0.03] d | 0.59 [0.09] c | 2.76 [0.21] a | 1.28 [0.02] b | 0.65 [0.09] c |
| 0.32 [0.07] c | 1.98 [0.16] a | 0.85 [0.01] b | 0.36 [0.07] c | ||
| NO | 0.01 [0.00] d | 0.04 [0.01] c | 0.27 [0.04] a | 0.10 [0.01] b | 0.06 [0.02] bc |
| 0.02 [0.01] c | 0.20 [0.03] a | 0.07 [0.01] b | 0.04 [0.01] bc | ||
| N2O+NO | 0.19 [0.03] d | 0.63 [0.08] c | 3.01 [0.24] a | 1.38 [0.03] b | 0.71 [0.11] c |
| 0.34 [0.06] c | 2.17 [0.19] a | 0.92 [0.02] b | 0.40 [0.08] c | ||
| Summer maize 1 | |||||
| N2O | 0.22 [0.04] b | 0.62 [0.10] b | 3.75 [1.82] a | 2.11 [0.26] b | 1.14 [0.09] b |
| 0.27 [0.07] b | 2.36 [1.21] a | 1.26 [0.17] ab | 0.62 [0.06] b | ||
| NO | 0.01 [0.00] b | 0.20 [0.09] ab | 0.39 [0.23] a | 0.19 [0.10] ab | 0.07 [0.02] b |
| 0.12 [0.06] a | 0.25 [0.15] a | 0.12 [0.07] a | 0.04 [0.01] a | ||
| N2O+NO | 0.23 [0.04] c | 0.82 [0.18] bc | 4.15 [1.67] a | 2.29 [0.34] b | 1.21 [0.11] bc |
| 0.39 [0.12] b | 2.61 [1.12] a | 1.38 [0.22] b | 0.66 [0.07] b | ||
| Annual 1 | |||||
| N2O | 0.40 [0.02] c | 1.21 [0.13] c | 6.51 [2.01] a | 3.39 [0.27] b | 1.79 [0.12] bc |
| 0.29 [0.05] b | 2.18 [0.72] a | 1.07 [0.10] b | 0.50 [0.04] b | ||
| NO | 0.02 [0.00] b | 0.24 [0.10] b | 0.66 [0.19] a | 0.28 [0.11] b | 0.14 [0.03] b |
| 0.08 [0.03] b | 0.23 [0.07] a | 0.09 [0.04] b | 0.04 [0.01] b | ||
| N2O+NO | 0.42 [0.02] c | 1.45 [0.17] c | 7.15 [1.89] a | 3.67 [0.36] b | 1.93 [0.14] c |
| 0.37 [0.06] b | 2.41 [0.68] a | 1.16 [0.13] b | 0.54 [0.05] b | ||
| CK | NPK | OM | OMNPK | CRNPK | |
|---|---|---|---|---|---|
| Winter wheat 1 | |||||
| N2O | 178 [29] c | 125 [19] c | 709 [53] a | 308 [4] b | 123 [17] c |
| NO | 8 [4] c | 8 [2] c | 68 [10] a | 24 [2] b | 12 [3] c |
| N2O+NO | 186 [28] c | 134 [17] c | 773 [62] a | 332 [6] b | 135 [20] c |
| Summer maize 1 | |||||
| N2O | 101 [18] b | 92 [15] b | 470 [227] a | 327 [40] ab | 186 [15] b |
| NO | 5 [2] b | 29 [13] ab | 49 [29] a | 29 [16] ab | 12 [3] ab |
| N2O+NO | 107 [19] c | 121 [26] c | 519 [210] a | 356 [52] ab | 198 [18] bc |
| Annual 1 | |||||
| N2O | 128 [6] c | 106 [12] c | 491 [152] a | 320 [26] b | 178 [12] c |
| NO | 6 [1] b | 21 [8] b | 50 [14] a | 27 [10] b | 14 [3] b |
| N2O+NO | 132 [7] c | 126 [15] c | 539 [143] a | 347 [34] b | 192 [14] c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, L.; Zhang, S.; Yao, Z.; Zhou, M.; Zhu, B. Organic–Inorganic Fertilization Sustains Crop Yields While Mitigating N2O and NO Emissions in Subtropical Wheat–Maize Systems. Agriculture 2025, 15, 1108. https://doi.org/10.3390/agriculture15101108
Liu Y, Hu L, Zhang S, Yao Z, Zhou M, Zhu B. Organic–Inorganic Fertilization Sustains Crop Yields While Mitigating N2O and NO Emissions in Subtropical Wheat–Maize Systems. Agriculture. 2025; 15(10):1108. https://doi.org/10.3390/agriculture15101108
Chicago/Turabian StyleLiu, Yan, Lei Hu, Shihang Zhang, Zhisheng Yao, Minghua Zhou, and Bo Zhu. 2025. "Organic–Inorganic Fertilization Sustains Crop Yields While Mitigating N2O and NO Emissions in Subtropical Wheat–Maize Systems" Agriculture 15, no. 10: 1108. https://doi.org/10.3390/agriculture15101108
APA StyleLiu, Y., Hu, L., Zhang, S., Yao, Z., Zhou, M., & Zhu, B. (2025). Organic–Inorganic Fertilization Sustains Crop Yields While Mitigating N2O and NO Emissions in Subtropical Wheat–Maize Systems. Agriculture, 15(10), 1108. https://doi.org/10.3390/agriculture15101108










